Abstract
Objective
To investigate the potential of Mindfulness-Based Stress Reduction (MBSR) as a treatment for chronic primary insomnia.
Design
Randomized controlled trial.
Setting
University health center.
Patients
30 adults with primary chronic insomnia based on DSM-IV-TR criteria were randomized 2:1 to MBSR or pharmacotherapy (PCT).
Interventions
MBSR, a program of mindfulness meditation training consisting of 8 weekly 2.5 hour classes and a day-long retreat, with ongoing home meditation practice expectations during 3 month follow-up; PCT, consisting of 3 mg of eszopiclone (LUNESTA™) nightly for 8 weeks, followed by 3 months of use as needed. A 10-minute sleep hygiene presentation was included in both interventions.
Main Outcomes
The Insomnia Severity Index (ISI), Pittsburgh Sleep Quality Index (PSQI), sleep diaries and wrist actigraphy collected pre-treatment, post-treatment (8 weeks), and at 5 months (self-reports only).
Results
Between baseline and 8-weeks, sleep onset latency measured by actigraphy decreased 8.9 minutes in the MBSR arm (P<.05). Large, significant improvements were found on the ISI, PSQI, and diary-measured total sleep time, sleep onset latency and sleep efficiency (Ps<.01, all) from baseline to 5 month follow-up in the MBSR arm. Changes of comparable magnitude were found in the PCT arm. 27 out of 30 patients completed their assigned treatment. This study provides initial evidence for the efficacy of MBSR as a viable treatment for chronic insomnia as measured by: sleep diary, actigraphy, well-validated sleep scales and measures of remission and clinical recovery.
Keywords: Chronic primary insomnia, mindfulness, meditation, sleep latency
Introduction
It is estimated that close to 1 in 10 adults in the United States has chronic insomnia, with higher rates among women, older adults and clinical populations.1-2 Chronic insomnia is associated with many co-morbidities, increases risks for depression, hypertension and heart disease, and has a pervasive, negative impact on individuals' quality of life.3-7 Insomnia also has a profoundly negative impact on the economy through lost productivity and increased health care costs.8-9 Although both cognitive behavioral therapy and pharmacotherapy have been shown to improve sleep outcomes, most people do not obtain effective insomnia treatment.3, 8, 10 Complementary and alternative medicine (CAM) approaches have the potential to reduce, at least in part, the gap between the prevalence of chronic insomnia and the number of patients effectively treated. In 2007, 1.4% of adults in the United States reported using CAM approaches to treat insomnia or difficulty sleeping.11 However, no CAM treatments have conclusively established efficacy and safety for treatment of chronic insomnia.1 Mindfulness-Based Stress Reduction (MBSR) is a mind-body based CAM intervention that had been identified as a potential therapy for insomnia, but lacks evidence from adequately powered and controlled clinical trials.12
MBSR and Sleep
The MBSR program was developed to facilitate adaptation to the stresses of living with chronic illness.13 The MBSR program teaches participants to learn how to focus their attention through a variety of meditative techniques. Participants are trained to perceive their immediate emotional and physical state, including pain or discomfort, and to let thoughts come and go in awareness with no attempt to change, suppress or elaborate on thoughts. Through mindfulness training, participants learn to view their thoughts as mental events and not facts. In this way, participants become exposed to the positive and negative content of their thoughts, and do not get absorbed in thought, caught up in planning for the future or worrying about the past. The practice of mindfulness throughout the day is posited to enable one to skillfully respond to stressors with appropriate actions, as opposed to reacting “on automatic pilot” with conditioned responses that can be emotionally arousing or harmful. By “breaking up” cycles of rumination and worry, mindfulness is hypothesized to reduce “verbal over-regulation” and facilitate the dis-engagement necessary to fall asleep.14
Mindfulness programs have been found to improve sleep quality in longitudinal studies of patients with medical or psychiatric illnesses.12 Patients with cancer15 and recipients of organ transplants16-17 reported significantly improved Pittsburgh Sleep Quality Index (PSQI) scores after attending an MBSR program, with medium to large effect sizes for MBSR (d =.60 to .65). Among the transplant recipients, findings supported a dose-response relationship between home meditation practice and sleep quality – more practice was associated with a greater decline in PSQI scores (correlation = -.47, p=.04).16-17 MBSR was used by Bootzin and colleagues in a multi-component intervention to improve sleep and reduce substance abuse recidivism among adolescents.18 Improvements in several sleep parameters measured by diary were found, and actigraphy results were marginally significant (p=.06) for improved total sleep time. Two studies of Mindfulness-based Cognitive Therapy (MBCT), a depression-relapse prevention program modeled after MBSR that shares its mindfulness training techniques, reported improved sleep outcomes for patients with mood and anxiety disorders.19 20 The impact of MBSR on patients with primary sleep disorders has not been investigated.
Research Objective
The objective of this study was to determine if MBSR could achieve clinically meaningful reductions in insomnia severity in patients with primary chronic insomnia. This trial was designed to be a pilot for a future full-scale comparative effectiveness trial of MBSR as a treatment for chronic insomnia. The main research questions for this pilot were:
Will MBSR impact sleep quality and quantity, as measured by sleep diary, actigraphy and standardized scales at 8 weeks, and if so, are impacts sustained at 5 month follow-up?
Are the magnitudes of MBSR's effects on sleep outcomes generally comparable to the impact of nightly use of an FDA-approved sedative hypnotic?
Will MBSR show benefit to health-related quality of life and reduce activity impairment among chronic insomnia patients?
Are relationships between sleep outcomes and meditation practice time consistent with a dose-response relationship?
Methods
Study Design and Sample Size
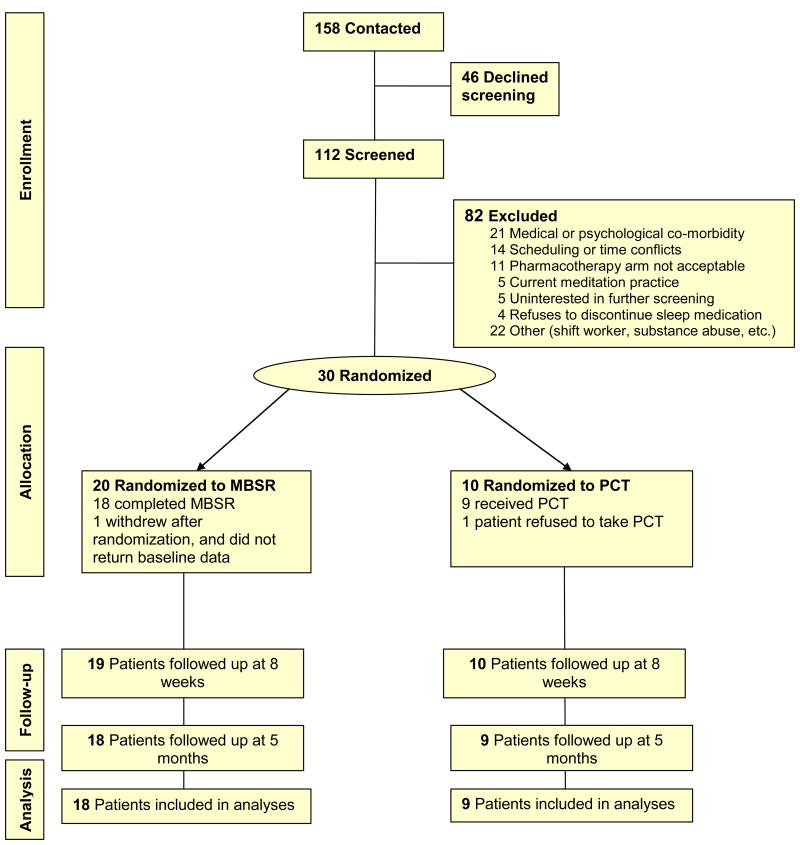
This was a 2-group randomized, controlled pilot study. Patients were stratified by gender, and then randomized 2:1 to MBSR (n=20) or pharmacotherapy (PCT, n=10) using computer-generated randomization schedules with small randomly permuted blocks to ensure balance within strata and across study arms. (See Figure 1) The study coordinator was notified by email of random assignments after baseline data collection, and then the coordinator notified the patient and other study personnel. Outcomes were measured at 3 time points: pre-randomization baseline; end of the active intervention period; and 5 month follow-up. The pilot sample was chosen to be sufficient to detect moderate to large treatment effects within the MBSR group (effect size = 0.68; assuming n= 19 due to 10% dropouts, a pre-post correlation = 0.50, 80% power, and two-sided .05 alpha).21 Based on the pooled baseline standard deviations, this effect size corresponds to 2.3, 1.7, 6.3 and 4.2 points on the Insomnia Severity Index, Pittsburgh Sleep Quality Index, SF-12 Physical and Mental Health Component Summaries, respectively or 18.9 minutes for sleep latency measured by sleep diary.
FIGURE 1. Participant Flow Diagram.
The flow of participants through the study, from enrollment to follow-up is detailed by treatment group. Patients included in the outcome analyses are those who completed Mindfulness-based Stress Reduction (MBSR) or were treated with PCT (eszopiclone).
The study protocol was approved by the institutional review panels of the University of Minnesota and the Hennepin County Medical Center, site of the Minnesota Regional Sleep Disorders Center, and all patients completed written informed consent.
Patients
Patients were recruited between July, 2007 and September, 2008 through newspapers, Internet and radio advertisements, and by clinician referral. Inclusion criteria were age 18 to 65, ability to read and speak English, and a diagnosis of primary chronic insomnia. Chronic insomnia was defined as difficulty initiating or maintaining sleep despite adequate opportunity for sleep, with related daytime dysfunction on 3 or more nights a week for the past 6 months or longer, consistent with the DSM-IV-TR (307.42)22 and International Classification of Sleep Disorders (ICSD-2).23 A 4-part screening protocol applied consensus diagnostic criteria for primary insomnia.24 Telephone screening was followed by a structured psychiatric interview (SCID-IV),25 completion of a screening sleep diary, and a history and physical examination conducted by a sleep physician.24, 26-27 Persons with medical conditions, mental disorders, or different sleep disorders suspected of being directly related to the insomnia were excluded. Those using prescription or non-prescription sleep aids prior to enrollment could be included if willing to discontinue use for the duration of the study. Patients (n=20) who reported use of prescription or over-the-counter sleep aids at the first stage of the screening process were required to discontinue use prior to continuation in screening. Washout periods varied, but the minimum period of discontinuation prior to baseline sleep data collection was 10 days. Of the 158 persons who initially contacted the study, 46 declined to be screened (Figure 1). Eighty-two persons were excluded: 69 during telephone screening; and 13 following the SCID or sleep physician examination. Among the exclusions were 11 people who would not accept the possibility of being randomized to pharmacotherapy.
Interventions
MBSR was provided through the Center for Spirituality and Healing at the University of Minnesota, and conducted using the standard format of 8 weekly, 2.5 hours classes, and a day-long retreat.28 Meditation techniques included the body-scan, standing, sitting and walking meditations, and gentle Hatha yoga. A 6-hour silent retreat was held on the weekend between weeks 6 and 7. Home practice expectations were 45 minutes of meditation per day at least 6 days a week for 8 weeks, followed by 20 minutes per day for 3 months. The MBSR teacher completed basic and advanced MBSR instructor training at the Center for Mindfulness in Medicine, Health Care and Society (www.umassmed.edu/cfm), and followed the class-by-class outline of activities, readings and homework assignments in the 2003 MBSR instructor's manual.29 Whereas readings on Sleep and Sleep Stress from Full Catastrophe Living13 are optional in the standard MBSR program, for this study those readings were assigned for week 2. To promote treatment fidelity, study staff distributed the assignments and class handouts in a workbook developed by the Center for Spirituality and Healing at the University of Minnesota (www.csh.umn.edu/). Participants were given audio recordings of guided meditations for home use.
Pharmacotherapy was selected as the control for this study because it is the most readily available, proven treatment for chronic insomnia, and the extensive testing required for FDA approval supports the reproducibility of efficacious impact when delivered in a standard dose. Our PCT arm was modeled on clinical practice. It is standard and common practice to prescribe ezopiclone on a nightly basis for a number of months during the initial management of chronic primary insomnia. The PCT treatment consisted of 3mg of eszopiclone nightly for 8 weeks, followed by use as needed for 3 months. Patients initially met in a small group with the sleep physician who gave instructions for properly taking medication and explained potential side effects. Patients were then given their first 30 day supply of 3mg eszopiclone tablets in a bottle with a Medication Event Monitoring System cap (MEMS Cap®). Refills were mailed. Patients were instructed to pour refill tablets into the MEMS® capped bottle when taking their next pill. The MEMS® cap records a date/time stamp when opened, but provides no feedback to the patient. Patients were told that the MEMS® cap records adherence.
Adherence was also tracked electronically in the MBSR arm. Participants were instructed to turn on a logger when they began a meditation or yoga session, and to switch the logger off at the end of the session. The logger, a pocket-sized, battery-operated recording device (HOBO ® data logger, Onset Computer Corporation, Pocasset, MA), stores a date/time stamp whenever it is switched on or off, and provides no feedback to the patient. Patients were told that the logger records practice time.
A 10-minute presentation about sleep hygiene was given at the first MBSR class and at the PCT group meeting with the sleep physician. Sleep hygiene booklets developed at the National Institutes of Health30 were distributed to all patients in order to standardize knowledge of sleep hygiene across treatment arms. Adverse events were monitored in both treatment arms. Monitoring calls were made weekly during the first 8 weeks, tapered to bi-weekly in month 3, and once in months 4 and 5. A call one month after study completion monitored for problems with drug discontinuation (PCT arm only). In the MBSR arm, monitoring calls also promoted regular meditation practice. The monitoring calls were made by study research staff, not the MBSR teacher or sleep physician. In cases where adverse events were reported in the PCT arm, the sleep physician was consulted. No adverse events were reported in the MBSR arm.
Measures
Sleep diaries, wrist actigraphy and self-report questionnaires were used to measure outcomes. Participants kept a standard sleep diary and wore a wrist actigraph on their non-dominant wrist for 14 days prior to the start of the study interventions, during the final two weeks of the active intervention period, and for two weeks during month 5 (sleep diary only). The sleep diary variables are averages at each interval and include: total sleep time (TST), sleep onset latency (SOL), wake after sleep onset (WASO), and sleep efficiency (SE, time asleep divided by time in bed). These variables were also objectively estimated using actigraphy recordings (Actiwatch® AW64 analyzed by Actiware Sleep v.3.4 software, Mini-Mitter Company, Inc., a Philips-Respironics Company, Bend, Oregon).26 Benchmarks for “poor sleep” are SOL or WASO of 30 minutes or more, TST less than 6 hours and SE under 85%.
Standardized measures of sleep and health-related quality of life were reported in questionnaires completed at home and returned by mail at pre-randomization baseline, 8-weeks and 5-months. The 7-item Insomnia Severity Index (ISI) assesses the current severity of insomnia symptoms, sleep dissatisfaction, daytime impacts and distress about sleep difficulties.31 Each ISI item is rated on a 5-point scale, with total scores ranging from 0 to 28. ISI clinical cut-points are defined as follows: no insomnia (0-7); sub-threshold insomnia (8-14); moderate insomnia (15-21); and severe insomnia (22-28). Response to treatment on the ISI has been defined as a change of 7 or more points from baseline, and remission as reduction to a score less than 8.32
The Pittsburgh Sleep Quality Index (PSQI) measures sleep quality and quantity, based on recall of sleep behaviors in the past month.33-34 The 19 PSQI questions measure 7 domains of sleep: quality, latency, duration, efficiency, disturbances, use of sleep medications, and daytime dysfunction. Domain scores are coded from 0 to 3, and an overall PSQI score is obtained by summing across domains. The overall PSQI score can range from 0 to 21, 3 points constitutes a meaningful change, and a score greater than 5 has been proposed to identify poor sleepers.
The Dysfunctional Beliefs and Attitudes about Sleep (DBAS-16) measures perceived consequences of insomnia, helplessness and worry about sleep, sleep expectations, and physical and emotional attributions that are hypothesized to perpetuate insomnia.27, 35 Sixteen statements are rated on a 0 to 10 scale, and averaged, with higher scores indicating more dysfunction. The Sleep Self-Efficacy Scale (SSES) consists of 9 items which measure confidence in perceived ability to engage in behaviors that influence sleep.36 Item responses were scored on a 0 to 10 scale and then averaged, with higher scores indicating greater confidence.37
Secondary outcomes include widely used, well-validated measures of anxiety, depression, health-related quality of life, and activity limitation. The 20-item State-Trait Anxiety Inventory - state version (STAI) was used to measure anxiety at the present time.38 Scores range from 20 to 80, and cut-points have been proposed for mild (<40), moderate (40 - 59), and severe (≥ 60) anxiety symptoms. Depression symptom intensity was measured by the 20 item Center for Epidemiologic Studies Depression Scale (CES-D). CES-D scores range from 0 to 60, and a score of 16 or higher has been proposed to identify clinically meaningful depression symptoms.39
Health-related quality of life was measured by mental and physical component summary scores (MCS and PCS) of the Short Form-12, version 2 (SF-12).40 The SF-12 items measure the 8 aspects of health and well-being that form the SF-36 health profile: physical functioning, role limitations due to physical health, bodily pain, general health perceptions, energy/fatigue, social functioning, role limitations due to emotional health, and mental health (psychological distress and emotional well-being). The SF-12 MCS and PCS are unbiased, close approximations to their SF-36 counterparts.41 Activity impairment was assessed by a question that asks “In the last 7 days, to what extent did insomnia affect your ability to carry out normal daily activities, not including your job?”, with responses marked on an 11-point scale, with 0= Insomnia did not affect my daily activities, to 10= Insomnia did not permit me to do daily activities at all.42 It is expressed as an impairment percentage, with higher scores indicating more impairment.
Statistical Methods
The balance between treatment groups on baseline variables was examined using descriptive statistics, Fisher's Exact tests and Mann-Whitney U tests. Changes from baseline to 8 weeks and to 5 months were tested within treatment groups using paired t-tests, or by Wilcoxon tests when normality could not be assumed. 43 Cohen's d effect sizes were estimated by the difference between follow-up and baseline values divided by the pooled standard deviation at baseline.21 Cohen's criteria for small, medium, and large treatment effects are .2, .5 and .8, respectively.44 Repeated measures ANOVAs were conducted to compare outcomes between groups over time, although only very large differences would be detectable in this pilot study. Clinical importance was estimated by examining “percent recovered” in conjunction with using the Reliable Change Index (RCI).45 The RCI requires that a patient's outcome score moves from the abnormal to the normal range, and moreover, that the amount of change exceeds measurement error, based on the reliability of the outcome scale.
For all efficacy analyses, 3 patients were omitted: 1 patient randomized to PCT who refused to take the study medication; 1 patient who withdrew after randomization to MBSR, failing to return baseline data and without attending any intervention sessions; and 1 patient who attended only 4 MBSR classes (attendance at 5 or more classes is the benchmark for MBSR course completion46). Sample sizes are indicated for each analysis; missing data were not imputed. Adherence and treatment satisfaction were summarized using descriptive statistics. Meditation practice time was correlated with change in primary outcomes using Spearman's rho correlations. Descriptive statistics were obtained using SPSS 17.0 (SPSS Inc., Chicago, IL). All other statistical analyses were conducted using SAS 9.1 (SAS Institute, Cary, NC).
Results
The sample consisted of 22 women and 8 men, who ranged in age from 19 to 65 years old and were well-educated. (Table 1) The majority (80%) were working full or part-time. History of insomnia complaints ranged from 1 to 30 years, with a median duration of 6 years. At screening, two-thirds of the patients were using sleep medications, either prescription or over-the-counter, and 20% had previously tried eszopiclone. Medical co-morbidities were common, with almost half the sample (46%) reporting two or more. Baseline characteristics were reasonably balanced across study arms, however the number of patients who reported a history of depression or an anxiety disorder was somewhat higher in the MBSR arm.
Table 1. Participant Characteristics at Randomization.
|
MBSR (n = 20) 1 |
PCT (n = 10) |
P valuea | |
|---|---|---|---|
| Age in years, median (range) | 47 (21-65) | 53.5 (29-59) | 0.07 |
| Female n (%) | 15 (75) | 7 (70) | 0.77 |
| Race n (%) | |||
| White | 20 (100) | 9 (90) | 0.33 |
| Black/African American | 0 (0) | 1 (10) | |
| Hispanic/Latino n (%) | 1 (5) | 1 (10) | 0.56 |
| Married n (%) | 11 (55) | 8 (80) | 0.18 |
| College degree n (%) | 18 (90) | 6 (60) | 0.14 |
| Employment n (%) | |||
| Full-time or part-time | 12 (75) | 9(90) | 0.63 |
| Other (retired, homemaker, student, etc.) | 5 (25) | 1 (10) | |
| No. of medical co-morbiditiesb n (%) | |||
| None | 5 (25) | 2 (20) | 0.72 |
| 1 | 4 (20) | 5 (50) | |
| ≥ 2 | 11 (55) | 3 (30) | |
| Insomnia duration in years, median (range) | 5 (1-22) | 9 (1-30) | 0.16 |
| Taking medication for sleep at start of screening n (%) | 15 (75) | 5 (56) c | 0.40 |
| Prior use of eszopiclone (LUNESTA™)n (%) | 4 (20) | 2 (20) | 0.69 |
| History of depression or anxiety disorder (diagnosed) n (%) | 9(47) | 1 (10) | 0.046 |
| Current use of medication for depression n (%) | 3 (16) c | 0 (0) | 0.53 |
Abbreviations: MBSR, Mindfulness-based Stress Reduction; PCT, Pharmacotherapy
P values from Fisher's Exact test or Mann-Whitney U test
Medical co-morbidities were recorded as pulmonary, cardiovascular, neurologic, gastrointestinal, endocrine, renal, hematologic, cancer, musculoskeletal or pain, or head, eyes, ears or throat.
One unknown
Sleep diaries completed for 14-days prior to randomization confirmed poor sleep: mean SOL = 44.5 minutes; mean WASO= 55.5 minutes; mean TST = 6.3 hours; and mean SE = 75.3%. Actigraphy-derived averages for this period were similar to the diary values for TST (mean 6.4 hours), WASO (mean 58.5 minutes) and SE (77%), and somewhat shorter than the diary values for SOL (means 34.6 vs. 44.5 minutes, Mann-Whitney U test, P=.05). Self-report scales also confirmed sleep problems at baseline. The average score on the ISI was consistent with moderate insomnia (mean =17.1, SD = 3.34, ISI range of 12 to 24), and all patients scored above 7, the cutoff for no insomnia. All participants were in the PSQI “poor sleep” range at baseline and the average PSQI score was 11.6 (SD=2.53, range from 8 to 18). No significant differences were found at baseline on actigraphy-derived sleep parameters or on the standardized sleep scales between treatment groups, however, based on diary reports, WASO was perceived to be somewhat shorter (46.6 vs. 72.6 minutes, P =0.11) and SE somewhat higher (77.3% vs. 71.7%, P = 0.07) in the group randomized to MBSR than in the PCT arm.
No statistically significant differences were found between MBSR and PCT groups over time on sleep or quality of life outcomes, based on repeated measures ANOVAs. Lack of difference does not confirm equivalence, however, as the sample size was not sufficient to establish the non-inferiority of MBSR to PCT for sleep treatment. Consistent with the pilot objectives, changes within each group are described below.
Sleep Diary Outcomes
Sleep diaries were completed every morning for 2 weeks at 3 time points: pre-treatment baseline, the end of the active intervention period, and the end of follow-up. Sleep diary results are shown in Table 2. In the MBSR arm, diary-based SOL, WASO, and SE significantly improved from baseline to the end of the active intervention period, providing medium-sized treatment effects (ds = -.57, -.46, and .61, respectively; Ps<.01, all). A treatment impact of MBSR on TST emerged by 5-month follow-up. Whereas the impact on WASO appeared to wane, it is notable that other treatment effects in the MBSR arm increased with longer follow-up; medium to large treatment effects for TST, SOL and SE (ds = .69, .79, and -.80, respectively; Ps<.01, all) by 5-months. In the PCT arm, diary-based TST, WASO, and SE significantly improved from baseline to the end of the active intervention period, and these improvements were sustained, with large treatment effects from baseline to 5 months (ds = .92, -.97, and 1.29, respectively). While not statistically significant, there is a suggestion that MBSR may impact SOL more than PCT (mean change at follow-up: -22.2 vs. -4.65 minutes for MBSR vs. PCT).
Table 2. Sleep Diary Parameters by Treatment Group and Time.
| Baseline | Baseline to end of active treatmenta | Baseline to 5 - month follow-upb | |||||
|---|---|---|---|---|---|---|---|
| Treatment Group | N | Mean ± SD | Change (95% CI) | d | Change (95% CI) | d | |
| TST (hrs) | MBSR | 17 | 6.31 ± 0.72 | 0.20 (-0.22, 0.63) | 0.25 | 0.56 (0.17, 0.95) | 0.69** |
| PCT | 9 | 6.18 ± 0.92 | 0.60 (0.16, 1.04) | 0.74** | 0.75 (0.04, 1.46) | 0.92* | |
| SOL (min) | MBSR | 17 | 47.52 ± 30.70 | -15.85 (-25.01, -6.70) | -0.57** | -22.2 (-33.33, - 11.10) | -0.80***c |
| PCT | 9 | 38.81 ± 21.74 | -10.03 (-20.47, 0.42) | -0.36 | -4.64 (-42.60, 33.33) | -0.16 | |
| WASO (min) | MBSR | 17 | 46.62 ± 21.35 | -14.80 (-23.15, -6.45) | -0.46** | -7.46 (-17.67, 2.74) | -0.23 |
| PCT | 9 | 72.16 ± 42.49 | -39.66 (-68.11, - 11.22) | -1.24** | -30.91 (-74.08, 12.25) | -0.97 | |
| SE (%) | MBSR | 17 | 77.29 ± 7.88 | 5.27 (1.61, 8.93) | 0.61** | 6.77 (2.53, 11.01) | 0.79** |
| PCT | 9 | 71.66 ± 9.07 | 9.57 (3.57, 15.57) | 1.11** | 11.12 (2.91, 19.34) | 1.29* | |
Abbreviations: MBSR, Mindfulness-based Stress Reduction; PCT, Pharmacotherapy; d= Cohen's d, an effect size; CI=Confidence Interval; TST=total sleep time; SOL=sleep onset latency; WASOwake after sleep onset; SE=sleep efficiency.
Sample excludes 3 patients: 1 refused to take study medication, 1 refused to attend MBSR, 1 completed fewer than 5 MBSR classes.
From baseline to the final 2 weeks of the active intervention period of weekly classes/nightly dosing (MBSR n=16, PCT n=9).
From baseline to the final 2 weeks of the 5 month follow-up period (MBSR n=16, PCT n=6).
P values based on Wilcoxon signed ranks test.
P<.05;
P<.01;
P<.001
Actigraphy Outcomes
Actigraphs were worn at two points, in time in tandem with completion of the sleep diaries at pre-randomization, and the end of the active intervention period. Actigraphy results are shown in Table 3. A statistically significant 8.9 minute decrease in actigraphy-derived SOL was found within the MBSR arm (d = -.31, P = .04), consistent with the perceptions of shorter times to fall asleep that these patients recorded in their diaries. Other actigraphy changes in the MBSR arm were small (ds < |.22|) and not statistically significant. In the PCT arm, however, actigraphy-derived TST and SE parameters significantly improved (ds = .63 and .52, respectively; Ps<.05, both), and while not statistically significant, the average WASO decreased 14.8 minutes.
Table 3. Actigraphy Sleep Parameters by Treatment Group and Time.
| Treatment Group | N | Baseline | End of active treatmenta | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | N | Mean ± SD | Changeb (95% CI) | d | |||
| TST (hrs) | MBSR | 18 | 6.34 ± 0.60 | 16 | 6.21 ± 0.82 | -0.12 (-0.44, 0.20) | -0.21 |
| PCT | 9 | 6.40 ± 0.60 | 8 | 6.95 ± 0.65 | 0.37 (0.03, 0.71) | 0.63* | |
| SOL (min) | MBSR | 18 | 34.25 ± 28.34 | 16 | 25.61 ± 19.96 | -8.88 (-19.45, 1.69) | -0.31* c |
| PCT | 9 | 35.17 ± 31.67 | 8 | 17.37 ± 9.43 | -7.98 (-19.43, 3.46) | -0.28 | |
| WASO (min) | MBSR | 18 | 57.17 ± 24.76 | 16 | 54.44 ± 25.10 | -2.79 (-10.68, 5.09) | -0.10 |
| PCT | 9 | 61.16 ± 38.33 | 8 | 47.72 ± 32.72 | -14.83 (-44.59, 14.93) | -0.51c | |
| SE (%) | MBSR | 18 | 77.75 ± 7.26 | 16 | 78.49 ± 8.98 | 0.88 (-2.34, 4.11) | 0.10 |
| PCT | 9 | 75.49 ± 12.53 | 8 | 83.50 ± 6.40 | 4.81 (2.18, 7.44) | 0.52** | |
Abbreviations: MBSR, Mindfulness-based Stress Reduction; PCT, Pharmacotherapy; d= Cohen's d, an effect size; CI=confidence interval; TST=total sleep time; SOL=sleep onset latency; WASO=wake after sleep onset; SE=sleep efficiency.
Sample excludes 3 patients: 1 refused to take study medication, 1 refused to attend MBSR, 1 completed fewer than 5 MBSR classes.
From week 6 to week 8, the final 2 weeks of the active (weekly classes/nightly dosing) intervention period.
Discrepancies between means at each time point and change scores are due to missing data.
P value based on Wilcoxon signed ranks test.
P<.05;
P<.01;
P<.001
Standardized Sleep Scales and Quality of Life Measures
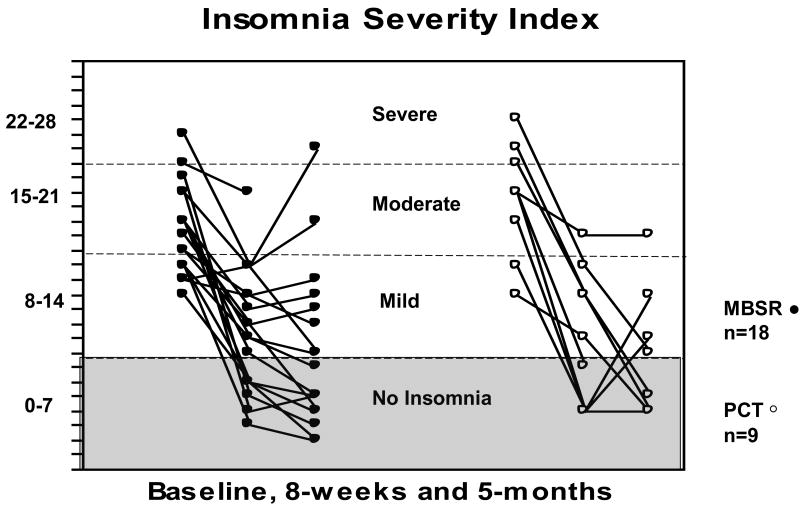
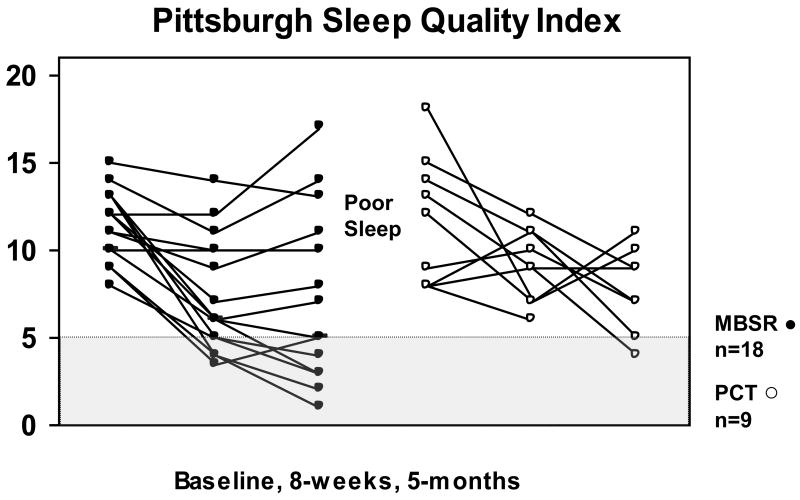
Standardized sleep scales are shown in Table 4. The MBSR group reported large, statistically significant improvements from baseline to follow-up on all self-reported sleep measures at 8-weeks and at 5-months (|ds| ranging from .8 to 2.4, Ps<0.01, all). Large treatment impacts were also noted in the PCT arm, although due to the smaller sample size, some results were not statistically significant. Figures 2 and 3 depict the individual trajectories of change for the ISI and PSQI by treatment group; reduced symptoms are visible by 8-weeks, and many patients reported continued improvement through the end of follow-up.
Table 4. Sleep Scales and Health-related Quality of Life by Treatment Group and Time.
| Treatment Group | Baseline | Baseline to 8-weeks | Baseline to 5-months | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Changea (95% CI) | d | Changeb (95% CI) | d | ||
| Standardized Sleep Scales | ||||||
| Insomnia Severity Index | MBSR | 16.44 ± 3.03 | -6.89 (-9.07, -4.71) | -2.03*** | -8.06 (-10.61, -5.50) | -2.38*** |
| PCT | 18.56 ± 3.78 | -9.44 (-12.59, -6.30) | -2.79** | -10.75 (-15.17, -6.33) | -3.18*** | |
| Pittsburgh Sleep Quality Index | MBSR | 11.50 ± 1.89 | -4.25 (-5.60, -2.90) | -1.68*** | -4.50 (-6.64, -2.36) | -1.78*** |
| PCT | 11.67 ± 3.64 | -2.56 (-5.73, 0.62) | -1.01 | -4.38 (-6.10, -2.65) | -1.73*** | |
| Sleep Self-Efficacy Scale | MBSR | 4.67 ± 1.75 | 2.19 (1.39, 2.98) | 1.19*** | 2.38 (1.47, 3.29) | 1.29*** |
| PCT | 4.02 ± 2.04 | 1.84 (1.03, 2.65) | 1.03** | 1.63 (-0.58, 3.83) | 0.88 | |
| Dysfunctional Beliefs about Sleep Scale | MBSR | 5.53 ± 1.49 | -1.05 (-1.75, -0.36) | -0.80** | -1.45 (-2.25, -0.65) | -1.03** |
| PCT | 5.22 ± 1.68 | -1.01 (-1.76, -0.25) | -0.69* | -1.45 (-2.97, 0.06) | -1.00 | |
| Health-related Quality of Life Measures | ||||||
| % Activity impairment | MBSR | 50.00 ± 24.73 | -29.41 (-41.67, -7.15) | -1.09*** | -21.67 (-35.12, -8.21) | -0.80** |
| PCT | 45.56 ± 32.45 | -20.00 (-46.35, 6.35) | -0.74 | -21.25 (-54.52, 12.02) | -0.79 | |
| State-trait Anxiety Inventory | MBSR | 33.94 ± 11.32 | -1.06 (-4.81, 2.69) | -0.09 | -3.89 (-9.70, 1.92) | -0.33 |
| PCT | 31.16 ± 12.69 | 0.18 (-5.39, 5.74) | 0.02 | -1.68 (-8.82, 5.47) | -0.14 | |
| Center for Epidem. Studies Depression Scale | MBSR | 10.86 ± 7.88 | -0.86 (-3.99, 2.27) | -0.09 | -2.42 (-6.26, 1.43) | -0.26 |
| PCT | 13.73 ± 12.15 | -3.62 (-6.73, -0.51) | -0.39* | -7.00 (-16.10, 2.10) | -0.75 | |
| SF-12 Mental Summary Score | MBSR | 45.13 ± 9.70 | 3.65 (-0.52, 7.81) | 0.39 | 4.60 (-0.54, 9.74) | 0.50 |
| PCT | 45.20 ± 8.83 | 3.11 (-3.28, 9.50) | 0.34 | 5.75 (-0.44, 11.94) | 0.62 | |
| SF-12 Physical Summary Score | MBSR | 52.89 ± 5.92 | -0.08 (-2.93, 2.76) | -0.01 | -2.17 (-5.17, 0.84) | -0.35 |
| PCT | 53.27 ± 7.04 | 0.65 (-4.70, 6.01) | 0.11 | -0.07 (-4.06, 3.91) | -0.01 | |
Abbreviations: MBSR, Mindfulness-based Stress Reduction; PCT, Pharmacotherapy; d= Cohen's d, an effect size; CI=Confidence Interval.
Sample sizes for baseline and for change to 8-weeks, MBSR n=18, PCT n=9.
Sample size for change to 5-months vary due to missing data, MBSR n=17-18, PCT n=7-8.
Sample excludes 3 patients: 1 refused to take study medication, 1 refused to attend MBSR, 1 completed fewer than 5 MBSR classes.
P<.05;
P<.01;
P<.001
FIGURE 2.
Scores on the Insomnia Severity Index are shown by patient and treatment arm, at baseline, 8-weeks and 5-months. Each line represents one patient. Patients in the MBSR arm are represented by solid dots, and patients in the PCT (eszopiclone) arm are represented by open dots. Criteria for severe, moderate, mild and no insomnia on the Insomnia Severity Index are marked by horizontal lines. The shaded area includes scores of 7 or lower, and corresponds to no insomnia on the Insomnia Severity Index.
FIGURE 3.
Scores on the Pittsburgh Sleep Quality Index are shown by patient and treatment arm, at baseline, 8-weeks and 5-months. Each line represents one patient. Patients in the MBSR arm are represented by solid dots, and patients in the PCT (eszopiclone) arm are represented by open dots. Scores of 5 or lower on the Pittsburgh Sleep Quality Index are in the shaded area. Scores that fall above the shaded area meet the criterion for poor sleep on the Pittsburgh Sleep Quality Index.
Health-related quality of life results are shown at the bottom of Table 4. Insomnia-related activity impairment was significantly reduced in the MBSR group at both 8-weeks and 5-months (Ps<0.01, ds= -1.1 and -.80). The impact on activity impairment in the PCT arm was similar, but not significant due to the smaller sample size. By 5-month follow-up, symptoms of anxiety and depression decreased and mental health scores increased in both groups, but these changes were not statistically significant. The improvement in mental health approached significance in both arms (Ps= .06 and .08) with mean changes of 4.6 to 5.8 points, exceeding accepted criteria for clinically important change.47
Recovery from Chronic Insomnia
All study participants met criteria for insomnia and poor sleep at baseline. Numbers of patients who experienced a reliable improvement and no longer met these criteria are shown in Table 5. By 5-months, over half the MBSR patients had recovered, based on the ISI and PSQI. Moreover, no patient experienced a clinically important deterioration in his or her insomnia, defined as an increase of 7 points on the ISI. Notably, about half the patients in the MBSR arm whose recorded sleep efficiency was below 85% at baseline had a reliable improvement by 5-month follow-up (7 of 13), and all but one of the MBSR patients whose total sleep time averaged less than 6 hours at baseline was reliably sleeping more than 6 hours per night at follow-up (4 of 5). Although not statistically significant, it is interesting to note that the rates of recovery from poor sleep, as measured by the PSQI, were considerably higher following MBSR than PCT at 8-weeks and at 5-months.
Table 5. Clinical Significance of Self-Reported Outcomes.
| Outcomes | Criteria for Normative Sleep | Cronbach alpha or test-retest reliabilitiesb | Treatment Group | Recovered based on the Reliable Change Indexa | |||
|---|---|---|---|---|---|---|---|
| 8 weeks | 5 months | ||||||
| % | n | % | n | ||||
| Insomnia Severity Index | 7 or lower | 0.77 | MBSR | 33% | 6 of 18 | 53% | 9 of 17 |
| PCT | 44% | 4 of 9 | 50% | 4 of 8 | |||
| Pittsburgh Sleep Quality Index | 5 or lower | 0.83 | MBSR | 28% | 5 of 18 | 56% | 10 of 18 |
| PCT | 0 | 0 of 9 | 25% | 2 of 8 | |||
| Total Sleep Time (diary) | Over 6 hrs | 0.86 | MBSR | 50% | 3 of 6 | 80% | 4 of 5 |
| PCT | 80% | 4 of 5 | 75% | 3 of 4 | |||
| Sleep Onset Latency (diary) | Less than 30 minutes | 0.85 | MBSR | 30% | 3 of 10 | 20% | 2 of 10 |
| PCT | 20% | 1 of 5 | 50% | 2 of 4 | |||
| Wake after Sleep Onset (diary) | Less than 30 minutes | 0.87 | MBSR | 36% | 4 of 11 | 18% | 2 of 11 |
| PCT | 43% | 3 of 7 | 40% | 2 of 5 | |||
| Sleep Efficiency (diary) | 85% or higher | 0.88 | MBSR | 38% | 5 of 13 | 54% | 7 of 13 |
| PCT | 25% | 2 of 8 | 33% | 2 of 6 | |||
Recovered is defined as not being in the normative range at baseline, having a reliably improved score at the end of treatment, and therefore moving to the normative range at follow-up. Reliable improvement is determined by exceeding the 95% confidence interval for an individual's score, based on the standard error of measurement, a function of the reliability of the scale or parameter.
Reliabilities for the Insomnia Severity Index and Pittsburgh Sleep Quality Index are Cronbach's alpha scores from the developers (33,35); reliabilities for the sleep diary parameters were test-retest correlations from Currie, Wilson and Curran (60).
Adherence and Treatment Satisfaction
The study was completed with 2 MBSR programs (n's = 9 and 11). Eighteen out of 20 patients (90%) attended at least 5 MBSR classes, meeting the benchmark for course completion. Ten MBSR patients attended all 8 classes, and 16 of 20 attended the all-day retreat. In their follow-up questionnaires, 14 of the 18 MBSR patients in the outcome analyses (78%) reported no use of sleep medications (prescription or over-the-counter); 2 reported using sleep medications less than once a week and 2 reported use once or twice a week.
Nine out of 10 patients randomized to PCT took their assigned medication. Based on analysis of the electronic pill monitors (MEMS Caps®), over the first 8 weeks, three patients were 100% compliant with nightly dosing; two had over 90% compliance; and four took eszopiclone 4 to 6 nights each week. By the final study month, two patients discontinued eszopiclone completely and these patients reported using no other sleep medications. Six PCT patients continued use of eszopiclone through the last month of follow-up: two maintained nightly dosing; three took a dose 4 to 6 nights a week; and one used eszopiclone an average of 2 nights a week. One patient did not submit medication use data at 5-months.
Meditation home practice time was calculated based on electronic loggers submitted by 17 patients who completed MBSR. The average home practice time was 23.7 (SD = 11.2) minutes per day during the 8 week active intervention period. This represents 61% of the home practice expectation during this period (e.g., 45 minutes per day, 6 days a week), however time spent meditating during MBSR classes would boost these averages. Sixteen MBSR patients reported practice throughout follow-up, with an average of 21.8 minutes of practice per day during months 3 thru 5, consistent with follow-up practice expectations of 20 minutes per day. The median number of days practiced in the final week of the study was 4 days. Reductions in Dysfunctional Beliefs about Sleep scores and Activity Limitation due to Insomnia from baseline to month 5 were significantly predicted by home practice during the active intervention period (Spearman's rho correlations = 0.62 and 0.71, respectively, Ps<.02). Correlations with changes in other measures were smaller and not significant.
Treatment satisfaction and treatment preferences were evaluated at 5-month follow-up. Treatment satisfaction was rated on a 0 to 10 point scale from very dissatisfied to very satisfied. The satisfaction scores in the MBSR group were high, with a mean of 8.8. The average satisfaction score in the PCT group was lower, with a mean of 6.1. Treatment preference was ascertained by recall, using the question, “When you joined this study which treatment did you hope to be assigned to?” In the MBSR arm, 14 indicated MBSR, 2 PCT, and 2 no preference. In the PCT arm, 5 indicated MBSR, 2 PCT, and 1 no preference. There were no unexpected, serious adverse events related to the interventions in this trial. One PCT patient was switched from eszopiclone to controlled-release zolpidem during the first month of treatment because of persistent complaints of an extremely unpleasant after-taste. Other side effects reported in the PCT arm included excessive sleepiness, headache and dizziness. No adverse events related to MBSR were reported.
Discussion
This study provides initial evidence for the efficacy of a complementary and alternative treatment modality, MBSR, as a viable treatment for chronic insomnia as measured by: sleep diary, well-validated sleep scales, actigraphy, and measures of remission and clinical recovery. Our results suggest that MBSR, when combined with a brief sleep hygiene presentation, is able to achieve reductions in insomnia symptoms and improvements in sleep quality comparable to regular use of an FDA-approved sedative hypnotic. To our knowledge, this is the first study to employ a rigorous randomized study design to compare the efficacy of MBSR to pharmacotherapy in patients with primary chronic insomnia.
Patients who completed 5 or more MBSR classes reported sleep diary changes that were large and clinically meaningful – total sleep time increased by over 30 minutes, sleep onset latency reduced by over 20 minutes and sleep efficiency increased to 84.5% by 5 month follow-up. Notably, a statistically significant reduction in actigraphy-derived sleep onset latency of about 9 minutes supported the patients' self-reports. Moreover, half of the patients randomized to MBSR met stringent criteria for recovery from insomnia at the end of the study, and average treatment satisfaction scores were high. Whereas patients in the PCT arm obtained similar benefits to sleep outcomes, their treatment satisfactions scores were not high, and several patients reported adverse events.
Impacts found following MBSR compare favorably to outcomes reported from trials of cognitive-behavioral therapy (CBT) for patients with chronic or persistent insomnia. Morin et al. recently reported the results of a trial of where adults with persistent insomnia were randomized to 6 weeks of group CBT. These patients improved from an average ISI score of 17.26 at baseline to an average of 8.11 at 6 month follow-up; sleep efficiency measured by diary improved from 69% to 82.4%, and large improvements in sleep onset latency and time awake after sleep onset were also found.32 Our finding of durable improvements to sleep outcomes from MBSR over follow-up is consistent with results reported by Edinger et al. from their seminal trial of CBT primary insomnia 48
Our findings build upon positive results from several longitudinal studies of mindfulness-based treatment approaches with insomnia patients. Three uncontrolled studies with a total of 56 patients and one waitlist-controlled trial with 52 patients reported reductions in insomnia symptoms and improvements on other sleep outcomes in patients with mood and/or anxiety disorders following a MBCT. Ree and Craigie20 included the ISI in a study of MBCT for psychiatric outpatients with insomnia, and reported significant improvement (ISI, d =.84) for 23 patients following the program, and benefits maintained at 3 month follow-up. Heidenrich et al.49 reported that 14 patients with refractory chronic insomnia co-morbid with other mental disorders showed pre- to post-MBCT improvements in total sleep time and sleep latency measured by sleep diary, and a decline in dysfunctional thoughts about insomnia. Yook et al.50 reported PSQI scores were significantly decreased among 19 patients with anxiety disorders and insomnia after an 8-week MBCT program. Britton et al.51 studied the sleep outcomes of 7 women with insomnia following an abbreviated MBSR program and found that WASO measured by sleep diary was reduced. In a subsequent waitlist-controlled trial, Britton et al.19 enrolled adults with insomnia co-morbid with depression, and randomized them to an 8-week MBCT program or a waitlist. Compared to controls (n=17), MBCT participants' sleep diary reports (n=25) indicated significantly shorter WASO, and trends for decreased SOL and decreased awakenings, adjusted for use of antidepressants.19 These studies, all of which found reductions in one or more measures of mood or cognition (depression, anxiety, worry or rumination) as well as sleep improvements, complement the growing literature on the health benefits of mindfulness training with MBSR or MBCT.28, 52 These findings, in conjunction with the results of the present study, suggest that mindfulness training has potentially broad application for improving insomnia and closely associated problems that may perpetuate insomnia - symptoms of anxiety and depression.
An exciting approach undertaken by Ong and colleagues53 adds mindfulness training to CBT for insomnia. These investigators developed a 6-week program that combined mindfulness training with CBT. Following the combined program, patients with psycho-physiological insomnia (N=30) reported lower total wake time (a combination of SOL, WASO and terminal wakefulness), increased SE (from 78.5% to 87.6%), reduced insomnia severity (ISI mean 14.9 pre-program, 9.6 post-program), and a reduction in dysfunctional beliefs about sleep. Long-term follow-up of a subset of patients (n=21) showed that benefits to total wake time, sleep efficiency and ISI scores were sustained.54 It would be fruitful in future studies to determine if CBT for insomnia combined with mindfulness is more effective than either approach alone.
Whereas the benefit of MBSR for sleep problems has been demonstrated, the biological and psychological mechanisms responsible for these benefits are not known. The impact of mindfulness training on physiologic and emotional hyperarousal, maladaptive sleep habits and dysfunctional attitudes and beliefs about sleep is discussed in the Sleep and Sleep Stress chapter of the text which introduced the MBSR program, Full Catastrophe Living.13 Suggested behaviors are consistent with the recommendations of sleep hygiene and cognitive behavioral programs and include establishing a mindful pre-sleep routine, not spending time awake in bed (e.g., getting up and doing yoga or something enjoyable if unable to sleep), and switching attention from wakefulness by focusing on the breath or practicing a meditation technique. Additional work will be needed to determine which elements of MBSR are responsible for its impact on sleep. Finally, although MBSR appears to compare favorably with conventional pharmacotherapy, the fact that at follow-up only half of the patients in this study met criteria for recovery from insomnia suggests that there is still considerable room for improvement in our approaches to treating insomnia.55-56
Strengths of our study include a rigorous randomized design, and multi-stage screening process to eliminate individuals likely to have insomnia due to another underlying disorder. Maintaining fidelity to the standard MBSR approach, with only the inclusion of a brief, standard sleep hygiene component should facilitate replication of our findings and enhance generalizability. In addition, the use of objective tracking technologies (MEMS caps® and Hobo® loggers) to obtain information on compliance substantially enriches the findings of this study. Limitations include the pilot sample size, which precluded establishing equivalence between MBSR and PCT arms,57 and lack of additional control groups, such as a medication placebo and a behavioral attention control, to exclude the possibility that non-specific factors such as expectancy, attention, or regression to the mean might account for the positive effects found. As eszopiclone is an FDA-approved medication for treatment of chronic insomnia, and outcomes were assessed by objective and subjective measures at several time points, we have confidence that non-specific factors are not fully responsible for the positive impacts shown.58 Other limitations include the homogeneity of participants in terms of educational attainment and race.
Prior research has shown that, in general, patients prefer non-pharmacologic therapies for their insomnia.59 In querying our study participants following the completion of the study regarding treatment satisfaction and preference, more participants preferred a treatment assignment to the MBSR group, and treatment satisfaction in this group was higher on average. This suggests that there may be a sizable proportion of individuals with chronic insomnia for whom CAM therapies such as MBSR are particularly desirable and preferred to pharmacologic interventions.
Conclusions
While the time commitment associated with participating in and practicing a behavioral intervention such as MBSR is more than with medication, our results suggest that this is not a deterrent to most of our participants. Given patient preferences, the side effects of pharmacotherapy, evidence of the efficacy of MBSR and the potential positive benefits of meditation that go beyond management of insomnia symptoms, it is important that health care providers be aware of the range of non-pharmacologic therapy approaches and that clinicians offer patients options that include MBSR. Future studies of MBSR for insomnia should employ larger sample sizes, and longer follow up to assess the durability of treatment interventions, and include design features that could reveal mechanisms of action and deduce the most effective components of this intervention.60
Acknowledgments
We would like to thank the meditation instructor, Terry Pearson, and the patients who participated in this study.
Financial support: Faculty development grant from the Academic Health Center, University of Minnesota and National Institutes of Health, National Center for Research Resources grant M01 RR00400.
Footnotes
Institutions where work was performed: University of Minnesota, Minneapolis, MN and Minnesota Regional Sleep Disorders Center, Hennepin County Medical Center, Minneapolis, MN
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Cynthia R. Gross, College of Pharmacy and School of Nursing, University of Minnesota.
Mary Jo Kreitzer, Center for Spirituality & Healing, University of Minnesota.
Maryanne Reilly-Spong, College of Pharmacy, University of Minnesota.
Melanie Wall, Division of Biostatistics, University of Minnesota.
Nicole Y. Winbush, Department of Family Practice and Community Health, University of Minnesota.
Robert Patterson, Physical Medicine and Rehabilitation, University of Minnesota.
Mark Mahowald, Minnesota Regional Sleep Disorders Center, Hennepin County Medical Center.
Michel Cramer-Bornemann, Minnesota Regional Sleep Disorders Center, Hennepin County Medical Center.
References
- 1.Buscemi N, Vandermeer B, Friesen C, et al. Summary, Evidence Report/Technology Assessment No. 125. (Prepared by the University of Alberta Evidence-based Practice Center, under Contract No C400000021.) AHRQ Publication No 05-E-021-1. Rockville, MD: Agency for Healthcare Research and Quality; Jun, 2005. Manifestations and management of chronic insomnia in adults. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohayon MM. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Medicine Reviews. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. National Institutes of Health State-of-the-Science Conference Final Statement on Manifestations and Management of Chronic Insomnia in Adults. Sleep. 2005;28:1049–57. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- 4.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262:1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 5.Mallon L, Broman JE, Hetta J. Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of a middle-aged Swedish population. J Intern Med. 2002;251:207–16. doi: 10.1046/j.1365-2796.2002.00941.x. [DOI] [PubMed] [Google Scholar]
- 6.Suka M, Yoshida K, Sugimori H. Persistent insomnia is a predictor of hypertension in Japanese male workers. J Occup Health. 2003;45:344–50. doi: 10.1539/joh.45.344. [DOI] [PubMed] [Google Scholar]
- 7.Zammit GK, Weiner J, Damato N, Sillup GP, McMillan CA. Quality of life in people with insomnia. Sleep. 1999;22(Suppl 2):S379–85. [PubMed] [Google Scholar]
- 8.Colten HR, Altevogt MB. Sleep Disorders and Sleep Deprivation: An unmet public health problem. Washington DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- 9.Ozminkowski R, Wang S, Walsh J. The direct and indirect costs of untreated insomnia in adults in the United States. Sleep. 2007;30:263–73. doi: 10.1093/sleep/30.3.263. [DOI] [PubMed] [Google Scholar]
- 10.Morin CM, LeBlanc M, Daley M, Gregoire JP, Merette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Medicine. 2006;7:123–30. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Barnes P, Powell-Griner E, McFann K, Nahin R. Complementary and Alternative Medicine Use Among Adults: United States,2002. Center for Disease Control Advance Data Report. 2004;343 http://nccam.nih.gov/news/camstats.htm. ed. [PubMed]
- 12.Winbush NY, Gross CR, Kreitzer MJ. The effects of mindfulness-based stress reduction on sleep disturbance: A systematic review. EXPLORE: The Journal of Science & Healing. 2007;3:585–91. doi: 10.1016/j.explore.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Kabat-Zinn J. Full Catastrophe Living: Using the wisdom of your body and mind to face stress, pain, and illness. New York: Dell Publishing; 1990. [Google Scholar]
- 14.Lundh LG. The role of acceptance and mindfulness in the treatment of insomnia. Journal of Cognitive Psychotherapy. 2005;19:29–40. [Google Scholar]
- 15.Carlson LE, Garland SN. Impact of mindfulness-based stress reduction (MBSR) on sleep, mood, stress and fatigue symptoms in cancer outpatients. Int J Behav Med. 2005;12:278–85. doi: 10.1207/s15327558ijbm1204_9. [DOI] [PubMed] [Google Scholar]
- 16.Gross CR, Kreitzer MJ, Russas V, Treesak C, Frazier PA, Hertz MI. Mindfulness meditation to reduce symptoms after organ transplant: A pilot study. Alternative Therapies in Health and Medicine. 2004;10:58–66. [PubMed] [Google Scholar]
- 17.Kreitzer MJ, Gross CR, Ye X, Russas V, Treesak C. Longitudinal impact of mindfulness meditation on illness burden in solid-organ transplant recipients. Prog Transplant. 2005;15:166–72. doi: 10.1177/152692480501500210. [DOI] [PubMed] [Google Scholar]
- 18.Bootzin RR, Stevens SJ. Adolescents, substance abuse, and the treatment of insomnia and daytime sleepiness. Clin Psychol Rev. 2005;25:629–44. doi: 10.1016/j.cpr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Britton W. Meditation and Depression A doctoral dissertation in Psychology. Tucson: University of Arizona; 2006. [Google Scholar]
- 20.Ree M, Craigie M. Outcomes following mindfulness-based cognitive therapy in a heterogenous sample of adult outpatients. Behaviour Change. 2007;24:70–86. [Google Scholar]
- 21.Dunlop WP, Cortina JM, Vaslow JB, Burke MJ. Meta analysis of experiments with matched groups or repeated measures designs. Psychological Methods. 1996;1:170–7. [Google Scholar]
- 22.American Psychological Association . Diagnostic and Statistical Manual of Mental Disorders. 4th Edition, Text Revision. Washington, DC: American Psychological Association; 2000. [Google Scholar]
- 23.American Academy of Sleep Medicine . International Classification of Sleep Disorders: 2nd Ed Diagnostic and Coding Manual: American Academy of Sleep Medicine. 2005. [Google Scholar]
- 24.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: Report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 25.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition (SCID I/P, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1997. [Google Scholar]
- 26.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–73. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 27.Morin CM. Measuring outcomes in randomized clinical trials of insomnia treatments. Sleep Medicine Reviews. 2003;7:263–79. doi: 10.1053/smrv.2002.0274. [DOI] [PubMed] [Google Scholar]
- 28.Baer R, editor. Mindfulness-Based Treatment Approaches: A clinician's guide to evidence-base and applications. Elsevier Academic Press; Amsterdam: 2006. [Google Scholar]
- 29.Santorelli SF, Kabat-Zinn J, editors. Mindfulness-based Stress Reduction Professional Training: MBSR Curriculum Guide and Supporting Materials. Worcester, MA: Center for Mindfulness in Medicine, Health Care and Society; 2002. [Google Scholar]
- 30.Patlak M. Report: National Institutes of Health; 2005. Report No: 06-5271. Your Guide to Healthy Sleep. [Google Scholar]
- 31.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 32.Morin CM, Vallieres A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia, A randomized controlled trial. JAMA. 2009;301:2005–15. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 34.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998;45:5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 35.Morin CM, Blais F, Savard J. Are changes in beliefs and attitudes about sleep related to sleep improvements in the treatment of insomnia? Behaviour Research and Therapy. 2002;40:741–52. doi: 10.1016/s0005-7967(01)00055-9. [DOI] [PubMed] [Google Scholar]
- 36.Lacks P. Behavioral Treatment for Persistent Insomnia. New York: Pergamon Press; 1987. [Google Scholar]
- 37.Edinger JD, Sampson WS. A primary care “friendly” cognitive behavioral insomnia therapy. Sleep. 2003;26:177–82. doi: 10.1093/sleep/26.2.177. [DOI] [PubMed] [Google Scholar]
- 38.Spielberger C. Manual for the State-Trait Anxiety Inventory (Form Y) Redwood City, CA: Mind Garden, Inc.; 1983. [Google Scholar]
- 39.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;3:385–401. [Google Scholar]
- 40.Ware JE, Jr, Kosinski MA, Turner-Bowker DM, Gandek B. How to score Version 2 of the SF-12 Health Survey. Boston MA: QualityMetric Inc and Health Assessment Lab; 2002. [Google Scholar]
- 41.Ware J, Kosinski M, Dewey J. How to score version 2 of the SF-36(R) Health Survey. Lincoln, RI: QualityMetric Incorporated; 2000. [Google Scholar]
- 42.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353–65. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 43.Royston PJ. Approximating the Shapiro-Wilk W-Test for non-normality Statistics and Computing. 1992;2:117–9. [Google Scholar]
- 44.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 45.Jacobson NS, Roberts LJ, Berns SB, McGlinchey JB. Methods for defining and determining the clinical significance of treatment effects: Description, application, and alternatives. Journal of Consulting and Clinical Psychology. 1999;67:300–7. doi: 10.1037//0022-006x.67.3.300. [DOI] [PubMed] [Google Scholar]
- 46.Roth B, Robbins D. Mindfulness-based stress reduction and health-related quality of life: Findings from a bilingual inner-city patient population. Psychosomatic Medicine. 2004;66:113–23. doi: 10.1097/01.psy.0000097337.00754.09. [DOI] [PubMed] [Google Scholar]
- 47.Gross CR, Wyrwich KW. Criteria for evaluating quality of life measurement tools. In: Verster JC, Pandi-Perumal SR, Streiner D, editors. Sleep and Quality of Life in Clinical Medicine. Totowa, NJ: Humana Press, Springer Publishing; 2008. pp. 19–28. [Google Scholar]
- 48.Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillan RE. Cognitive behavioral therapy for treatment of chronic primary insomnia: A randomized controlled trial. JAMA. 2001;285:1856–64. doi: 10.1001/jama.285.14.1856. [DOI] [PubMed] [Google Scholar]
- 49.Heidenreich T, Tuin I, Pflug B, Michal M, Michalak J. Letter to the editor: Mindfulness-based cognitive therapy for persistent insomnia: A pilot study Psychother Psychosom. Vol. 75. 2006. [DOI] [PubMed] [Google Scholar]
- 50.Yook K, Lee SH, Ryu M, et al. Usefulness of mindfulness-based cognitive therapy for treating insomnia in patients with anxiety disorders. A pilot study The Journal of Nervous and Mental Disease. 2008;196:501–3. doi: 10.1097/NMD.0b013e31817762ac. [DOI] [PubMed] [Google Scholar]
- 51.Britton W, Shapiro S, Penn P, Bootzin R. Treating insomnia with Mindfulness-Based Stress Reduction. Sleep. 2003;26:A309–A10. [Google Scholar]
- 52.Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. J Psychosom Res. 2004;57:35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- 53.Ong JC, Shapiro SL, Manber R. Combining mindfulness meditation with cognitive-behavior therapy for insomnia: A treatment-development study. Behavior Therapy. 2008;39:171–82. doi: 10.1016/j.beth.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ong JC, Shapiro SL, Manber R. Mindfulness meditation and cognitive behavioral therapy for insomnia: A naturalistic 12-month follow-up. EXPLORE. 2009;5:30–6. doi: 10.1016/j.explore.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benca RM. Diagnosis and treatment of chronic insomnia: A review. Psychiatry Services. 2005;56:332–43. doi: 10.1176/appi.ps.56.3.332. [DOI] [PubMed] [Google Scholar]
- 56.Edinger JD, Means MK. Cognitive-behavioral therapy for primary insomnia. Clin Psychol Rev. 2005;25:539–58. doi: 10.1016/j.cpr.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 57.Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJ. Reporting of noninferiority and equivalence randomized trials: An extension of the CONSORT statement. JAMA. 2006;295:1152–60. doi: 10.1001/jama.295.10.1152. [DOI] [PubMed] [Google Scholar]
- 58.Lindquist R, Wyman J, Talley K, Findorff M, Gross C. Design of control group conditions in clinical trials of behavioral interventions. Journal of Nursing Scholarship. 2007;39:214–21. doi: 10.1111/j.1547-5069.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- 59.Vincent N, Lionberg C. Treatment preference and patient satisfaction in chronic insomnia. SLEEP. 2001;24:411–17. doi: 10.1093/sleep/24.4.411. [DOI] [PubMed] [Google Scholar]
- 60.Currie SR, Wilson KG, Curran D. Clinical significance and predictors of treatment response to cognitive-behavioral therapy for insomnia secondary to chronic pain. J Behav Med. 2002;25:135–53. doi: 10.1023/a:1014832720903. [DOI] [PubMed] [Google Scholar]