Abstract
There will be over 220,000 people diagnosed with lung cancer and over 160,000 dying of lung cancer this year alone in the United States. In order to arrive at better control, prevention, diagnosis, and therapeutics for lung cancer, we must be able to personalize the approach towards lung cancer. Mind-mapping has existed for centuries for physicians to properly think about various “flows” of personalized medicine. We include here the epidemiology, diagnosis, histology, and treatment of lung cancer—specifically, non-small cell lung cancer. As we have new molecular signatures for lung cancer, this is further detailed. This review is not meant to be a comprehensive review, but rather its purpose is to highlight important aspects of lung cancer diagnosis, management, and personalized treatment options.
Keywords: lung cancer, mind-mapping, personalized medicine, non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer related death both in the United States and worldwide for men and women and although improved, the overall prognosis remains poor. Lung cancer treatment is often multimodal in scope employing the use of surgery, chemotherapy, and radiotherapy. The choice of which depends on a number of factors, related both to the patient and the tumor- including, the histology, stage, tumor biology, and overall health of the patient. This process is often referred to as treatment “planning” by the multidisciplinary team. This can be a complex endeavor involving the association of multiple unique patient and/or tumor related features. Mind-mapping was developed as a tool to condense material and illustrate non-linear relationships between related ideas.1 Mind-maps make use of color, pictures, and spatial orientation to facilitate the understanding of intra- and inter-relationships between concepts, that are felt to reflect the type of thinking involved in the clinical setting. This is already a concept being studied to facilitate this type of thinking with medical students.2-4 Advantages of the mind mapping technique include its ease of use and its ability to present complex information concisely.5 Furthermore, there is data to suggest that mind maps may improve fact recall as it may facilitate a deeper level of processing.3 We therefore hypothesize that it may be a valuable tool for physicians to utilize in order to understand and possibly guide decision making in the diagnosis and management of lung cancer. This review will focus on how individual patient and tumor characteristics give rise to therapeutic options currently employed in clinical practice that are personalized to maximize efficacy and reduce risk to the patient (see figure 1).
Figure 1. Mind-map overview of non-small cell lung cancer.
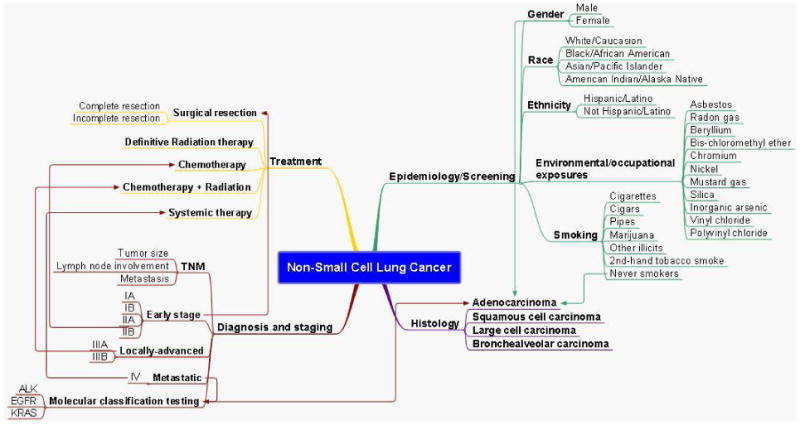
Epidemiology
The most common cancer to be diagnosed in the United States in 2010 will be lung cancer.6 There are estimated to be 222,520 cases diagnosed this year. The mortality burden from this disease is quite high, with an estimated 157,300 deaths expected in 2010.7 According to SEER 17 data, the age-adjusted incidence of cancer of the lung and bronchus was 62.5 per 100,000 persons per year from 2003-2007. The median age of diagnosis was 71 and the 5-year period of survival was 16.4%. The overall life time risk of developing lung cancer is about 1 in 14.
Between 2003 and 2007, approximately 14% of cases were of small cell lung cancer (SCLC). Of the remaining histologically confirmed cases, 85% were of non-small cell lung cancer (NSCLC). Among Whites, African-Americans, Asian/Pacific Islanders (API), American Indian/Alaska Native (AIAN), and Hispanics, AIANs had the highest prevalence of SCLC and APIs had the highest prevalence of NSCLC, though their absolute number of cases was relatively low.
Between 1999 and 2006, at the time of diagnosis, 56% of lung cancer cases had already spread to distant sites. 22% of cases were diagnosed at a stage of regional involvement and 15% of cases had only local involvement. The remaining cases were unstaged. These values are similar between males and females and between whites and African-Americans, as 60% of lung cancer cases among African-americans had distant involvement and 55% of white cases had distant involvement. While the burden of disease is quite high from lung cancer, the trends in incidence are encouraging. Between 1991 and 2007, the annual percent change rate declined by 0.8% annually in the SEER 9 dataset. This was the first period during which a significant decline in incidence was noted. While significant declines in incidence occurred for both small cell and non-small cell lung cancer, declines in small cell lung cancer incidence were much greater over this period of time (see figure 2).
Figure 2. Mind map of lung cancer epidemiology, screening, and risk factors.
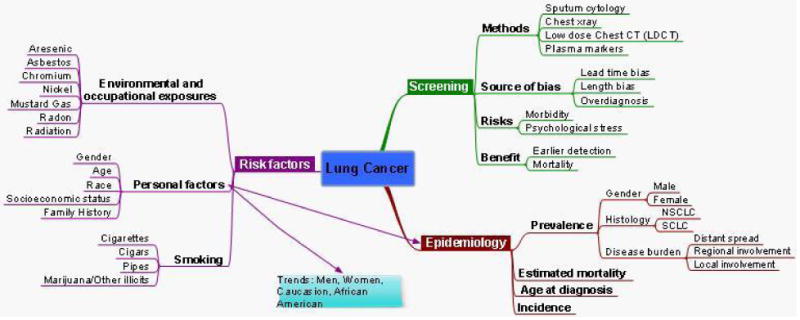
When stratified by race and gender, a few interesting trends emerge. Though the incidence of lung cancer remains lower among women than men, the data suggest that the two genders may be reaching parity. However, irrespective of the year, black men have had a significantly higher incidence of lung cancer than white men, while black women and white women have had similar incidence rates. Similarly, between 2003 and 2007, the mortality rates were higher among black men than white men (87.5 and 68.3 deaths per 100,000, respectively) and comparable among black and white women (39.6 and 41.6 deaths per 100,000, respectively). However, the decline in incidence has also been the greatest among black men with an average annual percent change of 3.4% versus 2.2% in white men, a gain of 0.3% among black women, and no change among white women between 1998 and 2007.
Risk factors
Smoking
The two major factors that play into individual risk of lung cancer are personal history of active cigarette smoking and age. Cigarette smoking is well established as the major cause of lung cancer, with statistics showing that smokers are 10-20 times more likely to develop lung cancer than nonsmokers.8 Since the pathway of smoking-caused lung carcinogenesis is a process that takes place during a period of several decades, the majority of the burden of smoking caused lung cancer occurs among the elderly. The age of individual risk is affected by the following factors: patient age, duration of smoking, intensity of smoking, age of initiation, and age of cessation (if relevant).9 Increased risk of lung cancer becomes apparent among smokers in their mid to late forties. After that, age-specific relative risk for the development of lung cancer rises steadily in smokers until it peaks at an age in the late seventies.10 Both cessation and reduction of smoking have been shown to cut down the risk of developing lung cancer.11,12 However, no randomized control trial has ever demonstrated decreased mortality attributed to lung cancer among cases as a result of cessation.13
Other forms of smoking, including cigars and pipes, are also associated with an increased risk of lung cancer, although the actual amount of the effect is unclear. While the issue of marijuana smoke as a cause of lung cancer has been controversial, recent data from the National Surveys on Drug Use and Health (NSDUH) suggests association between long-term duration of marijuana use and lung cancer.14
Finally, there is compelling evidence that second-hand smoke is a cause of lung cancer.15 Second-hand tobacco smoke, also known as environmental tobacco smoke (ETS), contains higher concentrations of many of the toxins found in cigarette smoke, such as formaldehyde, lead, arsenic, benzene, and radioactive polonium-210. It has been estimated that living or working in a place where smoking is permitted increases the non-smokers' risk of developing lung cancer by 20 – 30%.
Approximately 10% of patients with lung cancer in the USA are lifelong never smokers, women disproportionately more often than men. This warrants the consideration of environmental exposures as risk factors for lung cancer.
Environmental and occupational exposures
The International Agency for Research on Cancer (IARC) has identified several workplace substances that have been implicated or proven to be carcinogens in lung cancer. Although asbestos and radon gas are the best known occupational lung carcinogens, beryllium, bis-chloromethyl ether, chromium, nickel, mustard gas, silica, inorganic arsenic, vinyl chloride, polyvinyl chloride, and particulate air matter are all known or suspected occupational exposures associated with increased lung cancer risk.16,17
Asbestos is commonly implicated as a cause of lung cancer and mesothelioma. Historically, asbestos was used in thin cement and plastics as well as for insulation, roofing, fireproofing, and sound absorption. Other uses for asbestos include incorporation into brake shoes, clutch pads, ceiling and floor tiles, paints, coatings, adhesives, vermiculite-containing garden products and some talc-containing crayons. People exposed to asbestos in their workplace, their communities, or their homes may inhale tiny asbestos fibers that are released into the air when asbestos-containing products are disturbed. Several bans and restrictions have been placed in the US on asbestos use since the 1970s, thereby decreasing the health risks posed by this mineral.18 Asbestos-induced lung cancer, however, has a latency period of 20 years or longer between start of exposure and onset of disease, thereby allowing the impact of this risk factor to continue for some time.
Radon gas and its solid decay products occur naturally in the air worldwide. Radon itself originates from decay of uranium and thorium in the soil. When inhaled as a solid decay product, it becomes deposited into the lung, ultimately causing DNA damage that leads to lung cancer.16 Radon gas enters houses from the environment via openings communicating to outside air, resulting in much higher indoor concentrations than would be found outdoors. Radon levels are usually higher in basements, cellars and other areas in contact with soil. Even higher concentrations are found in places such as mines, caves, and water treatment facilities. Radon in homes contributes substantially to the incidence of lung cancers worldwide with a dose-response relationship that seems to be linear with no evidence of a threshold. It has been considered the second leading cause of lung cancer in the US, according to the 1998 report of the National Research Council's Committee on the Biological Effects of Ionizing Radiation (BEIR) VI, accounting for 15,000-22,000 cancer deaths per year.
Personal factors
Demographic factors including race, gender and socioeconomic status have been investigated with regards to their effect on individual susceptibility to lung cancer causing agents. The association between race, gender, and lung cancer incidence was discussed in depth above.
Socioeconomic status is inversely associated with lung cancer risk. This may be related to higher smoking prevalence, greater use of nonfiltered and high-tar cigarettes and lower quit rates among those of lower socioeconomic status. Individuals with lower SES also have a greater likelihood of exposure to risk factors such as unhealthy diets, environmental and occupational work exposures, and second-hand smoke.19
Even though smoking is the major cause, lung cancer still only occurs in a minority of smokers, suggesting that inherited factors may play a role. It is evident that patients with a previous personal or first-degree relative family history of lung cancer have a higher risk of developing lung cancer,20 although molecular basis underlying any familial increase in risk is not yet well characterized.
Patients with previous radiation treatment to the chest are at higher risk for lung cancer, especially if they smoke.21 Several benign lung diseases have also been associated with an increased risk of lung cancer. The strongest association has been shown with chronic obstructive pulmonary disease (COPD).22 However, pulmonary fibrosis and alpha-1-antitrypsin deficiency also increase the risk for lung cancer.23,24
Studies looking at the possible role of some vitamin supplementation in reducing lung cancer risk have not shown promise thus far. While beta-carotene was previously associated with a lower risk of lung cancer, recent studies have demonstrated increased risk of lung cancer in smokers who took beta-carotene supplements. It is unclear whether or not an increased consumption of fruit, certain vegetables and some micronutrients may be associated with a lower risk of lung cancer in both smokers and nonsmokers.25
Screening
The prognosis of lung cancer is markedly improved when cancers are resected in early stages. The ability to identify a subgroup of the population (i.e. those with a smoking history) at particularly high risk for lung cancer could make screening a cost effective endeavor. Several randomized control trials involving screening high risk patients with sputum cytology, chest x-ray, and low-dose computed tomography (LDCT) have been conducted, which have resulted in at best greater cancer detection but without lung-cancer specific mortality benefit.26-28 Two large randomized trials, the National Lung Study Trial (NLST) and the Dutch–Belgian randomized lung cancer screening trial (NELSON) which compare CT to CXR screening and CT to “usual care” respectively are currently ongoing, and should offer more conclusive data as to whether lung cancer screening by CT is able to reduce lung cancer mortality.29,30
A novel approach to lung cancer screening involves the use of partial wave spectroscopic (PWS) microscopy. The main principle of PWS is that the degree of disorder of the intracellular architecture of the cell can be measured based on the amount of wave length distortion. One field of research poses the theory that the genetic pathogenic changes of the focal neoplastic lesion in the lung can be reflected in the mucosa of the entire aerodigestive tract.31 A recent study hypothesized that the buccal epithelium may be used as a surrogate field for the detection of lung carcinogenesis through the use of PWS. Roy et al.32 analyzed normal buccal epithelium from 135 smokers with and without lung cancer. Using this technique, they were able to detect a >50% level of architectural disorder in patients harboring lung cancer compared to smokers who were lung cancer free. They concluded that the utility of this technique could be used as a “prescreening” tool to identify those patients at highest risk who might benefit from more invasive testing.
Along with the potential biases such as lead-time, length, and overdiagnosis which can explain the lack of mortality benefit seen in earlier studies, cancer screening comes with certain risks and morbidities. Results from the Nelson study indicate that short-term recipients of an indeterminate screening result experienced an increase in lung-cancer-specific distress.33 Furthermore, the risks of screening for lung cancer in the absence of a demonstrated mortality benefit include the morbidity of the work-up of benign nodules as well as the treatment of pathologically confirmed lung cancers that may have not manifested clinically during the lifespan of the patient.34,35 At this time, the challenges of finding a screening method with acceptable sensitivity and specificity as well as a significant mortality benefit render insufficient evidence to recommend screening.
Histology
Before a patient begins treatment for lung cancer, it is important to determine the pathological diagnosis as that can have varying implications on treatment options. In 2004, the World Health Organization (WHO) updated the classification of lung tumors.36
Although there are several classifications of NSCLC, the three major histologic subtypes are adenocarcinoma, constituting approximately 44% of cases, followed by squamous cell carcinoma at 21% of cases, and large cell carcinoma at 4% of cases. Approximately 30% of NSCLC cases could not be categorized as one of these three histologies.6 (see figure 3).
Figure 3. Mind map of lung cancer histology.
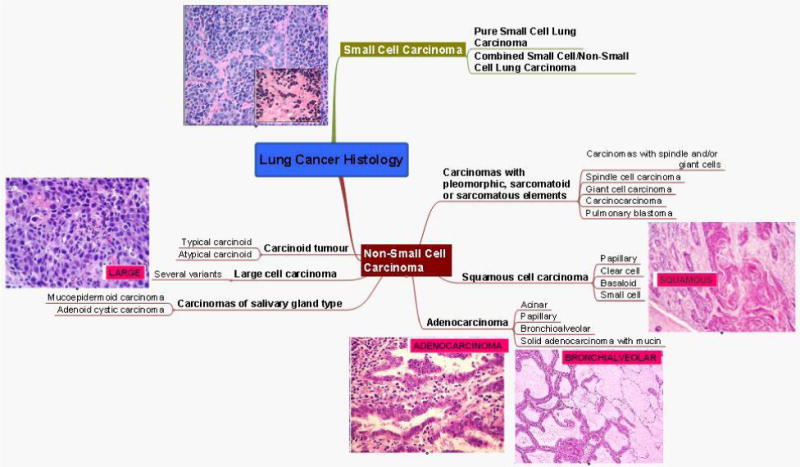
There are four major subtypes of adenocarcinoma based on their growth pattern, of which the glandular variety is the most common. Adenocarcinomas arise in the periphery the majority of the time, and are more commonly associated with K-Ras mutations.37 The microscopic features that are associated with adenocarcinoma include either neoplastic gland formation or intracytoplasmic mucin. Bronchioalveloar carcinoma (BAC) is considered a subtype of adenocarcinoma and may have a better prognosis based on a less aggressive tumor biology.38,39 The distinction between BAC and frank adenocarcinoma has become increasingly important as the incidence of BAC has been increasing over the last decades and the management and prognosis of these two entities are markedly differernt.41 The original histologic features that were described defining this subtype included; well-differentiated cytology, aerogenous and lymphatic spread, a “lepidic” growth pattern (along intact alveolar septa) and an origin distal to a grossly recognizable bronchi.42 This earlier classification had to comprise at least 75% of the tumor, however, in 2004 the WHO restricted the definition of BAC to those tumors in which the lepidic growth pattern comprises the entire tumor.
Squamous cell carcinomas (SCC) occur most commonly in the central area of the lung and tend to extend both intrabronchially as well as peribronchially which can result in distal pulmonary collapse, consolidation, and obstructive pneumonitits. The microscopic features of SCC include cells that keratinize or tend to form epithelial pearls or both. This histologic type is associated with the highest frequency of p53 mutations.40
Large cell carcinoma (LCC) usually presents as a large peripheral mass with prominent necrosis. The microscopic features of LCC include large nuclei and abundant pale staining cytoplasm arranged in sheets of non-differentiating polygonal cells. A lack of glandular or squamous differentiation, and/or small cell carcinoma cytologic features implies that the diagnosis of LCC is one of exclusion. The features and behavior of LCC parallel that of other poorly differentiated NSCLCs.
Staging
Clinical staging of NSCLC is commenced by a thorough history and physical exam and computed tomography (CT) scanning of the chest and upper abdomen. Regardless of clinical stage, pre-surgical planning is continued with combined CT/PET imaging, which has been shown not only to provide more accurate staging than either CT or PET alone, but to also help avoid inappropriate surgery.43 Any pathologic mediastinal lymphadenopathy, as defined by a short axis size >1cm and/or those found to be metabolically active on PET scan, discovered during this period is sampled. Mediastinoscopy is considered the gold standard for lymph node sampling of the mediastinum with an overall reported sensitivity and specificity of 87% and 100%, respectively.44 Transesophageal endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) and endobronchial ultrasound -guided transbronchial needle aspiration (EBUS) are a less invasive means to stage patients which has also proven to be effective.45 Bronchoscopy is also utilized in the preoperative staging for early stage central and peripheral NSCLC lesions to better assess the T stage of the tumor. As we need more tissues for genetic/proteomic analysis for decision making for each individual patient, it behooves us to consider obtaining as much tissue as possible for diagnosis, staging, and molecular markers. Brain MRI is recommended for more advanced stage disease as determined by clinical assessment to establish the absence/presence of distant metastases.46 Pulmonary function tests (PFTs) are performed at this time as well to establish the medical fitness of the patient to undergo surgical resection.
Staging in NSCLC is based on the tumor-node-metastasis (TNM) system. Staging may be determined using either a clinical staging system or a histopathologic staging system. The former relies on history and physical exam, laboratory evidence, radiologic testing, and tissue sampling. The latter staging system includes data from the resected tumor such as the histologic grade of the tumor, the tumor margins, and lymphovascular invasion. 47 If a clinical staging scheme is used, the TNM stage will be preceded by a “c”; similarly, if a pathologic staging scheme is used, TNM will be preceded by a “p”.
The American Joint Commission on Cancer (AJCC) updated their staging guidelines for NSCLC in January 2010 to the 7th ed. Major differences between the 6th and 7th editions include the addition of sub-divisions within the T1, T2, and M1 descriptors, new size cutoffs particularly for the T2 and T3 descriptors, the reclassification of malignant pleural effusion, and the reclassification of satellite nodules. By introducing these further subclassifications, more precise information will be available to patients and their physicians regarding estimated 5 year survival.48
Treatment
Stage IA-IIB NSCLC
Stage IA-IB NSCLC consists of small tumors (<5cm), which do not have lymph node metastases (T1a-T2a, N0). While stage IIA-IIB NSCLC consists of large tumors which may or may not invade the chest wall, diaphragm, main bronchus < 2cm from the carina, phrenic nerve, parietal pericardium, or have separate tumor nodules within the same lobe without having lymph node metastases (T1a-T3, N0); or consist of smaller tumors surrounded by lung (i.e. non-invasive) which have ipsilateral hilar and/or peribronchial lymph node metastases (T1a-T2b, N1). Surgical resection is considered to offer the best chance for cure for patients with early stage I-II NSCLC based on large series reported in the literature.49 Patients able to undergo resection are then offered anatomic lobectomy with lymph node dissection or sampling, whereas patients unfit for surgery are offered a more limited resection or radiation therapy (see figure 4).
Figure 4. Mind map of treatment for stage IA-IIB NSCLC.
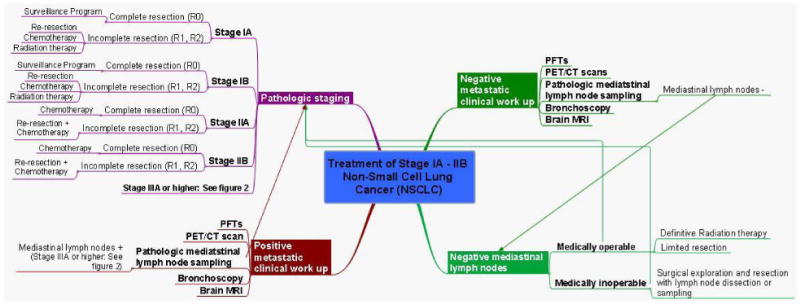
Patients with stage IIB (T3, N0) disease due to chest wall invasion are treated with en bloc resection followed by chemotherapy. A retrospective series of 212 patients in this subgroup demonstrated a 5-year survival rate of approximately 40%, regardless of use of adjuvant radiotherapy. This is in contrast to those found to have nodal involvement (T3, N1; stage IIIA), in which 5-year survival was only 12%.50
Patients with completely resected pathologic stage IA and IB disease are entered into a surveillance program as adjuvant chemotherapy is not currently recommended.46 Patients with incompletely resected pathologic stage IA and IB disease are then offered re-resection, radiation therapy, or adjuvant chemotherapy based on physiologic reserve. Patients with completely resected stage IIA and IIB disease are treated with a cisplatin based adjuvant therapy regimen combined with either vinorelbine or etoposide,51 while those patients who are incompletely resected are offered re-resection followed by chemotherapy.
Surgical Considerations
Complete resection is the goal of surgical treatment of NSCLC. Incomplete resection which leaves behind visible tumor (R2) offers no therapeutic advantage and can cause the postponement of subsequent therapy in addition to the pain and suffering it may cause the patient. With regards to the extent of resection, sleeve lobectomy or greater resection is recommended for patients with stage I and II NSCLC who have adequate physiologic reserve over lesser resections (sublobar or wedge resection).49 In patients who have poor physiologic reserve, sublobar or wedge resection is preferred over local therapies (cyrotherapy, radiofrequency ablation, localized radiation. Although less often required, bilobectomy or pneumonectomy should be performed if the extent of locoregional disease would preclude a complete resection by lobectomy. Most often this occurs when a tumor has spread across the minor fissure or approximates an incomplete fissure in the case of bilobectomy and/or central tumors that involve the main bronchus in the case of pneumonectomy. Video-assisted thoracoscopic procedures can be performed in early stage patients with acceptable results in surgeons familiar with the technique.52,53 Lymph node sampling with a minimum of three N2 lymph node stations or a formal lymph node dissection should be performed in patients with early stage I and II to increase the chance of finding occult disease on pathologic specimen.49
Stage IIIA NSCLC
Stage IIIA NSCLC patients are a heterogenous group and consist of tumors with ipsilateral mediastinal/hilar lymph node metastases and/or resectable chest wall or mediastinal involvement (T3-4, N0-2). For N2 disease discovered preoperatively, chemoradiotherapy with a sequential regimen of cisplatin and vinblastine followed by radiation is currently recommended, with the possibility of surgical resection only taking place in the context of a clinical trial.54 This recommendation is based on previously published randomized control trials demonstrating no significant survival benefit over preoperative chemotherapy followed by surgery or surgery alone vs. chemoradiation alone.55-57 Patients undergoing resection of stage IIIA (N2) disease should have a formal ipsilateral mediastinal lymph node dissection performed.46 For N2 disease discovered during surgical exploration, resection should only take place if the tumor and involved nodal station can be completely resected. If a complete resection cannot be completed due to advanced nodal disease, or technically due to the primary tumor, then the planned operation should be aborted.58 However, the anatomic site of N2 positivity may influence the decision to proceed with surgical resection. In one series, survival rates for N2 positivity in an inferior or superior location were 8% versus 25%. Furthermore, N2 metastasis confined to level 5 (aortopulmonary) in patients with left-upper-lobe NSCLC has been associated with a more favorable outcome, with 5-year survival rates ranging from 20% to 42% depending on the completeness of resection (see Figure 5).59,60
Figure 5. Mind map of treatment for stage IIIA and IIIB (T1-3, N2) NSCLC.
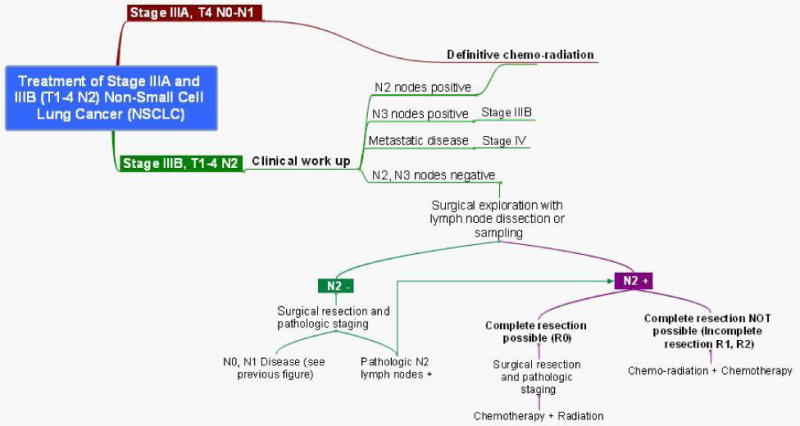
If N2 disease is discovered after surgical resection during pathologic staging, further treatment with sequential chemotherapy followed by radiation is offered to the patient in the presence of a complete resection. In the presence of incomplete resection, concurrent chemoradiation therapy with or without chemotherapy is offered depending on the institution offering treatment.58
Tumors which involve the chest wall or proximal airways (within 2cm of the carina) which have hilar, but not mediastinal, lymph node involvement are classified as stage IIIA (T3, N1), however, they have a much better prognosis than those patients with stage IIIA disease due to N2 nodal involvement.61-64 It is currently preferred that patients undergo surgical resection, if technically possible, as initial management, followed by adjuvant chemotherapy for those with a complete resection. Those undergoing incomplete resection are offered reresection followed by chemotherapy or chemoradiation followed by chemotherapy, depending on performance status. If complete resection is not thought to be technically possible based on preoperative imaging, then concurrent chemoradiation or chemotherapy should precede surgical resection.46
Patients with ipsilateral satellite nodules within the same lobe (T3, N0-N1) or another lobe (T4, N0-N1) are another subset of patients with stage IIIA which have shown to benefit from surgical resection followed by adjuvant chemotherapy.65-68 However, (T4, N0-N1) disease which has direct invasion of the heart, esophagus, great vessels, recurrent laryngeal nerve, vertebral body, or carina are considered unresectable disease and are treated with concurrent chemoradiation.69
Tumors which involve the apical portion of the chest and the lower portion of the brachial plexus, superior sulcus (Pancoast) tumors, often invade the first and/or second rib. Concurrent chemoradiation is currently recommended prior to surgical resection in order to obtain complete resection.49 Resection typically involves en bloc resection of the involved apical chest wall (which can include multiple ribs and the T1 nerve root) along with lobectomy. This is then followed by two additional postoperative courses of chemotherapy with a platinum-based doublet. This multimodal approach has been reported to result in complete resection rates of 68%-76% and 5-year survival rates of 44%-56%.70,71
Stage IIIB NSCLC
Stage IIIB NSCLC consists of patients with N3 disease (T1-3, N3) and patients with T4 disease which has mediastinal lymph node involvement (T4, N2-3). Clinical stage IIIB disease is evaluated by preoperative bronchoscopy, brain MRI, and lymph node sampling to confirm the presence or absence of N2-N3 disease. There is no role for surgical resection in patients with T4 disease and N2-N3 lymph node involvement (see figure 6). Series have reported 5-year survival rates of only 6% and 3% for clinical T4 and N3 disease respectively that underwent surgical resection.72 Concurrent radiotherapy with cisplatin based regimen is recommended for patients with good performance status, while radiation therapy alone is recommended for those with poor performance status.46
Figure 6. Mind map of treatment for Stage IIIB (T1-3,N3) and Stage IV NSCLC.
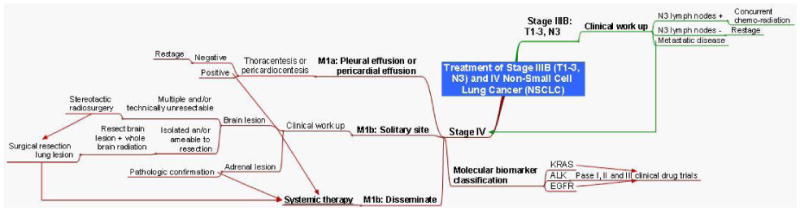
Stage IV NSCLC
Patients with more advanced stage IV disease as discovered during preoperative planning have limited options (see figure 6). Patients found to have pleural effusions (M1a) undergo pathologic confirmation with either a thoracentesis or pericardiocentesis. If the diagnosis is confirmed, local therapy with pleurodesis, catheter drainage, and a pericardial window is followed by systemic therapy. Patients with isolated brain metastases (M1b) have been shown to benefit from surgical resection followed by whole brain radiation.71-75 Patients with multiple brain metastases or surgically unresectable lesions can be treated with stereotactic radiosurgery alone or followed with whole brain radiation.76 Treatment of the primary can then follow with surgical resection for T1-2, N0-1 or T3, N0 disease followed by chemotherapy, whereas any concurrent T4 or N2, N3 disease is treated with systemic chemotherapy. Patients with pathologically confirmed adrenal metastases or disseminated metastatic disease are also treated with systemic chemotherapy. Patients with advanced or metastatic NSCLC should be tested for K-Ras, EGFR, and ALK mutations in that order, as they potentially occur mutually exclusive of one another.77 A platinum based two drug regimen either alone (in patients with non-squamous histology) or in combination with either bevacizumab (an anti-VEGF receptor monoclonal antibody) or cetuximab (an anti-EGF receptor monoclonal antibody) is recommended for patients with a performance status of 0-1 or 0-2 respectively.46 Whereas patients with a performance status of 3-4 do not benefit from cytotoxic therapy, except erlotinib for EGFR positive patients. Currently, data is emerging for maintenance therapy also with pemetrexed or erlotinib78. Futhermore, initial therapy with erlotinib is recommended for patients with advanced or metastatic NSCLC who are EGFR mutation positive.79
There have been a large number of genomic and proteomic studies that have been done for lung cancer over the past several years. A number of different markers have been evaluated in patients with lung cancer aimed at early disease detection, assigning prognosis, and predicting both rates of relapse and response to treatment.80 These include serum markers as well as molecular and genetic markers, and it is only recently that they are beginning to be integrated into clinical decision making. Since lung cancer is such a heterogenous disorder, no one signature will encompass all of the patients. Many of these markers are under investigation and will not be our focus; however, certain themes are emerging on the mutations within lung cancer. In particular, K-Ras can be abnormal in certain adenocarcinomas, as well as EGFR, and most recently ALK translocations. These molecular tests are helpful in determining if the individual patient can respond (or be resistant) to certain therapies. Described in depth below are these three molecules.
K-Ras
The K-Ras gene encodes a group of 21-kDa proteins, the p21 proteins, which are bound to GTP in their active state. Cell proliferation is initiated through Ras-dependent kinases that are inactivated when GTP-ase activating protein hydrolyzes GTP to guanosine diphosphate. Point mutations have been described at codons 12, 13 and 61, which result in a loss of intrinsic GTP-ase activity. It is estimated that approximately 20% of NSCLC have Ras mutations at codon 12, including 30–50% of adenocarcinomas.81-84 While an association between smoking and K-Ras mutations has been described, a recent report showed that the incidence of K-Ras mutations in patients with adenocarcinomas who had never smoked was 15%, compared with 25% in patients who were smokers.81,85,86 In addition, the K-Ras mutation profile in the nonsmokers was distinct from that found in patients who smoked, highlighting the biologic heterogeneity in this particular subset of patients.
The prognostic significance of K-Ras mutations was evaluated in a number of studies with conflicting results. Although limited by the retrospective nature of the included studies, a meta-analysis of 28 trials with a total of 3620 patients found that K-Ras mutations appeared to confer a worse prognosis in patients with NSCLC when detected by PCR, but not by immunohistochemistry (IHC), with a hazard ratio (HR) of 1.35 (95% confidence interval [CI]: 1.16–11.56). This negative prognostic value appeared to apply to patients with adenocarcinoma, with a HR of 1.59 (95% CI: 1.26–2.02), but not to those with squamous cell carcinoma.87 In other studies, this association is not seen, or is only demonstrated in small subsets of patients.88-90 Previous studies have not shown K-Ras mutational status to be predictive of a benefit from standard adjuvant chemotherapy in NSCLC.89,90 However, it is predictive of therapeutic benefit from EGFR tyrosine kinase inhibitors (TKIs). Miller et al.91 demonstrated a significant difference in the response rates of 80 patients with K-ras codon 12 and 13 mutations vs. those without who had been treated with first-line single-agent erlotinib (20/62, 32% vs 0/18: p < .01). Furthermore, there is evidence that the addition of erlotinib may decrease the therapeutic benefit of chemotherapy for those patients with K-ras codon 12 and 13 mutations undergoing treatment. 92
EGFR
The epidermal growth factor receptor (EGFR) is part of a family of tyrosine kinase receptors that regulate pathways involving tumor proliferation, invasion, metastasis, resistance to apoptosis and angiogenesis. An increased level of EGFR expression is most commonly seen in squamous cell carcinoma (50-80%), although it is expressed to some degree in all cells of epithelial origin.93 In two important studies, the presence of somatic mutations in the kinase domain of EGFR was shown to be associated with increased sensitivity to the EGFR tyrosine kinase inhibitors (TKI) gefitinib and erlotinib in patients with non-small cell lung cancer.94,95 These mutations have been shown to occur more frequently in East Asians, women, adenocarcinoma, and those who have never smoked, however, a recent study has shown that a significant number of men and smokers harbor this mutation as well.96,97 The EGFR mutations occur as in-frame insertions/duplications, point mutations, and in-frame deletions of the 4 kinase domains (exons 18-21), with the two most common being an in-frame deletion in exon 19 and the point mutation L858R in exon 21.96,98 The exact mechanism by which EGFR mutations promote lung cancer and sensitize tumors to treatment has not been fully explained, however, both in vivo and in vitro studies have demonstrated an oncogenic effect of prolonged receptor kinase activity on cell transformation.94,95,99-101 Several studies have shown that EGFR overexpression, as determined by immunohistochemistry, appears to confer a poor prognosis in patients with NSCLC.102-105 However, these results have not been confirmed in other studies.106-108 Given the conflicting data that are currently available, EGFR does not have a clear role as a prognostic marker in NSCLCs.
With the recent advances in the development of EGFR-targeted therapy, however, it has become increasingly important to define predictive markers that identify a subset of patients who are most likely to benefit from EGFR-TKI therapy. In four randomized trials, the addition of gefitinib or erlotinib did not confer a survival advantage when added to a platinum doublet as first-line treatment in patients with NSCLC.109-112 Subsequent trials of EGFR-TKIs in patients who have been previously treated have shown an improvement in both survival and quality of life when compared with placebo groups.113,114 The role of EGFR as a molecular predictive marker for response to therapy is less clear. Retrospective studies have suggested that there is an association between EGFR mutations and response to EGFR-TKIs that ultimately translates into an improvement in overall survival.115 However, in larger, randomized trials, this was not confirmed. 113
ALK
In a subset of patients with NSCLC, the anaplastic lymphoma kinase (ALK) and echinoderm microtubule-associated protein like 4 (EML4) gene have been recently found to undergo fusion as a result of an inversion on the short arm of chromosome 2 resulting in the novel oncogene EML4-ALK.116 This gene rearrangement occurs largely independent from EGFR or K-Ras mutations.117 Patients with this fusion oncogene tend to be younger, have adenocarcinoma with acinar histology, and be never or light smokers.118-122 The overall incidence of EML4-ALK rearrangement has been reported to be 4% - 7%. However, when screened for the clinical features mentioned above, the frequency increased to 33%.123 Patients harboring the fusion oncogene were retrospectively studied to examine its effect on both cytoxic chemotherapy and tyroskine kinase inhibitor therapy response. When comparing those who did and did not harbor the EML4-ALK fusion oncogene, there were no difference in response rates or time to progression in those treated with platinum-based combination therapy, whereas there was no clinical response and a time to progression of 5 months for those treated with EGFR TKIs.123 While these patients appear to be resistant to EGFR TKIs such as erlotinib and gefitinib, the small molecule tyrosine kinase inhibitor crizotinib has shown activity against cell lines containing the EML4-ALK fusion oncogene.116,119,124-126 A Phase II trial of NSCLC patients who harbored EML4-ALK demonstrated a radiographic response rate of 57% and a disease control rate of 87% at eight weeks after receiving crizotinib 250mg twice daily. Progression free survival at six months had an estimated probability of 72%.77 These results have led to a phase III trial comparing crioztinib to standard, single-agent docetaxel or pemetrexed in EML4-ALK positive NSCLC patients with metastatic disease who have received one prior line of chemotherapy (NCT00932893). 127
Conclusions
Lung cancer is a devastating illness for which the overall survival is still quite poor. As we develop better diagnostics, staging, and therapeutics, we will have impacted on the survival and the quality of life for our patients. In particular, the staging system has been revised, the diagnostics have incorporated several new techniques, and the therapeutics are ever expanding in their innovation. For early stage disease, better survival is being seen in terms of the types of surgical resections and adjuvant therapy. For late stage disease, molecular signatures are helping us predict which patients can respond to novel therapeutics. Mind maps graphically present complex information in a manner which promotes a more global understanding of concepts.128 As they have the potential to improve learning, it is anticipated that end users of the mind maps presented here will be quick to apply knowledge gained to clinical and research practices to help patients in various aspects of lung cancer diagnosis and treatment.
Acknowledgments
Funding Supported in part by NIH/National Cancer Institute (5R01CA125541-03, 3R01CA125541-03S109, 5R01CA129501-02, 3R01CA129501-02S109, 5P01HL058064-140009, 5R01CA100750-07), V-Foundation (Guy Geleerd Memorial Foundation), Kate McMullen Foundation, Respiratory Health Association of Metropolitan Chicago, and Mesothelioma Applied Research Foundation (Jeffrey P Hayes Memorial Grant).
References
- 1.Buzan T, Buzan B. The Mind Map Book. London, England: BBC Books; 1993. [Google Scholar]
- 2.D'Antoni AV, Zipp GP, Olson VG, Cahill TF. Does the mind map learning strategy facilitate information retrieval and critical thinking in medical students. BMC Med Educ. 2010 Sep 16;10:61. doi: 10.1186/1472-6920-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrand P, Hussain F, Hennessy E. The efficacy of the ‘mind map’ study technique. Med Educ. 2002 May;36(5):426–31. doi: 10.1046/j.1365-2923.2002.01205.x. [DOI] [PubMed] [Google Scholar]
- 4.Wickramasinghe A, Widanapathirana N, Kuruppu O, Liyanage I, Karunathilake I. Effectiveness of mind maps as a learning tool for medical students. South East Asian J Med Educ. 2007;1:30–32. [Google Scholar]
- 5.Eppler MJ. A comparison between concept maps, mind maps, conceptual diagrams, and visual metaphors as complementary tools for knowledge construction and sharing. Information Visualization. 2006;5:202–210. [Google Scholar]
- 6.Altekruse SF, Kosary CL, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2007. National Cancer Institute; Bethesda, MD: [9/28/10]. http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site, 2010. [Google Scholar]
- 7.http://www.cancer.gov/cancertopics/types/commoncancers
- 8.Health Service, Centers for Disease Control and Prevention; Washington, DC: 2010. [5/4/10]. Smoking and tobacco use—fact sheets. Available online: http://www.cdc.gov/tobacco/data_statistics/fact_sheets/ [Google Scholar]
- 9.Alberg AJ, Nonemaker J. Who Is at High Risk for Lung Cancer? Population-Level and Individual-Level Perspectives. Seminars in Respiratory & Critical Care Medicine. 2008 Jun;29(3):223–32. doi: 10.1055/s-2008-1076742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aisner J, et al., editors. Thoracic Oncology. Williams and Wilkins; Baltimore: 1996. p. 51. [Google Scholar]
- 11.U.S. Department of Health and Human Services . The Health Benefits of Smoking Cessation: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, CDC, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 1990. [5/4/10]. [Google Scholar]
- 12.Godtfredsen NS, Prescott E, Osler M. Effect of smoking reduction on lung cancer risk. JAMA. 2005;294:1505. doi: 10.1001/jama.294.12.1505. [DOI] [PubMed] [Google Scholar]
- 13.Shaten BJ, Kuller LH, Kjelsberg MO, Stamler J, Ockene JK, Cutler JA, Cohen JD. Lung cancer mortality after 16 years in MRFIT participants in intervention and usual-care groups. Multiple Risk Factor Intervention Trial. Ann Epidemiol. 1997 Feb;7(2):125–36. doi: 10.1016/s1047-2797(96)00123-8. [DOI] [PubMed] [Google Scholar]
- 14.Han B, Gfroerer JC, Colliver JD. Associations between duration of illicit drug use and health conditions: results from the 2005-2007 national surveys on drug use and health. Annals of Epidemiology. 2010 Apr;20(4):289–97. doi: 10.1016/j.annepidem.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Hackshaw AK, Law MR, Wald NJ. The accumulated evidence on lung cancer and environmental tobacco smoke. Br Med J. 1997;315:980. doi: 10.1136/bmj.315.7114.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brüske-Hohlfeld I. Environmental and Occupational Risk Factors for Lung Cancer. Methods in Molecular Biology. 2009;472:3–23. doi: 10.1007/978-1-60327-492-0_1. [DOI] [PubMed] [Google Scholar]
- 17.American Cancer Society What Causes Small Cell Lung Cancer? [5/19/2010]; Available online: http://www.cancer.org/
- 18.National Cancer Institute Asbestos Exposure and Cancer Risk. [5/20/10]; Available online: http://www.cancer.gov/cancertopics/factsheet/Risk/asbestos.
- 19.Van Loon AJM, Goldbohm RA, Kant IJ, Swan GMH, Kremer AM, Van de Brandt PA. Socioeconomic status and lung cancer incidence in men in the Netherlands: is there a role for occupational exposure? J Epidemiol Community Health. 1997;51:24–29. doi: 10.1136/jech.51.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matakidou A, Eisen T, Houlston RS. Systematic review of the relationship between family history and lung cancer risk. Br J Cancer. 2005 Oct 3;93(7):825–33. doi: 10.1038/sj.bjc.6602769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prochazka M, Hall P, Gagliardi G, Granath F, Nilsson BN, Shields PG, Tennis M, Czene K. Ionizing radiation and tobacco use increases the risk of a subsequent lung carcinoma in women with breast cancer: case-only design. J Clin Oncol. 2005 Oct 20;23(30):7467–74. doi: 10.1200/JCO.2005.01.7335. [DOI] [PubMed] [Google Scholar]
- 22.Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, Conway WA, Jr, Enright PL, Kanner RE, O'Hara P, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994 Nov 16;272(19):1497–505. [PubMed] [Google Scholar]
- 23.Yang P, Sun Z, Krowka MJ, Aubry MC, Bamlet WR, Wampfler JA, Thibodeau SN, Katzmann JA, Allen MS, Midthun DE, Marks RS, de Andrade M. Alpha1-antitrypsin deficiency carriers, tobacco smoke, chronic obstructive pulmonary disease, and lung cancer risk. Arch Intern Med. 2008 May 26;168(10):1097–103. doi: 10.1001/archinte.168.10.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hubbard R, Venn A, Lewis S, Britton J. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am J Respir Crit Care Med. 2000 Jan;161(1):5–8. doi: 10.1164/ajrccm.161.1.9906062. [DOI] [PubMed] [Google Scholar]
- 25.Goodman GE, Thornquist MD, Balmes J, et al. The beta-carotene and retinol efficacy trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping betacarotene and retinol supplements. J Natl Cancer Inst. 2004;96:1743–50. doi: 10.1093/jnci/djh320. [DOI] [PubMed] [Google Scholar]
- 26.Fontana RS, Sanderson DR, Taylor WF, Woolner LB, Miller WE, Muhm JR, Uhlenhopp MA. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Mayo Clinic study. Am Rev Respir Dis. 1984 Oct;130(4):561–5. doi: 10.1164/arrd.1984.130.4.561. [DOI] [PubMed] [Google Scholar]
- 27.Kubik A, Polak J. Lung cancer detection. Results of a randomized prospective study in Czechloslovakia. Cancer. 1986;57:2427. doi: 10.1002/1097-0142(19860615)57:12<2427::aid-cncr2820571230>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 28.Melamed MR, Flehinger BJ, Zaman MB, Heelan RT, Perchick WA, Martini N. Screening for early lung cancer. Results of the Memorial Sloan-Kettering study in New York. Chest. 1984 Jul;86(1):44–53. doi: 10.1378/chest.86.1.44. [DOI] [PubMed] [Google Scholar]
- 29.National Lung Screening Trial (NLST) [5/26/2010]; Available online: http://clinicaltrials.gov/
- 30.van Iersel CA, de Koning HJ, Draisma G, Mali WP, Scholten ET, Nackaerts K, et al. Int J Cancer. 4. Vol. 120. 2007. Risk-based selection from the general population in a screening trial: selection criteria, recruitment and power for the Dutch–Belgian randomised lung cancer multi-slice CT screening trial (NELSON) pp. 868–874. [DOI] [PubMed] [Google Scholar]
- 31.Steiling K, Ryan J, Brody JS, Spira A. The field of tissue injury in the lung and airway. Cancer Prev Res. 2008;1:396–403. doi: 10.1158/1940-6207.CAPR-08-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roy HK, Subramanian H, Damania D, Hensing TA, Rom WN, Pass HI, Ray D, Rogers JD, Bogojevic A, Shah M, Kuzniar T, Pradhan P, Backman V. Optical detection of buccal epithelial nanoarchitectural alterations in patients harboring lung cancer: implications for screening. Cancer Res. 2010 Oct 15;70(20):7748–54. doi: 10.1158/0008-5472.CAN-10-1686. Epub 2010 Oct 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Bergh KA, Essink-Bot ML, Borsboom GJ, et al. British Journal of Cancer. 1. Vol. 102. 2010. Jan 5, Short-term health-related quality of life consequences in a lung cancer CT screening trial (NELSON) pp. 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reich JM. A critical appraisal of overdiagnosis: estimates of its magnitude and implications for lung cancer screening. Thorax. 2008;63:377–83. doi: 10.1136/thx.2007.079673. [DOI] [PubMed] [Google Scholar]
- 35.Bach P. Overdiagnosis in lung cancer: different perspectives, definitions, implications. Thorax. 2008;63:298–300. doi: 10.1136/thx.2007.082990. [DOI] [PubMed] [Google Scholar]
- 36.Travis WD, Brambilla E, Muller-Hermlink HK, Harris CC, editors. Pathology and genetics of tumors of the lung, pleura, thymus and heart. IARC Press; Lyon: 2004. World Health Organization classification of tumors. [Google Scholar]
- 37.Rodenhuis S, Slebos RJC, Boot AJM, Evers SG, Mooi WJ, et al. Incidence and possible clinical significance of K-ras oncogene activation in adenocarcinoma of the human lung. Cancer Res. 1988;48:5738–5741. [PubMed] [Google Scholar]
- 38.Zell JA, Ou SH, Ziogas A, Anton-Culver H. Epidemiology of bronchioalveolar lung carcinoma: Evidence and implications of a multiclonal origin. Mod Pathol. 1994;7:633. [PubMed] [Google Scholar]
- 39.Breathnach OS, Kwiatkowski DJ, Finkelstein DM, Godleski J, Sugarbaker DJ, Johnson BE, Mentzer S. Bronchioloalveolar carcinoma of the lung: recurrences and survival in patients with stage I disease. J Thorac Cardiovasc Surg. 2001 Jan;121(1):42–7. doi: 10.1067/mtc.2001.110190. [DOI] [PubMed] [Google Scholar]
- 40.Wang YC, Chen CY, Chen SK, Cherng SH, Ho WL, Lee H. High frequency of deletion mutations in p53 gene from squamous cell lung cancer patients in Taiwan. Cancer Res. 1998 Jan 15;58(2):328–33. [PubMed] [Google Scholar]
- 41.Barsky SH, Cameron R, Osann KE, Tomita D, Holmes EC. Rising incidence of bronchioloalveolar lung carcinoma and its unique clinicopathologic features. Cancer. 1994 Feb 15;73(4):1163–70. doi: 10.1002/1097-0142(19940215)73:4<1163::aid-cncr2820730407>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 42.Liebow AA. Bronchiolo-alveolar carcinoma. Adv Intern Med. 1960;10:329. [PubMed] [Google Scholar]
- 43.Maziak De, Darling GE, Inculet RI, et al. Positron emission tomography in staging early lung cancer: a randomized trial. Ann Intern Med. 2009;151:221–228. doi: 10.7326/0003-4819-151-4-200908180-00132. [DOI] [PubMed] [Google Scholar]
- 44.Luke WP, Pearson FG, Todd TR, et al. Prospective evaluation of mediastinoscopy for assessment of carcinoma of the lung. J Thorac Cardiovasc Surg. 1986;91:53–56. [PubMed] [Google Scholar]
- 45.Vilmann P, Krasnick M, Larsen SS, Jacobsen GK, Clementsen P. Transesophageal endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) and endobronchial ultrasound -guided transbronchial needle aspiration (EBUS): a combined approach in the evaluation of mediastinal lesions. Endoscopy. 2005;37:833–839. doi: 10.1055/s-2005-870276. [DOI] [PubMed] [Google Scholar]
- 46.National Comprehensive Cancer Network (NCCN) Guidelines in Oncology: Non-Small Cell Carcinoma [cited 1; Available from: (http://www.nccn.org/professionals/physician_gls/PDF/nscl.pdf.)]
- 47.Schwartz AM, Henson DE. Diagnostic surgical pathology in lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:78S. doi: 10.1378/chest.07-1350. [DOI] [PubMed] [Google Scholar]
- 48.Ou SH, Zell JA, Anton-Culver H. Prognostic factors for survival of stage 1 nonsmall cell lung cancer patients: a population based analysis of 19, 702 stage 1 patients in the California Cancer Registry from 1989 to 2003. Cancer. 2007;110:1532–1541. doi: 10.1002/cncr.22938. [DOI] [PubMed] [Google Scholar]
- 49.Scott WJ, Howington J, Feigenberg S, Movsas B, Pisters K. Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:234S–242S. doi: 10.1378/chest.07-1378. [DOI] [PubMed] [Google Scholar]
- 50.Doddoli C, D'Journo B, Le Pimpec-Barthes F, et al. Lung cancer invading the chest wall: a plea for en-bloc resection but the need for new treatment strategies. Ann Thorac Surg. 2005;80:2032. doi: 10.1016/j.athoracsur.2005.03.088. [DOI] [PubMed] [Google Scholar]
- 51.Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 52.Daniels LJ, Balderson SS, Onaitis MW, et al. Thoracoscopic lobectomy: a safe and effective strategy for patients with stage I lung cancer. Ann Thorac Surg. 2002;74:860–864. doi: 10.1016/s0003-4975(02)03764-5. [DOI] [PubMed] [Google Scholar]
- 53.Mckenna RJ, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg. 2006;81:421–426. doi: 10.1016/j.athoracsur.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 54.Watanabe Y, Shimizu J, Hayashi Y, et al. Results of surgical treatment on patients with stage IIIA non-small lung cancer. Thorac Cardiovasc Surg. 1991;39:44–49. doi: 10.1055/s-2007-1013929. [DOI] [PubMed] [Google Scholar]
- 55.Johnstone DW, Byhardt RW, Ettinger D, et al. Phase III study comparing chemotherapy and radiotherapy with preoperative chemotherapy and surgical resection in patients with non-small cell lung cancer with spread to mediastinal lymph nodes (N2); final report of RTOG 89-01. Intern J Radiat Oncol Biol Phys. 2002;54:365–369. doi: 10.1016/s0360-3016(02)02943-7. [DOI] [PubMed] [Google Scholar]
- 56.Taylor NA, Liao ZX, Cox JD, et al. Equivalent outcome of patients with clinical stage IIIA non-small cell lung cancer treated with concurrent chemoradiation compared with induction chemotherapy followed by resection. Int J Radiat Oncol Biol Phys. 2004;58:204–212. doi: 10.1016/s0360-3016(03)01575-x. [DOI] [PubMed] [Google Scholar]
- 57.Van Meerbeeck JP, Kramer G, Van Schil PE, et al. A randomized trial of radical surgery versus thoracic radiotherapy in patients with stage IIIA-N2 non-small cell lung cancer after response to induction chemotherapy (EORTC 08941) J Clin Oncol. 2005;23(Suppl):7015. doi: 10.3816/clc.2000.n.020. abstract. [DOI] [PubMed] [Google Scholar]
- 58.Robinson LA, Ruckdeschel JC, Wagner H, Jr, Stevens CW, American College of Chest Physicians Treatment of non-small cell lung cancer-stage IIIA: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007 Sep;13224(3 Suppl):3S–265S. doi: 10.1378/chest.07-1379. [DOI] [PubMed] [Google Scholar]
- 59.Patterson GA, Piazza D, Pearson FG, Todd TR, Ginsberg RJ, Goldberg M, Waters P, Jones D, Ilves R, Cooper JD. Significance of metastatic disease in subaortic lymph nodes. Ann Thorac Surg. 1987 Feb;43(2):155–9. doi: 10.1016/s0003-4975(10)60386-4. [DOI] [PubMed] [Google Scholar]
- 60.Watanabe Y, Hayashi Y, Shimizu J, Oda M, Iwa T. Mediastinal nodal involvement and the prognosis of non-small cell lung cancer. Chest. 1991 Aug;100(2):422–8. doi: 10.1378/chest.100.2.422. [DOI] [PubMed] [Google Scholar]
- 61.Pairolero PC, Trastek VF, Payne WS. Treatment of bronchogenic carcinoma with chest wall invasion. Surg Clin North Am. 1987 Oct;67(5):959–64. doi: 10.1016/s0039-6109(16)44334-3. [DOI] [PubMed] [Google Scholar]
- 62.Piehler JM, Pairolero PC, Weiland LH, Offord KP, Payne WS, Bernatz PE. Bronchogenic carcinoma with chest wall invasion: factors affecting survival following en bloc resection. Ann Thorac Surg. 1982 Dec;34(6):684–91. doi: 10.1016/s0003-4975(10)60909-5. [DOI] [PubMed] [Google Scholar]
- 63.Harpole DH, Jr, Healey EA, DeCamp MM, Jr, Mentzer SJ, Strauss GM, Sugarbaker DJ. Chest wall invasive non-small cell lung cancer: patterns of failure and implications for a revised staging system. Ann Surg Oncol. 1996 May;3(3):261–9. doi: 10.1007/BF02306281. [DOI] [PubMed] [Google Scholar]
- 64.McCaughan BC, Martini N, Bains MS, McCormack PM. Chest wall invasion in carcinoma of the lung. Therapeutic and prognostic implications. J Thorac Cardiovasc Surg. 1985 Jun;89(6):836–41. [PubMed] [Google Scholar]
- 65.Port JL, Korst RJ, Lee PC, Kansler AL, Kerem Y, Altorki NK. Surgical resection for multifocal (T4) non-small cell lung cancer: is the T4 designation valid. Ann Thorac Surg. 2007 Feb;83(2):397–400. doi: 10.1016/j.athoracsur.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 66.Osaki T, Sugio K, Hanagiri T, Takenoyama M, Yamashita T, Sugaya M, Yasuda M, Yasumoto K. Survival and prognostic factors of surgically resected T4 non-small cell lung cancer. Ann Thorac Surg. 2003 Jun;75(6):1745–51. doi: 10.1016/s0003-4975(03)00037-7. [DOI] [PubMed] [Google Scholar]
- 67.Nagai K, Sohara Y, Tsuchiya R, Goya T, Miyaoka E. Japan Lung Cancer Registration Committee. Prognosis of resected non-small cell lung cancer patients with intrapulmonary metastases. J Thorac Oncol. 2007 Apr;2(4):282–6. doi: 10.1097/01.JTO.0000263709.15955.8a. [DOI] [PubMed] [Google Scholar]
- 68.de Perrot M, Fadel E, Mussot S, de Palma A, Chapelier A, Dartevelle P. Resection of locally advanced (T4) non-small cell lung cancer with cardiopulmonary bypass. Ann Thorac Surg. 2005 May;79(5):1691–6. doi: 10.1016/j.athoracsur.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 69.Albain KS, Rusch VW, Crowley JJ, Rice TW, Turrisi AT, 3rd, Weick JK, Lonchyna VA, Presant CA, McKenna RJ, Gandara DR, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995 Aug;13(8):1880–92. doi: 10.1200/JCO.1995.13.8.1880. [DOI] [PubMed] [Google Scholar]
- 70.Rusch VW, Giroux DJ, Kraut MJ, Crowley J, Hazuka M, Winton T, Johnson DH, Shulman L, Shepherd F, Deschamps C, Livingston RB, Gandara D. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160) J Clin Oncol. 2007 Jan 20;25(3):313–8. doi: 10.1200/JCO.2006.08.2826. [DOI] [PubMed] [Google Scholar]
- 71.Komaki R, Mountain CF, Holbert JM, Garden AS, Shallenberger R, Cox JD, Maor MH, Guinee VF, Samuels B. Superior sulcus tumors: treatment selection and results for 85 patients without metastasis (Mo) at presentation. Int J Radiat Oncol Biol Phys. 1990 Jul;19(1):31–6. doi: 10.1016/0360-3016(90)90130-c. [DOI] [PubMed] [Google Scholar]
- 72.Mountain CF. Expanded possibilities for surgical treatment of lung cancer: survival in stage IIIA disease. Chest. 1990;97111:1710. doi: 10.1378/chest.97.5.1045. [DOI] [PubMed] [Google Scholar]
- 73.Hu C, Chang EL, Hassenbusch SJ, 3rd, Allen PK, Woo SY, Mahajan A, Komaki R, Liao Z. Nonsmall cell lung cancer presenting with synchronous solitary brain metastasis. Cancer. 2006 May 1;106(9):1998–2004. doi: 10.1002/cncr.21818. [DOI] [PubMed] [Google Scholar]
- 74.Mintz A, Perry J, Spithoff K, Chambers A, Laperriere N. Management of single brain metastasis: a practice guideline. Curr Oncol. 2007 Aug;14(4):131–43. doi: 10.3747/co.2007.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS, Young B. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990 Feb 22;322(8):494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 76.Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, Kunieda E, Inomata T, Hayakawa K, Katoh N, Kobashi G. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006 Jun 7;295(21):2483–91. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 77.Bang Y, Kwak EL, Shaw AT, et al. Clinical activity of the oral ALK inhibitor PF-02341066 in ALK-positive patients with non-small cell lung cancer (abstract #3) J Clin Oncol. 2010;28:946s. [Google Scholar]
- 78.Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, Laack E, Wu YL, Bover I, Begbie S, Tzekova V, Cucevic B, Pereira JR, Yang SH, Madhavan J, Sugarman KP, Peterson P, John WJ, Krejcy K, Belani CP. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009 Oct 24;374(9699):1432–40. doi: 10.1016/S0140-6736(09)61497-5. Epub 2009 Sep 18. [DOI] [PubMed] [Google Scholar]
- 79.American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancer. Azzoli CG, Baker S, Jr, Temin S, Pao W, Aliff T, Brahmer J, Johnson DH, Laskin JL, Masters G, Milton D, Nordquist L, Pfister DG, Piantadosi S, Schiller JH, Smith R, Smith TJ, Strawn JR, Trent D, Giaccone G. J Clin Oncol. 2009 Dec 20;27(36):6251–66. doi: 10.1200/JCO.2009.23.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.West H, Lilenbaum R, Harpole D, Wozniak A, Sequist L. Molecular Analysis-based Treatment Strategies for the Management of Non-small Cell Lung Cancer. J Thorac Oncol. 2009;4:S1029–S1039. doi: 10.1097/JTO.0b013e3181b27170. [DOI] [PubMed] [Google Scholar]
- 81.Rodenhuis S, van de Wetering ML, Mooi WJ, Evers SG, van Zandwijk N, Bos JL. Mutational activation of the K-ras oncogene. A possible pathogenetic factor in adenocarcinoma of the lung. N Engl J Med. 1987;317(15):929–935. doi: 10.1056/NEJM198710083171504. [DOI] [PubMed] [Google Scholar]
- 82.Rodenhuis S, Slebos RJ, Boot AJ, et al. Incidence and possible clinical significance of K-ras oncogene activation in adenocarcinoma of the human lung. Cancer Res. 1988;48(20):5738–5741. [PubMed] [Google Scholar]
- 83.Mitsudomi T, Viallet J, Mulshine JL, Linnoila RI, Minna JD, Gazdar AF. Mutations of ras genes distinguish a subset of non-small-cell lung cancer cell lines from small-cell lung cancer cell lines. Oncogene. 1991;6(8):1353–1362. [PubMed] [Google Scholar]
- 84.Slebos RJ, Kibbelaar RE, Dalesio O, et al. K-ras oncogene activation as a prognostic marker in adenocarcinoma of the lung. N Engl J Med. 1990;323(9):561–565. doi: 10.1056/NEJM199008303230902. [DOI] [PubMed] [Google Scholar]
- 85.Nelson HH, Christiani DC, Mark EJ, Wiencke JK, Wain JC, Kelsey KT. Implications and prognostic value of K-ras mutation for early-stage lung cancer in women. J Natl Cancer Inst. 1999;91(23):2032–2038. doi: 10.1093/jnci/91.23.2032. [DOI] [PubMed] [Google Scholar]
- 86.Riely GJ, Kris MG, Rosenbaum D, et al. Frequency and distinctive spectrum of KRAS mutations in never smokers with lung adenocarcinoma. Clin Cancer Res. 2008;14(8):5731–5734. doi: 10.1158/1078-0432.CCR-08-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mascaux C, Iannino N, Martin B, et al. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer. 2005;92(1):131–139. doi: 10.1038/sj.bjc.6602258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schiller JH, Adak S, Feins RH, et al. Lack of prognostic significance of p53 and K-ras mutations in primary resected non-small-cell lung cancer on E4592: a Laboratory Ancillary Study on an Eastern Cooperative Oncology Group Prospective Randomized Trial of Postoperative Adjuvant Therapy. J Clin Oncol. 2001;19(2):448–457. doi: 10.1200/JCO.2001.19.2.448. [DOI] [PubMed] [Google Scholar]
- 89.Graziano SL, Gamble GP, Newman NB, et al. Prognostic significance of K-ras codon 12 mutations in patients with resected stage I and II non-small-cell lung cancer. J Clin Oncol. 1999;17(2):668–675. doi: 10.1200/JCO.1999.17.2.668. [DOI] [PubMed] [Google Scholar]
- 90.Tsao MS, Aviel-Ronen S, Ding K, et al. Prognostic and predictive importance of p53 and RAS for adjuvant chemotherapy in non small-cell lung cancer. J Clin Oncol. 2007;25(33):5240–5247. 5247. doi: 10.1200/JCO.2007.12.6953. [DOI] [PubMed] [Google Scholar]
- 91.Miller VA, Riely GJ, Zakowski MF, Li AR, Patel JD, Heelan RT, Kris MG, Sandler AB, Carbone DP, Tsao A, Herbst RS, Heller G, Ladanyi M, Pao W, Johnson DH. Molecular characteristics of bronchioloalveolar carcinoma and adenocarcinoma, bronchioloalveolar carcinoma subtype, predict response to erlotinib. J Clin Oncol. 2008 Mar 20;26(9):1472–8. doi: 10.1200/JCO.2007.13.0062. [DOI] [PubMed] [Google Scholar]
- 92.Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–5909. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 93.Hirsch FR, Scagliotti GV, Langer CJ, Varella-Garcia M, Franklin WA. Epidermal growth factor family of receptors in preneoplasia and lung cancer: perspectives for targeted therapies. Lung Cancer. 2003;41(Suppl 1):S29–42. doi: 10.1016/s0169-5002(03)00137-5. [DOI] [PubMed] [Google Scholar]
- 94.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 95.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 96.Shigematsu H, Gazdar AF. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. International Journal of Cancer. 2006;118:257–262. doi: 10.1002/ijc.21496. [DOI] [PubMed] [Google Scholar]
- 97.Pietanza M, D'Angelo SP, Johnson ML, Paik PK, Riely GJ, Miller VA, Zakowski MF, Rusch VW, Ladanyi M, Kris MG. EGFR mutations in men and cigarette smokers with lung adenocarcinoma. Clin Oncol. 2010;28:15s. doi: 10.1200/JCO.2010.32.6181. suppl; abstr 10538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mitsudomi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Science. 2007;98:1817–1824. doi: 10.1111/j.1349-7006.2007.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Greulich H, Chen TH, Feng W, Janne PA, Alvarez JV, Zappaterra M, et al. Oncogenic transformation by inhibitor-sensitive and -resistant EGFR mutants. PLoS Med. 2005;2:e313. doi: 10.1371/journal.pmed.0020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Amann J, Kalyankrishna S, Massion PP, et al. Aberrant epidermal growth factor receptor signaling and enhanced sensitivity to EGFRinhibitors in lung cancer. Cancer Res. 2005;65:226–35. [PubMed] [Google Scholar]
- 101.Ji H, Li D, Chen L, et al. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell. 2006;9:485–95. doi: 10.1016/j.ccr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 102.Cox G, Jones JL, O'Byrne KJ. Matrix metalloproteinase 9 and the epidermal growth factor signal pathway in operable non-small cell lung cancer. Clin Cancer Res. 2000;6(6):2349–2355. [PubMed] [Google Scholar]
- 103.Ohsaki Y, Tanno S, Fujita Y, et al. Epidermal growth factor receptor expression correlates with poor prognosis in non-small cell lung cancer patients with p53 overexpression. Oncol Rep. 2000;7(3):603–607. doi: 10.3892/or.7.3.603. [DOI] [PubMed] [Google Scholar]
- 104.Volm M, Rittgen W, Drings P. Prognostic value of ERBB-1, VEGF, cyclin A, FOS, JUN and MYC in patients with squamous cell lung carcinomas. Br J Cancer. 1998;77(4):663–669. doi: 10.1038/bjc.1998.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Parra HS, Cavina R, Latteri F, et al. Analysis of epidermal growth factor receptor expression as a predictive factor for response to gefitinib (‘Iressa’, ZD1839) in non-small-cell lung cancer. Br J Cancer. 2004;91(2):208–212. doi: 10.1038/sj.bjc.6601923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pastorino U, Andreola S, Tagliabue E, et al. Immunocytochemical markers in stage I lung cancer: relevance to prognosis. J Clin Oncol. 1997;15(8):2858–2865. doi: 10.1200/JCO.1997.15.8.2858. [DOI] [PubMed] [Google Scholar]
- 107.Pfeiffer P, Clausen PP, Andersen K, Rose C. Lack of prognostic significance of epidermal growth factor receptor and the oncoprotein p185HER-2 in patients with systemically untreated non-smallcell lung cancer: an immunohistochemical study on cryosections. Br J Cancer. 1996;74(1):86–91. doi: 10.1038/bjc.1996.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rusch V, Klimstra D, Venkatraman E, Pisters PW, Langenfeld J, Dmitrovsky E. Overexpression of the epidermal growth factor receptor and its ligand transforming growth factor α is frequent in resectable non-small cell lung cancer but does not predict tumor progression. Clin Cancer Res. 1997;3(4):515–522. [PubMed] [Google Scholar]
- 109.Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25(12):1545–1552. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 110.Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23(25):5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 111.Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial – INTACT 1. J Clin Oncol. 2004;22(5):777–784. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 112.Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial – INTACT 2. J Clin Oncol. 2004;22(5):785–794. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 113.Bezjak A, Tu D, Seymour L, et al. Symptom improvement in lung cancer patients treated with erlotinib: quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol. 2006;24(24):3831–3837. doi: 10.1200/JCO.2006.05.8073. [DOI] [PubMed] [Google Scholar]
- 114.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 115.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7(3):169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 116.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K, Hatanaka H, Bando M, Ohno S, Ishikawa Y, Aburatani H, Niki T, Sohara Y, Sugiyama Y, Mano H. Identification of the transforming EML4-ALK fusion gene in non-smallcell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 117.Takahashi T, Sonobe M, Koboyashi M, et al. Clinicopathologic features of non-small cell lung cancer with EML4-ALK fusion gene. Ann Surg Oncol. 2010;17:889. doi: 10.1245/s10434-009-0808-7. [DOI] [PubMed] [Google Scholar]
- 118.Wong DW, Leung EL, So KK, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer. 2009;115:1723–1733. doi: 10.1002/cncr.24181. [DOI] [PubMed] [Google Scholar]
- 119.Koivunen JP, Mermel C, Zejnullahu K, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14:4275–4283. doi: 10.1158/1078-0432.CCR-08-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 121.Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol. 2008;3:13–17. doi: 10.1097/JTO.0b013e31815e8b60. [DOI] [PubMed] [Google Scholar]
- 122.Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Mod Pathol. 2009;22:508–515. doi: 10.1038/modpathol.2009.2. [DOI] [PubMed] [Google Scholar]
- 123.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non–small-cell lung cancer harboring EML4-ALK. J Clin Oncol. 2009;27:4247–4253. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hwang SJ, Cheng LS, Lozano G, Amos CI, Gu X, Strong LC. Lung cancer risk in germline p53 mutation carriers: association between an inherited cancer predisposition, cigarette smoking, and cancer risk. Hum Genet. 2003;113:238–43. doi: 10.1007/s00439-003-0968-7. [DOI] [PubMed] [Google Scholar]
- 124.Soda M. A mouse model for EML4-ALK rearrangement in non-small cell lung cancer and non-tumor lung tissues. Am J Pathol. 2009;174:661. doi: 10.2353/ajpath.2009.080755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Christensen JG, Zou HY, Arango ME, Li Q, Lee JH, McDonnell SR, Yamazaki S, Alton GR, Mroczkowski B, Los G. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol Cancer Ther. 2007 Dec;6(12 Pt 1):3314–22. doi: 10.1158/1535-7163.MCT-07-0365. [DOI] [PubMed] [Google Scholar]
- 126.McDermott U, Iafrate AJ, Gray NS, Shioda T, Classon M, Maheswaran S, Zhou W, Choi HG, Smith SL, Dowell L, Ulkus LE, Kuhlmann G, Greninger P, Christensen JG, Haber DA, Settleman J. Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Res. 2008 May 1;68(9):3389–95. doi: 10.1158/0008-5472.CAN-07-6186. [DOI] [PubMed] [Google Scholar]
- 127.National Institutes of Health Clinical Trials database. [July 7th, 2010]; http://www.clinicaltrials.gov/
- 128.Mento AJ, Martinelli P, Jones RM. The Journal of Management Development. Vol. 18. Bradford; 1999. p. 390. [Google Scholar]


