Abstract
A trace element preconcentration procedure is described utilizing a minicolumn of yeast (Yamadazyma spartinae) immobilized TiO2 nanoparticles for determination of Cr, Cu, Fe, Mn, Ni and Zn from water samples by inductively coupled plasma atomic emission spectrometry. The elements were quantitatively retained on the column between pH 6 and 8. Elution was made with 5% v/v HNO3 solution. Recoveries ranged from 98 ± 2 (Cr) to 100 ± 4 (Zn) for preconcentration of 50 mL multielement solution (50 µg L−1). The column made up of 100 mg sorbent (yeast immobilized TiO2 NP) offers a capacity to preconcentrate up to 500 mL of sample solution to achieve an enrichment factor of 250 with 2 mL of 5% v/v HNO3 eluent. The detection limits obtained from preconcentration of 50 mL blank solutions (5% v/v HNO3, n =11) were 0.17, 0.45, 0.25, 0.15, 0.33 and 0.10 µg L−1 for Cr, Cu, Fe, Mn, Ni and Zn, respectively. Relative standard deviation (RSD) for five replicate analyses was better than 5%. The retention of the elements was not affected from up to 500 µg mL−1 Na+ and K+ (as chlorides), 100 µg mL−1 Ca2+ (as nitrate) and 50 µg mL−1 Mg2+ (as sulfate). The method was validated by analysis of freshwater standard reference material (SRM 1643e) and applied to the determination of the elements from tap water and lake water samples.
Keywords: Trace element, Preconcentration, TiO2 nanoparticles, Yamadazyma spartinae, Inductively coupled plasma atomic emission spectrometry, Water
1. Introduction
Heavy metal contamination has been a serious issue because of the adverse effects of heavy metals on human and environmental health, which in due course necessitates the development of rapid and sensitive methods for accurate determination of heavy metals [1, 2]. Inductively coupled plasma atomic emission spectrometry (ICP-AES) is widely recognized as a multi-element technique for the determination of elemental species, though direct determinations in environmental samples at trace levels is difficult due to insufficient detection limits of ICP-AES. This limitation can be overcome by the using enrichment methods in that metals ions of interest from solution solutions are selectively separated and concentrated into smaller volumes to achieve better detection by ICP-AES. This task can be achieved by using various methods, such as ion exchange [3, 4], cloud point extraction [5], electro-deposition [6], coprecipitation [7], membrane filtration [8], dispersive liquid–liquid micro extraction (DLLME) [9] flotation [10] and solid-phase extraction (SPE) [11–16]. Among these methods, SPE is the most frequently employed as it affords the highest attainable enrichment factors with relatively rapid separation and low cost [15].
In SPE applications, preconcentration of the metal ions in solutions is achieved by means of a suitable chelating agent that is either added into the sample solution (off-line) or immobilized onto a solid support material, such as activated alumina [16–20]. Various materials, including 1-nitroso-2-naphthol [17], 8-hydroxyquinoline [18], gallic acid [19] and boric acid [20] that are immobilized on solid supports have been successfully used in trace element preconcentration. A similar tactic to this approach is the immobilization of microorganisms (also known as biosorption) on a solid support and passing solutions through the column containing solid phase (biosorbent) [11, 21, 22]. In the last two decades, the biosorption process has been studied extensively using microbial biomass as biosorbents for metal removal and preconcentration of trace metals [21–29]. Metal ion uptake by biosorption may involve the contribution of diffusion, adsorption, chelation, complexation, and coordination or micro-precipitation mechanisms depending on the specific substrate (biomass). Biological microorganisms including fungi [22], yeast [25, 26], bacteria [27], and algae [28, 29] have been shown to efficiently accumulate metal ions from aqueous solutions. Recently, several review articles [30–32] are published that illustrate the latest developments in the biosorption of metals by microbial biomass for analyte preconcentration, matrix separation and speciation analysis. Various support materials, such as carbon nano tubes [32], nano alumina [33], iron oxide (Fe3O4) [34], silica [35] and titanium oxide (TiO2) nanoparticles [20, 21, 36] have been employed in biomass immobilization. The use of nanometer size solid support has the added advantage of increasing the active surface area, and thus is becoming increasingly popular in biosorption applications [20, 21, 32, 36].
Yeast are known to accumulate metal ions from solution to substantial levels as they exhibit high tolerance to heavy metals. In this studty, we investigated the performance characteristics of a new strain of yeast, Yamadayzma spartinae, for metal biosorption in an effort to develop a solid phase extraction method for determination of trace metals from water samples. This strain of yeast is highly resistant to toxicity of heavy metals, and thus offers potential for bioaccumulation, bioremediation and biosorption of metals ions from natural waters [37]. Dried biomass of Y. spartinae were immobilized on TiO2 nanoparticles, which was found to be highly selective for preconcentration of Cr, Cu, Fe, Mn, Ni and Zn from aqueous solutions. To achieve the quantitative determination of these elements, the experimental conditions, including load pH of sample solution, mass of solid phase, flow rate of sample solution, and the concentration and volume of eluent were optimized. The performance of the method was verified by analysis of freshwater standard reference material (SRM 1643e) and spiked natural water samples by ICP-AES.
2. Experimental
2.1. Instrumentation
A Perkin Elmer (Shelton, CT, USA) Optima 3300 DV ICP-AES instrument is used throughout. The instrument is optimized for sensitivity with 2 µg mL−1 Mn solution as needed. Data collection was achieved by ICP-WinLab software package (version 3.1). Elemental measurements were made in axial view mode using the recommended wavelengths. The operation conditions for the instrument are summarized in Table 1. An OAKTON 510 model digital pH meter with glass electrode was used to adjust the pH of the solutions.
Table 1.
Operating conditions for Perkin Elmer Optima 3300 DV ICP-AES instrument
| RF power (kW) | 1.5 |
| Nebulizer | Cross-flow |
| Spray chamber | Glass concentric |
| Plasma Ar (L min−1) | 16 |
| Auxiliary Ar (L min−1) | 0.4 |
| Nebulizer Ar (L min−1) | 0.6 |
| Sample uptake (mL min−1) | 1.5 |
| Delay time (s) | 45 |
| Scanning mode | Continuous, axial |
| Integration time (s) | Automatic (min. 0.5 – max. 5 s) |
| Readings/replicate | 3 |
| Wavelength (nm) | Cr: 267.716; Cu: 324.754; Fe: 259.940; Mn: 257.610; Ni: 231.604; Zn: 213.856 |
2.2. Reagents and standard solutions
Reagents of analytical and spectral purity were used. Deionized water obtained from Barnstead E-pure system (17.5 MΩ cm) was used to prepare all solutions. Standard stock solution of Cr, Cu, Fe, Mn, Ni and Zn (1,000 µg L−1) were purchased from Spex Certiprep (Metuchen, NJ). A 10 µg mL−1 multi-element solution was prepared in 2% v/v HNO3 from individual single element standard solutions and used throughout the study. Trace metal grade HCl and NH4OH were used for adjusting the pH of the sample solutions. Titanium oxide nano-powder (100 nm TiO2) purchased from Sigma Aldrich was used as solid support for immobilization of yeast biomass. The yeast culture (Yamadazyma spartinae) was purchased from ATCC™ (No: 18866) and kept frozen at −4 °C until use.
2.3. Water samples and preparation
Freshwater standard reference material (SRM 1643e) was purchased from National Institute of Standards & Technology, Gaithersburg, MD. Tap water samples were obtained from Jackson State University campus water. Lake water samples were collected from local lakes in Jackson MS, USA. Tap water and lake water samples were filtered through a 0.45 µm pore-size Millipore cellulose nitrate membrane to remove any fine particulate matter present and then acidified to 0.2% v/v HNO3.
2.4. Cultivation and preparation of biomass
A solid medium containing agar (Pateto Dekstro Agar (PDA-Oxoid), Potato extract 4.0 g L–1, Glucose 20.0 g L–1) was used for the cultivation of the laboratory strain of Y. spartinae prior to storage. Y. spartinae was stored in a refrigerator at 4 °C. The liquid medium to produce starter culture was prepared by dissolving 0.3 g malt extract, 0.3 g yeast extract, 1.0 g glucose, 0.5 g peptone in 100 mL water. This liquid medium was sterilized by autoclaving at 120 °C. Y. spartinae was inoculated into the liquid medium from the solid medium and incubated with string on an orbital shaker (250 rpm) for about 24 hours at the room temperature. For preparing the experimental culture, 200 mL of liquid medium was further prepared and inoculated with 10 mL of the starter culture and incubated on a shaker for 24 h at the room temperature. The yeast grown in the liquid medium was separated from the growth media by filtering through 0.45 µm filter paper (Whatman). The isolated biomass was washed with doubly distilled water and dried at 100±10 °C for about 1 h.
2.5. Preparation of column
Before immobilization of biomass, TiO2 nanopowder was cleaned by treating with 5 mL of 5% HNO3 and then with 5 mL of 5% HCl solution and finally washed with water to remove surface contamination. The immobilization of Y. spartinae was performed according to the procedure described elsewhere [27]. Briefly, 300 mg of powdered dried biomass was mixed with 3 g of decontaminated TiO2 nanopowder. The mixture was wetted with 2 mL of pure distilled water and thoroughly mixed. After mixing, the paste was heated in an oven at 110 ± 10 °C for approximately 1 h to dry the mixture. Higher temperatures were not used to avoid charring of biomass during drying. The wetting and drying steps were repeated to maximize the immobilization of the yeast biomass on TiO2 nanopowder and then used as a solid phase in the column without any further physical and chemical treatment.
2.6. Procedure
A glass column (15 cm×0.4 cm i.d.) equipped with a valve to control the solution flow rate and a 50-mL reservoir at the top was used to prepare analytical column. A small amount of glass wool was placed at the bottom of the glass column above the valve, and then 100 mg of Y. spartinae immobilized-TiO2 nanopowder was slurried in water, and uniformly dispersed onto the glass wool. Another piece of glass wool plug was placed at the top of the biosorbent. Total height of the biosorbent bed was approximately 1 cm in the glass column. Before use, 5 mL of 5% v/v HCl and 5 mL of 5% v/v HNO3 solution were passed through and then the column was neutralized with deionized water. The pH of the sample or standard solutions were adjusted off-line to the desired value with dilute HCl and NH4OH, or alternatively with dilute HCl and ammonium acetate solution (pH 10), and then passed through the column at a flow rate of 1.0 mL min−1. The retained elements were eluted into 5 mL of 5% v/v HNO3 solution at a flow rate of 0.5 mL min−1. All preconcentrated solutions were then analyzed by ICP-AES for the trace elements of interest.
Results and discussion
3.1. Effect of the pH
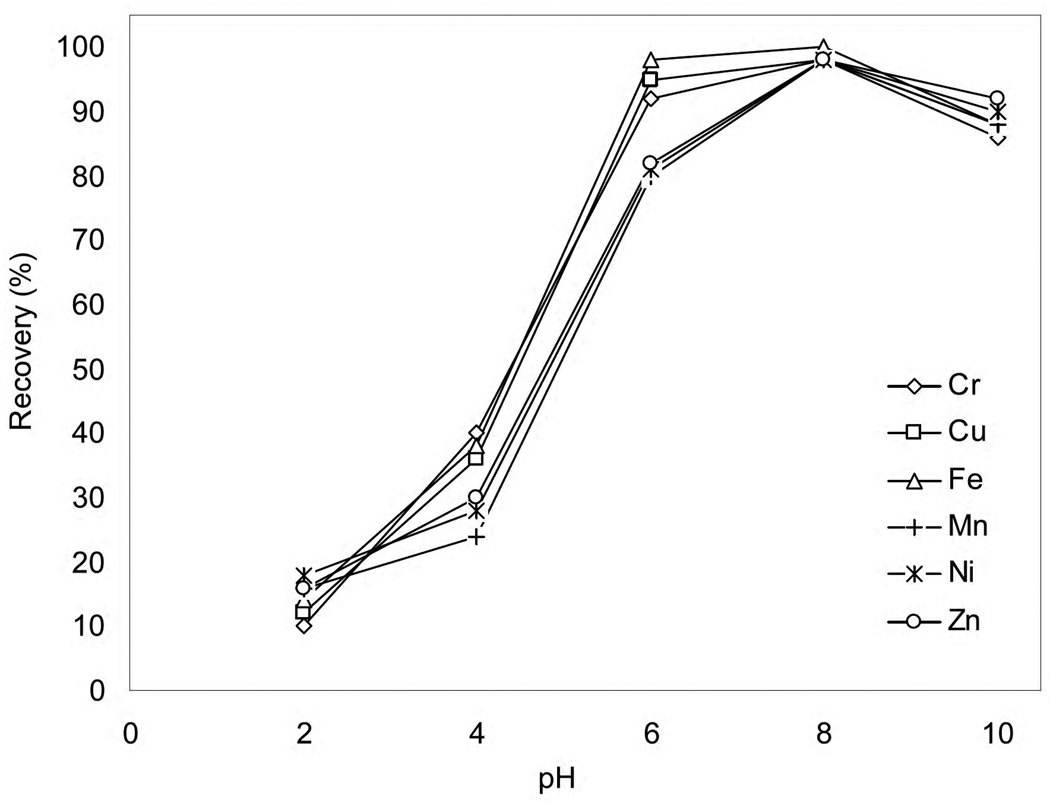
In highly acidic solutions, the binding sites on the column are protonated while alkaline conditions may lead to the precipitation of metal ions as hydroxides. The pH (acidity) of a solution, therefore, is an important variable that influences the retention process. The effect of solution pH on the retention pattern of the trace elements on Y. spartinae immobilized nano-TiO2 column is illustrated in Fig. 1. While Cr, Cu and Fe were retained quantitatively between pH 6 and 8, the recoveries for Mn, Ni and Zn were about 80% at pH 6. Highest signals for these elements were obtained at pH 8. Because the cell walls of the yeast are negatively charged, the decrease in recoveries with decreasing pH was due to the competition between protons and the metal ions for the adsorption sites on the column. Recoveries tended to decrease above pH 8.5 which could arise from the formation of anionic hydroxide complexes as well as the competition between the ligand of cell wall and ammonia. Based on the results from Fig. 1, a pH range of 7.8 to 8.2 appears to be the suitable to achieve quantitative retention of all elements of interest simultaneously.
Fig. 1.
The effect of pH on the recoveries for a 50 ng mL−1 solution (50 mL) of Cr, Cu, Fe, Mn, Ni and Zn. Mass of biosorbent = 100 mg; flow rate = 1 mL min−1; eluent = 5 mL, 5% (v/v) HNO3.
3.2. Effects of biosorbent mass and eluents on recovery
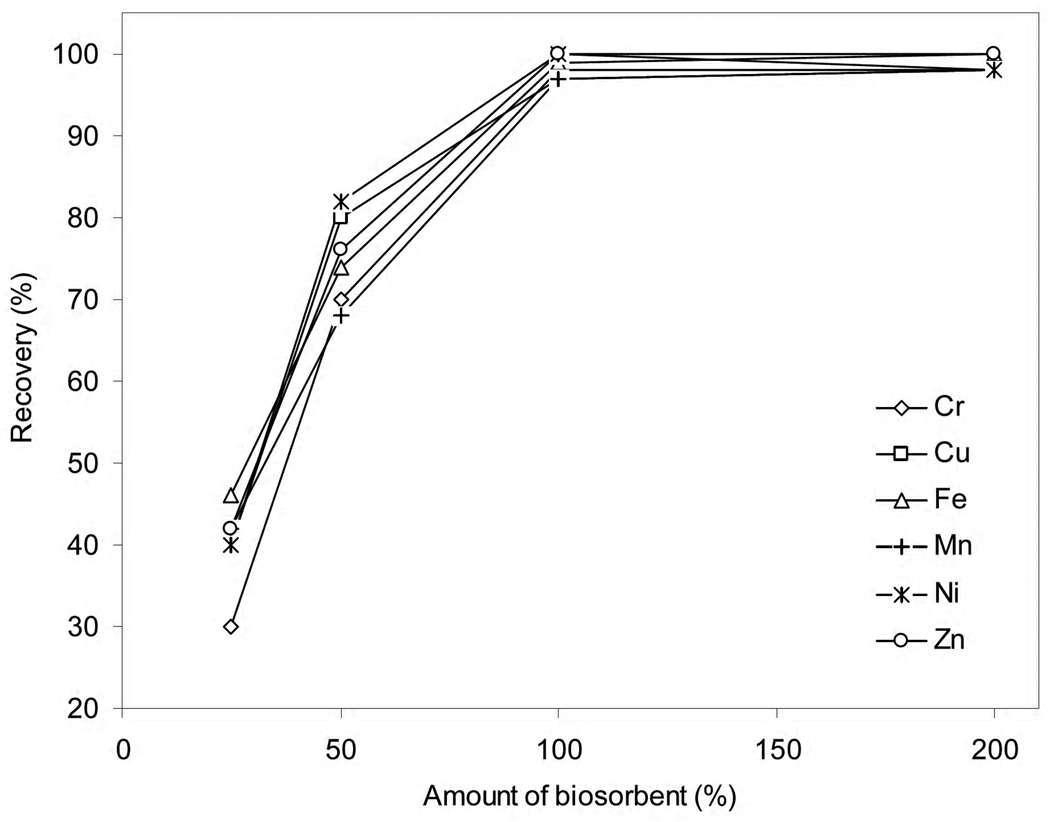
For quantitative determination, the amount of biosorbent is critical to provide sufficient interaction for the retention of the metal ions. Excess amount of biosorbent may not only decrease throughput by reducing the solution flow rate, but also it may lead to incomplete elution of the elements from the column by small volume of eluent, which in due course deteriorates both precision and accuracy. Thus, the amount of biosorbent placed into the column was optimized at pH 6 and 8. The results indicate that a minimum of 100 mg of sorbent should be used to achieve quantitative recoveries (Fig. 2). Retention was lower for smaller masses of biosorbent because of the weaker interaction and loss of the elements from the column during loading, and thus, the column was filled with 100 mg of biomass in further experiments. Higher masses of biosorbent did not show any improvement, but rather compromised the throughput as the flow rate was reduced substantially. Preliminary studies with nano-TiO2 support (100 mg) without biomass yielded inadequate retention (ca. 52–60%), especially at pH 6. At pH 8, recoveries were somewhat better around 75–80%, which was probably due to the retention of colloidal metal hydroxides on the column. These results in light of the better recoveries with increasing biosorbent mass (Fig. 2) confirm that both yeast biomass and nano-TiO2 support contribute to the retention of the elements.
Fig. 2.
The effect of amount of biomass on the recoveries for a 50 ng mL−1 solution (50 mL) of Cr, Cu, Fe, Mn, Ni and Zn. Mass of biosorbent = 100 mg; flow rate = 1 mL min−1; eluent = 2 mL, 5% (v/v) HNO3.
For elution, 1, 2 and 5 mL of 5% v/v HCl and 5% v/v HNO3 were studied. Recoveries were consistently higher with HNO3 when compared with same volume and concentration of HCl. When elution was performed with 1 mL of 5% HNO3, average recoveries ranged between 81% for Ni to 86% for Cu. For complete removal of the elements from the column, 2 mL of 5% HNO3 was sufficient, which afforded a preconcentration factor of 25 for a sample volume of 50 mL. Elemental recoveries for 5 mL HNO3 were not any significantly different from those of 2 mL, but yielded lower a preconcentration factor.
3.3. Effects of the flow rate and sample volume
In SPE utilizing an analytical column, the flow rate of the solution not only affects the retention of elements of interest but also the total analysis time. Under the optimum pH, the flow rate of the sample solution (50 µg L−1) was varied from 0.5 to 2.0 mL min−1 to determine the optimum range. All elements were quantitatively retained on the column when loaded up to 2 mL min−1. In the subsequent experiments, the flow rate was kept constant at 2.0 mL min−1.
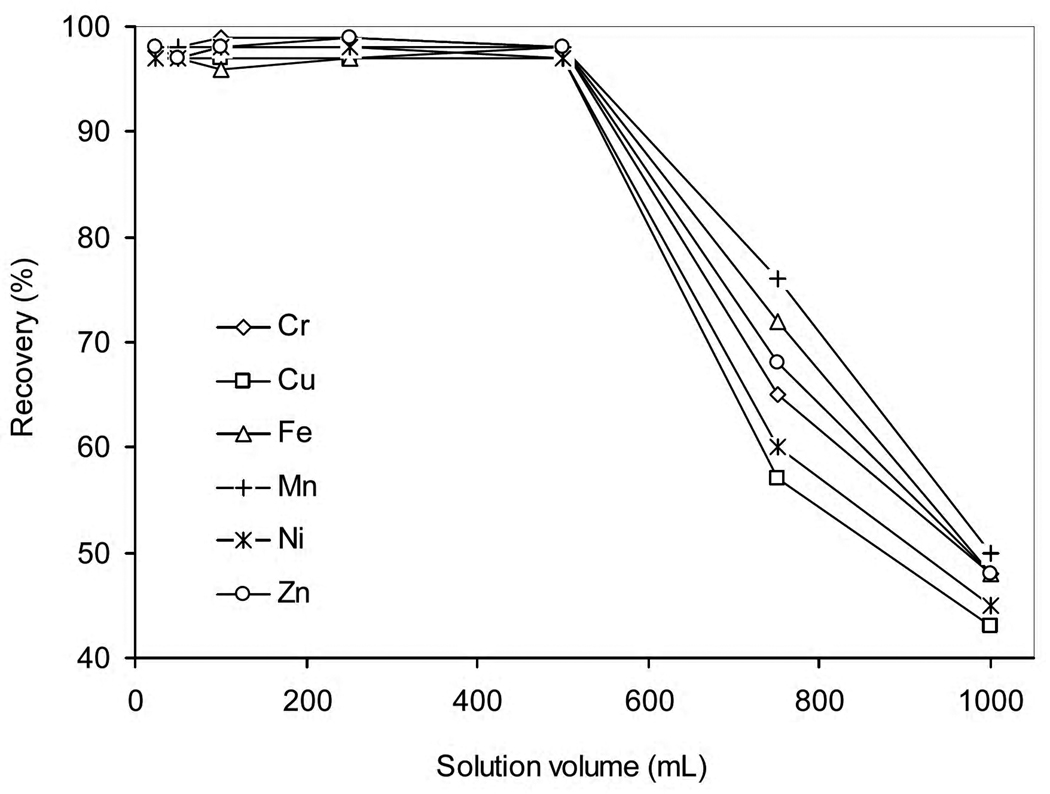
The capacity of the column was examined by loading 25, 50, 100, 250, 500, 750 and 1000 mL volumes of sample solutions containing 100, 50, 25, 10, 5, 3.33 and 2.5 µg L−1 of Cr, Cu, Fe, Mn, Ni and Zn, respectively. The recoveries for the elements of interest are shown in Fig. 3. All elements were quantitatively retained for up to 500 mL solution, which when eluted into 2 mL 5% HNO3 would yield a preconcentration factor of 250.
Fig. 3.
The effect of sample volume on elemental recoveries. Analyte mass was kept constant in each volume. Mass of biosorbent = 100 mg; flow rate = 2 mL min−1; eluent = 2 mL, 5% (v/v) HNO3.
3.4. Analytical performance and detection limits
Alkaline and alkaline earth elements increase the concentration of total dissolved solids and thereby interfere with the retention of the trace transition metals on the column. The influences of Na+, K+, Ca2+ and Mg2+ were investigated for high-purity (99.99%) salts of NaCl, KCl, Ca(NO3)2 and MgSO4. The tolerance limit, defined as the ion concentration causing a relative error of ±5%, was measured for different concentrations of each salt. The column exhibited high tolerance up to 500 µg mL−1 NaCl and KCl in solution. For salts of Ca and Mg, maximum concentrations were about 100 µg mL−1 for Ca(NO3)2 and 50 µg mL−1 for MgSO4. Concentrations above 100 µg mL−1 Ca(NO3)2 and 50 µg mL−1 MgSO4 reduced the retention performance below 85%. Still, these tolerance limits were adequate to provide accurate determination of Cr, Cu, Fe, Mn, Ni and Zn in natural water samples using Y. spartinae immobilized on nano-TiO2 sorbent.
Calibration was made with multi-element solutions containing 0, 1, 2, 5, and 10 µg L−1 in 50 mL volume. Each solution was passed through the column and collected in 2 mL of 5% v/v HNO3. The calibration graph was linear (r2 = 0.994–1.00) for Cr, Cu, Fe, Mn, Ni and Zn. The detection limits were calculated by 3s method for preconcentration of 50 mL blank solutions, (5% v/v HNO3, n = 11) processed as the calibration standards. The pH of the blank solutions were adjusted to pH 8 and passed through the column and then eluted into 2 mL of 5% v/v HNO3 yielding a enrichment factor of about 25. Under the optimum conditions, the detection limits were found to be 0.17, 0.45, 0.25, 0.15, 0.33 and 0.10 µg L−1 for Cr, Cu, Fe, Mn, Ni and Zn, respectively. The precision (% RSD) for three replicate measurements was better than 5% with mean recoveries of 98±2%, 99±2%, 100±2%, 99±2%, 99±4% and 100±4% for Cr, Cu, Fe, Mn, Ni and Zn, respectively, from preconcentration of 50 mL multielement solution (50 µg L−1) into 2 mL 5% v/v HNO3 (Table 2).
Table 2.
Recoveries for preconcentration of 50 mL of multi-element solution (50 µg L−1). Detection limits are obtained from preconcentration of 50 mL of 5% v/v HNO3 (n = 11)
| Element | Recovery (%) |
Detection limit (µg L−1) |
|---|---|---|
| Cr | 98 ± 2 | 0.17 |
| Cu | 99 ± 2 | 0.45 |
| Fe | 100 ± 2 | 0.25 |
| Mn | 99 ± 2 | 0.15 |
| Ni | 99±4 | 0.33 |
| Zn | 100 ± 4 | 0.10 |
Uncertainty at 95% confidence limit (n = 5)
The stability of the column was also tested by monitoring the changes in the elemental recoveries of Cr, Cu, Fe, Mn, Ni and Zn through several adsorption-elution cycles. In each cycle, 50 mL multi-element solution (50 µg L−1) was passed through the column and then stripped with 5 mL of 5% HNO3. This procedure was carried out ten times in a day followed by another ten runs in subsequent days. It was found that the column was stable up to 50–60 runs for Cr, Cu, Fe, Mn, Ni and Zn without any changes in the performance. Notable degradation occurred in retention when column was used over 70 cycles.
3.5. Analysis of real samples
The method was validated by determination of Cr, Cu, Fe, Mn, Ni and Zn an NIST freshwater standard reference material (SRM 1643e) and then applied to the determination of the concentration of the elements in tap water and lake water samples. For analysis of SRM 1643e, 20 mL sample solutions (n = 3) were passed through the column and then collected into 2 mL 5% HNO3. The experimental values agreed with the certified values within 95% confidence interval (Table 3). The relative error (−4 to −7%) was acceptable for quantitative trace analysis, though recoveries were 3 to 6% lower, unlike those from multielement solutions (98–100%) in deionized water. This reduction in recoveries could be induced by the matrix ions of about Ca (33.3 µg mL−1), Mg (8.0 µg mL−1) and Na (20.7 µg mL−1) in the SRM 1643e. The recoveries from tap water and lake water samples (Table 4) were also deemed accurate within 95% confidence interval suggesting that the preconcentration method by using Y. spartinae immobilized nano-TiO2 could be suitably used for determination of the trace elements in freshwater samples by ICP-AES.
Table 3.
Results for Cr, Cu, Fe, Mn, Ni and Zn from the analysis of freshwater standard reference material (SRM 1643e)
| Element | Certified value (µg L−1) |
Found (µg L−1)a |
Relative error (%) |
|---|---|---|---|
| Cr | 19.9±0.23 | 18.6±0.3 | −7 |
| Cu | 22.20±0.31 | 20.8±0.4 | −6 |
| Fe | 95.7±1.4 | 90.2±1.6 | −6 |
| Mn | 38.02±0.44 | 35.6±0.5 | −6 |
| Ni | 60.89±0.67 | 58.4±0.8 | −4 |
| Zn | 76.5±2.1 | 72.7±2.2 | −5 |
Mean of three determinations at 95 % confidence level .
Table 4.
Results for Cr, Cu, Fe, Mn, Ni and Zn from tap water and lake water samples
| Element | Added (µg L−1) |
Found (µg L−1) |
|
|---|---|---|---|
| Tap water | Lake water | ||
| Cr | - | 24.2±1.2 | 34.4±1.2 |
| 50 | 70.6±2.1 | 80.2±2.1 | |
| Cu | - | 30.6±1.3 | 40.6±1.6 |
| 50 | 74.6±2.2 | 84.4±2.6 | |
| Fe | - | 45.6±2.6 | 57.6±2.2 |
| 50 | 88.0±2.8 | 102±2.8 | |
| Mn | - | 35.4±1.2 | 42.4±1.6 |
| 50 | 80.2±2.6 | 88.2±2.8 | |
| Ni | - | 10.2±0.4 | 16.4±1.8 |
| 50 | 56.4±1.6 | 60.2±2.6 | |
| Zn | - | 24.0±0.4 | 34.2±1.8 |
| 50 | 70.0±1.4 | 80.0±3.2 | |
Mean of five determinations at 95 % confidence level.
4. Conclusion
The use Y. spartinae for solid phase extraction via immobilization on TiO2 nanopowder was demonstrated in this study. The method offers several advantages, including long column reusability up to 50–60 cycles without experiencing any significant deterioration, high capacity to achieve higher preconcentration factors and ability for trace metal preconcentration without using any chelating or complexing agent that minimizes the risk of reagent contamination. The retained trace elements can be completely eluted by dilute HNO3, which is also advantageous to minimize memory effects in subsequent samples but also allows rapid reconditioning of the column for next cycle.
Acknowledgements
This work is funded in part by grants from NIH-RCMI Program (Grant No G12RR013459) and NIH-ERDA Program (Grant No 5 G11 HD046519-05) to Jackson State University. The views expressed herein are those of authors and do not necessarily represent the official views of the NIH and any of its sub agencies.
References
- 1.Lesmana SO, Febriana N, Soetaredjo FE, Sunarso J, Ismadji S. Biochem. Eng. J. 2009;44:19. [Google Scholar]
- 2.Debelius B, Forja JM, Valls AD, Lubian LM. Ecotoxicol. Environ. Saf. 2009;72:1503. doi: 10.1016/j.ecoenv.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Bruno P, Caselli M, de Gennaro G, Ielpo P, Ladisa T, Placentino CM. Chromatographia. 2006;64:537. [Google Scholar]
- 4.Arslan Z, Paulson AJ. Anal. Bioanal. Chem. 2002;372:776. doi: 10.1007/s00216-002-1274-2. [DOI] [PubMed] [Google Scholar]
- 5.He Q, Hu Z, Jiang Y, Chang X, Tu Z, Zhang L. J. Hazard. Mater. 2010;175:710. doi: 10.1016/j.jhazmat.2009.10.067. [DOI] [PubMed] [Google Scholar]
- 6.Kalidhasan S, Ganesh M, Sricharan S, Rajesh N. J. Hazard. Mater. 2009;165:886. doi: 10.1016/j.jhazmat.2008.10.122. [DOI] [PubMed] [Google Scholar]
- 7.Oymak T, Tokalıoglu Ş, Yılmaz V, Kartal Ş, Aydın D. Food Chem. 2009;113:1314. [Google Scholar]
- 8.Divrikli U, Kartal AA, Soylak M, Elçi L. J. Hazard. Mater. 2007;145:459. doi: 10.1016/j.jhazmat.2006.11.040. [DOI] [PubMed] [Google Scholar]
- 9.Naseri MT, Hemmatkhaha P, Milani Hosseini MR, Assadi Y. Anal. Chim. Acta. 2008;610:135. doi: 10.1016/j.aca.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 10.Karimi H, Ghaedi M, Shokrollahi A, Rajabi HR, Soylak M, Karami B. J. Braz. Chem. Soc. 2007;18:1207. [Google Scholar]
- 11.Baytak S, Kendüzler E, Türker AR, Gök N. J. Hazard. Mater. 2008;153:975. doi: 10.1016/j.jhazmat.2007.09.049. [DOI] [PubMed] [Google Scholar]
- 12.Çiftçi H, Yalçın H, Eren E, Ölçücü A, Şekerci M. Desalination. 2010;256:48. [Google Scholar]
- 13.Karatepe A, Korkmaz E, Soylak M, Elçi L. J. Hazard. Mater. 2010;173:433. doi: 10.1016/j.jhazmat.2009.08.098. [DOI] [PubMed] [Google Scholar]
- 14.Özdemir S, Gül-Güven R, Kılınç E, Doğru M, Erdoğan S. Microchim. Acta. 2010;169:79. [Google Scholar]
- 15.Li Z, Chang X, Zou X, Zhu X, Nie R, Hu Z, Li R. Anal. Chim. Acta. 2009;632:272. doi: 10.1016/j.aca.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Baytak S. Acta Chim. Slov. 2007;54:385. [Google Scholar]
- 17.Mahmoud ME, Osman MM, Hafez OF, Elmelegy E. J. Hazard. Mater. 2010;173:349. doi: 10.1016/j.jhazmat.2009.08.089. [DOI] [PubMed] [Google Scholar]
- 18.Otero-Romani J, Moreda-Piñeiro A, Bermejo-Barrera P, Martin-Esteban A. Microchem. J. 2009;93:225. [Google Scholar]
- 19.Sharma RK, Pant P. J. Hazard. Mater. 2009;163:295. doi: 10.1016/j.jhazmat.2008.06.120. [DOI] [PubMed] [Google Scholar]
- 20.Kalfa OM, Yalçınkaya O, Türker AR. J. Hazard. Mater. 2009;166:455. doi: 10.1016/j.jhazmat.2008.11.112. [DOI] [PubMed] [Google Scholar]
- 21.Bakırcıoğlu Y, Bakırcıoğlu D, Akman S. J. Hazard. Mater. 2010;178:1015. doi: 10.1016/j.jhazmat.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 22.Baytak S, Türker AR. Clean- Soil, Air, Water. 2009;37:314. [Google Scholar]
- 23.Apiratikul R, Pavasant P. Bioresour. Technol. 2008;99:2766. doi: 10.1016/j.biortech.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 24.Mendil D, Tüzen M, Usta C, Soylak M. J. Hazard. Mater. 2008;150:357. doi: 10.1016/j.jhazmat.2007.04.116. [DOI] [PubMed] [Google Scholar]
- 25.Baytak S, Türker AR. Microchim. Acta. 2005;149:109. [Google Scholar]
- 26.Ohnuki T, Ozaki T, Yoshida T, Sakamoto F, Kozai N, Wakai E, Francis AJ, Iefuji H. Geochim. Cosmochim. Acta. 2005;69:5307. [Google Scholar]
- 27.Baytak S, Türker AR. Talanta. 2005;65:938. doi: 10.1016/j.talanta.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 28.Rajfur M, Kłos A, Waclawek M. Bioelectrochemistry. 2010;80:81. doi: 10.1016/j.bioelechem.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Vilar VJP, Botelho CMS, Boaventura RAR. Chem. Engineer. J. 2008;141:42. [Google Scholar]
- 30.Türker AR. Clean: Soil, Air, Water. 2007;35:548. [Google Scholar]
- 31.Wang J, Chen C. Biotechnol. Adv. 2009;27:195. doi: 10.1016/j.biotechadv.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Pyrzynska K, Bystrzejewski M. Colloids and Surfaces A: Physicochem. Eng. Aspects. 2010;362:102. [Google Scholar]
- 33.Ezoddin M, Shemirani F, Abdi K, Saghezchi MK, Jamali MR. J. Hazard. Mater. 2010;178:900. doi: 10.1016/j.jhazmat.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 34.Faraji M, Yamini Y, Saleh A, Rezaee M, Ghambarian M, Hassani R. Anal. Chim. Acta. 2010;659:172. doi: 10.1016/j.aca.2009.11.053. [DOI] [PubMed] [Google Scholar]
- 35.Cui Y, Chang X, Zhu X, Jiang N, Hu Z, Lian N. Microchem. J. 2007;86:23. [Google Scholar]
- 36.Chen D, Hu B, He M, Huang C. Microchem. J. 2010;95:90. [Google Scholar]
- 37.Miller G, Arslan Z. Personal Communication. [Google Scholar]