Abstract
The role of inducible NO synthase (iNOS) in allergic airway inflammation remains elusive. We tested the hypothesis that iNOS plays different roles during acute versus chronic airway inflammation. Acute and chronic mouse models of OVA-induced airway inflammation were used to conduct the study. We showed that iNOS deletion was associated with a reduction in eosinophilia, mucus hypersecretion, and IL-5 and IL-13 production upon the acute protocol. Such protection was completely abolished upon the chronic protocol. Interestingly, pulmonary fibrosis observed in wild-type mice under the chronic protocol was completely absent in iNOS−/− mice despite persistent IL-5 and IL-13 production, suggesting that these cytokines were insufficient for pulmonary fibrosis. Such protection was associated with reduced collagen synthesis and indirect but severe TGF-β modulation as confirmed using primary lung smooth muscle cells. Although activation of matrix metalloproteinase-2/-9 exhibited little change, the large tissue inhibitor of metalloproteinase-2 (TIMP-2) increase detected in wild-type mice was absent in the iNOS−/− counterparts. The regulatory effect of iNOS on TIMP-2 may be mediated by peroxynitrite, as the latter reversed TIMP-2 expression in iNOS−/− lung smooth muscle cells and fibroblasts, suggesting that the iNOS–TIMP-2 link may explain the protective effect of iNOS-knockout against pulmonary fibrosis. Analysis of lung sections from chronically OVA-exposed iNOS−/− mice revealed evidence of residual but significant protein nitration, prevalent oxidative DNA damage, and poly(ADP-ribose) polymerase-1 activation. Such tissue damage, inflammatory cell recruitment, and mucus hypersecretion may be associated with substantial arginase expression and activity. The results in this study exemplify the complexity of the role of iNOS in asthma and the preservation of its potential as a therapeutic a target.
Despite the alarming increased rate in prevalence and morbidity of asthma worldwide (1), the complexity of the chronic inflammatory disease of the airways remains a serious hindrance to establishing a clear understanding of the disease. It is well accepted that the increased amount of NO in exhaled air in individuals with asthma reflects disease severity (2, 3). On one hand, NO is considered as a major beneficial player in airway function, as it controls vascular and bronchial tone and neuroendocrine regulation of airway mediator release (3). On the other hand, NO rapidly oxidizes sulfhydryl groups and mediates nitration and hydroxylation of aromatic compounds including tyrosine and guanosine (4) after combining with superoxide to form the highly reactive peroxynitrite (ONOO−). ONOO− not only participates in cell killing and tissue injury in airway inflammation (4, 5), but also influences the function and/or expression of many inflammatory factors (6). Three distinct forms of NO synthase (NOS) have been identified to produce NO using L-arginine as a substrate, which include neuronal NOS, endothelial NOS, and inducible NOS (iNOS). iNOS is rapidly induced upon exposure to a variety of inflammatory agents including allergens, oxidants, or cytokines (5, 7). It is important to note that arginases compete for L-arginine, which may influence the function of iNOS and the outcome of NO production (8). Similar to iNOS, exposure to allergens also induces expression of arginases (8). The role of arginases in asthma remains unsettled, as several studies reported that inhibition of these enzymes could exert either anti- or proinflammatory effects (9–12).
An increasing number of conflicting reports have described crucial and dispensable roles for iNOS in the pathogenesis of allergen-induced airway inflammation (comprehensively reviewed in Ref. 13). Although inhibition of iNOS-mediated production of NO appeared to be a viable therapeutic target to prevent manifestation of asthma symptoms upon exposure to allergens (14–16), such potential has been challenged by the observation that selective inhibition of iNOS does not affect airway inflammatory cell numbers or airway hyperresponsiveness after allergen challenge in subjects with asthma (17). However, the association between asthma protection and susceptibility with polymorphisms in the iNOS gene maintains the viability of the importance of this gene in the pathogenesis of the disease and ultimately its therapeutic potential. Undoubtedly, additional studies are necessary to fully understand the complexities of the role(s) of iNOS and its byproducts during airway inflammation and associated pathologies.
Given the intricacies of the role of iNOS, we surmised that the function of the protein may be context dependent and may play different roles during acute versus chronic inflammation. Accordingly, in this study, we investigated the function of iNOS in airway inflammation and fibrosis and the influence of its inhibition by gene deletion on the different traits of such inflammation using an acute and a chronic protocol of allergen (OVA) exposure as well as a primary cell culture system with lung smooth muscle cells.
Materials and Methods
Animals, sensitization, and challenge protocols
C57BL/6J wild-type (WT) and iNOS−/− mice (B6.129P2-Nos2tm1Lau/J) from The Jackson Laboratory (Bar Harbor, ME) were bred in a specific-pathogen free facility at Louisiana State University Health Sciences Center, New Orleans, LA, and allowed unlimited access to sterilized chow and water. Maintenance, experimental protocols, and procedures were all approved by the Louisiana State University Health Sciences Center Institutional Animal Care and Use Committee. Six- to eight-week-old male mice were sensitized to 100 μg grade V chicken OVA (Sigma-Aldrich, St. Louis, MO) mixed with 2 mg aluminum hydroxide in saline by i.p. injection twice, once a week as previously described (18). Mice were then challenged with aerosolized 3% OVA for 30 min once for the acute protocol or twice a week for 6 wk or as indicated in the figure legends for the chronic protocol.
Organ recovery, tissue staining and quantitation, and Bioplex assay for cytokine measurement
Animals were sacrificed by CO2 asphyxiation, and lungs were fixed with formalin for histological analysis, subjected to bronchoalveolar lavage (BAL), or homogenized to prepare protein extracts. Formalin-fixed lungs were sectioned and subjected to H&E, periodic acid-Schiff (PAS) or trichrome staining using standard protocols, or to immunohistochemistry (IHC) with the appropriate Abs as described previously (19). Histological staining (collagen by trichrome or mucus by PAS) and immunoreactivity for 8-oxo-dG, nitrotyrosine, and poly(ADP-ribose) (PAR) were assessed in images of stained sections captured with a high-resolution Nikon digital camera (DXM1200F, Nikon, Melville, NY) and analyzed using Image-Pro Plus software (version 6.0 for Windows, Silver Spring, MD). Briefly, images of representative areas were obtained using a ×40 objective under the same camera settings (exposure time and light intensity). The measurement parameters included density mean, area sum, and integrated OD. The software system allows a computerized assessment of the density of the staining (special stain or immunoreactivity) as a sum of the values for intensity of all the pixels of a counted region in an analyzed area as well as the total area in an unbiased manner. Threshold range of the colors of positive staining (red for mucus, blue for collagen, or brown for immunoreactivity) was selected in such a way that both faint and strong signals were detected without a high level of background. Density of stain or immunoreactivity was then determined for all areas with a positive signal. The results were expressed as percent of values detected in tissue sections from control (unchallenged) mice.
Protein extracts were subjected to immunoblot analysis as described below.
BAL fluids were used for assessment of IL-5 and IL-13 using a Single-plex assay kit (Bio-Rad, Hercules, CA) as per instructions of the manufacturer and as described (18).
Zymography
Protein extracts were prepared by homogenizing lungs in lysis buffer (1:4 w/v) (20) (50 μg) and were immediately assayed for matrix metalloproteinase (MMP) activity with SDS-PAGE gelatin using commercial gels (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions.
Cell culture, RT-PCR, and immunoblot analysis
Lung smooth muscle cells (LSMCs) were isolated using the explants method as described (21). Lung fibroblasts were isolated from naive mice essentially as described (22). Cells were then seeded on culture dishes and used for experiments at passage 4 to 5. Cells were treated with PMA (Sigma-Aldrich), TNF (Roche, Basel, Switzerland), IL-13 (Sigma-Aldrich), a combination of TNF and IL-13, LPS (Alexis, San Diego, CA), the NO donor S-nitroso-N-acetylpenicillamine (SNAP; Alexis), or ONOO− (Alexis) for the indicated time points, after which cells were collected for total RNA or protein isolation. Immunoblot analyses were conducted essentially as described (23). Nitrocellulose filters were then probed with Abs to iNOS (Millipore, Billerica, MA), arginase-1 (Santa Cruz Biotechnology, Santa Cruz, CA), arginase-2 (Santa Cruz Biotechnology), tissue inhibitor of metalloproteinase-2 (TIMP-2; Abcam, Cambridge, MA), or actin (Santa Cruz Biotechnololgy). RNA was extracted from LSMCs using standard methods, and cDNA was generated using reverse transcriptase III (Invitrogen). Oligonucleotide primers to specifically amplify a fragment of TGF-β1, type 1 collagen (Col-1), or β-actin were all purchased from Integrated DNA Technologies (Coralville, IA). The resulting PCR products were subjected to agarose electrophoresis.
Arginase activity
Total lung extracts from the different experimental groups were tested for arginase activity by conversion of L-arginine to L-ornithine or to urea, as previously described (24).
Data analysis
All data are expressed as means ± SEM of values from at least six mice per group unless stated otherwise. Prism software (GraphPad, San Diego, CA) was used to analyze the differences between experimental groups by two-way ANOVA followed by Bonferroni posttests to compare replicate means by row.
Results
Differential roles for iNOS during allergen (OVA)-induced airway inflammation in mice: acute versus chronic inflammation
To further clarify the role of iNOS in the pathogenesis of airway inflammation, we conducted a comparative study on the effects of iNOS gene deletion upon a single exposure (the acute model) versus repeated exposures (the chronic model) to OVA. Fig. 1A shows that acute exposure of WT mice to aerosolized OVA promoted massive eosinophilia, a moderate increase in macrophages, and a slight increase in neutrophils compared with levels in unchallenged mice, which is consistent with our recently published work (25). The recruitment of these cells was significantly reduced in iNOS−/− mice subjected to similar acute OVA exposure (p < 0.01). In sharp contrast, iNOS gene deletion did not appear to block airway inflammation in response to repeated exposures to OVA when compared with WT mice (Fig. 1B). Fig. 1C depicts examples of such differences. These results suggest that iNOS may be a critical factor for the full manifestation of inflammation upon acute exposure to allergens and that its inhibition by gene deletion may be effective in partially blocking acute inflammation. However, the enzyme does not appear to be necessary for the establishment of a chronic inflammatory response.
FIGURE 1.
Differential effects of iNOS gene deletion during airway inflammation in response to acute or chronic exposure to OVA in mice. C57BL/6J WT or C57BL/6J iNOS−/− mice were subjected to the acute or the chronic challenge protocol or left untreated; mice were sacrificed 48 h after the last challenge. Lungs were either subjected to BAL (A and B) or formalin fixation (C). A and B, Cells of BAL fluids were differentially stained, and total, Eos, MQ, and Neut were counted. Data are expressed as total number of cells per mouse. Data are means ± SD of values from at least six mice per group. C, H&E stain of lung sections from the different experimental groups; scale bars, 4 μm. Original magnification ×20. Arrows indicate sites of inflammation. *Difference from unchallenged mice, p < 0.01; #difference from WT mice challenged with OVA, p < 0.01. Eos, eosinophils; MQ, macrophages; Neut, neutrophils.
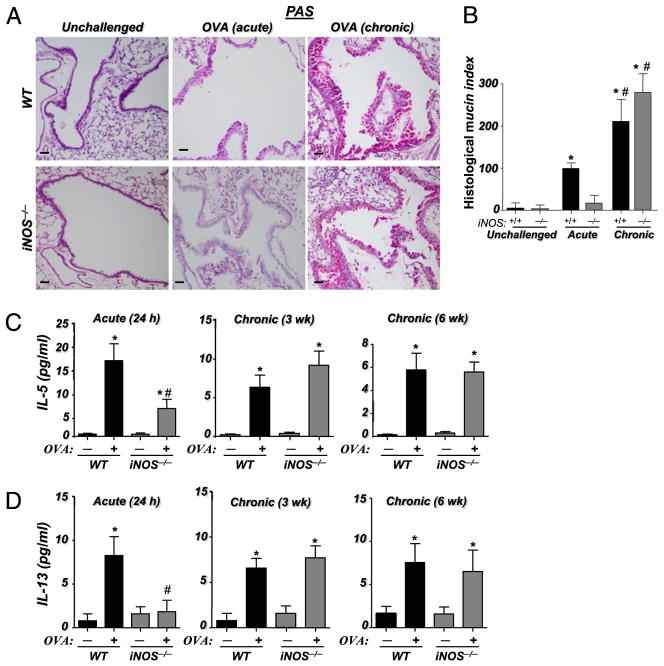
iNOS expression is required for airway mucus hypersecretion and production of IL-5 and IL-13 upon acute, but not chronic, exposure to OVA
Hyperproduction of mucus by goblet cells is an important feature of asthma-associated airway inflammation that has been correlated with the production of Th2 cytokines, namely IL-13 (for review, see Ref. 26). Recently, using poly(ADP-ribose) polymerase-1 (PARP-1)−/− mice, we showed that iNOS may be required for full manifestation of mucus production upon OVA exposure (25). Fig. 2A and 2B show that iNOS gene deletion drastically reduced mucus production upon an acute challenge to OVA. Conversely, iNOS gene deletion exerted no decreasing effect on mucus production upon chronic exposure to OVA, suggesting that the enzyme may be dispensable for chronic inflammation.
FIGURE 2.
Differential requirement of iNOS expression for mucus hypersecretion and IL-13 production upon acute and chronic exposure to OVA in mice. A, Lung sections from the different experimental groups were subjected to PAS staining. Scale bars, 4 μm. Original magnification ×20. B, The extent of mucus production (histological mucin index) was assessed as described in Materials and Methods previously. *Difference from respective untreated mice, p < 0.01; #difference from WT mice subjected to the acute protocol, p < 0.01. Assessment of BAL fluids from the different experimental groups for IL-5 (C) and IL-13 (D). Data are given as means ± SEM of values obtained from at least six mice per group. *Difference from unchallenged mice, p < 0.01; #difference from WT mice subjected to the acute protocol, p < 0.01.
A number of reports have provided evidence that support a critical role for IL-5 and IL-13 in mucus hypersecretion and lung remodeling during allergen-induced lung inflammation (for review, see Ref. 27). Accordingly, we examined the effects of iNOS deletion on IL-5 and IL-13 production upon acute or chronic exposure to OVA. Fig. 2C and 2D show a significant elevation in the production of IL-5 and IL-13 (p < 0.01) upon both acute and chronic exposure to OVA in WT mice. Although IL-13 production was reduced to baseline and IL-5 to markedly lower levels in iNOS−/− mice upon an acute exposure to OVA, the levels of both cytokines remained high and unaffected when iNOS−/− mice were subjected to chronic exposure to the allergen. Fig. 2C and 2D also show that iNOS gene deletion failed to negatively affect IL-5 and IL-13 production as early as 3 wk of repeated exposure to OVA, suggesting that the inhibitory effect on these cytokines that was observed may be strictly associated with only an acute exposure to the allergen. These results are consistent with the effect of iNOS gene deletion on eosinophilia mucus production. It is important to note that although iNOS seems to be dispensable for allergen-induced chronic inflammation, given the complexity of allergen-induced airway inflammation, these results do not categorically rule out a potential contribution for this enzyme in all aspects of chronic airway inflammation.
Expression of iNOS may play a crucial role in lung fibrosis and TGF-β expression upon chronic exposure to OVA
Collagen deposition is a hallmark of lung remodeling upon chronic exposure to allergens. Given the observation that iNOS gene deletion neither blocked airway inflammation nor reduced mucus production upon chronic exposure to OVA, we wished to examine whether a similar trend was also evident in pulmonary fibrosis. Fig. 3A and 3B show that chronic OVA challenge induced substantial and significant collagen deposition in lungs of WT mice. Surprisingly, such collagen deposition was significantly less in iNOS−/− mice subjected to similar repeated exposures to OVA. Collagen deposition was not noticeable in either WT or iNOS−/− mice subjected to acute OVA exposure compared with their respective controls (data not shown). These results imply that iNOS may play a critical role in promoting collagen deposition after the establishment of inflammation. More importantly, these results suggest that the production of IL-13 was insufficient to promote collagen deposition and accumulation upon repeated exposures to OVA.
FIGURE 3.
Expression of iNOS may play a crucial role in lung fibrosis and TGF-β expression upon chronic exposure to OVA. A, Trichrome staining of lung sections from the different experimental groups. Scale bars, 4 μm. Original magnification ×20. B, Assessment of collagen deposition as described in Materials and Methods. C, Lung sections from the different experimental groups were subjected to IHC staining with Abs to mouse TGF-β. Original magnification ×20. D, WT or iNOS−/− LSMCs were treated with 20 ng/ml PMA for 12 or 24 h or with 20 ng/ml TNF, 20 ng/ml IL-13, or a combination of TNF or IL-13 for 12 h. Total RNA was subjected to RT-PCR for TGF-β1 using specific primers. E, RNA isolated from PMA-treated LSMCs was subjected to RT-PCR for Col-1 using specific primers. β-actin was used as an internal control for the PCR reactions. *Difference from respective untreated mice, p < 0.01; #difference from WT mice subjected to the chronic protocol, p < 0.01.
The role of TGF-β in lung fibrosis is well established in a number of respiratory pathologies including asthma (28). To determine whether the lack of collagen deposition in lungs of iNOS−/− mice that were chronically exposed to OVA was associated with reduced levels of TGF-β, lung sections were subjected to immunohistochemical analysis with Abs against the cytokine. Fig. 3C shows that although lung sections from OVA-challenged WT mice displayed pronounced and widespread TGF-β immunoreactivity, those from OVA-challenged iNOS−/− mice exhibited very little TGF-β immunoreactivity, suggesting a potential role for iNOS in the process leading to TGF-β production and that the lack of lung fibrosis may be due, in part, to reduced levels of TGF-β independently of IL-13.
To determine whether iNOS plays a direct role in the pathway leading to TGF-β gene expression, we examined whether iNOS gene deletion negatively affects expression of the cytokine in an in vitro system using LSMCs derived from WT or iNOS−/− mice with broad (PMA) or specific inducers (TNF with or without IL-13) of TGF-β. LSMCs were treated with the inducers for at least 12 h to allow iNOS expression to occur in WT cells (data not shown), after which TGF-β expression was assessed by RT-PCR. Fig. 3D shows that TGF-β expression induced by PMA or TNF alone or in combination with IL-13 was unaffected by iNOS gene deletion in treated LSMCs (Fig. 3D), suggesting that iNOS may not play a crucial role in TGF-β expression. In contrast, whereas PMA did not cause an increase in Col-1 expression, deletion of iNOS moderately reduced Col-1 expression (Fig. 3E). Altogether, these results suggest that iNOS may not directly control expression of TGF-β but appears to be relatively important for maintaining homeostasis of Col-1 in lung tissue.
The protective effect of iNOS gene deletion against chronic OVA exposure-mediated lung fibrosis is associated with a severe reduction in TIMP-2 expression
The lack of a regulatory effect on TGF-β and the moderate reducing effect on Col-1 synthesis by iNOS gene deletion did not explain the severe reduction in collagen deposition in iNOS−/− mice under the chronic protocol. Thus, we surmised that the observed effect might be associated predominantly with alteration in factors necessary for tissue matrix homeostasis, namely MMPs and TIMPs. Fig. 4A shows that chronic OVA exposure was associated with an increase in the native and the active forms of MMP-2 and MMP-9 in lung tissue of WT mice as assessed by zymography. It is noteworthy that the increase in active MMP-2 was much more pronounced in this experimental group. Interestingly, although the active forms of MMP-2 and MMP-9 in lung tissues of OVA-challenged iNOS−/− mice exhibited little to no change, the increase in the native form of MMP-9 was substantial as compared with that detected in the WT counterparts.
FIGURE 4.
Effects of iNOS gene deletion on MMP-2/-9 expression and activity and TIMP-2 expression. Lung extracts (50 μg) from WT or iNOS−/− mice (n ≥ 4) that were subjected to the chronic protocol or left untreated were subjected to zymography and visualized by Coomassie blue staining (A) or subjected to immunoblot analysis with Abs to TIMP-2 or actin (B). Asterisk (*) indicates a non-specific band that remained after blot stripping. C, WT or iNOS−/− LSMCs were treated with 1 μg/ml LPS for 6, 12, or 24 h. Protein extracts were subjected to immunoblot analysis with Abs to TIMP-2, iNOS, or actin. D, Cells were treated with LPS for 24 h, after which protein extracts were subjected to immunoblot analysis with Abs to nitrotyrosine, TIMP-2, iNOS, or actin. Ea, Cells were treated with 10 μM ONOO−, after which protein extracts were subjected to immunoblot analysis with Abs to TIMP-2 or actin. Eb, Cells were treated with LPS in the presence or absence of 10 μM SNAP for 24, or 48 h, after which protein extracts were subjected to immunoblot analysis with Abs to TIMP-2 or actin. F, MLFs derived from WT or iNOS−/− mice were treated with 1 μg/ml LPS for 12 or 24 h, after which protein extracts were subjected to immunoblot analysis with Abs to TIMP-2, iNOS, or actin. G, WT or iNOS−/− MLFs were treated with 10 μM ONOO− for 48 h or left untreated, after which protein extracts were subjected to immunoblot analysis with Abs to TIMP-2 or actin. MLFs, primary lung fibroblasts.
Although these results show an association between iNOS gene deletion and elevation of MMP-2 and MMP-9 expression but not their respective activation, these results do not provide a clear explanation for the prevention or blockage of collagen deposition in lungs of chronically OVA-exposed iNOS−/− mice. Accordingly, we examined the effect of iNOS gene deletion on the fate of TIMPs, endogenous physiological inhibitors of MMPs that tightly regulate the proteolytic potential of these proteases (29). TIMP-2, a member of the small TIMP family, specifically inhibits both active and latent forms of MMPs (29). Further, the expression status of this inhibitor mirrors several pathological situations, including lung remodeling (30). An examination of the fate of TIMP-2 in the different experimental groups revealed that OVA challenge induced a substantial amount of TIMP-2 in the lungs of WT animals, whereas such an increase was largely absent in the iNOS counterparts (Fig. 4B). The defect in TIMP-2 expression appeared to be direct, because induction of this protein was markedly reduced in primary iNOS−/− LSMCs upon exposure to LPS (Fig. 4C).
To fully confirm the involvement of iNOS and its byproducts in the process of TIMP-2 expression, we tested the hypothesis that if ONOO− mediates of TIMP-2 expression, then a re-establishment of TIMP-2 expression may be achieved by direct treatment of iNOS−/− LSMCs with the reactive nitrogen species. The expression of TIMP-2 in LPS-treated WT cells and lack thereof in LPS-treated iNOS−/− cells coincided with the levels of nitrosative stress as assessed by immunoblot analysis with Abs to nitrotyrosine (Fig. 4D). Fig. 4Ea shows that ONOO− treatment induced a similar time-dependent increase in TIMP-2 accumulation in both WT and iNOS−/− LSMCs, strengthening the potential connection between iNOS and TIMP-2 expression. This notion was further supported by the ability of the NO donor SNAP to re-establish TIMP-2 expression in iNOS−/− LSMCs upon LPS treatment (Fig. 4Eb). The severe reduction in TIMP-2 expression upon LPS treatment (Fig. 4F) and its reversal by ONOO− (Fig. 4G) were also observed in primary fibroblasts derived from lungs of iNOS−/− mice as compared with the WT counterparts. Altogether, these results strongly suggest that the lack of a robust deposition of collagen in the lungs of iNOS−/− mice upon chronic OVA challenge may be related to a continuous and uninhibited MMP activity because of the compromised expression of TIMP-2 in these tissues as a result of iNOS gene deletion. Such an iNOS–TIM-P-2 link may provide an explanation to the protective effect of iNOS knockout against pulmonary fibrosis, but it does not, however, shed any light on the persistence of inflammation and mucus production.
Persistence of mucus production and inflammation is associated with extensive oxidative DNA damage and PARP-1 activation in lung tissue of chronically OVA-exposed iNOS−/− mice
We recently showed that iNOS gene deletion completely blocked oxidative DNA damage, as assessed by 8-oxo-dG immunoreactivity, in lungs of mice that were subjected to an acute exposure to OVA (31). It is noteworthy that oxidative DNA damage, such as the formation of 8-oxo-dG, is being increasingly associated with iNOS enzymatic activity in conditions other than allergen-induced inflammation (32–34). Interestingly, iNOS gene deletion was accompanied by only a moderate reduction in oxidative DNA damage upon chronic exposure to OVA (Fig. 5A), which may suggest that iNOS expression may be an important but not the sole participant in mediating oxidative DNA damage upon chronic exposure to allergens. Indeed, an assessment of nitrotyrosine immunoreactivity revealed that iNOS gene deletion resulted in severely reduced protein nitration upon chronic exposure to OVA as compared with signals in WT counterparts, but not a complete block (Fig. 5B). The low level of protein nitration observed in OVA-challenged iNOS−/− mice may be due to activities of reactive nitrogen species-generating enzymes other than iNOS.
FIGURE 5.
Association between persistence of inflammation and mucus production with extensive oxidative DNA damage and PARP-1 activation in lung tissue of chronically OVA-exposed iNOS−/− mice. Lung sections from mice under the chronic protocol were subjected to IHC staining with Abs to 8-oxo-dG (A), to Nitro-Y (B), or to PAR moieties of PARP-1–modified proteins (PAR) (C) and observed by light microscopy. In this figure, only OVA-challenged groups are shown; the control groups exhibited little to no immunoreactivity with the different Abs (data not shown). The extent immunoreactivity was then assessed. *Difference from respective untreated mice, p < 0.01; #difference from WT mice subjected to the chronic protocol, p < 0.01. Scale bars, 4 μm. Original magnification ×20. Nitro-Y, nitrotyrosine.
As previously reported by us, the occurrence of protein nitration and oxidative DNA damage was accompanied by PARP-1 activation in an acute model of asthma (31). To determine whether the presence of oxidative DNA damage in lungs of OVA-challenged iNOS−/− mice coincided with PARP-1 activation, we subjected lung sections from the different experimental groups to IHC with Abs to the PAR moiety of PARP-1-modified proteins. PAR immunoreactivity in lung sections of OVA-challenged iNOS−/− mice was similar to that observed in the WT counterparts (Fig. 5C). Altogether, these results suggest that the persistence of oxidative DNA damage and PARP-1 activation may be important participants in the process leading to recruitment of inflammatory cells and mucus production in lungs OVA-challenged of iNOS−/− mice.
iNOS gene deletion enhances arginase-2 but not arginase-1 without affecting the overall arginase activity in lungs of chronically OVA-challenged iNOS−/− mice
Recently, Maaringh et al. (35) suggested that L-arginine deficiency as a result of arginase activity may lead to increased formation of ONOO−, which ultimately participates in allergen-induced airway inflammation as well as hyperresponsiveness. To examine whether the persistence of protein nitration and oxidative DNA damage and associated inflammation and mucus production in lungs of OVA-challenged iNOS−/− mice with arginase expression and activity, we subjected protein extracts isolated from the different experimental groups to immunoblot analysis with Abs to arginase-1 or arginase-2. Fig. 6A shows that the basal levels of arginase-1 were relatively similar in lungs of control WT and iNOS−/− mice, which drastically increased upon chronic exposure to OVA both in the WT and iNOS−/− mice, albeit the increase in the latter mice was less pronounced. Arginase-2 was slightly higher in lungs of control iNOS−/− mice as compared with the WT control animals. The level of arginase-2 was slightly increased in lungs of WT mice upon chronic exposure to OVA. Interestingly, however, arginase-2 expression levels increased substantially in chronically OVA-challenged iNOS−/− mice. A quantitative assessment of the data is presented in Fig. 6B. A measurement of arginase activity in lung extracts from the different experimental groups revealed that iNOS gene deletion did not affect the overall arginase activity upon chronic exposure to OVA as measured by conversion of L-arginine to L-ornithine (Fig. 6C) or to urea (Fig. 6D), which suggests that the iNOS gene deletion-associated dramatic increase in arginase-2 did not influence the overall tissue activity of these enzymes. Altogether, these results suggest that the high and persistent levels of arginase activity in the lungs of mice that were chronically exposed to OVA may be the source for oxidative tissue damage detected in the iNOS−/− mice and may be the underlying cause for the inability of iNOS gene deletion to exert a protective effect upon chronic exposure to OVA in our experimental model.
FIGURE 6.

Effects of iNOS gene deletion on expression of arginase-1 and arginase-2 and overall arginase activity in lungs of chronically OVA-challenged mice. A, Lung extracts from WT or iNOS−/− mice (n = 8) under the chronic protocol were subjected to immunoblot analysis with Abs to arginase-1, arginase-2, or actin. B, Signals of the immunoblots were quantified using National Institutes of Health ImageJ software (Bethesda, MD). *Difference from respective untreated mice, p < 0.01; #difference from WT mice subjected to the chronic protocol, p < 0.01. Lung extracts (20 μg) from the different experimental groups were assayed for arginase activity by conversion of L-arginine to L-ornithine (C) or to urea (D). *Difference from respective untreated mice, p < 0.01.
Discussion
The exact role of iNOS in allergic airway inflammation remains elusive despite the continuous and increasing body of evidence pertaining to its potential function. Many conflicting reports have described critical and dispensable roles of iNOS in the pathogenesis of the disease using both experimental models as well as human subjects [reviewed elegantly by Mathrani et al. (13)]. These conflicting findings were complicated even more after the recent clinical study by Singh et al. (17) reporting that selective inhibition of iNOS neither blocked airway inflammation nor improved airway hyperresponsiveness after allergen challenge in subjects with asthma despite its ability to effectively reduce exhaled breath NO levels. We recently showed that iNOS may be required for oxidative DNA damage and full manifestation of mucus production; however, the expression of the protein may be dispensable for eosinophilia after IL-5 production. Such dispensability may explain, in part, the reported ineffectiveness of specific iNOS inhibitors in blocking allergen-induced inflammation in humans. Given the intricacies of the role of iNOS, we surmised that the function of the protein may be context dependent and play different roles during acute versus chronic inflammation.
The results of our study show that iNOS deletion was associated with reduction in eosinophilia and blockage of mucus and IL-13 production upon an acute exposure to OVA. Such protective effects were completely abolished upon repeated exposure to the allergen, suggesting completely different roles for iNOS during acute and chronic allergic inflammation. Accordingly, NO and/or its byproducts may function as initiators of the allergic inflammatory response. Accordingly, reduced levels of NO would delay the onset of the response to allergens. Such possibility may explain the difference in the responses of iNOS−/− mice to the acute versus chronic exposure to OVA. Indeed, very recently, Brahmachari and Pahan (36) reported that suppression of regulatory T cells by IL-12p40 can be mediated by NO. Of note, the role of these T cells in controlling allergic diseases, such as asthma, is well established (37). Additionally, NO production and nitrotyrosine formation were reported to affect the Ag presentation and modify the immune response (38).
The finding that iNOS and its byproduct ONOO− may play a crucial role in lung fibrosis was rather interesting, especially because such protection occurred in the presence of inflammation and persistent IL-13 production. It appears that the role of iNOS in lung fibrosis may be linked to TIMP-2 expression and persistent MMP-2/-9 activity that would continuously degrade the collagen that may be produced as a result of inflammation. Our results suggest that iNOS is involved, through its byproduct ONOO−, in the regulation of TIMP-2 expression. The protective effect of iNOS knockout against OVA-induced lung fibrosis also was associated with a severe reduction in TGF-β. Surprisingly, this effect did not seem to be direct, as revealed by our in vitro studies using primary LSMCs. Although the iNOS–TIMP-2 link may explain the protective effect of iNOS knockout against lung fibrosis, it does not explain the persistence of inflammation and mucus production. Immunohistochemical analysis of lung sections from chronically OVA-exposed iNOS−/− mice revealed evidence of residual but significant nitrotyrosine immunoreactivity, which indicates that oxidative DNA damage also may be present in these tissues. Indeed, prevalent oxidative DNA damage was observed that coincided with substantial PARP-1 activation. Such tissue damage, concomitant inflammation, and mucus hypersecretion may be the result of substantial expression and activity of arginase-1 and arginase-2 in the lungs of iNOS−/− mice. At present, it is unclear how iNOS gene deletion could be associated with a differential effect on the expression of arginase-1 and arginase-2. Although the effect of iNOS gene deletion on arginase-2 is well in keeping with that reported by Last’s group (12) using a shorter chronic exposure protocol, the increase in arginase-1 observed by this group was not apparent in our model. This discrepancy may be attributed to the difference in the duration of the chronic exposure protocol. It is important to note that despite the effect of iNOS gene deletion on the expression of arginases, the overall catalytic activity of these enzymes was not affected. A beneficial potential of arginase inhibition by N (ω)-hydroxy-nor-L-arginine was recently demonstrated, as it significantly decreased airway hyperresponsiveness and eosinophilia upon a chronic exposure to OVA (12). Altogether, these results provide further evidence for the involvement of iNOS in allergen-induced pathology and may still preserve the potential of iNOS as a therapeutic target for the treatment of some asthma-related symptoms.
The protective effect of iNOS gene knockout against airway inflammatory traits induced by an acute exposure to an allergen is consistent with numerous reports using either genetic or pharmacological inhibition of iNOS (reviewed in Ref. 13). The mechanism by which iNOS inhibition protects against acute inflammation may be associated with the prevention of NO overproduction and its conversion into ONOO−. However, asthma is a chronic inflammatory disease, and an acute effect may not be sufficient to dampen or block the symptoms of the disease. In terms of inflammation, our finding that the inability of iNOS gene knockout to be protective against airway inflammatory cell recruitment and mucus hypersecretion upon repeated exposure to OVA is consistent with that reported by Last’s group (13). Our results are also consistent with the effect of a selective iNOS inhibitor in human asthma, because the drug did not block airway inflammation (17). Interestingly, however, Kenyon et al. (39) reported that iNOS−/− mice are more susceptible to both inflammation and fibrosis using an exposure protocol (4–10 wk) in which inflammation appears to be driven primarily by macrophages. In our chronic OVA exposure model, eosinophilia was persistent, with clear production of IL-5 and IL-13, collagen deposition, and mucus production, which is consistent with the traits reported by numerous groups (40–43) using C57BL/6 mice under protocols comparable to ours.
Airway inflammation may not be sufficient for airway remodeling, as iNOS gene deletion failed to block the infiltration of inflammatory cells and mucus production but severely abrogated the deposition of collagen in the chronic model. The absence of clear lung fibrosis in chronically OVA-challenged iNOS−/− mice was evident even in the presence substantial levels of oxidative DNA damage, PARP-1 activation, and arginases. Further, IL-13 has always been associated with allergic airway inflammation by promoting both mucus hypersecretion and remodeling (44). Our results show that IL-13 could not promote lung fibrosis in the absence of iNOS, and the mechanisms by which this cytokine induces mucus hypersecretion and collagen deposition may be completely distinct. These observations lend additional support to the notion that inflammation may not be a contributing factor in lung fibrosis [reviewed by Wilson and Wynn (45)]. Epithelial to mesenchymal transition is increasingly regarded as an important process contributing to lung fibrosis. Recently, several groups (46–50) showed that TGF-β may be a critical player in driving mesenchymal transition during airway inflammation. Interestingly, such transition was not inhibited by corticosteroid (50). It is tempting to speculate that the decrease in TGF-β expression observed in iNOS−/− mice that were chronically exposed to OVA is related to the markedly reduced manifestation of fibrosis in these mice compared with their WT counterparts.
It is well accepted that collagen deposition in a variety of tissues is tightly regulated by a delicate balance between MMPs and TIMPs. OVA challenge has been shown to increase expression of MMPs (51), as was observed in our model. Our results show that iNOS gene deletion appeared to increase expression of MMP-9 as compared with levels detected in the WT counterparts without actually increasing the abundance of the active form of the protease. Because NO was reported to possibly be required for the activation of MMP-9 in vitro (52) or in a cell culture system of airway epithelium wound healing (53), it is plausible that the increase in MMP-9 in iNOS−/− mice under the chronic protocol did not translate into an actual increase in active MMP-9 because of a lack in NO. Interestingly, iNOS gene deletion dramatically reduced the expression of TIMP-2, suggesting that the absence of the inhibitor may tilt the balance toward more MMP activity, thus promoting the degradation of matrix at a faster rate in the lungs of iNOS−/− mice as compared with the WT counterparts. The imbalance between TIMP-2 and MMP levels in the lungs of iNOS−/− mice under the chronic protocol may be both detrimental and beneficial. Because MMP activity is crucial for the continuous establishment of inflammation and recruitment of inflammatory cells, such increased MMP activity may be a contributing factor for the persistence of inflammation in the lungs of iNOS−/− mice. In contrast, persistent inflammation may be an inducing factor for lung fibrosis, and the absence of TIMP-2 may prevent the accumulation of collagen deposits—hence, the protection against lung fibrosis by iNOS gene knockout. We also provide evidence for a potential link between iNOS and TIMP-2 expression as the byproduct, ONOO−, promoted expression of TIMP-2 in iNOS−/− LSMCs. The mechanism by which ONOO− induces TIMP-2 expression remains to be determined; it may involve induction of a required transcription factor for, or neutralization of a suppressor of, TIMP-2 gene expression.
It is important to note that our results also reveal a novel relationship between iNOS and IL-13. What is known about this relationship is that IL-13 exerts a modulatory effect on iNOS expression (54). However, our results show that iNOS is required for IL-13 expression upon an acute exposure to OVA. This relationship may involve ONOO− because this radical has been shown to induce expression of other interleukins in the lung (55) as well as in cell culture systems (56). The reason behind the disappearance of the requirement of iNOS for IL-13 expression upon chronic exposure to OVA is unclear. Furthermore, the persistent production of IL-13 may explain the full manifestation of mucus production in chronically OVA-challenged iNOS−/− mice. The strong relationship between mucus hypersecretion and IL-13 was established by the fact that such a pathological event is attenuated in mice lacking IL-13 (57). Thus, one could speculate that the small but persistent levels of protein nitration, oxidative DNA damage, and PARP-1 activation detected in the chronic model may be contributing factors. The presence of these levels of protein nitration and oxidative DNA damage may be reminiscent of a low level of NO-producing activity, such as that mediated by the other isoforms of NOS. An important question presents itself: if a small amount of NO was produced in OVA-challenged iNOS−/− mice, how did it become a source for reactive nitrogen species, resulting in protein nitration and oxidative DNA damage? Recently, L-arginine deficiency a result of arginase activity was associated with increased formation of ONOO− (35), ultimately participating in allergen-induced airway inflammation as well as hyperresponsiveness. Our results suggest that the high levels of arginase activity in the lungs of iNOS−/− mice under the chronic protocol may be the source of oxidative tissue damage detected in these mice and may be the underlying cause for the inability of iNOS gene deletion to exert a protective effect upon chronic exposure to OVA in our experimental model. Interestingly, in pioneering work by Janssen-Heininger’s group (9, 58), pharmacological inhibition of arginases was associated with an increase in S-nitrosylated and nitrated proteins possibly through availability of a larger pool of L-arginine to iNOS upon exposure to allergen. Such an increase in nitrosative stress was reported to lead to enhanced activation of NF-κB and subsequent increase in NF-κB–dependent gene expression.
Taken together, our results support the existence of an important role for iNOS in asthma pathogenesis, and continuing efforts to investigate the exact role of iNOS in lung inflammation and fibrosis may provide a clearer view on how to use iNOS as a therapeutic target. Possible approaches may not block inflammation but rather prevent lung fibrosis, a critical aspect of asthma.
Acknowledgments
This work was supported in part by Grant HL072889 from the National Institutes of Health, Grant RSG-116608 from the American Cancer Society, and funds from the Louisiana Cancer Research Consortium (New Orleans, LA) to A.H.B.
Abbreviations used in this paper
- BAL
bronchoalveolar lavage
- Col-1
type 1 collagen
- Eos
eosinophils
- IHC
immunohistochemistry
- iNOS
inducible NO synthase
- LSMC
lung smooth muscle cell
- MLFs
primary lung fibroblasts
- MMP
matrix metalloproteinase
- MQ
macrophages
- Neut
neutrophils
- Nitro-Y
nitrotyrosine
- NOS
NO synthase
- ONOO−
peroxynitrite
- PAR
poly(ADP)-ribose
- PARP-1
poly(ADP-ribose) polymerase-1
- PAS
periodic acid-Schiff
- SNAP
S-nitroso-N-acetylpenicillamine
- TIMP-2
tissue inhibitor of metalloproteinase-2
- WT
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, Casale TB, Chanez P, Enright PL, Gibson PG, de Jongste JC, et al. A new perspective on concepts of asthma severity and control. Eur Respir J. 2008;32:545–554. doi: 10.1183/09031936.00155307. [DOI] [PubMed] [Google Scholar]
- 2.DeNicola LR, Kissoon N, Duckworth LJ, Blake KV, Murphy SP, Silkoff PE. Exhaled nitric oxide as an indicator of severity of asthmatic inflammation. Pediatr Emerg Care. 2000;16:290–295. doi: 10.1097/00006565-200008000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Ricciardolo FL, Nijkamp FP, Folkerts G. Nitric oxide synthase (NOS) as therapeutic target for asthma and chronic obstructive pulmonary disease. Curr Drug Targets. 2006;7:721–735. doi: 10.2174/138945006777435290. [DOI] [PubMed] [Google Scholar]
- 4.Kirkham P, Rahman I. Oxidative stress in asthma and COPD: antioxidants as a therapeutic strategy. Pharmacol Ther. 2006;111:476–494. doi: 10.1016/j.pharmthera.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 5.MacNee W. Oxidative stress and lung inflammation in airways disease. Eur J Pharmacol. 2001;429:195–207. doi: 10.1016/s0014-2999(01)01320-6. [DOI] [PubMed] [Google Scholar]
- 6.Beckman JS. Understanding peroxynitrite biochemistry and its potential for treating human diseases. Arch Biochem Biophys. 2009;484:114–116. doi: 10.1016/j.abb.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogg JC. The pathology of asthma. APMIS. 1997;105:735–745. doi: 10.1111/j.1699-0463.1997.tb05079.x. [DOI] [PubMed] [Google Scholar]
- 8.Bratt JM, Franzi LM, Linderholm AL, Last MS, Kenyon NJ, Last JA. Arginase enzymes in isolated airways from normal and nitric oxide synthase 2-knockout mice exposed to ovalbumin. Toxicol Appl Pharmacol. 2009;234:273–280. doi: 10.1016/j.taap.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ckless K, Lampert A, Reiss J, Kasahara D, Poynter ME, Irvin CG, Lundblad LK, Norton R, van der Vliet A, Janssen-Heininger YM. Inhibition of arginase activity enhances inflammation in mice with allergic airway disease, in association with increases in protein S-nitrosylation and tyrosine nitration. J Immunol. 2008;181:4255–4264. doi: 10.4049/jimmunol.181.6.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maarsingh H, Zuidhof AB, Bos IS, van Duin M, Boucher JL, Zaagsma J, Meurs H. Arginase inhibition protects against allergen-induced airway obstruction, hyperresponsiveness, and inflammation. Am J Respir Crit Care Med. 2008;178:565–573. doi: 10.1164/rccm.200710-1588OC. [DOI] [PubMed] [Google Scholar]
- 11.Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, Smith AM, Thompson RW, Cheever AW, Murray PJ, Wynn TA. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5:e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bratt JM, Franzi LM, Linderholm AL, O’Roark EM, Kenyon NJ, Last JA. Arginase inhibition in airways from normal and nitric oxide synthase 2-knockout mice exposed to ovalbumin. Toxicol Appl Pharmacol. 2010;242:1–8. doi: 10.1016/j.taap.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathrani VC, Kenyon NJ, Zeki A, Last JA. Mouse models of asthma: can they give us mechanistic insights into the role of nitric oxide? Curr Med Chem. 2007;14:2204–2213. doi: 10.2174/092986707781389628. [DOI] [PubMed] [Google Scholar]
- 14.Hansel TT, Kharitonov SA, Donnelly LE, Erin EM, Currie MG, Moore WM, Manning PT, Recker DP, Barnes PJ. A selective inhibitor of inducible nitric oxide synthase inhibits exhaled breath nitric oxide in healthy volunteers and asthmatics. FASEB J. 2003;17:1298–1300. doi: 10.1096/fj.02-0633fje. [DOI] [PubMed] [Google Scholar]
- 15.Mulrennan SA, Redington AE. Nitric oxide synthase inhibition: therapeutic potential in asthma. Treat Respir Med. 2004;3:79–88. doi: 10.2165/00151829-200403020-00002. [DOI] [PubMed] [Google Scholar]
- 16.Brindicci C, Ito K, Barnes PJ, Kharitonov SA. Effect of an inducible nitric oxide synthase inhibitor on differential flow-exhaled nitric oxide in asthmatic patients and healthy volunteers. Chest. 2007;132:581–588. doi: 10.1378/chest.06-3046. [DOI] [PubMed] [Google Scholar]
- 17.Singh D, Richards D, Knowles RG, Schwartz S, Woodcock A, Langley S, O’Connor BJ. Selective inducible nitric oxide synthase inhibition has no effect on allergen challenge in asthma. Am J Respir Crit Care Med. 2007;176:988–993. doi: 10.1164/rccm.200704-588OC. [DOI] [PubMed] [Google Scholar]
- 18.Naura AS, Hans CP, Zerfaoui M, You D, Cormier SA, Oumouna M, Boulares AH. Post-allergen challenge inhibition of poly(ADP-ribose) polymerase harbors therapeutic potential for treatment of allergic airway inflammation. Clin Exp Allergy. 2008;38:839–846. doi: 10.1111/j.1365-2222.2008.02943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boulares AH, Zoltoski AJ, Sherif ZA, Jolly P, Massaro D, Smulson ME. Gene knockout or pharmacological inhibition of poly (ADP-ribose) polymerase-1 prevents lung inflammation in a murine model of asthma. Am J Respir Cell Mol Biol. 2003;28:322–329. doi: 10.1165/rcmb.2001-0015OC. [DOI] [PubMed] [Google Scholar]
- 20.Boulares AH, Zoltoski AJ, Yakovlev A, Xu M, Smulson ME. Roles of DNA fragmentation factor and poly(ADP-ribose) polymerase in an amplification phase of tumor necrosis factor-induced apoptosis. J Biol Chem. 2001;276:38185–38192. doi: 10.1074/jbc.M100629200. [DOI] [PubMed] [Google Scholar]
- 21.Naura AS, Hans CP, Zerfaoui M, Errami Y, Ju J, Kim H, Matrougui K, Kim JG, Boulares AH. High-fat diet induces lung remodeling in ApoE-deficient mice: an association with an increase in circulatory and lung inflammatory factors. Lab Invest. 2009;89:1243–1251. doi: 10.1038/labinvest.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Saux CJ, Teeters K, Miyasato SK, Hoffmann PR, Bollt O, Douet V, Shohet RV, Broide DH, Tam EK. Down-regulation of caveolin-1, an inhibitor of transforming growth factor-beta signaling, in acute allergen-induced airway remodeling. J Biol Chem. 2008;283:5760–5768. doi: 10.1074/jbc.M701572200. [DOI] [PubMed] [Google Scholar]
- 23.Boulares AH, Yakovlev AG, Ivanova V, Stoica BA, Wang G, Iyer S, Smulson M. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J Biol Chem. 1999;274:22932–22940. doi: 10.1074/jbc.274.33.22932. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 25.Naura AS, Datta R, Hans CP, Zerfaoui M, Rezk BM, Errami Y, Oumouna M, Matrougui K, Boulares AH. Reciprocal regulation of iNOS and PARP-1 during allergen-induced eosinophilia. Eur Respir J. 2009;33:252–262. doi: 10.1183/09031936.00089008. [DOI] [PubMed] [Google Scholar]
- 26.Rogers DF. Physiology of airway mucus secretion and pathophysiology of hypersecretion. Respir Care. 2007;52:1134–1146. discussion 1146–1149. [PubMed] [Google Scholar]
- 27.Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008;118:3546–3556. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper PR, Panettieri RA., Jr Steroids completely reverse albuterol-induced beta(2)-adrenergic receptor tolerance in human small airways. J Allergy Clin Immunol. 2008;122:734–740. doi: 10.1016/j.jaci.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 29.Stetler-Stevenson WG. Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal. 2008;1:re6. doi: 10.1126/scisignal.127re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong S, Belvisi MG, Birrell MA. MMP/TIMP expression profiles in distinct lung disease models: implications for possible future therapies. Respir Res. 2009;10:72. doi: 10.1186/1465-9921-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hans CP, Feng Y, Naura AS, Zerfaoui M, Rezk BM, Xia H, Kaye AD, Matrougui K, Lazartigues E, Boulares AH. Protective effects of PARP-1 knockout on dyslipidemia-induced autonomic and vascular dysfunction in ApoE mice: effects on eNOS and oxidative stress. PLoS ONE. 2009;4:e7430. doi: 10.1371/journal.pone.0007430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murata M, Kawanishi S. Oxidative DNA damage induced by nitrotyrosine, a biomarker of inflammation. Biochem Biophys Res Commun. 2004;316:123–128. doi: 10.1016/j.bbrc.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 33.Pinlaor S, Hiraku Y, Ma N, Yongvanit P, Semba R, Oikawa S, Murata M, Sripa B, Sithithaworn P, Kawanishi S. Mechanism of NO-mediated oxidative and nitrative DNA damage in hamsters infected with Opisthorchis viverrini: a model of inflammation-mediated carcinogenesis. Nitric Oxide. 2004;11:175–183. doi: 10.1016/j.niox.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Pinlaor S, Hiraku Y, Yongvanit P, Tada-Oikawa S, Ma N, Pinlaor P, Sithithaworn P, Sripa B, Murata M, Oikawa S, Kawanishi S. iNOS-dependent DNA damage via NF-kappaB expression in hamsters infected with Opisthorchis viverrini and its suppression by the antihelminthic drug praziquantel. Int J Cancer. 2006;119:1067–1072. doi: 10.1002/ijc.21893. [DOI] [PubMed] [Google Scholar]
- 35.Maarsingh H, Zaagsma J, Meurs H. Arginase: a key enzyme in the pathophysiology of allergic asthma opening novel therapeutic perspectives. Br J Pharmacol. 2009;158:652–664. doi: 10.1111/j.1476-5381.2009.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brahmachari S, Pahan K. Suppression of regulatory T cells by IL-12p40 homodimer via nitric oxide. J Immunol. 2009;183:2045–2058. doi: 10.4049/jimmunol.0800276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ray A, Khare A, Krishnamoorthy N, Qi Z, Ray P. Regulatory T cells in many flavors control asthma. Mucosal Immunol. 2010;3:216–229. doi: 10.1038/mi.2010.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernández-Ruiz V, López-Moratalla N, González A. Production of nitric oxide and self-nitration of proteins during monocyte differentiation to dendritic cells. J Physiol Biochem. 2005;61:517–525. doi: 10.1007/BF03168377. [DOI] [PubMed] [Google Scholar]
- 39.Kenyon NJ, Gohil K, Last JA. Susceptibility to ovalbumin-induced airway inflammation and fibrosis in inducible nitric oxide synthetase-deficient mice: mechanisms and consequences. Toxicol Appl Pharmacol. 2003;191:2–11. doi: 10.1016/s0041-008x(03)00227-8. [DOI] [PubMed] [Google Scholar]
- 40.Jain VV, Kitagaki K, Businga T, Hussain I, George C, O’shaughnessy P, Kline JN. CpG-oligodeoxynucleotides inhibit airway remodeling in a murine model of chronic asthma. J Allergy Clin Immunol. 2002;110:867–872. doi: 10.1067/mai.2002.129371. [DOI] [PubMed] [Google Scholar]
- 41.Simeone-Penney MC, Severgnini M, Tu P, Homer RJ, Mariani TJ, Cohn L, Simon AR. Airway epithelial STAT3 is required for allergic inflammation in a murine model of asthma. J Immunol. 2007;178:6191–6199. doi: 10.4049/jimmunol.178.10.6191. [DOI] [PubMed] [Google Scholar]
- 42.Pinelli V, Marchica CL, Ludwig MS. Allergen-induced asthma in C57Bl/6 mice: hyper-responsiveness, inflammation and remodelling. Respir Physiol Neurobiol. 2009;169:36–43. doi: 10.1016/j.resp.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Pae S, Cho JY, Dayan S, Miller M, Pemberton AD, Broide DH. Chronic allergen challenge induces bronchial mast cell accumulation in BALB/c but not C57BL/6 mice and is independent of IL-9. Immunogenetics. 2010 doi: 10.1007/s00251-010-0452-1. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Homer RJ, Elias JA. Airway remodeling in asthma: therapeutic implications of mechanisms. Physiology (Bethesda) 2005;20:28–35. doi: 10.1152/physiol.00035.2004. [DOI] [PubMed] [Google Scholar]
- 45.Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2009;2:103–121. doi: 10.1038/mi.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alcorn JF, Guala AS, van der Velden J, McElhinney B, Irvin CG, Davis RJ, Janssen-Heininger YM. Jun N-terminal kinase 1 regulates epithelial-to-mesenchymal transition induced by TGF-beta1. J Cell Sci. 2008;121:1036–1045. doi: 10.1242/jcs.019455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang M, Zhang Z, Pan HY, Wang DX, Deng ZT, Ye XL. TGF-beta1 induces human bronchial epithelial cell-to-mesenchymal transition in vitro. Lung. 2009;187:187–194. doi: 10.1007/s00408-009-9139-5. [DOI] [PubMed] [Google Scholar]
- 48.Heijink IH, Postma DS, Noordhoek JA, Broekema M, Kapus A. House dust mite-promoted epithelial-to-mesenchymal transition in human bronchial epithelium. Am J Respir Cell Mol Biol. 2010;42:69–79. doi: 10.1165/rcmb.2008-0449OC. [DOI] [PubMed] [Google Scholar]
- 49.Hackett TL, Warner SM, Stefanowicz D, Shaheen F, Pechkovsky DV, Murray LA, Argentieri R, Kicic A, Stick SM, Bai TR, Knight DA. Induction of epithelial-mesenchymal transition in primary airway epithelial cells from patients with asthma by transforming growth factor-beta1. Am J Respir Crit Care Med. 2009;180:122–133. doi: 10.1164/rccm.200811-1730OC. [DOI] [PubMed] [Google Scholar]
- 50.Doerner AM, Zuraw BL. TGF-beta1 induced epithelial to mesenchymal transition (EMT) in human bronchial epithelial cells is enhanced by IL-1beta but not abrogated by corticosteroids. Respir Res. 2009;10:100. doi: 10.1186/1465-9921-10-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jung YW, Zindl CL, Lai JF, Weaver CT, Chaplin DD. MMP induced by Gr-1+ cells are crucial for recruitment of Th cells into the airways. Eur J Immunol. 2009;39:2281–2292. doi: 10.1002/eji.200838985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 53.Bove PF, Wesley UV, Greul AK, Hristova M, Dostmann WR, van der Vliet A. Nitric oxide promotes airway epithelial wound repair through enhanced activation of MMP-9. Am J Respir Cell Mol Biol. 2007;36:138–146. doi: 10.1165/rcmb.2006-0253SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berkman N, Robichaud A, Robbins RA, Roesems G, Haddad EB, Barnes PJ, Chung KF. Inhibition of inducible nitric oxide synthase expression by interleukin-4 and interleukin-13 in human lung epithelial cells. Immunology. 1996;89:363–367. doi: 10.1046/j.1365-2567.1996.d01-745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cuthbertson BH, Galley HF, Webster NR. Effect of inhaled nitric oxide on key mediators of the inflammatory response in patients with acute lung injury. Crit Care Med. 2000;28:1736–1741. doi: 10.1097/00003246-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 56.Poveda L, Hottiger M, Boos N, Wuertz K. Peroxynitrite induces gene expression in intervertebral disc cells. Spine. 2009;34:1127–1133. doi: 10.1097/BRS.0b013e31819f2330. [DOI] [PubMed] [Google Scholar]
- 57.Webb DC, McKenzie AN, Koskinen AM, Yang M, Mattes J, Foster PS. Integrated signals between IL-13, IL-4, and IL-5 regulate airways hyperreactivity. J Immunol. 2000;165:108–113. doi: 10.4049/jimmunol.165.1.108. [DOI] [PubMed] [Google Scholar]
- 58.Janssen-Heininger YM, Poynter ME, Aesif SW, Pantano C, Ather JL, Reynaert NL, Ckless K, Anathy V, van der Velden J, Irvin CG, van der Vliet A. Nuclear factor kappaB, airway epithelium, and asthma: avenues for redox control. Proc Am Thorac Soc. 2009;6:249–255. doi: 10.1513/pats.200806-054RM. [DOI] [PMC free article] [PubMed] [Google Scholar]