Abstract
In the arms race of host–microbe coevolution, successful microbial pathogens have evolved ingenious ways in which to evade host immunity. In this Review, we focus on ‘crosstalk manipulation’ — the microbial strategies that instigate, subvert or disrupt the molecular signalling crosstalk between receptors of innate immunity. This proactive interference undermines host defences and contributes to microbial adaptive fitness and persistent infections. Understanding how pathogens exploit host receptor crosstalk mechanisms and infiltrate the host signalling network is essential for developing interventions to redirect the host response to protective immunity.
Frontline defence cells, such as neutrophils, macrophages, and dendritic cells (DCs), detect invading pathogens through germline-encoded pattern recognition receptors (PRRs) and alert the mammalian immune system through both extracellular and intracellular activation cascades, such as, respectively, the complement and Toll-like receptor (TLR) pathways. The aim is to elicit innate antimicrobial and inflammatory responses and initiate adaptive immunity for the control or elimination of infection1,2. PRRs recognize relatively invariant microbial structures, often referred to as pathogen-associated molecular patterns (PAMPs), which are shared by related groups of microorganisms3. Different PRRs generally recognize distinct PAMPs, a concept that is best illustrated by the diverse ligand specificities of TLRs, which are the prototypical and best characterized PRR family1,4. The broad but distinct specificities of the PRRs and their ability to form functional multi-receptor complexes in lipid rafts5,6 allow for the generation of large combinatorial repertoires. This further diversifies the recognition and signalling capacities of cooperating PRRs and, at least in principle, enables the host to detect almost any type of infection, discriminate between different pathogens and mount a context-relevant immune response.
Sentinel cells receive a variety of ‘input messages’ from their environment, including those communicated by pathogen-sensing PRRs. The cell needs to appropriately process and integrate this information, which is relayed intracellularly through nonlinear signalling cascades. A systematic analysis of intracellular signalling crosstalk has sh However, chronic infectionsown that a large number of pathways converge on a relatively limited set of interaction mechanisms, which include both synergistic and antagonistic interactions7. Synergistic pathways greatly increase the sensitivity of detection, in that several individually weak stimuli can combine to elicit a vigorous cellular response. On the other hand, antagonistic pathways increase the specificity of the host response by restraining it and preventing collateral tissue damage. Signalling crosstalk is therefore important for the normal function of the immune system: It can synergistically activate host defences to clear infections or can antagonistically dampen unwarranted host responses2,8–11. Two characteristic examples are the cooperation of TLR2 with the C-type lectin dectin-1 to stimulate anti-fungal immunity12 and the homeostatic suppression of TLR-induced pro-inflammatory responses by the glucocorticoid and adenosine receptors13,14. Briefly stated, coordinated signalling crosstalk can contribute to maintain a fine balance between protective immunity and inflammatory pathology.
However, chronic infections and disease can ensue when bacterial, viral, or eukaryotic parasitic pathogens successfully evade, neutralize or subvert immune detection, signal transduction or effector killing functions15–17. Microbial pathogens that disable host defences target innate immunity preferentially16. In part, this is because the innate defences of the host are the first to be encountered by pathogens. In addition, by subverting innate immunity, pathogens can also undermine the overall host defence system, given the instructive role of innate immunity in the development of the adaptive immune response3.
One way in which pathogens could undermine host immunity to promote their adaptive fitness is through manipulation of crosstalk interactions between innate immune receptors. Indeed, despite the physiological significance of innate receptor crosstalk for immunity and homeostasis, undesirable outcomes can arise when these same mechanisms come under the control of pathogens. At least in principle, pathogens could induce antagonistic pathways leading to immune suppression. Furthermore, pathogens might induce synergistic interactions to skew the host immune response away from protective immunity — for example, driving the response towards a T helper 2 (TH2) cell response when protective immunity is mediated by TH1 cells. A growing body of literature indicates that diverse pathogens use specific virulence factors (Box 1) to exploit mechanisms of PRR cooperation, either at the receptor level or in downstream signalling (Supplementary information S1 table).
In this Review, we focus on microbial strategies that instigate, subvert or disrupt innate immune receptor crosstalk (‘crosstalk manipulation’), thereby contributing to microbial adaptive fitness and persisting infections. We do not cover general immune evasion strategies of pathogens16,18–20 or the other ingenious tactics that interfere with intracellular signalling pathways through the direct targeting of signalling intermediates (such as the inactivation of signalling molecules through cleavage or dephosphorylation by virulence proteins)21–23, as these topics have been extensively covered in excellent recent reviews. The objective of this Review is to summarize and discuss the relevant literature identifying virulence factors and hijacked receptors that mediate ‘crosstalk manipulation’ and to organize distinct microbial tactics into common themes. The following themes are discussed below: pathogens can coopt host inhibitory receptors (normally involved in homeostatic crosstalk), sometimes by expressing host-ligand mimetics24–28 (Fig. 1); instigate crosstalk pathways for the synergistic induction of immunosuppressive mediators, such as interleukin-10 (IL-10)29,30 (Fig. 2) or cAMP31,32 (Fig. 3); induce inside-out signalling to transactivate safe uptake pathways (intended for apoptotic cells)33–36 (Fig. 4); selectively inhibit TH1 cell-mediated immunity by capitalizing on complement-TLR regulatory crosstalk37–39 (Fig. 5); exploit TLR–TLR cross-inhibition40,41; and disrupt functional receptor interactions that are required for cooperative protective signalling42–44 (Table 1). Through these diverse mechanisms, pathogens hack into the host receptor crosstalk network to dysregulate innate immunity for their own benefit.
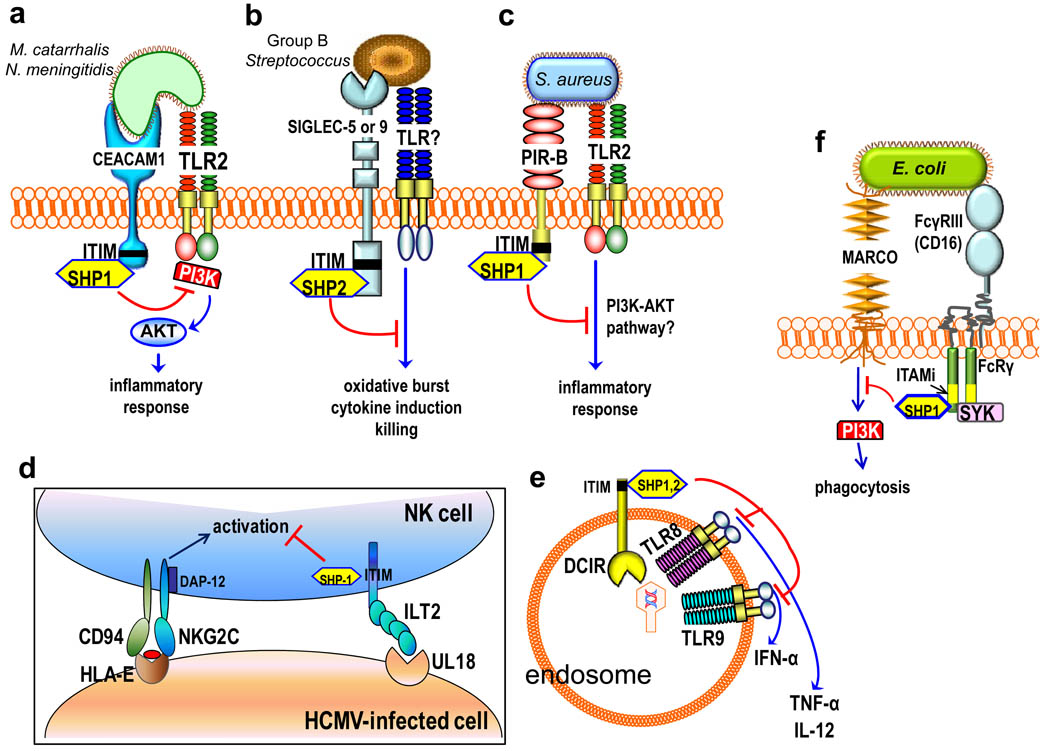
Figure 1. Inhibition of cell activation by pathogen-ligated ITIM-bearing or ITAMi-coupled receptors.
a| Moraxella catarrhalis and Neisseria meningitidis use specific virulence proteins to activate carcinoembryonic antigen–related cell adhesion molecule 1 (CEACAM1), which co-associates with and inhibits Toll-like receptor-2 (TLR2) signalling. The underlying crosstalk involves phosphorylation of the CEACAM1 immunoreceptor tyrosine-based inhibitory motif (ITIM), which recruits Src homology 2 domain-containing protein-tyrosine phosphatase 1 (SHP1); this suppresses the phosphorylation of phosphatidylinositol-3 kinase (PI3K) and downstream activation of the AKT-mediated pro-inflammatory pathway. b| Serotypes of Group B Streptococcus (GBS) bind sialic acid-recognizing immunoglobulin-superfamily lectins (Siglecs), either through molecular mimicry of host sialylated glycans or through a cell wall-anchored protein. GBS activation of ITIM-bearing Siglec-5 or Siglec-9 activates inhibitory SHP2–dependent signals that interfere with cellular activation and antimicrobial functions. c| Staphylococcus aureus uses the ITIM-containing paired Ig-like receptor B (PIR-B) to crosstalk with and inhibit the TLR2-induced inflammatory response, possibly by inhibiting the PI3K–AKT pathway. d| Human cytomegalovirus (HCMV) expresses an MHC class I homologue, UL18, which interacts with ILT2 and activates ITIM-dependent and SHP1–mediated signalling. This inhibits natural killer (NK) cell activating receptors, such as the NK group 2, member C (NKG2C)–CD94 complex, and interferes with NK cell-mediated cytolysis of the HCMV-infected cell. e| Upon activation by viruses, the ITIM-bearing DC immunoreceptor (DCIR) becomes internalized into endosomes and inhibits endosomal TLRs — specifically, it inhibits production of TLR8-induced interleukin-12 (IL-12) and TLR9-induced interferon-α (IFNα) in conventional and plasmacytoid dendritic cells, respectively. f| Escherichia coli evades macrophage receptor with collagenous structure (MARCO)-dependent phagocytic killing through an inhibitory crosstalk with FcγRIII. Specifically, nonopsonized E. coli binds with low-affinity to FcγRIII and induces partial phosphorylation of the FcRγ ITAM (ITAMi), leading to weak mobilization of spleen tyrosine kinase (SYK) but strong recruitment of SHP1. SHP1 dephosphorylates PI3K and impairs MARCO-dependent phagocytosis.
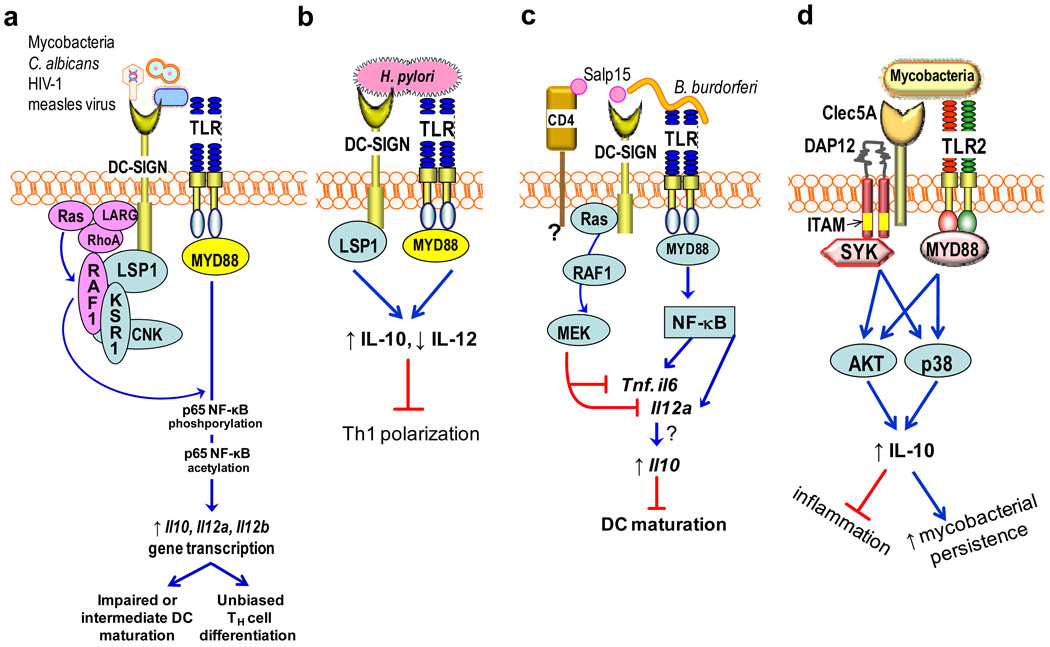
Figure 2. Pathogen-induced receptor crosstalk to stimulate IL-10 induction.
a| The indicated pathogens express mannose-containing ligands that bind DC-specific intercellular adhesion molecule-grabbing non-integrin (DC-SIGN) and induce crosstalk with Toll-like receptors (TLRs) through RAF1 signalling. Induction of RAF1 signalling involves the participation of the LSP1–KSR1–CNK scaffolding complex and upstream activators (LARG, Ras, and RhoA) and mediates phosphorylation and acetylation of TLR-activated nuclear factor-κB (NF-κB) p65 subunit. This causes enhanced transcription of the Il10, Il12a and Il12b genes due to the enhanced DNA-binding affinity and transcriptional activity of acetylated p65. b| Helicobacter pylori binds DC-SIGN through fucose-containing lipopolysaccharide Lewis antigens and activates LSP1-dependent but RAF1-independent signalling, leading to increased IL-10, decreased IL-12 and the inhibition of TH1 cell development. c| Borrelia burgdorferi uses the salivary protein Salp15 of its tick vector to induce DC-SIGN–TLR crosstalk. Here, DC-SIGN-induced RAF1 signalling does not lead to p65 acetylation but stimulates MEK signalling, which promotes Il6 and Tnf mRNA decay and impairs nucleosome remodeling at the Il12a promoter. This divergent RAF1 pathway might be attributed to possible Salp15 binding to CD4, which might participate in the crosstalk. This DC-SIGN–TLR crosstalk does not destabilize Il10 mRNA, but, rather, IL-10 production is synergistically enhanced and leads to inhibition of DC maturation. d| In neutrophils, mycobacteria interact with a C-type lectin (possibly Clec5A) linked to immunoreceptor tyrosine-based activation motif (ITAM)-bearing DAP12. This interaction induces spleen tyrosine kinase (SYK)-dependent crosstalk with the TLR2–MYD88 (myeloid differentiation primary response protein 88) pathway, which synergistically upregulates IL-10 through sustained phosphorylation of AKT and p38 mitogen-activated protein kinase (MAPK). This decreases lung inflammation but increases the persistence of a high mycobacterial burden in a mouse lung infection model. CNK, connector enhancer of KSR; KSR1, kinase suppressor of Ras-1; LARG, leukemia-associated RhoA guanine-nucleotide-exchange factor; LSP1, leukocyte-specific protein-1.
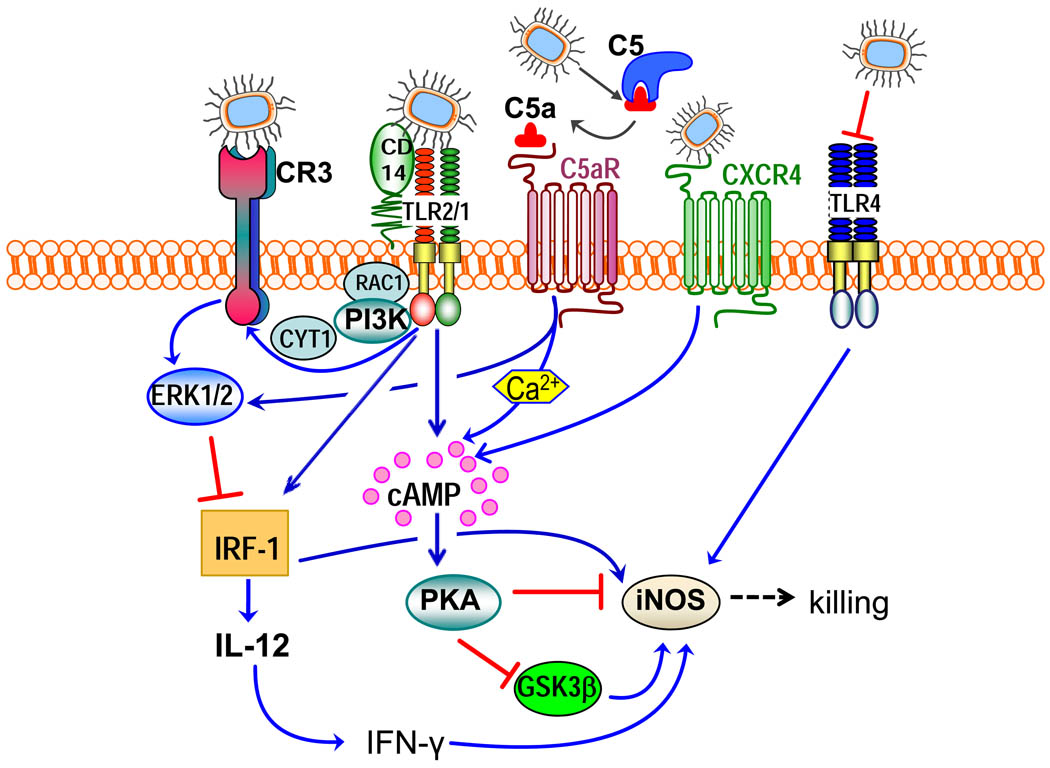
Figure 3. Integration of subversive crosstalk pathways leading to inhibition of pathogen killing.
Porphyromonas gingivalis interacts with Toll-like receptor (TLR)-2 (specifically with the CD14–TLR2–TLR1 complex) and TLR4. The latter receptor is blocked by the bacterium’s atypical lipopolysaccharide (TLR4 antagonist) and thus cannot induce protective responses. The TLR2 response is proactively modified through crosstalk with other receptors that are regulated by P. gingivalis. P. gingivalis controls C5a receptor (C5aR) by virtue of Arg-specific cysteine proteinases, which attack C5 and release biologically active C5a. C5a stimulates intracellular Ca2+ signalling which synergistically enhances the otherwise weak cAMP responses induced by TLR2 activation alone. Maximal cAMP induction requires the participation of CXC-chemokine receptor 4 (CXCR4), which is activated directly by the pathogen’s fimbriae and coassociates with both TLR2 and C5aR in lipid rafts. The ensuing activation of the cAMP-dependent protein kinase A (PKA) inactivates glycogen synthase kinase-3β (GSK3β) and impairs the inducible nitric oxide synthase (iNOS)-dependent killing of the pathogen in macrophages. An additional pathway induced downstream of TLR2 is an inside-out signalling pathway, mediated by RAC1, phosphatidylinositol-3 kinase (PI3K) and cytohesin 1 (CYT1), which transactivates complement receptor-3 (CR3). Activated CR3 binds P. gingivalis and induces extracellular signal-regulated kinase-1 (ERK1)/ERK2 signalling, which in turn selectively downregulates IL-12 p35 and p40 mRNA expression through suppression of interferon regulatory factor 1 (IRF1). This inhibitory ERK1/ERK2 pathway is also activated downstream of the C5aR. Inhibition of bioactive IL-12, and secondarily IFNγ, leads to impaired immune clearance of P. gingivalis.
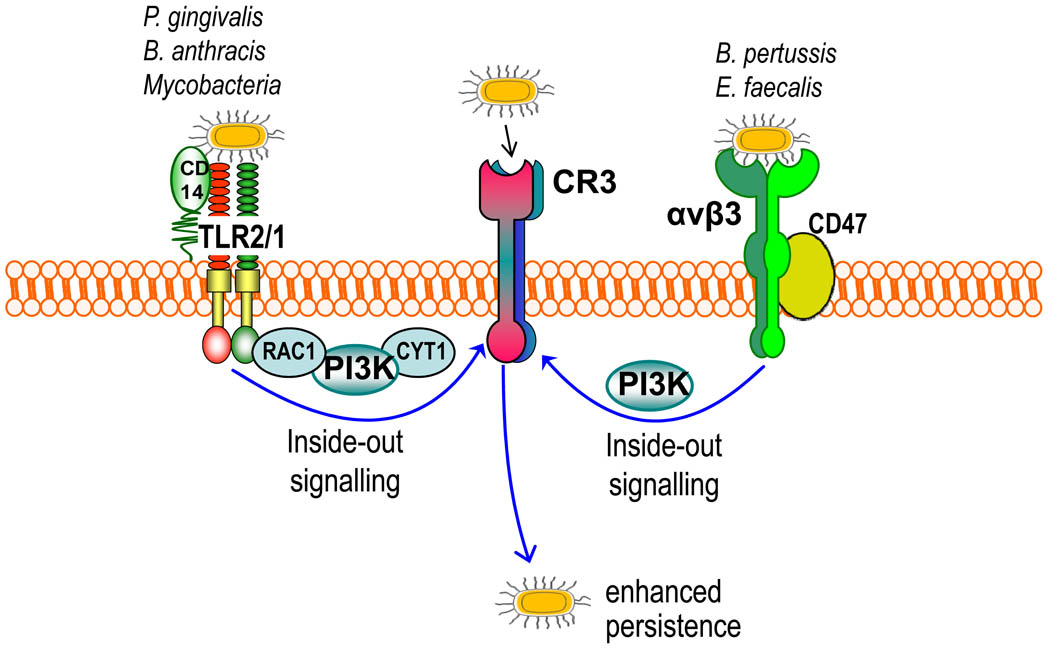
Figure 4. Pathogen-induced transactivation of CR3-mediated internalization.
Certain bacteria (such as Porphyromonas gingivalis, Mycobacterium tuberculosis and Bacillus anthracis) bind CD14 and induce Toll-like receptor-2 (TLR2)–TLR1 inside-out signalling for activating and binding complement receptor-3 (CR3), which leads to a relatively ‘safe’ uptake of these organisms by macrophages. The signalling pathway that activates the high-affinity state of CR3 is mediated by RAC1, phosphatidylinositol-3 kinase (PI3K) and cytohesin 1 (CYT1). Enterococcus faecalis and Bordetella pertussis stimulate their uptake by CR3 through an alternative inside-out signalling pathway. This mechanism is activated by the interaction of these bacteria with a receptor complex comprising the αvβ3 integrin and CD47, and is dependent on PI3K signalling. Similarly, CR3-mediated uptake of these bacteria inhibits their intracellular killing and promotes their persistence in the mammalian host.
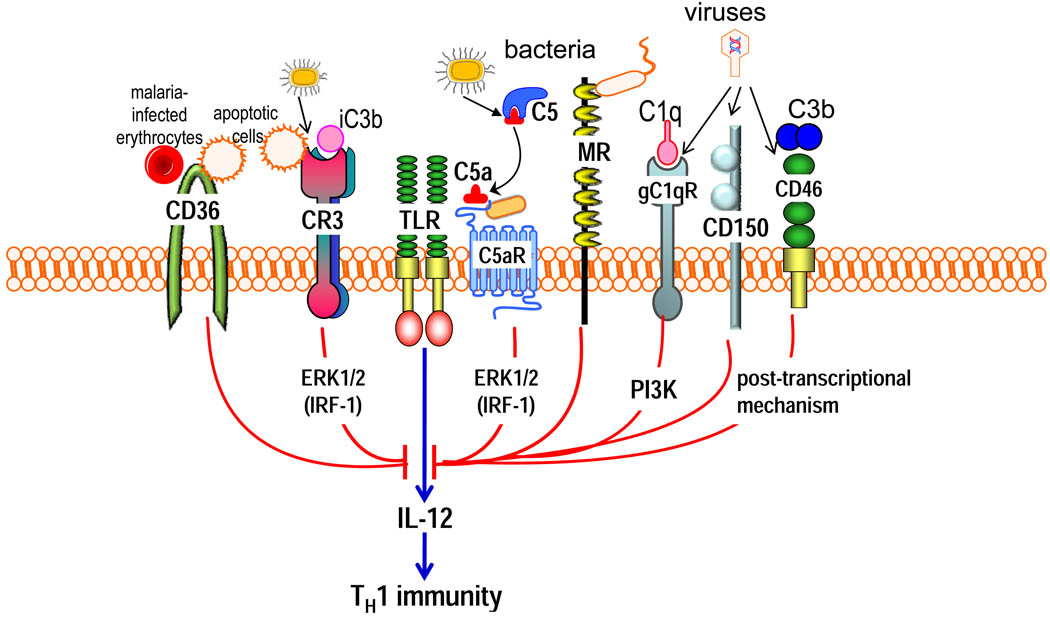
Figure 5. Selective inhibition of TLR-induced IL-12 production by pathogen-instigated PRR crosstalk.
The crosstalk between anaphylatoxin receptors (particularly C5a receptor [C5aR]) or other complement receptors (such as complement receptor-3 [CR3], gC1q receptor [gC1qR] and CD46) and Toll-like receptors (TLRs) selectively inhibits the induction of IL-12. Relatively little is known regarding the pathways mediating this selective inhibition; signalling molecules that have been implicated, such as extracellular signal-regulated kinase-1 (ERK1) and ERK2 and phosphatidylinositol-3 kinase (PI3K), are shown downstream of the corresponding receptors. At least for ERK1 and ERK2, the selectivity of IL-12 inhibition is attributed to the suppression of a crucial transcription factor, the interferon regulatory factor 1 (IRF1). Posttranscriptional mechanisms might also contribute to IL-12 inhibition. Activation of these complement receptors, or other innate immune receptors (such as CD36, mannose receptor and CD150) that share the ability to downregulate IL-12, by their natural ligands might have a homeostatic function. However, these same receptors can be activated by bacterial, viral or parasitic pathogens, which can thereby downregulate TLR-induced IL-12 production to interfere with host defences (such as the inhibition of TH1 cell-mediated immunity). Although microbial molecules that act as ligands for C5aR have been described, this receptor can come under pathogen control also through the enzymatic generation of high levels of C5a by microbial C5 convertase-like enzymes.
Table 1.
Microbial disruption of cooperative interactions between innate immune receptors
| Pathogens | Virulence molecules and their targets |
Crosstalking Receptors (R) |
Cell type | Cellular response without interference |
Mechanism and outcome of disruption |
Refs | |
|---|---|---|---|---|---|---|---|
| R1 | R2 | ||||||
| Coxiella burnetii | Uncertain; possible involvement of smooth-type LPS, which is thought to target CD47 | αvβ3/CD47 | CR3 | Monocytes | αvβ3/CD47-induced inside-out signalling activates CR3 (lectin site) phagocytosis, leading to intracellular killing* | Pathogen taken up by αvβ3, leading to intracellular survival; mechanism unclear, perhaps smooth-type LPS interferes with the co-signalling function of CD47 | 42 |
| Group A Streptococcus | Mac, a CD11b mimetic, binds CD16 | CD16 | CR3 | Neutrophils | Opsonophagocytosis, oxidative burst, and killing | Mac blocks CD16-CR3 interactions for outside-in signalling, thereby inhibiting the neutrophil antimicrobial response | 43 |
| Filarial nematodes | Screted glycoprotein ES-62 forms complex with TLR4 | TLR4 | FcεRI | Mast cells | FcεRI-mediated mast cell degranulation | Sequestration and degradation of PKC-α, required for the coupling of FcεRI to PLD, thereby inhibiting mast cell activation | 44 |
Refers to avirulent C. burnetii which expresses rough-type, rather than smooth-type, LPS.
CR3, complement receptor-3; LPS, lipopolysaccharide; TLR4, Toll-like receptor 4; FcεRI, Fcε receptor type I; PKC-α, protein kinase C-α; PLD, phospholipase D.
Co-option of inhibitory receptors
A distinct set of host inhibitory immune receptors signal through immunoreceptor tyrosine-based inhibitory motifs (ITIMs), which recruit phosphatases — Src homology 2 domain-containing protein-tyrosine phosphatase 1 (SHP1), SHP2 or Src homology 2 domain–containing inositol phosphatase (SHIP) — that attenuate signalling induced by juxtaposed receptors through the dephosphorylation of signalling intermediates8. ITIM-bearing receptors often, but not exclusively, interact with and inhibit immunoreceptor tyrosine-based activation motif (ITAM)-coupled receptors, such as the Fcγ receptors (FcγRs) and triggering receptor expressed on myeloid cells 1 (TREM1)45. ITAMs are found in the cytoplasmic domain of certain transmembrane adaptors (such as the FcR common γ-chain and DAP12) and generally mediate activating signals through the activation of spleen tyrosine kinase (SYK). However, ITAM-mediated cell activation requires high-avidity ligation of the ITAM-coupled receptors, whereas low-avidity (or tonic) ligation generates inhibitory signals mediated by SHP146,47. ITAMs acting in an inhibitory mode are referred to as ITAMi47.
Several microorganisms exploit ITIM-bearing or ITAMi-coupled receptors, which they may co-ligate with targeted receptors24–27. The resulting juxtaposition of ITIM-bearing or ITAMi-coupled receptors with the targeted receptors (such as TLRs or phagocytic receptors) allows the induction of inhibitory crosstalk that suppresses cellular activation and/or phagocytosis. For example, Moraxella catarrhalis and Neisseria meningitidis use virulence proteins (ubiquitous surface protein A1 and opacity-associated protein, respectively) to bind and activate carcinoembryonic antigen–related cell adhesion molecule 1 (CEACAM1) on respiratory epithelia resulting in inhibition of TLR2 signalling. The potential significance of this inhibitory effect is underscored by the heavy involvement of TLR2 in the inflammatory and antimicrobial responses of pulmonary epithelial cells. At the molecular level, TLR2 co-associates with CEACAM1 following their co-activation by these respiratory pathogens, although the microbial TLR2 ligands involved were not identified26. Subsequently, phosphorylation of the cytoplasmic ITIM of activated CEACAM1 recruits SHP1, which limits phosphorylation of the p85α regulatory subunit of phosphatidylinositol-3 kinase (PI3K) that is recruited to the TLR2 cytoplasmic domain26 (Fig. 1a). This in turn inhibits the activation of the PI3K–AKT pathway and decreases the production of interleukin-8 (IL-8) and granulocyte–macrophage colony-stimulating factor, which regulate the development, mobilization and activation of granulocytes in response to respiratory infections. These data indicate a mechanism of immune evasion through CEACAM1 exploitation, which might be shared by other CEACAM1-interacting pathogens such as Neisseria gonorrhoeae and enteropathogenic Escherichia coli and Salmonella strains. However, the lack of appropriate animal models (M. catarrhalis and N. meningitidis infections are specific to humans) does not allow for in vivo confirmation.
Group B Streptococcus (GBS) can bind sialic acid-recognizing immunoglobulin-superfamily lectins (Siglecs) on leukocytes in a sialic acid-dependent or -independent manner, depending on the serotype. For example, the sialylated capsular polysaccharide of serotype III GBS mimics host sialylated glycans and co-opts Siglec-924, and the cell wall-anchored β protein of serotype Ia and III GBS strains binds Siglec-525. GBS engagement of ITIM-bearing Siglec-5 or Siglec-9 activates inhibitory SHP2–dependent signals that interfere with cellular activation (Fig. 1b). This leads to the inhibition of several antimicrobial functions in leukocytes, including phagocytosis, induction of the oxidative burst and formation of extracellular DNA traps, which allows GBS to escape killing by monocytes and/or neutrophils24,25. The receptor(s) that crosstalk with Siglecs in this immune evasion mechanism were not identified, but could be TLRs, as Siglec-E, the mouse orthologue of Siglec-9, downregulates TLR4 signalling through SHP248.
An evasion strategy analogous to that of GBS might be used by Staphylococcus aureus in mouse macrophages, in which the TLR2-induced inflammatory response is counteracted by co-activation of another ITIM-containing receptor, the murine paired Ig-like receptor B (PIR-B)49 (Fig. 1c). The identity of the PIR-B–interacting ligand(s) of S. aureus is uncertain, although polyanionic molecules, such as dextran sulphate and polyinosinic acid, competitively inhibited the binding of this bacterium to PIR-B49. PIR-B, which under physiological conditions regulates activation of the ITAM-coupled PIR-A, has human orthologues — Ig-like transcript 2 (ILT2) and ILT5 — that are also bound by S. aureus and other bacteria49. Whether ILT receptors (also known as leukocyte Ig-like receptors, LIRs) in humans are exploited by bacterial pathogens remains to be investigated. However, ILT2 is exploited by human cytomegalovirus (HCMV) to suppress natural killer (NK) cell-mediated cytolysis, through SHP1–dependent crosstalk signals that interfere with activating NK cell receptors28,50(Fig. 1d). Intriguingly, activation of ILT2 and phosphorylation of its ITIM is mediated by UL18, a viral glycoprotein that not only mimics but also outcompetes MHC class I molecules as it binds ILT2 with >1000-fold higher affinity50. HIV-1 binds DC immunoreceptor (DCIR), an ITIM-containing C-type lectin, which promotes HIV-1 infection of DCs51. Because endocytosed DCIR crosstalks with and inhibits production of TLR8-induced IL-12 and TLR9-induced interferon-α (IFNα) in conventional and plasmacytoid DCs, respectively52,53 (Fig. 1e), this pathway might contribute to immune evasion by HIV-1 or other DCIR-binding viruses.
E. coli evades phagocytic killing by inducing ITAMi signals through FcγRIII (CD16) and its ITAM-bearing FcRγ signalling adaptor, which crosstalk with the class A scavenger receptor MARCO (macrophage receptor with collagenous structure)27 (Fig. 1f). Specifically, E. coli binds FcγRIII directly in a non-opsonic manner (without antibody) and this low-avidity interaction induces FcRγ phosphorylation, followed by recruitment of SHP1 and dephosphorylation of PI3K, which is thereby unable to support MARCO-mediated phagocytosis of E. coli27. Mice deficient in FcγRIII or FcRγ have increased survival in models of sepsis induced by cecal ligation and puncture, which is attributed, in part, to their enhanced ability to clear E. coli27.
It is conceivable that at least some of these inhibitory receptors could be used by the immune system to limit and control unnecessary inflammatory responses, as is the case with commensal Gram-negative bacteria in the gut54. In this context, the interaction of a receptor such as PIR-B with a commensal organism might avoid a vigorous host response, an outcome that is mutually beneficial for both the host and the microorganism. Although it might sound counterintuitive to suggest that commensals and pathogens share immune evasion mechanisms, the latter express additional factors, such as invasins and/or toxins, which enable them to breach epithelial barriers and invade host cells.
Synergistic induction of immunosuppressive mediators
Many pathogens capitalize on the immunosuppressive properties of IL-10 and cAMP to undermine aspects of innate immune defence55–58. Although IL-10– and cAMP–dependent pathways have important regulatory functions in maintaining homeostasis of the immune system, excessive and sustained production of these mediators, for example instigated by pathogens, impairs the killing capacity of phagocytes. The functions that are suppressed by IL-10 and cAMP include the production of reactive oxygen or nitrogen intermediates and of pro-inflammatory cytokines, and phagolysosomal fusion and acidification. However, differences between the effects of IL-10 and cAMP do exist in that IL-10 additionally inhibits the induction of costimulatory molecule expression by antigen-presenting cells, whereas cAMP inhibits degranulation of neutrophils more consistently than does IL-1055,58–60. Moreover, cAMP upregulates rather than inhibits IL-6 production32,58. Because cAMP is generated through enzymatic action (by adenylate cyclase), its levels can increase markedly within minutes of stimulation and, in fact, cAMP subsequently enhances transcription of the Il10 gene61.
Some microorganisms have genetically encoded mechanisms for regulating cAMP or IL-10 production. Specifically, bacteria such as Bordetella pertussis and Bacillus anthracis express their own adenylate cyclase enzyme for unregulated production of cAMP56,57, and certain viruses (such as Epstein–Barr virus, Orf virus and equine herpesvirus 2) encode their own version (viral homologue) of IL-1055. Alternatively, other pathogens can elicit IL-10 or cAMP through the induction of synergistic crosstalk pathways29–32,62,63 (S1 table).
Induction of IL-10
Several important pathogens including Mycobacterium tuberculosis, M. leprae, Helicobacter pylori, Candida albicans, measles virus and HIV-1, induce crosstalk between the C-type lectin DC-SIGN (DC-specific intercellular adhesion molecule-grabbing non-integrin) and TLRs, which leads to high levels of IL-10 production in DCs29,62,63 (Fig. 2a). For example, mycobacterial mannosylated lipoarabinomannan (ManLAM) binds DC-SIGN and induces a complex pathway that activates the serine/threonine kinase RAF1 (Fig. 2a). RAF1, in turn, induces Ser276 phosphorylation and subsequently acetylation of the p65 subunit of NF-κB on several lysines. The acetylation of p65 requires the activation of both DC-SIGN and TLR signalling and is mediated by the related acetyltransferases CREB-binding protein and p300, which get recruited to p65 by binding to its phosphorylated Ser276. This modification causes prolonged and enhanced transcription of the Il10 gene by NF-κB; the underlying mechanism involves the enhanced DNA-binding affinity and transcriptional activity of acetylated p65 and its prolonged presence in the nucleus29. The same pathway was later shown to induce enhanced transcription of the Il12a and Il12b genes64. The upregulation of both TH1-promoting (IL-12) and TH1-repressing (IL-10) cytokines is consistent with earlier observations that mycobacteria induce IL-10-producing TH cells without a TH1/TH2 bias64,65. The interaction of mycobacteria with DC-SIGN has also been associated with impaired or intermediate-stage maturation of DCs62,66, although it is not clear whether this represents immune evasion or a host mechanism to reduce inflammatory pathology.
On the other hand, the interaction of H. pylori with DC-SIGN through fucose-containing lipopolysaccharide Lewis antigens leads to increased IL-10 and reduced IL-12 production and, eventually, to inhibition of TH1 cell development63,64 (Fig. 2b). The two pathways (Fig. 2a and b) are therefore divergent; indeed, in contrast to mannose-containing DC-SIGN ligands (such as mycobacterial ManLAM, fungal mannan, and HIV-1 gp120), fucose-containing DC-SIGN ligands (such as Lewis X of H. pylori) proactively exclude RAF1 and other select components from the DC-SIGN signalling complex and thereby modify downstream signal transduction64.
The Lyme disease spirochete Borrelia burgdorferi induces DC-SIGN crosstalk with TLR2, although the DC-SIGN ligand is contributed by its tick vector (Ixodes scapularis)67 (Fig. 2c). Specifically, TLR2 and DC-SIGN are co-activated, respectively, by B. burgdorferi lipoproteins and I. scapularis salivary protein Salp15, which is captured by the outer surface protein C of the bacterium. Although the composition of the DC-SIGN signalling complex was not reported to the same detail as in the study discussed above64, Salp15 contains mannose structures and induces RAF1 signalling67. However, in this case, RAF1 activation does not lead to acetylation of the NF-κB p65 subunit, as described for ManLAM. It is thought that because Salp15 can also bind CD4, this receptor might participate in the DC-SIGN–TLR2 crosstalk and alter the RAF1-dependent signalling pathway (Fig. 2c). In this pathway, Salp15-induced RAF1 activation stimulates MEK signalling, which promotes Il6 and Tnf mRNA decay and impairs nucleosome remodeling and hence transcriptional activation at the Il12a promoter. The same pathway does not destabilize Il10 mRNA; in fact, co-activation of DCs with B. burgdorferi and Salp15 synergistically enhances IL-10 production67. Since IL-12 inhibits IL-10 production, it is possible that the observed inhibition of IL-12 could secondarily contribute to increased IL-10 levels. In terms of biological relevance, the consequences of Salp15 utilization by B. burgdorferi include inhibition of TLR-dependent maturation of DCs and their capacity to activate T cells, which is advantageous both for the arthropod vector and the bacterial pathogen67.
In neutrophils, mycobacteria use another C-type lectin (potentially Clec5A), which is coupled to the ITAM-containing adaptor protein DAP12 to induce SYK-dependent crosstalk with the TLR2–MYD88 (myeloid differentiation primary response protein 88) pathway. This crosstalk synergistically upregulates IL-10 production through rapid and sustained phosphorylation of two kinases: AKT and the p38 mitogen-activated protein kinase (MAPK)30 (Fig. 2d). In a chronic infection model in mice, neutrophil-derived IL-10 decreased lung inflammation but contributed to the persistence of a high mycobacterial burden30.
Induction of cAMP
Porphyromonas gingivalis is a periodontal pathogen that is also implicated in systemic conditions such as atherosclerosis and rheumatoid arthritis68,69. This Gram-negative bacterium uses an array of virulence factors to evade immune elimination and chronically persist in the human host70. Recent evidence indicates that P. gingivalis achieves this partly by subverting immune receptor crosstalk signalling31,32 (Fig. 3). Specifically, P. gingivalis induces the recruitment and co-association in macrophage lipid rafts of TLR2 and two G protein-coupled receptors (GPCR), the CXC-chemokine receptor 4 (CXCR4) and the complement C5a receptor (C5aR), leading to induction of high and sustained levels of cAMP31,32. P. gingivalis activates TLR2 through its surface fimbriae and lipoproteins, but, notably, it does not rely on immunological means for C5aR activation. Indeed, it can generate C5a through its own C5 convertase-like enzymatic activity, mediated by Arg-specific cysteine proteinases (the RgpA and RgpB gingipains)31. In addition, P. gingivalis can directly activate CXCR4, through its surface fimbriae (albeit involving different epitopes from those mediating TLR2 binding71,72), without requiring CXCL12 as a ligand32. Recognition of P. gingivalis by TLR2 alone induces a weak cAMP response, whereas CXCR4 or C5aR by themselves fail to induce cAMP. Strikingly, however, P. gingivalis-stimulated TLR2 cooperates with activated C5aR and CXCR4 to synergistically increase cAMP production; this, in turn, greatly increases cAMP-dependent protein kinase A signalling, which inactivates glycogen synthase kinase-3β and hence impairs inducible nitric oxide synthase (iNOS)-dependent killing of bacteria in vitro and in vivo31,32 (Fig. 3). The C5aR–TLR2 crosstalk depends on Gαi-coupled C5aR signalling and the mobilization of intracellular calcium31, which potentiates concurrent cAMP signalling and hence PKA activation7. Although the C5aR–TLR2 and CXCR4–TLR2 crosstalks can proceed independently of each other, maximal cAMP induction requires the cooperation of all three receptors31. P. gingivalis interacts with at least one other TLR, TLR4, although the ligands involved are atypical lipopolysaccharide molecules that only weakly activate TLR4 (5-acyl monophosphate lipid A structure) or even antagonize TLR4 (4-acyl monophosphate lipid A)73 (Fig. 3).
The Gs protein-coupled A2A and A2B adenosine receptors (A2AR and A2BR) respond to extracellular adenosine and increase intracellular cAMP levels. Activated A2AR (which has higher affinity for adenosine than does A2BR) crosstalks with and inhibits TLR-induced inflammation14. Intriguingly, Staphylococcus aureus expresses cell wall-associated adenosine synthase A (AdsA), which converts adenosine monophosphate to adenosine74. The pathogen exploits the immunosuppressive properties of the adenosine it generates to ‘disable’ phagocytes in the blood and escape immune clearance. AdsA-deficient mutants of S. aureus have a survival disadvantage in blood that can be reversed by the addition of exogenous adenosine. The authors of this paper also identified another 10 species of Gram-positive bacteria (such as B. anthracis, Clostridium perfringens and Listeria monocytogenes) that express homologues of the adenosine synthase domain of AdsA. Study of B. anthracis showed that it also uses AdsA to escape phagocytic clearance, which suggests that additional AdsA-expressing bacteria might share this evasion mechanism74. Given that A2AR signalling inhibits TH1 and TH17 cell development, while promoting the generation of adaptive regulatory T cells75, AdsA-expressing pathogens might also be able to manipulate T cell immunity.
Inside-out signalling and outside-in persistence
Complement receptor 3 (CR3) is a versatile β2 integrin (CD11b–CD18) that binds multiple ligands or counterreceptors (such as iC3b and intercellular adhesion molecule 1) and contributes to the phagocytosis of apoptotic cells, leukocyte trafficking and the regulation of cytokine production2. Such adhesive activity is tightly regulated; whereas CR3 has a low-affinity conformation in resting cells, a rapid and transient shift to a high-affinity state can be triggered through inside-out signalling by chemokine or anaphylatoxin receptors76. TLRs can also induce inside-out signalling for CR3 activation, as originally shown for TLR277,78 and recently confirmed for TLR479. The TLR2 inside-out signalling pathway proceeds through RAC1, PI3K and cytohesin-177,78,80 (Fig. 4). Unlike for TLR4, however, the TLR2 inside-out pathway does not depend on MYD8881; this is because PI3K can be recruited directly to the TLR2 cytoplasmic tail which, unlike TLR4, contains PI3K-binding motifs82. In terms of physiological significance, the ability of pathogen-sensing receptors, such as TLRs, to transactivate CR3 might contribute to leukocyte recruitment to sites of infection80.
Although CR3 is a phagocytic receptor, it is not linked to vigorous microbicidal mechanisms such as those activated by FcγR-mediated phagocytosis83,84 and, under certain conditions, CR3-derived phagosomes do not fuse with lysosomes85. This is possibly related to the physiological role of CR3 in the uptake of apoptotic cells, which are not normally recognized as a danger that warrants a strong host response2. Not surprisingly, therefore, the TLR2–CR3 crosstalk pathway is a target of immune subversion by several pathogens. P. gingivalis, M. tuberculosis and B. anthracis use, respectively, their fimbriae, lipoarabinomannan and the BclA glycoprotein to interact with the CD14–TLR2 receptor complex and induce TLR2-mediated transactivation of CR333,77,78 (Fig. 4). This mechanism allows these bacteria to hijack the phagocytic functions of CR3 for a relatively safe ‘outside-in’ entry into macrophages. The subversive effect of P. gingivalis is evident from the finding that CR3-deficient macrophages are superior to wild-type controls in intracellular killing of this pathogen34,86. Moreover, compared with CR3-deficient mice, wild-type mice have increased susceptibility to infection with B. anthracis spores; this is attributed to the ‘safe storage’ of the spores within macrophages after uptake by CR3 and their carriage to sites of spore germination and bacterial growth33. Similarly, the ability of M. tuberculosis to survive in macrophages may depend, at least in part, on its ability to stimulate TLR2-induced CR3-mediated phagocytosis78, although an additional step involves the recruitment of coronin-1 to CR3-derived phagosomes, which prevents their fusion with lysosomes85.
Two other organisms, Enterococcus faecalis and B. pertussis activate CR3-mediated phagocytosis through alternative inside-out signalling pathways. Specifically, the ‘aggregation substance’ glycoprotein of E. faecalis and the filamentous hemagglutinin of B. pertussis interact with a signalling complex comprising the αvβ3 integrin and the integrin-associated protein CD47, leading to CR3 transactivation in macrophages and neutrophils36,87 (Fig. 4). CR3-mediated uptake of E. faecalis leads to inhibition of the oxidative burst and promotes the survival of the bacterium35,88. B. pertussis takes advantage of CR3-mediated phagocytosis to escape immune clearance in vivo89; the pathogen is readily cleared if it is phagocytosed through the FcγRIII89 which, unlike CR3, is coupled to potent microbicidal mechanisms83,84.
Interestingly, at least in two cases, TLR2 inside-out signalling is activated by the same virulence proteins (namely P. gingivalis fimbriae34 and B. pertussis filamentous hemagglutinin36) that also bind transactivated CR3 for nonopsonic phagocytosis. Given the role of CR3 in the phagocytosis of iC3b-coated apoptotic cells, those pathogens targeting CR3 as a ‘preferred’ portal of entry (Fig. 4) may have actually co-opted a homeostatic, anti-inflammatory mechanism to evade innate immunity.
Subversive complement–TLR crosstalk to modulate T cell immunity
Complement and TLRs are rapidly co-activated in response to infection and common PAMPs (such as lipopolysaccharide and CpG DNA) function as both complement activators and TLR ligands2. In fact, the early innate immune response is shaped, to a large extent, by bidirectional crosstalk between the two systems11. For example, activation of the complement anaphylatoxin receptors (the GPCRs C3aR and C5aR) synergistically enhances TLR-induced production of pro-inflammatory and antimicrobial mediators2,90,91. The signalling pathways involved in complement-TLR4 crosstalk converge at the level of MAPKs, specifically extracellular signal-regulated kinase-1 (ERK1), ERK2 and JUN N-terminal kinase (JNK)90. This synergy could potentially enhance innate resistance to infection, and similar crosstalk effects explain, at least in part, why pharmacological inhibition of C5aR protects against sepsis induced by high doses of lipopolysaccharide. In a reciprocal reinforcing manner, TLR activation induces the expression of complement components and/or receptors2. Moreover, TLR signalling decreases the desensitization of GPCRs by downregulating the expression of G-protein-coupled receptor kinases that induce receptor phosphorylation and internalization92. This TLR activity would be expected to prolong the activation of C3aR and C5aR.
Regulation of IL-12 production
The crosstalk between anaphylatoxin receptors (particularly C5aR) and TLRs also induces specific antagonistic effects, at least in macrophages, that selectively affect the induction of IL-12 family cytokines. The underlying mechanism of C5aR–TLR crosstalk, which depends on ERK1/ERK2 and PI3K signalling, suppresses the activation of interferon regulatory factor 1 (IRF1) and IRF8, which preferentially regulate the expression of IL-12 and related cytokines such as IL-2337,93. The C5aR-ERK1/2-IRF1 pathway preferentially inhibits IL-12p70 production, while the C5aR-PI3K-IRF8 pathway primarily suppresses IL-2337. Similar inhibitory effects on IL-12 induction are seen when other complement receptors (gC1qR, CD46 and CR3) are co-activated with TLR4 or TLR2 in mouse macrophages or human monocytes39,94,95. The activation of these complement receptors by their natural ligands, such as C3b and C5a that are produced during the complement cascade or by non-complement host enzymes (such as thrombin and kallikrein)2, may represent regulatory mechanisms to attenuate T cell-mediated inflammation, given the important role of IL-12 in TH1 cell differentiation and activation96. For example, inhibition of IL-12p70 by C5aR–TLR4 crosstalk can suppress TH1 cell-mediated pathology. Moreover, inhibited IL-12p70 production following the interaction of macrophage CR3 with iC3b-coated apoptotic cells may prevent unwarranted inflammation and TH1 cell activation during apoptotic cell phagocytosis2. Conversely, C1q deficiency in humans and mice causes inflammatory autoimmune pathology97 although it is uncertain if, and to what extent, this results from lack of C1q-gC1qR–mediated homeostatic regulation of T cells through crosstalk with TLR signalling pathways in antigen-presenting cells.
The significance of these antagonistic crosstalk interactions becomes more evident in the context of microbial pathogenesis, where complement receptors seem to modify TLR signalling and skew the TH cell response in ways that interfere with protective immunity37–39. Certain non-complement innate receptors are also implicated in the selective inhibition of TLR-induced IL-12 production98, and examples of pathogens that exploit complement and non-complement receptors are given below (Fig. 5).
Leishmania major, an intracellular parasite of macrophages, seems to benefit from complement activation and C5aR-induced inhibition of TH1 cell immunity37. This conclusion is based on the finding that BALB/c mice, which are normally susceptible to cutaneous leishmaniasis, acquire TH1 cell-dependent resistance to L. major infection when C5aR is genetically ablated37. Microbial C5 convertase-like enzymes that generate C5a, such as the gingipains of P. gingivalis, have been implicated in selective downregulation of IL-12 and inhibition of IL-12–dependent immune clearance in vivo99. Similar evasive strategies, involving alternative complement receptors, are used by additional pathogens. These include measles virus, human herpesvirus 6 and adenovirus (Groups B and D), which interact with CD46 through specific virulence proteins/ligands39,100–103 (S1 table). Measles virus also inhibits TLR4-induced IL-12 production in DCs, although here the inhibitory signal is delivered by CD150 (also known as signalling lymphocyte activation molecule; SLAM)104. In both cases, however, the virus uses its hemagglutinin to bind CD150 or CD46. CD46 is used as a receptor by additional pathogens (such as Neisseria gonorrhoeae, Neisseria meningitidis and Streptococcus pyogenes)105, which raises the possibility that these organisms might also inhibit IL-12 production.
Hepatitis C virus uses its core protein to bind gC1qR on macrophages or DCs and thereby inhibit IL-12 production and TH1 cell immunity, which is thought to be an important mechanism whereby the virus can establish persistent infections38. This evasion mechanism might be shared by other pathogens, such as L. monocytogenes and S. aureus, which can also interact with gC1qR using specific virulence proteins106,107. Mycobacteria can downregulate TLR4-induced IL-12 production through the mannose receptor, although this mechanism has broader anti-inflammatory effects108,109.
Plasmodium falciparum selectively inhibits IL-12 production and suppresses DC maturation and T cell activation through interactions with CD36. Such interactions with CD36 are mediated by the P. falciparum erythrocyte membrane protein 1 (PfEMP1), which is expressed on infected erythrocytes110,111 and also interacts with TLR2112. Although PfEMP1–modulated DCs secrete high levels of IL-10, their inability to produce IL-12 is an IL-10-independent effect110,111. More recently, PfEMP1 was also implicated in specific suppression of the early induction of IFNγ, although this involves a CD36-independent mechanism113. Moreover, the role of CD36 in malarial pathogenesis is complex and multifactorial as suggested by a report that CD36-deficient mice have defective clearance of the parasite112.
Several microorganisms express virulence proteins that interact directly (in a nonopsonic way) with CR3, although specific CR3-mediated inhibition of IL-12 production has been documented for only a few cases. These include Histoplasma capsulatum, B. pertussis and P. gingivalis95,114–116 which use specific virulence proteins to inhibit IL-12 (S1 table). CR3-dependent immune subversion was confirmed in vivo for P. gingivalis, which uses its fimbriae to bind CR3, activate ERK1 and ERK2 signalling, and thereby inhibit TLR2-induced IL-12 production116 (Fig. 3). This allows P. gingivalis to survive in wild-type but not CR3-deficient mice (or normal mice in which CR3 is pharmacologically inhibited), which produce higher levels of IL-12 and, secondarily, IFNγ. However, the host protective effect of CR3 inhibition can be reversed by antibody-mediated neutralization of IL-12116.
TLR–TLR interplay
The capacity of TLR signalling pathways for cross-regulation9 could potentially be exploited by certain pathogens. This could be achieved through the induction of conflicting signals by distinct pathogen-expressed TLR ligands, and recent papers lend support to this concept. For example, M. tuberculosis expresses lipoproteins and glycolipids, which act in an inhibitory mode through TLR2 leading to impaired bacterial CpG DNA-induced IFNα/β production and MHC class I-restricted antigen cross-presentation through TLR9 signalling40. This crosstalk mechanism might explain why IFNα/β is not an important factor in host immunity to mycobacteria40. Hepatitis C virus uses its core protein to activate TLR2-mediated production of inhibitory cytokines (such as IL-10) by human monocytes that suppress TLR9-induced IFNα production by plasmacytoid DCs41. This mechanism involves transcellular crosstalk as human plasmacytoid DCs express TLR9 but not TLR2. By contrast, mouse myeloid DCs, which were used in the study of M. tuberculosis40, express both TLR2 and TLR9; in this system, direct intracellular TLR2–TLR9 signalling crosstalk is mainly responsible for TLR2-mediated inhibition of TLR9-induced antiviral immunity.
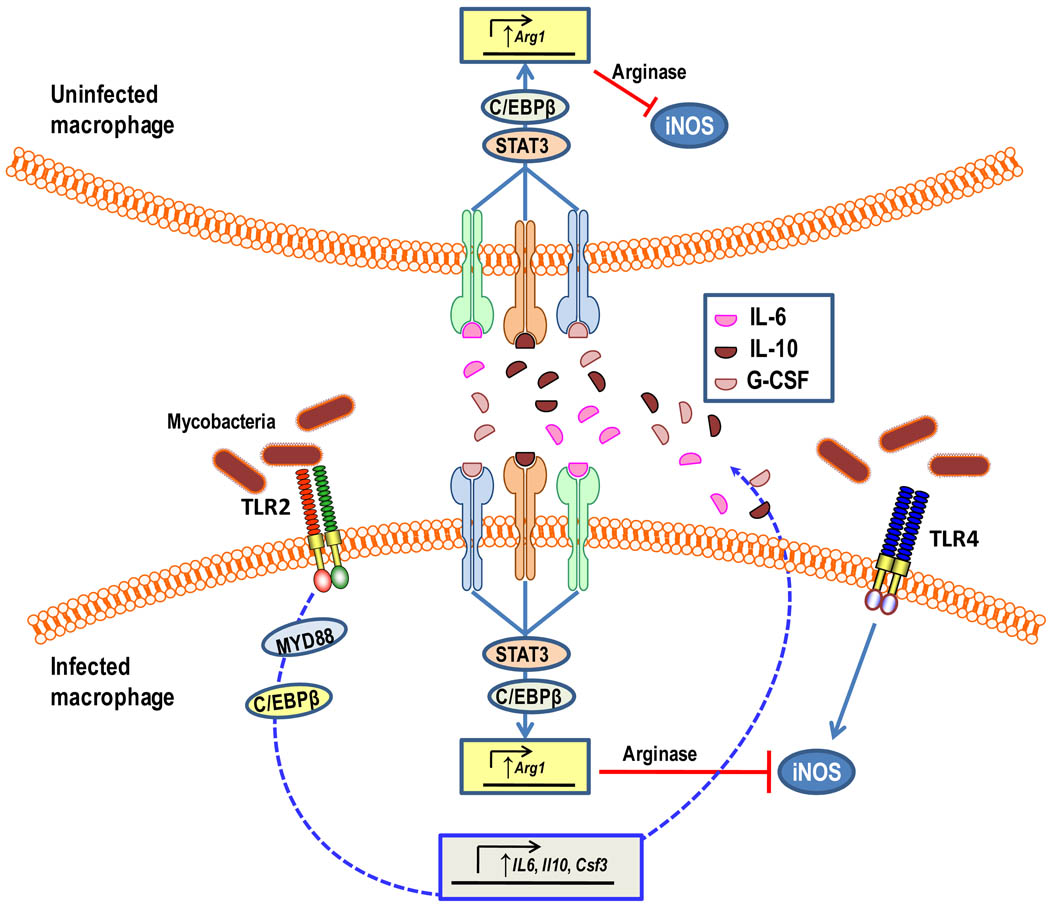
M. tuberculosis and Toxoplasma gondii were shown to promote their survival and ability to cause disease in mouse models through MyD88-dependent induction of macrophage arginase 1 (ARG1), which in turn inhibits nitric oxide production by competing with iNOS for the common substrate arginine117. MYD88-mediated ARG1 expression in mycobacteria-infected macrophages depends, in part, on TLR2 activation, whereas strong induction of nitric oxide production is mediated by TLR4 activation117. In a follow-up paper, the same group showed that the TLR–MyD88-mediated production of ARG1 did not involve direct MYD88 signalling to ARG1 expression but was controlled by the MYD88-dependent production of IL-6, IL-10 and granulocyte colony-stimulating factor acting though the signal transducer and activator of transcription-3 (STAT3)118 (Fig. 6). The implication of these findings is that ARG1 can be induced in a paracrine manner; therefore, mycobacteria can ‘instruct’ both infected and uninfected macrophages to decrease nitric oxide production, thereby rendering these cells permissive to their intracellular lifestyle. Interestingly, ARG1 can also be expressed in ‘alternatively activated’ macrophages through a STAT6-dependent pathway in the context of TH2 immunity118. This might affect the iNOS-dependent killing of pathogens like Francisella tularensis, which induces the production of IL-4 and IL-13 and thus activates macrophages through the alternative pathway119.
Figure 6. MyD88-dependent arginase induction prevents nitric oxide production in both mycobacteria-infected and -uninfected macrophages.
Activation of myeloid differentiation primary response protein 88 (MYD88) signalling by mycobacteria (at least in part through TLR2) induces CCAAT/enhancer-binding protein β (C/EBPβ)-mediated induction of interleukin-6 (IL-6), IL-10, and granulocyte colony-stimulating factor (GCSF) production. These signal transducer and activator of transcription-3 (STAT3)-activating cytokines act in both autocrine and paracrine manners to induce arginase 1 (Arg1) expression which is partially dependent on C/EBPβ. The produced arginase can inhibit inducible nitric oxide synthase (iNOS) activity through competition for the common substrate arginine. The MYD88 pathway for arginase production was shown to confer a survival benefit for mycobacteria in vivo and is thought to counteract pathways that activate nitric oxide production, such as TLR4.
C. albicans expresses ligands for both TLR2 and TLR4. Whereas TLR4 confers protection against infection120, TLR2 suppresses the candidacidal capacity of macrophages and promotes host susceptibility to invasive candidiasis121. The immunosuppressive effect of TLR2 is mediated through induction of high levels of IL-10121. Although a direct, cell-intrinsic TLR2–TLR4 cross-inhibition pathway has not yet been shown, C. albicans seems to exploit TLR2 to counteract potential TLR4-dependent immunity. Pathogenic Yersinia spp. also induce TLR2-dependent IL-10 production and cause immunosuppression by means of the secreted virulence protein LcrV122. An intriguing question is how pathogen-stimulated TLR2 can induce immunosuppressive levels of IL-10, whereas several TLR2 ligands, including synthetic lipopeptides, induce an overall pro-inflammatory response. It is plausible that complex microbial structures activate TLR2 in tandem with functionally associated coreceptor(s), such as certain C-type lectins29,30,64, and that high levels of IL-10 are actually induced by the resulting receptor crosstalk between TLR2 and a particular coreceptor. This notion is consistent with the inability of mycobacteria to induce high levels of IL-10 in neutrophils through the TLR–MYD88 pathway, unless they co-activate a C-type lectin–Syk–dependent pathway in parallel30.
Disruption of cooperative interactions between innate receptors
Pathogens have also evolved tactics to disrupt productive cooperation between certain innate immune receptors (Table 1). Integrins are an important target in this regard, owing to their capacity to engage in dynamic physical and/or functional interactions with several other receptors in lipid rafts123. Indeed, Coxiella burnetii impairs the crosstalk between the αvβ3–CD47 integrin signalling complex and CR3 that is required for activation of CR342. This prevents CR3-mediated uptake and post-phagocytic killing of this bacterium in monocytes. The exact mechanism is unclear, although it is thought that the smooth-type lipopolysaccharide of virulent C. burnetii interferes with the co-signalling function of CD47, which remains intact when monocytes are exposed to rough-type lipopolysaccharide–expressing avirulent C. burnetii42. The avoidance of CR3-mediated phagocytosis by C. burnetii contrasts with other bacteria that voluntarily activate CR3-mediqated phagocytosis (Fig. 4). However, the phagocytosis of C. burnetii is mediated by the carboxy-terminal lectin sites of CR3 which, unlike the amino-terminal I domain used by P. gingivalis, E. faecalis and other CR3-exploitative bacteria, are linked to induction of the oxidative burst124,125. Group A Streptococcus secretes a CD11b mimetic (Mac-1–like protein) that binds CD16 and blocks its productive interaction with CR3. The disruption of this functional co-association inhibits cooperative outside-in signalling and impairs opsonophagocytosis, oxidative burst, and killing of the bacteria43.
Pathogens may also have developed ways to disrupt productive interactions between non-integrin receptors. TLRs are functionally linked to FcRs with important implications in immunity but also inflammatory pathology8,126. Filarial nematodes express a secreted glycoprotein known as ES-62 that forms a complex with TLR4, leading to the sequestration and degradation of protein kinase C-α, which is required for the coupling of FcεRI to phospholipase D and mast cell degranulation44. Whether this mechanism is used by the parasite to evade mast cell-mediated immunity has not been specifically addressed, although ES-62 could be exploited as a potential therapeutic in allergy.
A number of viruses and parasites encode soluble molecules that mimic host receptors. Such molecules include the myxoma virus M-T7 glycoprotein, which scavenges IFNγ and sequesters C-, CC-and CXC-chemokines, and the Schistosoma mansoni chemokine-binding protein that binds CC-, CXC- and CX3C-chemokines127. These and other decoys contribute to immune evasion by preventing the interaction of cytokines or chemokines with their signalling receptors127. It is conceivable– though not yet specifically addressed– that such decoys may act to disrupt crosstalk interactions between the affected chemokine receptors and PRRs.
Concluding remarks and future perspectives
Receptor crosstalk in innate immunity is crucial to coordinate microbe-sensing signals and allow the host to tailor an appropriate immune response. However, many pathogens subvert these functions, often by taking control of host regulatory receptors. This can be achieved by mimicking host ligands/counterreceptors24,28,43 or host enzymes that generate such ligands31,74. Moreover, pathogens may ‘voluntarily’ interact with TLRs (or other innate receptors) through virulence molecules that have evolved to recognize such receptors25,32,122,128,129. It is clear that these interactions do not represent pattern recognition but rather involve subversive action that promotes the pathogens’ adaptive fitness. Future research may identify additional virulence factors and hijacked receptors, particularly among those with regulatory roles. For example, it would provide a significant survival advantage if any pathogen has evolved to interact with regulatory Toll/IL-1 receptor homology domain-containing transmembrane proteins, such as SIGIRR and ST2L, which are involved in negative crosstalk with the IL-1 receptor and/or TLRs9. Some of the evasion mechanisms discussed in this Review need to be substantiated in appropriate animal models, which are not always available. Continued research and improved animal models to identify mechanisms of ‘crosstalk manipulation’ is essential to develop approaches in which to counteract immune subversion by pathogens. Indeed, antagonistic blockade of hijacked receptors or neutralization of the virulence factors involved may offer promising options to control infection and associated immunopathology.
Supplementary Material
Acknowledgements
The authors regret that several important studies could only be cited indirectly through comprehensive reviews, owing to space and reference number limitations. Work in the authors’ laboratories is supported by US Public Health Service Grants DE015254, DE017138, DE018292 and DE021580 (to G.H.) and CA112162, AI68730, AI30040, AI72106, EB3968 and GM62134 (to J.D.L.).
Glossary
- Pattern recognition receptor
(PRR). A host receptor that can sense pathogen-associated molecular patterns and initiate signalling cascades that lead to an innate immune response. These can be membrane bound (such as Toll-like receptors) or soluble cytoplasmic receptors (such as NOD-like receptors).
- Pathogen-associated molecular pattern
(PAMP). A conserved molecular pattern that is found in pathogens but not mammalian cells. Examples include terminally mannosylated and polymannosylated compounds, which bind the mannose receptor, and various microbial products, such as bacterial lipopolysaccharides, hypomethylated DNA, flagellin, and double-stranded RNA, which bind Toll-like receptors.
- Lipid rafts
Membrane microdomains rich in cholesterol, sphingolipids and glycosylphosphatidylinositol-anchored proteins, which partition receptors for various cellular signalling and trafficking processes.
- Inside-out signalling
The process by which integrins (such ascomplement receptor 3) become activated (assume a high-affinity binding state) through intracellular signalling initiated by other receptors, such as anaphylatoxin receptors or Toll-like receptors. By contrast, outside-in signalling refers to intracellular signalling initiated by the activated and ligated integrins.
- Immunoreceptor tyrosine-based inhibitory motif
(ITIM). A structural motif containing a tyrosine residue that is found in the cytoplasmic tails of several inhibitory receptors, such as FcγRIIB and paired immunoglobulin-like receptor B (PIRB). The prototype six-amino-acid ITIM sequence is (Ile/Val/Leu/Ser)-X-Tyr-X-X-(Leu/Val), where X denotes any amino acid. Ligand-induced clustering of these inhibitory receptors results in tyrosine phosphorylation, often by SRC-family tyrosine kinases, which provides a docking site for the recruitment of cytoplasmic phosphatases that have an SRC homology 2 (SH2) domain, such as the SH2 domain-containing protein-tyrosine phosphatase 1 (SHP1).
- Immunoreceptor tyrosine-based activation motif
(ITAM). A structural motif containing two tyrosine residues that is found in the cytoplasmic tails of several signalling adaptor molecules. The motif has the form YXX(L/I)X6–12YXX(L/I), where X denotes any amino acid. The tyrosine residues are targets for phosphorylation by SRC-family protein tyrosine kinases and subsequent binding of proteins that contain SRC homology 2 (SH2) domains, such as the spleen tyrosine kinase (SYK).
- Oxidative burst
The process in phagocytic cells by which molecular oxygen is reduced by the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase system to produce reactive oxygen species, such as hydrogen peroxide and hydroxyl radicals. These are toxic oxidants that can destroy targeted microbes (for example in the phagosome lumen).
- Extracellular DNA traps
Often referred to by the acronym NETs (neutrophil extracellular traps). Upon activation (for example through Toll-like or Fcγ receptors), neutrophils release nuclear content such as chromatin (DNA, histone, and other proteins). This forms a web-like scaffold for the exposure of released antimicrobial molecules at high local concentrations, resulting in the trapping and extracellular killing of bacteria.
- G protein-coupled receptors
(GPCRs). Also known as seven-transmembrane domain receptors, this large group of receptors can bind a diverse set of molecules (such as chemokines, complement anaphylatoxins, hormones and neurotransmitters) and can induce intracellular signalling by coupling to heterotrimeric GTP-regulated signalling proteins.
- Anaphylatoxins
The pro-inflammatory fragments C3a and C5a that are generated during the activation of the complement system. These mediate a variety of inflammatory responses through their corresponding G-protein–coupled receptors, such as chemotaxis, oxidative burst and histamine release (from mast cells), but they can also (especially C5a) regulate other innate systems (such as TLRs) through crosstalk signalling pathways.
Biographies
GEORGE HAJISHENGALLIS
George Hajishengallis is Professor and Distinguished University Scholar at the University of Louisville Health Sciences Center. His Ph.D. studies at the University of Alabama in Birmingham focused on mucosal immunology and the development of novel adjuvants. His current interest lies at the interface of immunity and microbial pathogenesis, focusing on Toll-like receptors and complement and how their respective signaling pathways crosstalk in health and disease.
JOHN D. LAMBRIS
John D. Lambris is the Dr Ralph and Sallie Weaver Professor of Research Medicine at the Department of Pathology and Laboratory Medicine of the University of Pennsylvania, Philadelphia, USA. After earning his Ph.D. in Biochemistry from the University of Patras, Greece, he dedicated his research to various aspects of the complement system. In his laboratory at the University of Pennsylvania, he applies ideas and methods that are embodied in engineering, computer science, physics, chemistry and other fields to address today's challenges in complement research. His current research efforts include the development of small-size complement inhibitors, the crosstalk of complement with other pathways and complement evasion by bacteria and viruses. He has been the president of the International Complement Society and is the founder and executive director of the Aegean Conferences.
References
- 1.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 2. Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nature Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. A comprehensive review of the complement system, summarizing recent and emerging evidence that complement engages in reciprocal crosstalk interactions with other immune and physiological systems (such as TLRs and coagulation), aiming to fine-tune the host response to infection and other insults.
- 3.Medzhitov R. Toll-like receptors and innate immunity. Nature Rev. Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 4.Beutler BA. TLRs and innate immunity. Blood. 2009;113:1399–1407. doi: 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Triantafilou K, Triantafilou M, Dedrick RL. A CD14-independent LPS receptor cluster. Nature Immunol. 2001;2:338–345. doi: 10.1038/86342. [DOI] [PubMed] [Google Scholar]
- 6.Hajishengallis G, et al. Differential interactions of fimbriae and lipopolysaccharide from Porphyromonas gingivalis with the Toll-like receptor 2-centred pattern recognition apparatus. Cell. Microbiol. 2006;8:1557–1570. doi: 10.1111/j.1462-5822.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- 7. Natarajan M, Lin KM, Hsueh RC, Sternweis PC, Ranganathan R. A global analysis of cross-talk in a mammalian cellular signalling network. Nature Cell Biol. 2006;8:571–580. doi: 10.1038/ncb1418. Systematic analysis of intracellular signalling crosstalk showing that a large number of signalling pathways converge on a relatively limited set of interaction mechanisms, including both synergistic and antagonistic interactions.
- 8.Ivashkiv LB. Cross-regulation of signaling by ITAM-associated receptors. Nature Immunol. 2009;10:340–347. doi: 10.1038/ni.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee MS, Kim YJ. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu. Rev. Biochem. 2007;76:447–480. doi: 10.1146/annurev.biochem.76.060605.122847. [DOI] [PubMed] [Google Scholar]
- 10.Zak DE, Aderem A. Systems biology of innate immunity. Immunol. Rev. 2009;227:264–282. doi: 10.1111/j.1600-065X.2008.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hajishengallis G, Lambris JD. Crosstalk pathways between Toll-like receptors and the complement system. Trends Immunol. 2010;31:154–163. doi: 10.1016/j.it.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodridge HS, Underhill DM. Fungal recognition by TLR2 and dectin-1. Handb Exp Pharmacol. 2008:87–109. doi: 10.1007/978-3-540-72167-3_5. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa S, et al. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell. 2005;122:707–721. doi: 10.1016/j.cell.2005.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lukashev D, Ohta A, Apasov S, Chen JF, Sitkovsky M. Cutting edge: Physiologic attenuation of proinflammatory transcription by the Gs protein-coupled A2A adenosine receptor in vivo. J. Immunol. 2004;173:21–24. doi: 10.4049/jimmunol.173.1.21. [DOI] [PubMed] [Google Scholar]
- 15.Lambris JD, Ricklin D, Geisbrecht BV. Complement evasion by human pathogens. Nature Rev. Microbiol. 2008;6:132–142. doi: 10.1038/nrmicro1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finlay BB, McFadden G. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell. 2006;124:767–782. doi: 10.1016/j.cell.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 17.Bowie AG, Unterholzner L. Viral evasion and subversion of pattern-recognition receptor signalling. Nature Rev. Immunol. 2008;8:911–922. doi: 10.1038/nri2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diacovich L, Gorvel JP. Bacterial manipulation of innate immunity to promote infection. Nature Rev. Microbiol. 2010;8:117–128. doi: 10.1038/nrmicro2295. [DOI] [PubMed] [Google Scholar]
- 19.Flannagan RS, Cosio G, Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nature Rev. Microbiol. 2009;7:355–366. doi: 10.1038/nrmicro2128. [DOI] [PubMed] [Google Scholar]
- 20.Sansonetti PJ, Di Santo JP. Debugging how bacteria manipulate the immune response. Immunity. 2007;26:149–161. doi: 10.1016/j.immuni.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Brodsky IE, Medzhitov R. Targeting of immune signalling networks by bacterial pathogens. Nature Cell Biol. 2009;11:521–526. doi: 10.1038/ncb0509-521. [DOI] [PubMed] [Google Scholar]
- 22.Bhavsar AP, Guttman JA, Finlay BB. Manipulation of host-cell pathways by bacterial pathogens. Nature. 2007;449:827–834. doi: 10.1038/nature06247. [DOI] [PubMed] [Google Scholar]
- 23.Roy CR, Mocarski ES. Pathogen subversion of cell-intrinsic innate immunity. Nature Immunol. 2007;8:1179–1187. doi: 10.1038/ni1528. [DOI] [PubMed] [Google Scholar]
- 24.Carlin AF, et al. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood. 2009;113:3333–3336. doi: 10.1182/blood-2008-11-187302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlin AF, et al. Group B Streptococcus suppression of phagocyte functions by protein-mediated engagement of human Siglec-5. J. Exp. Med. 2009;206:1691–1699. doi: 10.1084/jem.20090691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slevogt H, et al. CEACAM1 inhibits Toll-like receptor 2-triggered antibacterial responses of human pulmonary epithelial cells. Nature Immunol. 2008;9:1270–1278. doi: 10.1038/ni.1661. [DOI] [PubMed] [Google Scholar]
- 27. Pinheiro da Silva F, et al. CD16 promotes Escherichia coli sepsis through an FcRγ inhibitory pathway that prevents phagocytosis and facilitates inflammation. Nature Med. 2007;13:1368–1374. doi: 10.1038/nm1665. This paper provided the first demonstration that inhibitory ITAMs (ITAMi) can be exploited by bacteria, in this case to induce a crosstalk that inhibits phagocytosis, leading to uncontrolled E. coli infection and sepsis.
- 28.Reyburn HT, et al. The class I MHC homologue of human cytomegalovirus inhibits attack by natural killer cells. Nature. 1997;386:514–517. doi: 10.1038/386514a0. [DOI] [PubMed] [Google Scholar]
- 29.Gringhuis SI, et al. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-κB. Immunity. 2007;26:605–616. doi: 10.1016/j.immuni.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 30. Zhang X, Majlessi L, Deriaud E, Leclerc C, Lo-Man R. Coactivation of Syk kinase and MyD88 adaptor protein pathways by bacteria promotes regulatory properties of neutrophils. Immunity. 2009;31:761–771. doi: 10.1016/j.immuni.2009.09.016. References 29 and 30 identified TLR coreceptors (C-type lectins) and elucidated the underlying signalling pathways that explain how inflammatory TLRs can induce high levels of the anti-inflammatory cytokine IL-10.
- 31. Wang M, et al. Microbial hijacking of complement-Toll-like receptor crosstalk. Sci. Signal. 2010;3:ra11. doi: 10.1126/scisignal.2000697. This study was the first to show that anaphylatoxin generation by a virulence enzyme is exploited by the pathogen to induce subversive complement-TLR crosstalk that impairs protective immunity.
- 32.Hajishengallis G, Wang M, Liang S, Triantafilou M, Triantafilou K. Pathogen induction of CXCR4/TLR2 cross-talk impairs host defense function. Proc. Natl. Acad. Sci. U S A. 2008;105:13532–13537. doi: 10.1073/pnas.0803852105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliva C, Turnbough CL, Jr, Kearney JF. CD14-Mac-1 interactions in Bacillus anthracis spore internalization by macrophages. Proc. Natl. Acad. Sci. U S A. 2009;106:13957–13962. doi: 10.1073/pnas.0902392106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang M, et al. Fimbrial proteins of Porphyromonas gingivalis mediate in vivo virulence and exploit TLR2 and complement receptor 3 to persist in macrophages. J. Immunol. 2007;179:2349–2358. doi: 10.4049/jimmunol.179.4.2349. [DOI] [PubMed] [Google Scholar]
- 35.Sumuth SD, et al. Aggregation substance promotes adherence, phagocytosis, and intracellular survival of Enterococcus faecalis within human macrophages and suppresses respiratory burst. Infect. Immun. 2000;68:4900–4906. doi: 10.1128/iai.68.9.4900-4906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishibashi Y, Claus S, Relman DA. Bordetella pertussis filamentous hemagglutinin interacts with a leukocyte signal transduction complex and stimulates bacterial adherence to monocyte CR3 (CD11b/CD18) J. Exp. Med. 1994;180:1225–1233. doi: 10.1084/jem.180.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hawlisch H, et al. C5a negatively regulates toll-like receptor 4-induced immune responses. Immunity. 2005;22:415–426. doi: 10.1016/j.immuni.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 38.Waggoner SN, Hall CH, Hahn YS. HCV core protein interaction with gC1q receptor inhibits Th1 differentiation of CD4+ T cells via suppression of dendritic cell IL-12 production. J. Leukoc. Biol. 2007;82:1407–1419. doi: 10.1189/jlb.0507268. [DOI] [PubMed] [Google Scholar]
- 39. Karp CL, et al. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 1996;273:228–231. doi: 10.1126/science.273.5272.228. The first paper to show that a complement receptor (CD46) can downregulate IL-12 induction by TLR agonists, even though the concept of mammalian TLRs had not yet been established.
- 40.Simmons DP, et al. Mycobacterium tuberculosis and TLR2 agonists inhibit induction of Type I IFN and Class I MHC antigen cross processing by TLR9. J. Immunol. 2010;185:2405–2415. doi: 10.4049/jimmunol.0904005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dolganiuc A, et al. Hepatitis C virus (HCV) core protein-induced, monocyte-mediated mechanisms of reduced IFN-α and plasmacytoid dendritic cell loss in chronic HCV infection. J. Immunol. 2006;177:6758–6768. doi: 10.4049/jimmunol.177.10.6758. [DOI] [PubMed] [Google Scholar]
- 42.Capo C, et al. Subversion of monocyte functions by Coxiella burnetii: impairment of the cross-talk between αvβ3 integrin and CR3. J. Immunol. 1999;163:6078–6085. [PubMed] [Google Scholar]
- 43. Lei B, et al. Evasion of human innate and acquired immunity by a bacterial homolog of CD11b that inhibits opsonophagocytosis. Nature Med. 2001;7:1298–1305. doi: 10.1038/nm1201-1298. This study presents a novel concept according to which a virulence protein that mimics an innate immune receptor disrupts its cooperative interactions with another receptor, resulting in inhibition of crucial antimicrobial responses.
- 44.Melendez AJ, et al. Inhibition of FcεRI-mediated mast cell responses by ES-62, a product of parasitic filarial nematodes. Nature Med. 2007;13:1375–1381. doi: 10.1038/nm1654. [DOI] [PubMed] [Google Scholar]
- 45.Turnbull IR, Colonna M. Activating and inhibitory functions of DAP12. Nature Rev. Immunol. 2007;7:155–161. doi: 10.1038/nri2014. [DOI] [PubMed] [Google Scholar]
- 46.Wang L, et al. Indirect inhibition of Toll-like receptor and type I interferon responses by ITAM-coupled receptors and integrins. Immunity. 2010;32:518–530. doi: 10.1016/j.immuni.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pinheiro da Silva F, Aloulou M, Benhamou M, Monteiro RC. Inhibitory ITAMs: a matter of life and death. Trends Immunol. 2008;29:366–373. doi: 10.1016/j.it.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Boyd CR, et al. Siglec-E is up-regulated and phosphorylated following lipopolysaccharide stimulation in order to limit TLR-driven cytokine production. J. Immunol. 2009;183:7703–7709. doi: 10.4049/jimmunol.0902780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakayama M, et al. Paired Ig-like receptors bind to bacteria and shape TLR-mediated cytokine production. J. Immunol. 2007;178:4250–4259. doi: 10.4049/jimmunol.178.7.4250. [DOI] [PubMed] [Google Scholar]
- 50.Chapman TL, Heikeman AP, Bjorkman PJ. The inhibitory receptor LIR-1 uses a common binding interaction to recognize class I MHC molecules and the viral homolog UL18. Immunity. 1999;11:603–613. doi: 10.1016/s1074-7613(00)80135-1. [DOI] [PubMed] [Google Scholar]
- 51.Lambert AA, Gilbert C, Richard M, Beaulieu AD, Tremblay MJ. The C-type lectin surface receptor DCIR acts as a new attachment factor for HIV-1 in dendritic cells and contributes to trans- and cis-infection pathways. Blood. 2008;112:1299–1307. doi: 10.1182/blood-2008-01-136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meyer-Wentrup F, et al. DCIR is endocytosed into human dendritic cells and inhibits TLR8-mediated cytokine production. J. Leukoc. Biol. 2009;85:518–525. doi: 10.1189/jlb.0608352. [DOI] [PubMed] [Google Scholar]
- 53.Meyer-Wentrup F, et al. Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-α production. Blood. 2008;111:4245–4253. doi: 10.1182/blood-2007-03-081398. [DOI] [PubMed] [Google Scholar]
- 54.Cerf-Bensussan N, Gaboriau-Routhiau V. The immune system and the gut microbiota: friends or foes? Nature Rev. Immunol. 2010;10:735–744. doi: 10.1038/nri2850. [DOI] [PubMed] [Google Scholar]
- 55.Redpath S, Ghazal P, Gascoigne NR. Hijacking and exploitation of IL-10 by intracellular pathogens. Trends Microbiol. 2001;9:86–92. doi: 10.1016/s0966-842x(00)01919-3. [DOI] [PubMed] [Google Scholar]
- 56.Confer D, Eaton J. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982;217:948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- 57.Turk BE. Manipulation of host signalling pathways by anthrax toxins. Biochem J. 2007;402:405–417. doi: 10.1042/BJ20061891. [DOI] [PubMed] [Google Scholar]
- 58.Vojtova J, Kamanova J, Sebo P. Bordetella adenylate cyclase toxin: a swift saboteur of host defense. Curr. Opin. Microbiol. 2006;9:69–75. doi: 10.1016/j.mib.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 59.Conti P, et al. IL-10, an inflammatory/inhibitory cytokine, but not always. Immunol. Lett. 2003;86:123–129. doi: 10.1016/s0165-2478(03)00002-6. [DOI] [PubMed] [Google Scholar]
- 60.Lavelle EC, et al. Effects of cholera toxin on innate and adaptive immunity and its application as an immunomodulatory agent. J. Leukoc. Biol. 2004;75:756–763. doi: 10.1189/jlb.1103534. [DOI] [PubMed] [Google Scholar]
- 61.Brenner S, et al. cAMP-induced Interleukin-10 promoter activation depends on CCAAT/enhancer-binding protein expression and monocytic differentiation. J. Biol. Chem. 2003;278:5597–5604. doi: 10.1074/jbc.M207448200. [DOI] [PubMed] [Google Scholar]
- 62.Geijtenbeek TB, et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bergman MP, et al. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J. Exp. Med. 2004;200:979–990. doi: 10.1084/jem.20041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Geijtenbeek TB. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nature Immunol. 2009;10:1081–1088. doi: 10.1038/ni.1778. This paper has characterized the composition of the DC-SIGN signalling complex which depends on the sugar specificity of the activating DC-SIGN ligand, which thereby can determine the nature of the induced signalling pathway.
- 65.Madura Larsen J, et al. BCG stimulated dendritic cells induce an interleukin-10 producing T-cell population with no T helper 1 or T helper 2 bias in vitro. Immunology. 2007;121:276–282. doi: 10.1111/j.1365-2567.2007.02575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dulphy N, et al. Intermediate maturation of Mycobacterium tuberculosis LAM-activated human dendritic cells. Cell. Microbiol. 2007;9:1412–1425. doi: 10.1111/j.1462-5822.2006.00881.x. [DOI] [PubMed] [Google Scholar]
- 67. Hovius JW, et al. Salp15 binding to DC-SIGN inhibits cytokine expression by impairing both nucleosome remodeling and mRNA stabilization. PLoS Pathog. 2008;4(e31) doi: 10.1371/journal.ppat.0040031. This paper presents the interesting concept of subversive receptor crosstalk being mediated by a pathogen and its arthropod vector, which contribute distinct agonists for TLRs and DC-SIGN, respectively.
- 68.Lundberg K, Wegner N, Yucel-Lindberg T, Venables PJ. Periodontitis in RA-the citrullinated enolase connection. Nature Rev. Rheumatol. 2010 Sep 7; doi: 10.1038/nrrheum.2010.139. [DOI] [PubMed] [Google Scholar]
- 69.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 70.Hajishengallis G. Porphyromonas gingivalis-host interactions: open war or intelligent guerilla tactics? Microbes Infect. 2009;11:637–645. doi: 10.1016/j.micinf.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pierce DL, et al. Host adhesive activities and virulence of novel fimbrial proteins of Porphyromonas gingivalis. Infect. Immun. 2009;77:3294–3301. doi: 10.1128/IAI.00262-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hajishengallis G, Ratti P, Harokopakis E. Peptide mapping of bacterial fimbrial epitopes interacting with pattern recognition receptors. J. Biol. Chem. 2005;280:38902–38913. doi: 10.1074/jbc.M507326200. [DOI] [PubMed] [Google Scholar]
- 73.Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nature Rev. Microbiol. 2010;8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 74.Thammavongsa V, Kern JW, Missiakas DM, Schneewind O. Staphylococcus aureus synthesizes adenosine to escape host immune responses. J. Exp. Med. 2009;206:2417, 2427. doi: 10.1084/jem.20090097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zarek PE, et al. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abram CL, Lowell CA. The ins and outs of leukocyte integrin signaling. Annu. Rev. Immunol. 2009;27:339–362. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harokopakis E, Hajishengallis G. Integrin activation by bacterial fimbriae through a pathway involving CD14, Toll-like receptor 2, and phosphatidylinositol-3-kinase. Eur. J. Immunol. 2005;35:1201–1210. doi: 10.1002/eji.200425883. [DOI] [PubMed] [Google Scholar]
- 78. Sendide K, et al. Cross-talk between CD14 and complement receptor 3 promotes phagocytosis of mycobacteria: regulation by phosphatidylinositol 3-kinase and cytohesin-1. J. Immunol. 2005;174:4210–4219. doi: 10.4049/jimmunol.174.7.4210. References 77 and 78 were the first to describe TLR pathways that transactivate integrins through inside-out signalling.
- 79.Han C, et al. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nature Immunol. 2010;11:734–742. doi: 10.1038/ni.1908. [DOI] [PubMed] [Google Scholar]
- 80.Harokopakis E, Albzreh MH, Martin MH, Hajishengallis G. TLR2 transmodulates monocyte adhesion and transmigration via Rac1- and PI3K-mediated inside-out signaling in response to Porphyromonas gingivalis fimbriae. J. Immunol. 2006;176:7645–7656. doi: 10.4049/jimmunol.176.12.7645. [DOI] [PubMed] [Google Scholar]
- 81.Hajishengallis G, Wang M, Liang S. Induction of distinct TLR2-mediated proinflammatory and proadhesive signaling pathways in response to Porphyromonas gingivalis fimbriae. J. Immunol. 2009;182:6690–6696. doi: 10.4049/jimmunol.0900524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arbibe L, et al. Toll-like receptor 2-mediated NF-κB activation requires a Rac1-dependent pathway. Nature Immunol. 2000;1:533–540. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- 83.Lowell CA. Rewiring phagocytic signal transduction. Immunity. 2006;24:243–245. doi: 10.1016/j.immuni.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 84.Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282:1717–1721. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- 85.Gatfield J, Pieters J. Essential role for cholesterol in entry of mycobacteria into macrophages. Science. 2000;288:1647–1650. doi: 10.1126/science.288.5471.1647. [DOI] [PubMed] [Google Scholar]
- 86.Hajishengallis G, Wang M, Harokopakis E, Triantafilou M, Triantafilou K. Porphyromonas gingivalis fimbriae proactively modulate β2 integrin adhesive activity and promote binding to and internalization by macrophages. Infect. Immun. 2006;74:5658–5666. doi: 10.1128/IAI.00784-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vanek NN, et al. Enterococcus faecalis aggregation substance promotes opsonin-independent binding to human neutrophils via a complement receptor type 3-mediated mechanism. FEMS Immunol. Med. Microbiol. 1999;26:49–60. doi: 10.1111/j.1574-695X.1999.tb01371.x. [DOI] [PubMed] [Google Scholar]
- 88.Rakita RM, et al. Enterococcus faecalis bearing aggregation substance is resistant to killing by human neutrophils despite phagocytosis and neutrophil activation. Infect. Immun. 1999;67:6067–6075. doi: 10.1128/iai.67.11.6067-6075.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hellwig SM, et al. Targeting to Fcγ receptors, but not CR3 (CD11b/CD18), increases clearance of Bordetella pertussis. J. Infect. Dis. 2001;183:871–879. doi: 10.1086/319266. An elegant in vivo demonstration of the differential outcomes of FcγR- and CR3-mediated phagocytosis, with the latter being relatively ineffective in bacterial clearance; this explains why several pathogens proactively induce their uptake by CR3.
- 90.Zhang X, et al. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood. 2007;110:228–236. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lappegård KT, et al. Human genetic deficiencies reveal the roles of complement in the inflammatory network: lessons from nature. Proc. Natl. Acad. Sci. U S A. 2009;106:15861–15866. doi: 10.1073/pnas.0903613106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fan J, Malik AB. Toll-like receptor-4 (TLR4) signaling augments chemokine-induced neutrophil migration by modulating cell surface expression of chemokine receptors. Nature Med. 2003;9:315–321. doi: 10.1038/nm832. [DOI] [PubMed] [Google Scholar]
- 93.la Sala A, Gadina M, Kelsall BL. G(i)-protein-dependent inhibition of IL-12 production is mediated by activation of the phosphatidylinositol 3-kinase-protein 3 kinase B/Akt pathway and JNK. J. Immunol. 2005;175:2994–2999. doi: 10.4049/jimmunol.175.5.2994. [DOI] [PubMed] [Google Scholar]
- 94.Waggoner SN, Cruise MW, Kassel R, Hahn YS. gC1q receptor ligation selectively down-regulates human IL-12 production through activation of the phosphoinositide 3-kinase pathway. J. Immunol. 2005;175:4706–4714. doi: 10.4049/jimmunol.175.7.4706. [DOI] [PubMed] [Google Scholar]
- 95.Marth T, Kelsall BL. Regulation of interleukin-12 by complement receptor 3 signaling. J. Exp. Med. 1997;185:1987–1995. doi: 10.1084/jem.185.11.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Goriely S, Neurath MF, Goldman M. How microorganisms tip the balance between interleukin-12 family members. Nature Rev. Immunol. 2008;8:81–86. doi: 10.1038/nri2225. [DOI] [PubMed] [Google Scholar]
- 97.Manderson AP, Botto M, Walport MJ. The role of complement in the development of systemic lupus erythematosus. Annu. Rev. Immunol. 2004;22:431–456. doi: 10.1146/annurev.immunol.22.012703.104549. [DOI] [PubMed] [Google Scholar]
- 98.Sutterwala FS, Noel GJ, Clynes R, Mosser DM. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J. Exp. Med. 1997;185:1977–1985. doi: 10.1084/jem.185.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liang S, et al. The C5a receptor impairs IL-12–dependent clearance of Porphyromonas gingivalis and is required for induction of periodontal bone loss. J. Immunol. 2010 doi: 10.4049/jimmunol.1003252. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smith A, et al. Selective suppression of IL-12 production by human herpesvirus 6. Blood. 2003;102:2877–2884. doi: 10.1182/blood-2002-10-3152. [DOI] [PubMed] [Google Scholar]
- 101.Santoro F, et al. Interaction of glycoprotein H of human herpesvirus 6 with the cellular receptor CD46. J. Biol. Chem. 2003;278:25964–25969. doi: 10.1074/jbc.M302373200. [DOI] [PubMed] [Google Scholar]
- 102.Iacobelli-Martinez M, Nepomuceno RR, Connolly J, Nemerow GR. CD46-utilizing adenoviruses inhibit C/EBPβ–dependent expression of proinflammatory cytokines. J. Virol. 2005;79:11259–11268. doi: 10.1128/JVI.79.17.11259-11268.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gaggar A, Shayakhmetov DM, Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nature Med. 2003;9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- 104.Hahm B, Cho JH, Oldstone MB. Measles virus-dendritic cell interaction via SLAM inhibits innate immunity: selective signaling through TLR4 but not other TLRs mediates suppression of IL-12 synthesis. Virology. 2007;358:251–257. doi: 10.1016/j.virol.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 105.Gill DB, Atkinson JP. CD46 in Neisseria pathogenesis. Trends Mol. Med. 2004;10:459–465. doi: 10.1016/j.molmed.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 106.Braun L, Ghebrehiwet B, Cossart P. gC1q-R/p32, a C1q-binding protein, is a receptor for the InlB invasion protein of Listeria monocytogenes. EMBO J. 2000;19:1458–1466. doi: 10.1093/emboj/19.7.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nguyen T, Ghebrehiwet B, Peerschke EIB. Staphylococcus aureus protein A recognizes platelet gC1qR/p33: a novel mechanism for staphylococcal interactions with platelets. Infect. Immun. 2000;68:2061–2068. doi: 10.1128/iai.68.4.2061-2068.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nigou J, Zelle-Rieser C, Gilleron M, Thurnher M, Puzo G. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J. Immunol. 2001;166:7477–7485. doi: 10.4049/jimmunol.166.12.7477. [DOI] [PubMed] [Google Scholar]
- 109.Chieppa M, et al. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J. Immunol. 2003;171:4552–4560. doi: 10.4049/jimmunol.171.9.4552. [DOI] [PubMed] [Google Scholar]
- 110.Urban BC, Willcox N, Roberts DJ. A role for CD36 in the regulation of dendritic cell function. Proc. Natl. Acad. Sci. U S A. 2001;98:8750–8755. doi: 10.1073/pnas.151028698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Urban BC, et al. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature. 1999;400:73–77. doi: 10.1038/21900. [DOI] [PubMed] [Google Scholar]
- 112.Patel SN, et al. Disruption of CD36 impairs cytokine response to Plasmodium falciparum glycosylphosphatidylinositol and confers susceptibility to severe and fatal malaria in vivo. J. Immunol. 2007;178:3954–3961. doi: 10.4049/jimmunol.178.6.3954. [DOI] [PubMed] [Google Scholar]
- 113.D'Ombrain MC, et al. Plasmodium falciparum erythrocyte membrane protein-1 specifically suppresses early production of host interferon-γ. Cell Host Microbe. 2007;2:130–138. doi: 10.1016/j.chom.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 114.Long KH, Gomez FJ, Morris RE, Newman SL. Identification of heat shock protein 60 as the ligand on Histoplasma capsulatum that mediates binding to CD18 receptors on human macrophages. J. Immunol. 2003;170:487–494. doi: 10.4049/jimmunol.170.1.487. [DOI] [PubMed] [Google Scholar]
- 115.McGuirk P, Mills KH. Direct anti-inflammatory effect of a bacterial virulence factor: IL-10-dependent suppression of IL-12 production by filamentous hemagglutinin from Bordetella pertussis. Eur. J. Immunol. 2000;30:415–422. doi: 10.1002/1521-4141(200002)30:2<415::AID-IMMU415>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 116.Hajishengallis G, Shakhatreh M-AK, Wang M, Liang S. Complement receptor 3 blockade promotes IL-12-mediated clearance of Porphyromonas gingivalis and negates its virulence in vivo. J. Immunol. 2007;179:2359–2367. doi: 10.4049/jimmunol.179.4.2359. [DOI] [PubMed] [Google Scholar]
- 117.El Kasmi KC, et al. Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nature Immunol. 2008;9:1399–1406. doi: 10.1038/ni.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Qualls JE, et al. Arginine usage in mycobacteria-infected macrophages depends on autocrine-paracrine cytokine signaling. Sci. Signal. 2010;3:ra62. doi: 10.1126/scisignal.2000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shirey KA, Cole LE, Keegan AD, Vogel SN. Francisella tularensis live vaccine strain induces macrophage alternative activation as a survival mechanism. J. Immunol. 2008;181:4159–4167. doi: 10.4049/jimmunol.181.6.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Netea MG, et al. The role of toll-like receptor (TLR) 2 and TLR4 in the host defense against disseminated candidiasis. J. Infect. Dis. 2002;185:1483–1489. doi: 10.1086/340511. [DOI] [PubMed] [Google Scholar]
- 121.Netea MG, et al. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J. Immunol. 2004;172:3712–3718. doi: 10.4049/jimmunol.172.6.3712. [DOI] [PubMed] [Google Scholar]
- 122. Sing A, et al. A hypervariable N-terminal region of Yersinia LcrV determines Toll-like receptor 2-mediated IL-10 induction and mouse virulence. Proc. Natl. Acad. Sci. U S A. 2005;102:16049–16054. doi: 10.1073/pnas.0504728102. This study provided molecular evidence that virulence proteins might have evolved to interact with and exploit TLRs; this exploitative interaction contrasts with the concept of ‘pattern recognition’, which aims to promote innate immunity through TLR recognition of conserved microbial structures.
- 123.Petty HR, Worth RG, Todd RF., 3rd Interactions of integrins with their partner proteins in leukocyte membranes. Immunol. Res. 2002;25:75–95. doi: 10.1385/IR:25:1:75. [DOI] [PubMed] [Google Scholar]
- 124.Le Cabec V, Cols C, Maridonneau-Parini I. Nonopsonic phagocytosis of zymosan and Mycobacterium kansasii by CR3 (CD11b/CD18) involves distinct molecular determinants and is or is not coupled with NADPH oxidase activation. Infect. Immun. 2000;68:4736–4745. doi: 10.1128/iai.68.8.4736-4745.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xia Y, et al. Function of the lectin domain of Mac-1/Complement Receptor Type 3 (CD11b/CD18) in regulating neutrophil adhesion. J. Immunol. 2002;169:6417–6426. doi: 10.4049/jimmunol.169.11.6417. [DOI] [PubMed] [Google Scholar]
- 126.Rittirsch D, et al. Cross-talk between TLR4 and FcγReceptorIII (CD16) pathways. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000464. e1000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mantovani A, Bonecchi R, Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nature Rev. Immunol. 2006;6:907–918. doi: 10.1038/nri1964. [DOI] [PubMed] [Google Scholar]
- 128.Pathak SK, et al. Direct extracellular interaction between the early secreted antigen ESAT-6 of Mycobacterium tuberculosis and TLR2 inhibits TLR signaling in macrophages. Nature Immunol. 2007;8:610–618. doi: 10.1038/ni1468. [DOI] [PubMed] [Google Scholar]
- 129.Postma B, et al. Chemotaxis inhibitory protein of Staphylococcus aureus binds specifically to the C5a and formylated peptide receptor. J. Immunol. 2004;172:6994–7001. doi: 10.4049/jimmunol.172.11.6994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.