Abstract
Introduction
This study investigated the effect of knee position on quadriceps force steadiness and activation strategies.
Methods
Quadriceps force steadiness was evaluated in twenty-two volunteers at two knee positions by testing their ability to regulate submaximal force. Muscle activation strategies were studied in both time and frequency domains using surface electromyography.
Results
Quadriceps force fluctuations and the associated agonist and antagonist activity were significantly higher at 90° than at 30° of flexion (P < 0.05). The quadriceps median frequency recorded at 30° was significantly higher than at 90° of flexion (P < 0.05). Regression analyses revealed that force steadiness was related to quadriceps activation and median frequency (P < 0.001), but not to hamstring coactivation (P > 0.05).
Discussion
The results indicate that knee position significantly affects quadriceps force steadiness and activation strategies. This finding may have important implications for designing a force control testing protocol and interpreting test results.
Keywords: Force control, muscle length, coactivation, neuromuscular control, EMG
INTRODUCTION
When subjects attempt to exert a constant force, the force is not constant, but it fluctuates about some average value.1 The magnitude of these force fluctuations provides meaningful information on motor coordination and movement control.2–4. Accordingly, force steadiness is a parameter that researchers have used to study adaptations in neuromotor control with aging, disuse, and training.2,5–7 Previous research indicates that force steadiness is impaired in elderly individuals, in people with neuromotor dysfunction, and after prolonged disuse.1,2,5,8,9 This impairment has been associated with movement dysfunction and an increased risk of falls in elderly people.8,10 Testing force steadiness may provide meaningful insight on neuromuscular plasticity after injury and in response to rehabilitation.
Force steadiness is commonly evaluated by testing the ability of an individual to perform steady submaximal contractions and is known to be influenced by several factors, such as a person’s age and activity level, the muscle group being tested, the type and intensity of the contraction, and the activation patterns of the agonist and antagonist muscles.1,5,6,11–13 It is well known that the force output of a muscle can be modulated by varying the number of motor units recruited or by changing the firing frequency of the recruited motor units. As a result, the way in which motor units are recruited and activated may affect force steadiness.1,13–15 Motor unit recruitment and discharge properties were the primary focus of early studies on force fluctuations in submaximal contractions; however, recent evidence suggests that force steadiness is more complicated than originally thought and depends on multiple features of motor units.1,15
Scientists have studied muscle force fluctuation as a function of contraction intensity using both experimental and theoretical/modeling approaches.15–18 The results of these investigations indicate that fluctuations in muscle force typically rise with increases in contraction intensity when expressed in absolute terms (standard deviation of the exerted force) and appear as a polynomial or sigmoidal function of the force level when expressed in relative terms (coefficient of variation of the exerted force). This finding has been attributed, in part, to the adaptations in motor unit recruitment and discharge properties with variation in the level of excitation.
The nervous system modulates the recruitment and rate coding properties of the motor units to compensate for altered contractile properties with changes in muscle length. For example, researchers have reported that when muscles are at shorter lengths they exhibit lower motor unit recruitment thresholds and higher discharge rates than when they are longer.19 There is also evidence suggesting that Ia afferent input to the α-motor neuron, a known factor in low frequency force fluctuations, is reduced at shorter versus longer muscle lengths. Therefore, it is reasonable that the muscle length (or joint position) used when assessing force steadiness significantly affects test results. Current evidence related to the effect of muscle length on force steadiness is limited and conflicting.12,20,21
The knee joint is one of the largest and most architecturally complex joints in the human body. The inherent vulnerability of the knee to serious injuries and degenerative joint disease is well established.22,23 As a result, there is significant interest in characterizing neuromuscular adaptations that occur after knee joint trauma and with rehabilitation. Assessing quadriceps muscle force steadiness after an injury or intervention may provide valuable information about neuromuscular plasticity. When planning such studies, an understanding of the effect of quadriceps muscle length on force steadiness would be helpful in selecting the knee position(s) to use when assessing quadriceps force steadiness and when interpreting the results of steadiness tests. Therefore, the purpose of this study was to evaluate the effect of knee angle on quadriceps muscle force steadiness and associated activation strategies across a range of submaximal torques (2% to 50% maximum). It was hypothesized that less force steadiness would be observed when the muscle was longer (i.e., greater force fluctuations would be observed at 90° of knee flexion than at 30° of knee flexion). We also hypothesized that quadriceps activation would be higher at 90° degrees of knee flexion than at 30° of flexion based on previous research.24
MATERIALS AND METHODS
Subjects
Twenty-two active young people with no history of serious lower extremity pathology participated in this study. The sample consisted of 11 males and 11 females who averaged 26.4 ± 4.2 years of age, 1.73 ± 0.11 meters in height, and 73.5 ± 14.6 kilograms in mass. All but one subject was right leg dominant by self-report of preferred kicking leg. Subjects were excluded if they had a history of significant knee injury or surgery, pain during resisted knee extension, pain or instability during functional activities, fracture of the pelvis, femur, tibia, fibula, or patella within the past two years, history of lower body nerve injury, lumbar radiculopathy, neurological disorder, or diabetes. Subjects provided written informed consent to participation by signing a form approved by the University of Iowa Human Subjects Research Institutional Review Board.
Isometric Force Control Testing
Subjects were asked to refrain from strenuous activity during the 24 hours preceding testing. Quadriceps muscle force steadiness was assessed in the dominant leg of each subject at 30° and at 90° of knee flexion using an isometric testing approach.25,26 The order of test angle presentation was randomized a priori to minimize potential effects associated with order of testing. Subjects began by riding a stationary bicycle for five minutes. Surface electromyography (EMG) preamplifiers (model 544, Therapeutics Unlimited, Iowa City, IA; 35x differential gain, 22 mm inter-electrode distance, 8 mm electrode diameter, 87 dB common-mode rejection at 60 Hz, input impedance > 25 MΩ, noise < 2 µV RMS) were applied over the muscle bellies of vastus lateralis, vastus medialis, rectus femoris, semitendinosus, and biceps femoris longus. The placement sites of the electrodes were standardized according to the recommendations of Perotto & Delagi.27 A common ground electrode was placed over the skin of the patella. Subjects were then asked to sit on a raised platform placed on the chair of a HUMAC NORM isokinetic dynamometer (Computer Sports Medicine, Inc., Stoughton, MA). The platform was used to minimize the potential for noise associated with pressure on the EMG preamplifiers fixed over the hamstring muscles. Subjects were stabilized to the testing system in standard fashion using the device’s seatbelts and straps (thigh, chest, and pelvic). The lateral epicondyle of the femur (theoretical knee joint axis) was aligned with the axis of the dynamometer. The test system’s torque arm pad was attached to the distal shank approximately 7.5 cm proximal to the medial malleolus. The hip was positioned at approximately 90° of flexion. Subjects then performed four 5-second submaximal isometric contractions (50 to 85% of perceived maximum) and one maximum voluntary isometric contraction (MVIC) of the knee extensors and flexors in an alternating fashion. This was done to familiarize subjects with the strength testing procedures. After the practice trials, subjects performed three MVICs of their knee extensors and flexors in alternating order. These maximal trials were used to obtain maximum torque and muscle activity data for use when setting the target torques and normalizing the EMG data. Three minutes of rest was provided between each like trial to minimize fatigue. Loud verbal encouragement and visual feedback of the real-time torque signals were provided during the MVICs to facilitate maximal effort.
After three minutes rest, quadriceps muscle force steadiness was tested by having subjects match linear torque targets of 2%, 10%, 20%, 30%, and 50% maximum that were displayed on the LCD monitor placed in front of them. Subjects were instructed to match the targets as precisely as possible by positioning their real-time torque curves over the linear torque target by applying loads against the torque arm of the isokinetic dynamometer (Figure 1). Subjects performed two practice trials at each torque level just before performing the test trials. The order in which the torque levels were presented was randomized to minimize systematic effects. Ten seconds of data were collected in each test trial after relative stability had been achieved in the subjects’ torque curves.5,28–30 Two experimental trials were performed at each torque level with 30 seconds rest between trials. The average of the two trials was used in analysis. Torque targets were always projected to the middle of the monitor screen. The visual gain of the force feedback (i.e., the distance moved on the screen per unit of torque) was consistent during testing.15,31 After testing had been completed at each of the five torque levels, the subject’s knee was repositioned at the second angle, and testing was repeated in an identical fashion starting with the MVIC trials.
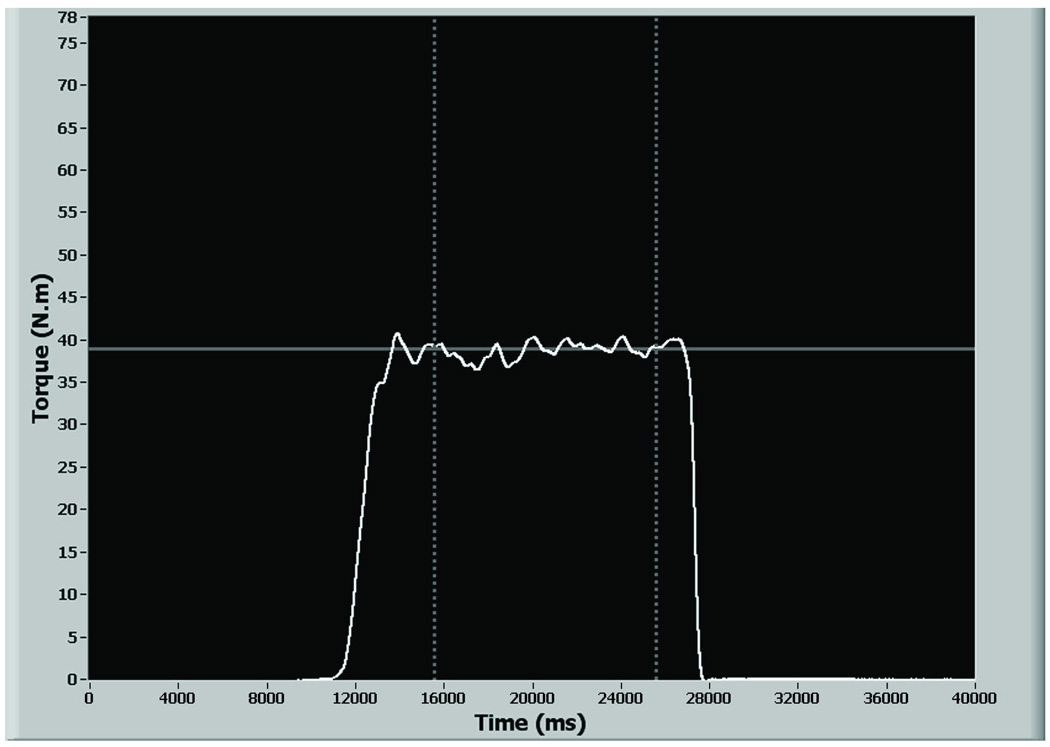
Figure 1.
Screen shot of the LCD monitor depicting the linear torque target, and real time feedback of the torque signals. The subject’s objective is to position his/her real time torque curves (white line) over the linear torque target (continuous grey line). EMG and torque data were recorded for ten seconds after the subject achieved stability of his/her torque responses when matching the target (broken grey lines).
Signal Sampling, Conditioning, and Processing
All data were sampled and processed using custom-written programs in LabVIEW version 7.0 (National Instruments Corp., Austin, TX, USA). The EMG and torque signals were sampled at a frequency of 1000 Hz using a personal computer with a 16-bit A-to-D conversion board (NI PCI-6032E, National Instruments Corp., Austin, TX, USA). The torque signals were low-pass filtered at 4 Hz using a 3rd order analog Butterworth filter and then converted to torque values (N·m) using a calibrated conversion factor that was obtained from a prior onsite validation testing.15,29 Force steadiness was quantified by determining the standard deviation, coefficient of variation, and percent absolute error of the recorded torque curve. The coefficient of variation was calculated by expressing the standard deviation of the exerted torque as a percentage of the mean of the exerted torque (Equation 1)
| [1] |
The percent absolute error was determined by calculating the mean of the absolute differences between the exerted and the target torque and expressing it as a percentage of the target torque (Equation 2).
| [2] |
The EMG signals were conditioned using an 8th order analog Butterworth low-pass filter with a 500 Hz cut-off frequency and analyzed in both the time and the frequency domain. Time-domain analysis was performed to compute the magnitudes of agonist and antagonist muscle activity during the steadiness tests. The EMG signals were baseline removed, rectified, and smoothened using a recursive 8th order digital Butterworth filter with a 6 Hz cut-off (linear envelope). Normalization of the EMG recordings was performed using maximal EMG values recorded at the respective angles (30° or 90°) during MVIC testing. The magnitudes of antagonistic medial and lateral hamstring activity present during testing were determined by presenting the values recorded during knee extensor trials as a percentage of peak values recorded from the respective muscles during flexion MVICs.
There is evidence indicating that the median frequency content of the surface EMG signal decreases significantly with motor unit synchronization.32,33 Therefore, the median frequency of the quadriceps EMG power spectrum was determined to obtain an indirect estimation of motor unit synchronization with changes in knee flexion angle. Frequency-domain analysis was performed to determine the median frequency of the agonist quadriceps muscle EMG power spectrum during testing. The analog-filtered raw EMG signals recorded during the steadiness test trials were used in the power-spectral analysis. After digitally filtering the EMG signals (Butterworth digital recursive filter, 20 to 500Hz cutoff), a spectral analysis using a Fast Fourier Transformation (Hamming window) was performed to determine the median frequency of the power spectrum. Spectral analysis was not performed on the EMG signals recorded during force control testing at 2% target load, as pilot analysis revealed that the signal-to-noise ratio in the EMG power spectrum was low at this level.
Data Analyses
Statistical analyses were performed using SPSS for Windows version 17.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were calculated for each variable. Two-factor (knee joint angle & target torque) repeated measures analysis of variance was performed to test for significant differences in force steadiness (standard deviation, coefficient of variation, and percent absolute error) and muscle activation (time and frequency domain EMG) data recorded at 30° and 90° of knee flexion. Significant main or interaction effects were followed by post hoc analyses using paired t-test with Sidak correction for multiple comparisons. A Greenhouse-Geisser correction was employed when the assumption of sphericity was violated. Multiple linear regression analysis was used to determine the factors (quadriceps activity, quadriceps median frequency, and antagonistic hamstring activity) that were significantly associated with force steadiness. The activation values (mean amplitude, median frequency) of vastus medialis, vastus lateralis, and rectus femoris were averaged to obtain mean quadriceps activity and median frequency of the quadriceps EMG power spectrum.34 The activation values of medial and lateral hamstring muscles were averaged to determine hamstrings coactivation during isometric force control testing. These averaged values were used for the purpose of regression analysis. A significance level of α = 0.05 was set for all statistical analyses.
RESULTS
Effect of Knee Angle on Quadriceps Muscle Force Steadiness
Standard Deviation of the Torque Curve
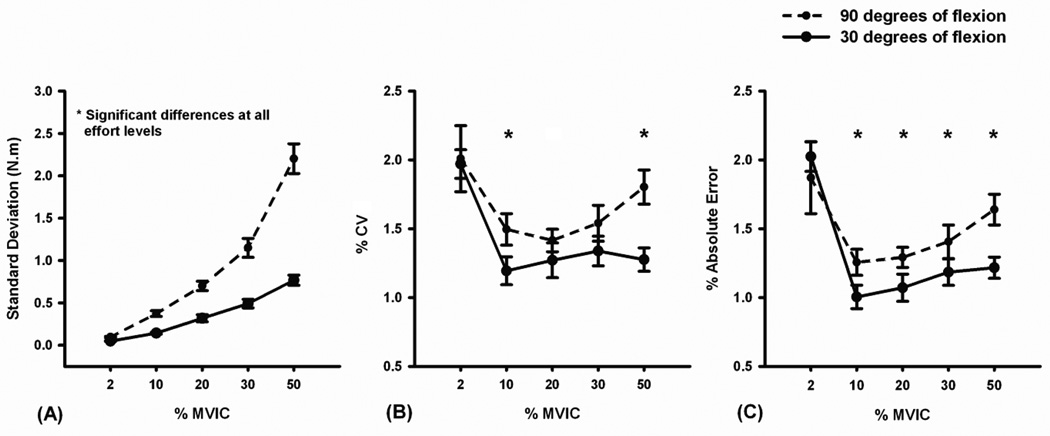
The standard deviation of the exerted torque increased linearly as a function of target torque level at both 90° (standard deviation = 0.044 × %MVIC − 0.079, r2 = 0.724, P < 0.001) and 30° (standard deviation = 0.015 × %MVIC + 0.010, r2 = 0.657, P < 0.001; Figure 2a) of knee flexion. Significant main effects were observed for knee joint angle (P < 0.001) and torque level (P < 0.001). There was also a significant interaction between knee joint angle and torque level (P < 0.001). Post-hoc analyses revealed that the mean standard deviation of the exerted torque was significantly greater at 90° than at 30° of knee flexion at all torque levels (P < 0.001, Figure 2a). Standard deviation values at each of the torque levels were significantly different from each other at both knee angles tested (P < 0.001, Figure 2a).
Figure 2.
Mean fluctuations in quadriceps muscle force (force steadiness) during submaximal isometric force control testing at 30° (solid) and 90° (broken) of knee flexion. Force steadiness was quantified by calculating the (A) standard deviation, (B) coefficient of variation (Standard deviations normalized to the mean of the exerted force), and (C) percent absolute error of the subjects’ torque curves at various torque targets during isometric force control testing. Quadriceps force steadiness was significantly affected (*) by knee joint angle. Error bars represent standard error of the mean.
Coefficient of Variation of the Torque Curve
Significant main effects were observed for knee joint angle (P = 0.001) and target torque level (P < 0.001). The torque curves were less steady at 90° than at 30° of knee flexion (Figures 3a & 3b). Statistically higher coefficients of variation were observed at the 10% (P = 0.02) and 50% (P < 0.001) targets (Figure 2b). Post-hoc analyses indicated that the mean coefficient of variation at the 2% torque level was significantly greater than at the 10% (P = 0.001), 20% (P = 0.007), 30% (P = 0.041), and 50% (P = 0.05) torque levels. There was no significant interaction between knee joint angle and target torque for coefficient of variation (P = 0.197).
Figure 3.
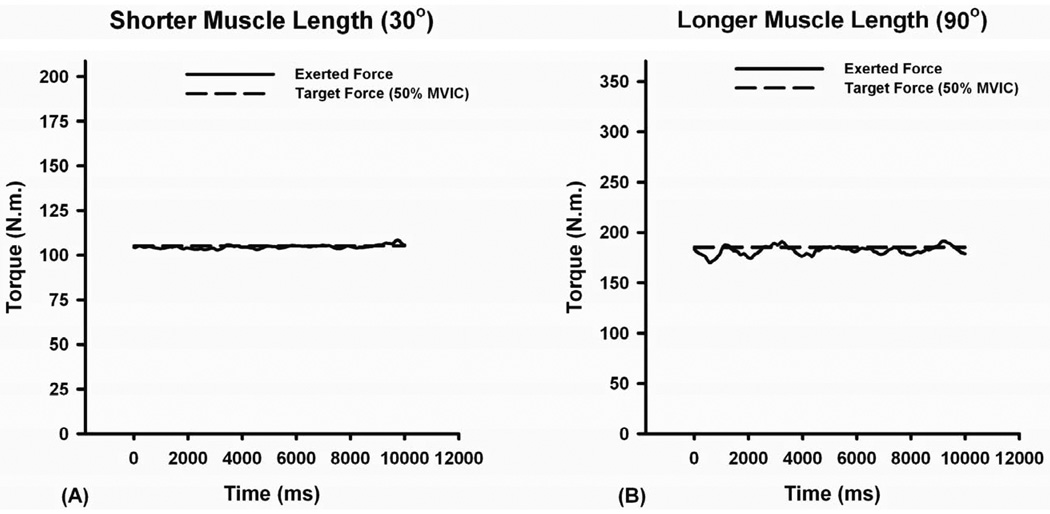
A representative example depicting the effect of muscle length on quadriceps muscle force steadiness. The fluctuations in quadriceps muscle force were typically lower at (A) shorter muscle length (30°) when compared to (B) longer muscle length (90°). The broken lines represent the target torque that the subjects attempted to match and the continuous lines represent the actual exerted torque.
Percent Absolute Error of the Torque Curve
Significant main effects were observed for knee joint angle (P = 0.001) and target torque level (P < 0.001). Percent absolute error values were significantly greater at 90° than at 30° of knee flexion at the 10% (P = 0.012), 20% (P = 0.013), 30% (P = 0.019), and the 50% (P = 0.001) target torques (Figure 2c). Post-hoc analyses revealed that the percent absolute error at the 2% target was significantly greater than that observed at the 10% (P < 0.001), 20% (P = 0.002), 30% (P = 0.021), and the 50% (P = 0.041) torque levels. Post-hoc analyses also indicated that the percent absolute error at the 50% target was significantly greater than those observed at 10% (P < 0.001) and 20% (P = 0.017) torque levels. There was no significant interaction between knee joint angle and target torque level (P = 0.164).
Effect of Knee Angle on Quadriceps and Hamstring Activation Strategies
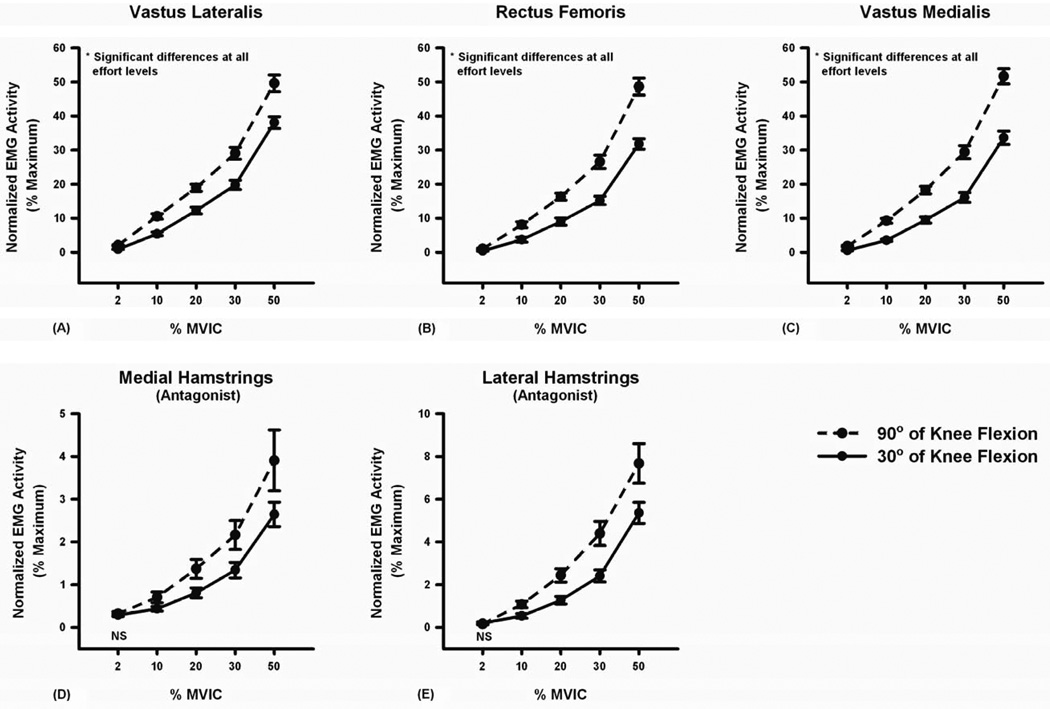
The magnitudes of quadriceps agonist activity increased linearly as a function of torque at both 90° and 30° of knee flexion (P < 0.001, Figure 4). However, the rate of increase (slope) was larger at 90° than at 30° of knee flexion (Table 1). A similar effect was observed for antagonistic hamstring muscle activity (Figure 4 and Table 1). Significant main effects were seen for knee joint angle (P < 0.001 to P = 0.020) and torque level (P < 0.001) with respect to agonist and antagonist muscle activity. A significant interaction was observed between knee joint angle and target torque level with both agonist and antagonist muscle activity (P < 0.001 to P = 0.047). Post-hoc analyses of the interaction indicated that quadriceps activation during testing at 90° of knee flexion was significantly higher than at 30° of knee flexion at each torque level (P < 0.001 to P = 0.031, Figure 4). Similar patterns of activation were observed for antagonistic hamstring muscles at all torque levels (P = 0.001 to P = 0.049) with the exception of the 2% target torque (medial hamstrings: P = 0.514, lateral hamstrings: P = 0.429) (Figure 4). Although antagonistic hamstring muscle activity differed significantly across knee joint angles during submaximal contractions, it did not differ across knee joint angles during maximal contractions (medial hamstrings: 7.2% vs. 7.3%, P = 0.915, lateral hamstrings: 15.1% vs. 15.1%, P = 0.957).
Figure 4.
Mean quadriceps (agonist) and hamstring (antagonist) muscle activity at various torque magnitudes (2% to 50% MVIC) during isometric force control testing. The knee angle used in testing had a significant effect on quadriceps and hamstring muscle activation (P < 0.05). The magnitudes of (A) vastus lateralis, (B) rectus femoris, and (C) vastus medialis muscle activity during submaximal isometric knee extensor contraction at 90° of knee flexion (broken) were significantly higher than those at 30° of knee flexion (solid). The magnitudes of (D) medial hamstring and (E) lateral hamstring muscle activity at 90° of knee flexion were also significantly higher than those at 30° of knee flexion except at 2% MVIC. Error bars represent standard error of the mean. NS indicates no significant difference at α = 0.05.
Table 1.
Slopes of agonist (quadriceps) and antagonist (hamstrings) muscles during isometric force control testing
| Angle | Muscle | β1 | β0 | R2 | P-Value |
|---|---|---|---|---|---|
| 90° | VL | 0.982 | 0.033 | 0.855 | P < 0.001 |
| RF | 0.991 | −2.075 | 0.842 | P < 0.001 | |
| VM | 1.041 | −1.246 | 0.871 | P < 0.001 | |
| MH | 0.076 | −0.002 | 0.356 | P < 0.001 | |
| LH | 0.160 | −0.430 | 0.565 | P < 0.001 | |
| 30° | VL | 0.778 | −2.138 | 0.862 | P < 0.001 |
| RF | 0.657 | −2.673 | 0.827 | P < 0.001 | |
| VM | 0.696 | −2.926 | 0.810 | P < 0.001 | |
| MH | 0.050 | −0.018 | 0.549 | P < 0.001 | |
| LH | 0.109 | −0.490 | 0.666 | P < 0.001 |
β1 Slope, β0 Intercept, R2 Coefficient of determination, VL: vastus lateralis, RF: rectus femoris, VM: vastus medialis, ST: semitendinosus, BFL: long head of the biceps femoris.
Effect of Knee Angle on Quadriceps Median Frequency
The median frequency of the quadriceps muscles differed significantly across knee angles and torque levels (P < 0.001 to P = 0.028). There was a significant interaction between knee joint angle and target torque level (P = 0.008 to P = 0.031). Post-hoc analyses of the interaction effects indicated that the median frequencies of the vastus lateralis and rectus femoris muscles were significantly higher at 30° than at 90° of knee flexion at each torque level (P < 0.001 to P = 0.002, Table 2). The median frequency of the vastus medialis was significantly higher at 30° than 90° of knee flexion at all of the target torque levels (P < 0.001 to P = 0.034, Table 2), but 10% (P = 0.120). Post-hoc analyses demonstrated that the median frequency of the quadriceps muscles was not different across torque levels at 90° of flexion (P = 0.361 to P =1.000); however, it was at 30° of knee flexion (P < 0.05, Table 2).
Table 2.
Median frequency of the quadriceps muscle EMG power spectrum at 30° and 90° of knee flexion during isometric knee extensor contractions at various torque magnitudes (10% to 50% MVIC)
| Muscle | Angle | Target Torque | Mean ± SE |
|---|---|---|---|
| Vastus Lateralis | 90° | 10 | 75.32 ± 2.42* |
| 20 | 76.42 ± 2.36* | ||
| 30 | 77.09 ± 2.38* | ||
| 50 | 77.02 ± 2.41* | ||
| 30° | 10 | 85.50 ± 2.98 | |
| 20 | 91.03 ± 3.32 | ||
| 30 | 94.09 ± 3.50§ | ||
| 50 | 95.46 ± 3.37§ | ||
| Rectus Femoris | 90° | 10 | 78.72 ± 2.72* |
| 20 | 78.96 ± 2.56* | ||
| 30 | 80.20 ± 2.64* | ||
| 50 | 79.07 ± 2.49* | ||
| 30° | 10 | 100.35 ± 3.22 | |
| 20 | 98.14 ± 2.29 | ||
| 30 | 104.08 ± 2.20† | ||
| 50 | 108.29 ± 3.14† | ||
| Vastus Medialis | 90° | 10 | 71.16 ± 2.25 |
| 20 | 71.40 ± 2.25* | ||
| 30 | 73.14 ± 2.21* | ||
| 50 | 73.22 ± 2.15* | ||
| 30° | 10 | 75.36 ± 1.78 | |
| 20 | 76.06 ± 1.63 | ||
| 30 | 78.63 ± 1.80† | ||
| 50 | 82.62 ± 2.21†‡ | ||
Values are group means ± standard error of the mean.
indicate P < 0.05 compared with 30° of knee flexion.
indicate P < 0.05 compared with 10% target torque.
indicate P < 0.05 compared with 20% target torque.
indicate P < 0.05 compared with 30% target torque.
Association between Force Steadiness and Activation Parameters
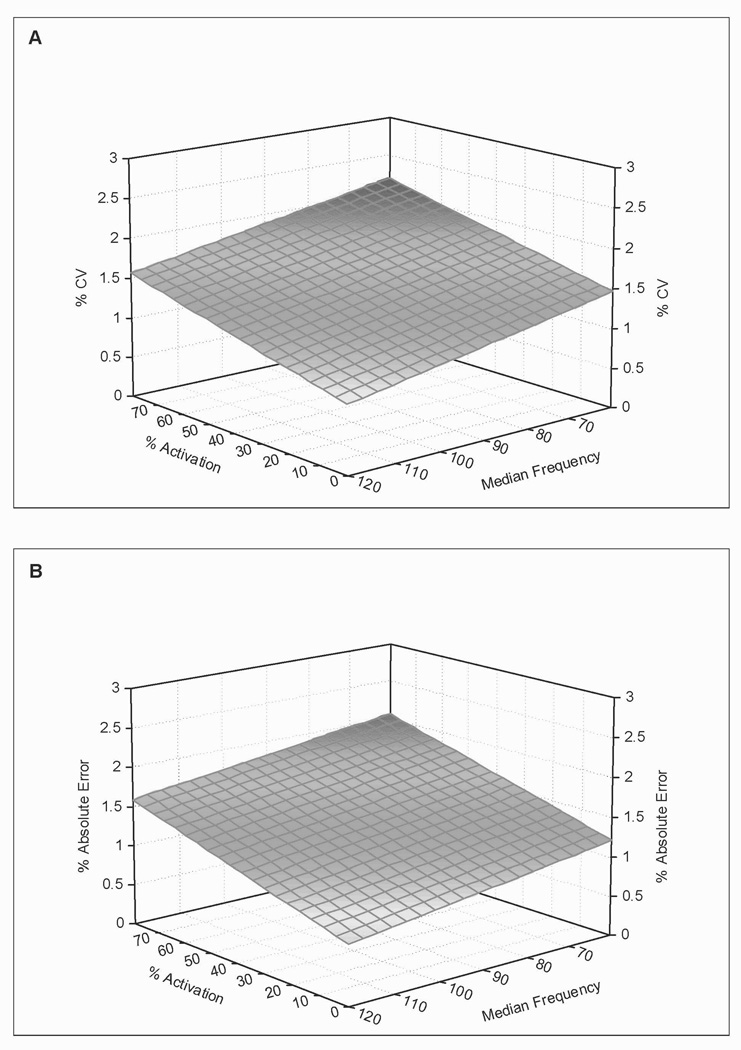
Regression analyses defined a linear association between quadriceps muscle force steadiness and quadriceps activation in the time and frequency domains (P < 0.001, Figure 5). Quadriceps activation level and median frequency together explained 13% of the variations in coefficient of variation (coefficient of variation = −0.01 × median frequency + 0.009 × activation + 2.09) and 16% of the variations in percent absolute error (Error = −0.008 × median frequency + 0.01 × activation + 1.68). Antagonist muscle activity did not contribute significantly to the observed coefficient of variation (R2 = 0.002, P = 0.568) or percent absolute error (R2 = 0.004, P = 0.358).
Figure 5.
Coefficient of variation (a) and percent absolute error (b) are plotted as a function of quadriceps muscle activation (% MVIC) and median frequency of the quadriceps muscle EMG power spectrum. Note that quadriceps force steadiness is positively associated with the levels of quadriceps muscle activation and negatively associated with quadriceps median frequency.
DISCUSSION
The purpose of this study was to evaluate the effect of muscle length (knee angle) on quadriceps muscle force steadiness test results and muscle activation strategies across a range of submaximal torques. The quadriceps muscle group is important for healthy human function, as it helps to maintain upright posture and generates the force required to extend the knee when performing tasks such as rising from a chair, walking, lifting a heavy object, and recreational activities that involve running or jumping. The quadriceps muscle also acts to decelerate the mass of the body when lowering oneself into a chair, walking downhill or down stairs, and when landing from a jump or slowing down during gait. It is well established that quadriceps strength and function are profoundly impacted by knee joint pathology and surgery.35–37 Quadriceps weakness and atrophy have also been associated with the risk for falls and the pathogenesis of knee osteoarthritis.38–41 Assessing quadriceps force steadiness may provide important insight into neuromuscular plasticity after injury, with interventions such as surgery or rehabilitation, or with changes in physiological state that occur with aging or reduced physical activity.2,5,25,30,42,43 Such insight may have meaningful implications for knee rehabilitation and interventions aimed at mitigating the effects of aging and disuse. When designing studies including quadriceps force steadiness tests, investigators must determine the knee angle(s) that will be used in testing. This decision may be driven by several factors including what is safe for the patient (e.g., loading of the anterior cruciate ligament graft is an important consideration in the early periods after anterior cruciate ligament reconstruction),44,45 patient comfort (e.g., patients with patellofemoral pain syndromes may have painful arcs of motion that need to be avoided during testing),46 and joint mobility with aging, osteoarthritis, or with other changes in physiological state. An understanding of how muscle length affects quadriceps force steadiness and activation strategies is helpful in understanding normal neuromotor behavior, which is requisite to understanding altered behavior. Our findings indicate that muscle length has a significant effect on quadriceps force steadiness and activation. Therefore, it is important that investigators consider muscle length when designing force steadiness studies, interpreting results of studies with more than one test angle, and when comparing results across studies.
We tested the effects of muscle length on force steadiness and activation at two knee angles: 1) 30° of flexion where the quadriceps muscle is relatively short, and 2) 90° of flexion where it is long. These knee positions were selected because they were likely to identify differences associated with muscle length and because they are knee positions that are commonly used when testing quadriceps muscle function.24,47,48 As hypothesized, force fluctuations and activation were significantly greater when quadriceps force steadiness was tested at 90° of flexion. The increased force fluctuations were accompanied by higher quadriceps and hamstring muscle activation and lower median frequency of the quadriceps muscle EMG power spectrum. A statistically significant association was observed between quadriceps force steadiness and the amplitude and median frequency of the quadriceps muscle EMG pattern. This was not true for hamstring muscle coactivity.
The finding of significantly greater force fluctuations at longer muscle lengths is consistent with the observations of Bigland-Ritchie et al.20 who reported greater fluctuations in tibialis anterior muscle force at longer muscle length. There are, however, some inconsistent findings in the literature.20,21 Differences in test methodology and sample characteristics are the likely sources of these differences. There are several plausible explanations for the observed effect of muscle length on force steadiness. The most straightforward relates to the differences in quadriceps muscle activation observed at the two knee positions. The magnitudes of quadriceps muscle activity at 90° were significantly higher than those observed at 30° of knee flexion. This finding was consistent across subjects and effort levels for all of the quadriceps muscles tested. The higher normalized EMG amplitudes observed at 90° indicate that a larger proportion of motor units are recruited and/or the motor units are firing at a faster rate in this position. This may be related to an increased central drive or a gain in the excitability of the α-motor neuron pool. The magnitude of noise in the neural control signal is known to increase with the value of the control signal (signal-dependent noise).49 Based on this signal-dependent noise phenomenon, it has been theorized that the greater noise associated with greater excitatory input to the α-motor neuron would result in greater fluctuations in muscle force.13 This theory is supported by experimental findings that show increased motor unit activity associated with task modification or intervention is often accompanied by an increase in muscle force fluctuations.34,50 Shinohara et al.50 have demonstrated that tonic vibration reflex-induced increases in force fluctuations are accompanied by increases in the average EMG activity recorded during force control testing. Shinohara et al.7 have also reported that increases in plantar flexor and knee extensor muscle force fluctuations observed after prolonged bed rest are accompanied by higher medial gastrocnemius and vastus lateralis muscle activity. More recently, Missenard et al.34 indicated that force fluctuations after a fatiguing task are not altered when muscle activation is experimentally controlled. Our finding of higher quadriceps muscle activity and reduced force steadiness at 90° of knee flexion further supports this association between muscle activation and force steadiness.
Researchers have demonstrated that increases in motor unit synchronization reduce force steadiness.14,33,51 Therefore, it is reasonable that the differences in quadriceps force steadiness observed at 30° and 90° of knee flexion may be associated with different levels of motor unit synchronization in the two positions. To our knowledge the influence of muscle length on motor unit synchronization has not been specifically investigated. We investigated the role of motor unit synchronization indirectly by assessing the median frequency of the quadriceps EMG power spectrum. Researchers have previously demonstrated that that motor unit synchronization lowers the median frequency of the surface EMG signal.32,33 We observed significantly lower median frequencies of quadriceps muscle activity at 90° of knee flexion. Regression analyses revealed that quadriceps median frequency and force steadiness were inversely related. Based on these findings and the available literature, it is reasonable that our observation of reduced force steadiness at 90° of flexion is associated with the presence of higher motor unit synchronization in this position. It should be noted, however, that the correlation coefficients for this inverse relationship were significant, but weak. Therefore, there are factors other than quadriceps activity and median frequency contributing to the observed knee position based differences in force steadiness. We also acknowledge that mechanical factors such as altered conduction distance associated with muscle fiber length changes, changes in the detection area under an electrode, and variation in the proximity of the recording electrode to the tendinous portion of the muscle can affect the median frequency of the EMG signal.52 These facts should be taken into account when considering the degree to which the lower median frequency observed at longer muscle lengths should be attributed to motor unit synchronization. A direct investigation of the role of motor unit synchronization in the muscle length dependence of force steadiness with fine wire or needle EMG is needed to clarify this issue.
The Ia afferent inputs from muscle spindles have been specifically linked to low frequency oscillations in muscle force.53,54 Findings from simulation experiments indicate that modeling results best match experimental findings when a low frequency oscillation in excitation is included in the model.15 This suggests that a gain in excitatory Ia afferent input would result in increased force fluctuations. This idea is supported by evidence indicating that selective inhibition of the excitatory Ia afferent inputs to the α-motor neuron pool by tendon vibration results in decreased force fluctuations.54 There is also evidence suggesting that Ia afferent input increases when a muscle is lengthened.55,56 Therefore, it is possible that the gain in excitatory Ia afferent inputs may have enhanced the force fluctuations at 90° of knee flexion by modulating the low frequency oscillation in the motor unit discharges.
Coactivation of the antagonist muscles is an important neuromotor strategy used to regulate force and control joint motion. Evidence suggests that adaptations in antagonist muscle coactivation with training may improve force steadiness.11 Our subjects displayed significantly lower levels of hamstring muscle coactivation at 30° flexion than they exhibited at 90° of flexion. Although greater force steadiness was observed at 30° of knee flexion, the relationship between antagonistic hamstring activity and quadriceps muscle force steadiness was not statistically significant. Researchers investigating the effects of intentional increases or decreases in antagonist muscle activity on force steadiness have previously reported that coactivation of the antagonist muscles has little effect on force steadiness.13 Further support for this lack of association is provided by others.17,28 Hence, there is significant evidence indicating that antagonist muscle coactivation contributes little to muscle length-dependent alterations in force steadiness during submaximal contractions.
The observed differences in magnitude of quadriceps muscle activity also provide evidence of length dependence in the EMG-moment relationships of the quadriceps muscle. It is well known that altering joint angle, and therefore muscle length, has a significant impact on muscle force and activation parameters. We tested maximum torque levels at both 30° and 90° of knee flexion to account for length-related changes in the biomechanical and physiological properties of the knee muscles. The target torques used in testing and the recorded EMG values were normalized to the MVIC values obtained at the respective joint positions. Despite this careful testing approach, we observed a significant difference in the magnitude of quadriceps muscle activity at 30° and 90° of knee flexion. This finding is contrary to observations in studies of the upper limb that demonstrate no significant effect of elbow angle on EMG-moment relationships of the elbow flexor and extensor muscles.57,58 Together these findings suggest that the relationship between muscle activation and joint angle may be muscle or joint specific. The mechanisms contributing to the observed differences in the quadriceps muscle EMG-moment relationships are currently unknown. Despite this fact, the results may have important implications for electrophysiological and biomechanical studies, as muscle activation dynamics and EMG-moment relationships are important inputs to neuromusculoskeletal models.59,60
Our reason for investigating the effect of knee joint position on quadriceps force steadiness was primarily related to our desire to use these tests when evaluating quadriceps muscle plasticity in applied physiology and rehabilitation research studies. An understanding of how knee joint position affects quadriceps force steadiness is important when designing test protocols and interpreting force steadiness studies. Our selection of contraction intensities (2% to 50% maximum) was based on the literature and the fact that most of what humans do during daily life involves submaximal levels of muscle activation.5,61 The coefficient of variation and absolute percent error of the subjects’ torque curves observed at the 2% torque level were different than those observed at the other levels of torque (Figure 2B & 2C). We used a single torque sensor during testing. Therefore, the 2% torque level had the lowest signal-to-noise ratio, which may partially explain the different patterns at this level. However, we think it is more likely that the observed differences at the 2% torque level are a product of basic quadriceps muscle physiology. The quadriceps is a very large muscle group that is architecturally designed to generate force and power. It has large motor units that are made up of large muscle fibers. Hence, the force increments associated with recruitment of additional motor units and increases in rate coding are larger than with smaller muscle groups. Although it is physiologically relevant to study force control of most upper extremity muscles at such a low level of force, the physiologic and clinical relevance of quadriceps control at 2% maximum is questionable considering its architecture and neuromechanical function. Accordingly, the observed deviation in force steadiness patterns at the 2% torque level may simply be a product of requiring the quadriceps to function in a manner for which it is not well designed. After carefully considering our test results and quadriceps muscle design/function, we no longer test quadriceps force steadiness at 2% maximum and instead use 10% maximum as the low force test when testing quadriceps force steadiness.
In summary, we observed significantly lower quadriceps force steadiness at 90° of knee flexion where the muscle is long than at 30° of flexion where the muscle is relatively short. The lower steadiness at 90° of knee flexion was accompanied by greater quadriceps activation and lower median frequency of the quadriceps muscle EMG power spectrum suggesting that the observed differences in force steadiness are related to motor unit activation and firing strategies. These findings are practically meaningful for applied physiologists and rehabilitation scientists when designing studies including quadriceps force steadiness tests and when interpreting the results of these tests and comparing them with those from reports in the literature. Because quadriceps force steadiness and activation are muscle length-dependent it is important to compare results recorded at similar knee joint angles. Investigators should consider performing quadriceps force steadiness testing at more than one knee angle if they expect to compare their results to data recorded at a divergent knee angles or they would like their findings to have broad applicability with respect to studies in the literature or populations of interest that may vary in regards to suitable knee test positions.
Acknowledgement
Supported in part by NIH Grant K12 HD055931 and the Davee Foundation. The authors would like to thank Rajiv Ranganathan, PhD for his critical review of the manuscript.
Abbreviations
- VL
Vastus Lateralis
- RF
Rectus Femoris
- MVIC
Maximum Voluntary Isometric Contraction
- SD
Standard Deviation
- CV
Coefficient of Variation
Footnotes
This study was presented at the 1) 2009 Marilyn Gossman Graduate Student Seminar held during the Combined Sections Meeting of the American Physical Therapy Association, Nashville, TN and 2) 56th Annual Meeting of American College of Sports Medicine, Seattle, WA
References
- 1.Enoka RM, Christou EA, Hunter SK, Kornatz KW, Semmler JG, Taylor AM, Tracy BL. Mechanisms that contribute to differences in motor performance between young and old adults. J Electromyogr Kinesiol. 2003;13(1):1–12. doi: 10.1016/s1050-6411(02)00084-6. [DOI] [PubMed] [Google Scholar]
- 2.Clark BC, Pierce JR, Manini TM, Ploutz-Snyder LL. Effect of prolonged unweighting of human skeletal muscle on neuromotor force control. Eur J Appl Physiol. 2007;100(1):53–62. doi: 10.1007/s00421-007-0399-6. [DOI] [PubMed] [Google Scholar]
- 3.Seynnes O, Hue OA, Garrandes F, Colson SS, Bernard PL, Legros P, Fiatarone Singh MA. Force steadiness in the lower extremities as an independent predictor of functional performance in older women. J Aging Phys Act. 2005;13(4):395–408. doi: 10.1123/japa.13.4.395. [DOI] [PubMed] [Google Scholar]
- 4.Christou EA, Shinohara M, Enoka RM. Fluctuations in acceleration during voluntary contractions lead to greater impairment of movement accuracy in old adults. J Appl Physiol. 2003;95(1):373–384. doi: 10.1152/japplphysiol.00060.2003. [DOI] [PubMed] [Google Scholar]
- 5.Tracy BL, Enoka RM. Older adults are less steady during submaximal isometric contractions with the knee extensor muscles. J Appl Physiol. 2002;92(3):1004–1012. doi: 10.1152/japplphysiol.00954.2001. [DOI] [PubMed] [Google Scholar]
- 6.Yoshitake Y, Kouzaki M, Fukuoka H, Fukunaga T, Shinohara M. Modulation of muscle activity and force fluctuations in the plantarflexors after bedrest depends on knee position. Muscle Nerve. 2007;35(6):745–755. doi: 10.1002/mus.20764. [DOI] [PubMed] [Google Scholar]
- 7.Shinohara M, Yoshitake Y, Kouzaki M, Fukuoka H, Fukunaga T. Strength training counteracts motor performance losses during bed rest. J Appl Physiol. 2003;95(4):1485–1492. doi: 10.1152/japplphysiol.01173.2002. [DOI] [PubMed] [Google Scholar]
- 8.Braendvik SM, Elvrum AK, Vereijken B, Roeleveld K. Relationship between neuromuscular body functions and upper extremity activity in children with cerebral palsy. Dev Med Child Neurol. 2010;52(2):e29–e34. doi: 10.1111/j.1469-8749.2009.03490.x. [DOI] [PubMed] [Google Scholar]
- 9.Vaillancourt DE, Larsson L, Newell KM. Effects of aging on force variability, single motor unit discharge patterns, and the structure of 10, 20, and 40 Hz EMG activity. Neurobiol Aging. 2003;24(1):25–35. doi: 10.1016/s0197-4580(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 10.Carville SF, Perry MC, Rutherford OM, Smith IC, Newham DJ. Steadiness of quadriceps contractions in young and older adults with and without a history of falling. Eur J Appl Physiol. 2007;100(5):527–533. doi: 10.1007/s00421-006-0245-2. [DOI] [PubMed] [Google Scholar]
- 11.Patten C, Kamen G. Adaptations in motor unit discharge activity with force control training in young and older human adults. Eur J Appl Physiol. 2000;83(2–3):128–143. doi: 10.1007/s004210000271. [DOI] [PubMed] [Google Scholar]
- 12.Salonikidis K, Amiridis IG, Oxyzoglou N, de Villareal ES, Zafeiridis A, Kellis E. Force variability during isometric wrist flexion in highly skilled and sedentary individuals. Eur J Appl Physiol. 2009;107(6):715–722. doi: 10.1007/s00421-009-1184-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinohara M, Yoshitake Y, Kouzaki M. Alterations in synergistic muscle activation impact fluctuations in net force. Med Sci Sports Exerc. 2009;41(1):191–197. doi: 10.1249/MSS.0b013e318183c0d9. [DOI] [PubMed] [Google Scholar]
- 14.Christakos CN, Papadimitriou NA, Erimaki S. Parallel neuronal mechanisms underlying physiological force tremor in steady muscle contractions of humans. J Neurophysiol. 2006;95(1):53–66. doi: 10.1152/jn.00051.2005. [DOI] [PubMed] [Google Scholar]
- 15.Taylor AM, Christou EA, Enoka RM. Multiple features of motor-unit activity influence force fluctuations during isometric contractions. J Neurophysiol. 2003;90(2):1350–1361. doi: 10.1152/jn.00056.2003. [DOI] [PubMed] [Google Scholar]
- 16.Christou EA, Grossman M, Carlton LG. Modeling variability of force during isometric contractions of the quadriceps femoris. J Mot Behav. 2002;34(1):67–81. doi: 10.1080/00222890209601932. [DOI] [PubMed] [Google Scholar]
- 17.Danion F, Gallea C. The relation between force magnitude, force steadiness, and muscle co-contraction in the thumb during precision grip. Neurosci Lett. 2004;368(2):176–180. doi: 10.1016/j.neulet.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Slifkin AB, Newell KM. Noise, information transmission, and force variability. J Exp Psychol Hum Percept Perform. 1999;25(3):837–851. doi: 10.1037//0096-1523.25.3.837. [DOI] [PubMed] [Google Scholar]
- 19.Pasquet B, Carpentier A, Duchateau J. Change in muscle fascicle length influences the recruitment and discharge rate of motor units during isometric contractions. J Neurophysiol. 2005;94(5):3126–3133. doi: 10.1152/jn.00537.2005. [DOI] [PubMed] [Google Scholar]
- 20.Bigland-Ritchie BR, Furbush FH, Gandevia SC, Thomas CK. Voluntary discharge frequencies of human motoneurons at different muscle lengths. Muscle Nerve. 1992;15(2):130–137. doi: 10.1002/mus.880150203. [DOI] [PubMed] [Google Scholar]
- 21.Sosnoff JJ, Voudrie SJ, Ebersole KT. The effect of knee joint angle on torque control. J Mot Behav. 2010;42(1):5–10. doi: 10.1080/00222890903269237. [DOI] [PubMed] [Google Scholar]
- 22.Louw QA, Manilall J, Grimmer KA. Epidemiology of knee injuries among adolescents: a systematic review. Br J Sports Med. 2008;42(1):2–10. doi: 10.1136/bjsm.2007.035360. [DOI] [PubMed] [Google Scholar]
- 23.Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman BN, Aliabadi P, Levy D. The incidence and natural history of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1995;38(10):1500–1505. doi: 10.1002/art.1780381017. [DOI] [PubMed] [Google Scholar]
- 24.Krishnan C, Huston K, Amendola A, Williams GN. Quadriceps and hamstrings muscle control in athletic males and females. J Orthop Res. 2008;26(6):800–808. doi: 10.1002/jor.20592. [DOI] [PubMed] [Google Scholar]
- 25.Enoka RM, Burnett RA, Graves AE, Kornatz KW, Laidlaw DH. Task- and age-dependent variations in steadiness. Prog Brain Res. 1999;123:389–395. doi: 10.1016/s0079-6123(08)62873-3. [DOI] [PubMed] [Google Scholar]
- 26.Bilodeau M, Keen DA, Sweeney PJ, Shields RW, Enoka RM. Strength training can improve steadiness in persons with essential tremor. Muscle Nerve. 2000;23(5):771–778. doi: 10.1002/(sici)1097-4598(200005)23:5<771::aid-mus15>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 27.Perotto A, Delagi EF. Anatomical guide for the electromyographer : The limbs and trunk. Springfield, Ill., USA: Charles C. Thomas; 1994. 309 pp. [Google Scholar]
- 28.Burnett RA, Laidlaw DH, Enoka RM. Coactivation of the antagonist muscle does not covary with steadiness in old adults. J Appl Physiol. 2000;89(1):61–71. doi: 10.1152/jappl.2000.89.1.61. [DOI] [PubMed] [Google Scholar]
- 29.Tracy BL. Force control is impaired in the ankle plantarflexors of elderly adults. Eur J Appl Physiol. 2007;101(5):629–636. doi: 10.1007/s00421-007-0538-0. [DOI] [PubMed] [Google Scholar]
- 30.Tracy BL, Enoka RM. Steadiness training with light loads in the knee extensors of elderly adults. Med Sci Sports Exerc. 2006;38(4):735–745. doi: 10.1249/01.mss.0000194082.85358.c4. [DOI] [PubMed] [Google Scholar]
- 31.De Serres SJ, Kutzscher DV, Enoka RM. Sensitivity of the force display influences performance on a force-matching task. Lake Louise Canada: 2000. pp. 19–23. [Google Scholar]
- 32.Kleine BU, Stegeman DF, Mund D, Anders C. Influence of motoneuron firing synchronization on SEMG characteristics in dependence of electrode position. J Appl Physiol. 2001;91(4):1588–1599. doi: 10.1152/jappl.2001.91.4.1588. [DOI] [PubMed] [Google Scholar]
- 33.Yao W, Fuglevand RJ, Enoka RM. Motor-unit synchronization increases EMG amplitude and decreases force steadiness of simulated contractions. J Neurophysiol. 2000;83(1):441–452. doi: 10.1152/jn.2000.83.1.441. [DOI] [PubMed] [Google Scholar]
- 34.Missenard O, Mottet D, Perrey S. Factors responsible for force steadiness impairment with fatigue. Muscle Nerve. 2009;40(6):1019–1032. doi: 10.1002/mus.21331. [DOI] [PubMed] [Google Scholar]
- 35.Palmieri-Smith RM, Thomas AC, Karvonen-Gutierrez C, Sowers MF. Isometric quadriceps strength in women with mild, moderate, and severe knee osteoarthritis. Am J Phys Med Rehabil. 2010;89(7):541–548. doi: 10.1097/PHM.0b013e3181ddd5c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petterson S, Barrance P, Marmon A, Handling T, Buchanan T, Snyder-Mackler L. Time Course of Quad Strength, Area and Activation After Knee Arthroplasty and Strength Training. Med Sci Sports Exerc. 2010 doi: 10.1249/MSS.0b013e3181eb639a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams GN, Snyder-Mackler L, Barrance PJ, Buchanan TS. Quadriceps femoris muscle morphology and function after ACL injury: a differential response in copers versus non-copers. J Biomech. 2005;38(4):685–693. doi: 10.1016/j.jbiomech.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Hurley MV. The role of muscle weakness in the pathogenesis of osteoarthritis. Rheum Dis Clin North Am. 1999;25(2):283–298. vi. doi: 10.1016/s0889-857x(05)70068-5. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen ND, Pongchaiyakul C, Center JR, Eisman JA, Nguyen TV. Identification of high-risk individuals for hip fracture: a 14-year prospective study. J Bone Miner Res. 2005;20(11):1921–1928. doi: 10.1359/JBMR.050520. [DOI] [PubMed] [Google Scholar]
- 40.Palmieri-Smith RM, Thomas AC. A neuromuscular mechanism of posttraumatic osteoarthritis associated with ACL injury. Exerc Sport Sci Rev. 2009;37(3):147–153. doi: 10.1097/JES.0b013e3181aa6669. [DOI] [PubMed] [Google Scholar]
- 41.Segal NA, Glass NA, Torner J, Yang M, Felson DT, Sharma L, Nevitt M, Lewis CE. Quadriceps weakness predicts risk for knee joint space narrowing in women in the MOST cohort. Osteoarthritis Cartilage. 2010;18(6):769–775. doi: 10.1016/j.joca.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bryant AL, Pua YH, Clark RA. Morphology of knee extension torque-time curves following anterior cruciate ligament injury and reconstruction. J Bone Joint Surg Am. 2009;91(6):1424–1431. doi: 10.2106/JBJS.H.01335. [DOI] [PubMed] [Google Scholar]
- 43.Pua YH, Clark RA, Bryant AL. Physical function in hip osteoarthritis: relationship to isometric knee extensor steadiness. Arch Phys Med Rehabil. 2010;91(7):1110–1116. doi: 10.1016/j.apmr.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Beynnon BD, Fleming BC. Anterior cruciate ligament strain in-vivo: a review of previous work. J Biomech. 1998;31(6):519–525. doi: 10.1016/s0021-9290(98)00044-x. [DOI] [PubMed] [Google Scholar]
- 45.Tagesson S, Oberg B, Kvist J. Tibial translation and muscle activation during rehabilitation exercises 5 weeks after anterior cruciate ligament reconstruction. Scand J Med Sci Sports. 2010;20(1):154–164. doi: 10.1111/j.1600-0838.2009.00903.x. [DOI] [PubMed] [Google Scholar]
- 46.Witvrouw E, Werner S, Mikkelsen C, Van Tiggelen D, Vanden Berghe L, Cerulli G. Clinical classification of patellofemoral pain syndrome: guidelines for non-operative treatment. Knee Surg Sports Traumatol Arthrosc. 2005;13(2):122–130. doi: 10.1007/s00167-004-0577-6. [DOI] [PubMed] [Google Scholar]
- 47.Palmieri-Smith RM, Thomas AC, Karvonen-Gutierrez C, Sowers M. A Clinical Trial of Neuromuscular Electrical Stimulation in Improving Quadriceps Muscle Strength and Activation Among Women With Mild and Moderate Osteoarthritis. Phys Ther. 2010 doi: 10.2522/ptj.20090330. [DOI] [PubMed] [Google Scholar]
- 48.Williams GN, Buchanan TS, Barrance PJ, Axe MJ, Snyder-Mackler L. Quadriceps weakness, atrophy, and activation failure in predicted noncopers after anterior cruciate ligament injury. Am J Sports Med. 2005;33(3):402–407. doi: 10.1177/0363546504268042. [DOI] [PubMed] [Google Scholar]
- 49.Harris CM, Wolpert DM. Signal-dependent noise determines motor planning. Nature. 1998;394(5):780–784. doi: 10.1038/29528. [DOI] [PubMed] [Google Scholar]
- 50.Shinohara M, Moritz CT, Pascoe MA, Enoka RM. Prolonged muscle vibration increases stretch reflex amplitude, motor unit discharge rate, and force fluctuations in a hand muscle. J Appl Physiol. 2005;99(5):1835–1842. doi: 10.1152/japplphysiol.00312.2005. [DOI] [PubMed] [Google Scholar]
- 51.Christou EA, Tracy BL, Enoka RM. Steadiness of lengthening contractions. In: Latash ML, editor. Progress in motor control: structure-function relations in voluntary movements. Volume 2. Champaign, USA: Human Kinetics; 2002. [Google Scholar]
- 52.Roy SH, De Luca CJ, Schneider J. Effects of electrode location on myoelectric conduction velocity and median frequency estimates. J Appl Physiol. 1986;61(4):1510–1517. doi: 10.1152/jappl.1986.61.4.1510. [DOI] [PubMed] [Google Scholar]
- 53.Yoshitake Y, Shinohara M, Kouzaki M, Fukunaga T. Fluctuations in plantar flexion force are reduced after prolonged tendon vibration. J Appl Physiol. 2004;97(6):2090–2097. doi: 10.1152/japplphysiol.00560.2004. [DOI] [PubMed] [Google Scholar]
- 54.Shinohara M. Effects of prolonged vibration on motor unit activity and motor performance. Med Sci Sports Exerc. 2005;37(12):2120–2125. doi: 10.1249/01.mss.0000178106.68569.7e. [DOI] [PubMed] [Google Scholar]
- 55.Bishop BP. Neural control of motor activities. In: Slaughter M, editor. Basic concepts in neuroscience: A student's survival guide. The McGraw-Hill Companies; 2002. [Google Scholar]
- 56.Houk JC, Rymer WZ, Crago PE. Dependence of dynamic response of spindle receptors on muscle length and velocity. J Neurophysiol. 1981;46(1):143–166. doi: 10.1152/jn.1981.46.1.143. [DOI] [PubMed] [Google Scholar]
- 57.Doheny EP, Lowery MM, Fitzpatrick DP, O'Malley MJ. Effect of elbow joint angle on force-EMG relationships in human elbow flexor and extensor muscles. J Electromyogr Kinesiol. 2008;18(5):760–770. doi: 10.1016/j.jelekin.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 58.Solomonow M, Guzzi A, Baratta R, Shoji H, D'Ambrosia R. EMG-force model of the elbows antagonistic muscle pair. The effect of joint position, gravity and recruitment. Am J Phys Med. 1986;65(5):223–244. [PubMed] [Google Scholar]
- 59.Buchanan TS, Lloyd DG, Manal K, Besier TF. Neuromusculoskeletal modeling: estimation of muscle forces and joint moments and movements from measurements of neural command. J Appl Biomech. 2004;20(4):367–395. doi: 10.1123/jab.20.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krishnan C, Williams GN. Error associated with antagonist muscle activity in isometric knee strength testing. Eur J Appl Physiol. 2010;109(3):527–536. doi: 10.1007/s00421-010-1391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tracy BL, Byrnes WC, Enoka RM. Strength training reduces force fluctuations during anisometric contractions of the quadriceps femoris muscles in old adults. J Appl Physiol. 2004;96(4):1530–1540. doi: 10.1152/japplphysiol.00861.2003. [DOI] [PubMed] [Google Scholar]