Abstract
Objective
Peroxisome proliferator-activated receptor-gamma (PPARγ) has been reported to decrease vascular lesion formation. However, the critical role of vascular smooth muscle cell (VSMC) PPARγ in vascular lesion formation following transplantation is not well understood. In this study, we investigated the role of VSMC PPARγ-mediated signaling in transplantation-associated vascular lesion formation.
Methods and Results
Carotid arteries from vascular smooth muscle cell-selective PPARγ knockout (SMPG KO) and wild-type mice were transplanted to CBA/CaJ recipient mice. The recipient mice received a control diet or pioglitazone-containing diet. Pioglitazone reduced vascular lesion formation in transplanted wild-type, but not in SMPG KO carotid arteries. Histological analysis suggested PPARγ attenuates vascular lesion formation through anti-inflammatory signaling, as evidenced by the increase of intimal inflammatory cells and tumor necrosis factor-alpha (TNF-α) expression in SMPG KO allografts. Intravital microscopy revealed increased inflammatory cell rolling and attachment to endothelial cells in small blood vessels of SMPG KO mice following cytokine stimulation. SMPG KO mice, as shown by Western blotting, have elevated vascular cell adhesion molecule-1 (VCAM-1) expression. Furthermore, immunohistochemistry demonstrated SMPG KO allografts have increased VCAM-1.
Conclusion
Loss of PPARγ in VSMC promotes transplantation-associated vascular lesion formation through increased VCAM-1 expression. VSMC PPARγ also mediates pioglitazone-reduced vascular lesion formation.
Keywords: PPARγ, vascular lesion formation, vascular smooth muscle cells, VCAM-1, anti-inflammatory
Introduction
Peroxisome proliferator-activated receptor-gamma (PPARγ) is a transcription factor with pleiotropic effects in many cells. PPARγ is expressed in cardiovascular cells, including endothelial cells (EC), vascular smooth muscle cells (VSMC), inflammatory cells, and cardiomyocytes1. Recent studies have implicated PPARγ as having an important role in the regulation of blood pressure2. Also, PPARγ has functional significance against the development of other cardiovascular diseases such as cardiac hypertrophy3, 4 and atherosclerosis5, 6.
PPARγ activation occurs through interaction with natural or synthetic ligands1. Thiazolidinediones (TZDs), a class of insulin-sensitizing agents currently used for maintaining normal glycemia in diabetic patients, are synthetic PPARγ ligands that regulate gene expression through activation of PPARγ signaling cascades7. The first clinical trial to study the effects of TZDs on cardiovascular outcome, the PROactive study, found that pioglitazone was important for secondary prevention of cardiovascular endpoints8. Thus, there is a necessity to further explore the role of PPARγ in the cardiovascular system.
A role for PPARγ has been implicated in anti-inflammatory signaling9, 10. PPARγ expression potentiates the efficiency of ligands to reduce transcriptional activity of pro-inflammatory genes9. For example, PPARγ-mediated signaling pathways involve the downregulation of adhesion molecules11, 12, proteins that mediate leukocyte rolling on and adhesion to endothelial cells13. In the context of clinical allograft transplantation, this has important significance since increased cardiovascular cell adhesion molecule expression is associated with elevated inflammatory responses shortly after organ or vessel transplantation14. In patients undergoing transplantation, increased inflammatory signaling has been implicated in allograft rejection15. These host immune responses often lead to intragraft vascular lesion formation15, considered to be a limiting factor for long-term patient survival16. Pioglitazone has been found to significantly reduce pro-inflammatory pathways responsible for facilitating acute and chronic allograft rejection17. However, with respect to allograft transplantation, it is unclear whether pioglitazone-induced anti-inflammatory signaling is PPARγ-dependent. Therefore, it is critical to understand the effect of vascular cell PPARγ on inflammatory signaling pathways associated with allograft rejection and transplant-related vascular lesion formation.
In this study, we utilized a VSMC-selective PPARγ knockout mouse model to study the impact of VSMC PPARγ on vascular lesion formation after carotid allograft transplantation. We employed an experimental in vivo model for transplant vasculopathy and demonstrated for the first time that VSMC PPARγ is an important contributor to the protection against transplant-associated vascular lesion formation. Next, we established a signaling mechanism by which the contribution of PPARγ to anti-inflammatory signaling in vascular smooth muscle cells results in decreased vascular lesion progression. Our novel findings outline the basis for selecting VSMC PPARγ as a molecular target for clinical therapeutic intervention following transplantation.
Methods
For a detailed description of Methods, please see supplemental materials (available online at http://atvb.ahajournals.org)
Animals
The animals that served as donors of carotid vessels include: 1) VSMC-selective PPARγ conditional knockout mice (SMPG KO) with a genotype of SM22α-Cre-knock-in heterozygous and PPARγ-floxed homozygous (SM22αCre/+/PPARγflox/flox), and 2) wild-type mice (SM22αCre/+/PPARγflox−/flox−). The generation of SMPG KO and wild-type mice has been previously described18.
Statistical Analysis
Statistical analysis was performed using a standard one-way ANOVA test. Data are expressed as mean ± SEM. P-values < 0.05 were considered statistically significant.
Results
VSMC-selective PPARγ deletion promotes vascular lesion formation following transplantation
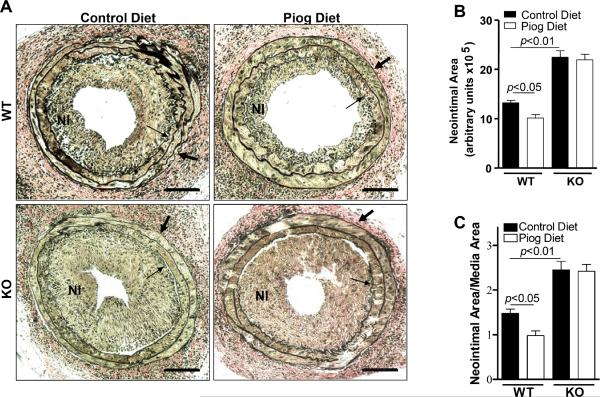
SMPG KO mice show evidence of deleted PPARγ in VSMC18 while expression patterns of other PPAR isoforms, PPARdelta (PPARδ) and PPARalpha (PPARα), are unchanged (Supplemental Figure I). Therefore, to examine the effects of VSMC PPARγ in transplantation-associated vascular lesion formation, we utilized a transplantation experimental model in which SMPG KO or wild-type mice served as donors of carotid arteries (Supplemental Figure II). CBA/CaJ mice served as recipients for SMPG KO and wild-type allografts (Supplemental Figure II). The recipient mice were administered either a pioglitazone-containing or control diet. Figure 1A shows representative sections of carotid allografts four weeks after transplantation. Elastic van Gieson staining provided evidence that SMPG KO allografts had a greater neointimal area and a greater neointimal area/medial area compared with wild-type allografts irrespective of diet (Figures 1A, 1B, and 1C) (P < 0.01, ANOVA). Also, the neointimal area and neointimal area/medial area in wild-type allografts were further reduced when CBA/CaJ recipient mice were administered a pioglitazone-containing diet (Figures 1A, 1B and 1C) (P < 0.05, ANOVA). However, pioglitazone did not decrease the neointimal area or the neointimal area/medial area in SMPG KO allografts (Figures 1A, 1B, and 1C). These results demonstrated the effect of pioglitazone on transplantation-associated vascular lesion formation is VSMC PPARγ-dependent. Moreover, these findings clearly demonstrated the significance of VSMC PPARγ in preventing vascular lesion development following transplantation.
Figure 1. Effects of VSMC PPARγ on vascular lesion formation.
(A) Representative histological sections of carotid arteries from VSMC-selective PPARγ knockout (SMPG KO) and wild-type mice that were transplanted to CBA/CaJ recipient mice administered either a pioglitazone-treated diet or normal diet. Scale bars= 50 μm. Thin arrows demonstrate internal elastic layer and thick arrows demonstrate external elastic layer. (B) The effect of pioglitazone on the neointimal area of SMPG KO and wild-type mouse allografts. (C) The effect of pioglitazone on the neointimal area/medial area of SMPG KO and wild-type mouse allografts. n=6 for SMPG KO group and n=7 for wild-type group. Results are mean ± SEM. P-values < 0.05 were considered statistically significant.
VSMC-selective PPARγ deletion increases inflammatory cell attachment
Next, we began to elucidate mechanisms by which PPARγ attenuates vascular lesion formation in wild-type vessels transplanted to recipient mice. Histological sections of SMPG KO and wild-type allografts were stained with hematoxylin and eosin since hematoxylin confirms the spherical shaped morphology of monocyte nuclei. Representative histological sections demonstrated that the lumens of SMPG KO allografts had greater inflammatory cell attachment when compared with wild-type allografts two weeks after carotid artery transplantation (Figure 2A and 2B). Next, analysis of tissue sections after hematoxylin and eosin staining showed that SMPG KO allografts two weeks post-transplantation had a greater inflammatory cell count per section compared with wild-type allograft sections (Figure 2C) (P < 0.01, ANOVA). Also, SMPG KO allografts demonstrated greater inflammatory infiltration in the medial layer versus transplanted wild-type vessels (Figure 2A and 2B). Immunohistochemical staining for CD14, a monocyte marker, confirmed the presence of inflammatory cells in the vascular lesions and medial layer of transplanted vessels (Supplemental Figure III).
Figure 2. VSMC PPARγ deletion increases inflammatory cell attachment.
Representative hematoxylin and eosin staining of VSMC-selective PPARγ knockout (SMPG KO) and wild-type mouse carotid arteries that were transplanted to CBA/CaJ recipient mice fed a normal diet. Two weeks after transplantation, there was greater inflammatory cell accumulation in the lumens of SMPG KO allografts (B) compared with wild-type allografts (A). n=6 for SMPG KO group and n=7 for wild-type group. The inflammatory cell number for SMPG KO tissue was greater compared with the inflammatory cell number for wild-type tissue (C). Immunohistochemical staining for TNF-α expression revealed a greater area positive for cytokine expression in SMPG KO (E, F) compared with wild-type vessels (D, F). n=3 for each group in immunohistochemical study. Two slides per animal were analyzed for TNF-α expression. Scale bars= 50 μm. Results are mean ± SEM. P-values < 0.05 were considered statistically significant.
Next, histological sections of SMPG KO and wild-type allografts two weeks post-transplantation were stained for immunohistochemical detection of TNF-α expression (Figure 2D and 2E). Evaluation of immunohistochemical staining revealed a greater increase in TNF-α expression for transplanted SMPG KO carotid arteries (Figure 2F) (P < 0.01, ANOVA).
In addition, there was a greater loss of medial smooth muscle cells in SMPG KO vessels two weeks after transplantation (Figure 2A and 2B). Therefore, to investigate the origin of lesional cells following transplantation, carotid arteries from enhanced green fluorescent protein (CAG-EGFP) transgenic mice were transplanted to CBA/CaJ mice and vice versa. When CAG-EGFP carotid arteries were transplanted to CBA/CaJ mice, there was little evidence of EGFP expression in the vascular lesions of CAG-EGFP allografts (Supplemental Figure IV). Conversely, we found that newly-formed vascular lesions of CBA/CaJ allografts were positive for EGFP following transplantation of CBA/CaJ carotid arteries to transgenic CAG-EGFP mice (Supplemental Figure IV). Our results, consistent with other reports19 suggested that cells in the vascular lesions of transplanted vessels are mainly derived from the host and not from the graft medial layer. Thus, the loss of medial VSMC in allografts two weeks after transplantation indicated that VSMC PPARγ inhibits the early pro-inflammatory response immediately following transplantation. These findings show the critical contribution of VSMC PPARγ in attenuating inflammatory cell signaling mechanisms that result in acute organ rejection following transplantation.
VSMC PPARγ deletion increases monocyte adhesion in vivo
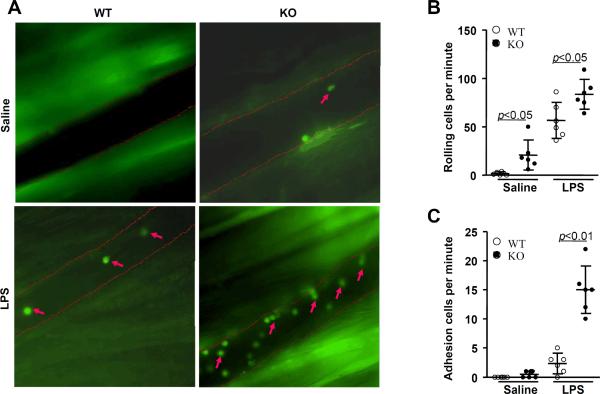
Based on our results in Figure 2, we decided to further demonstrate an inverse relationship between VSMC PPARγ expression and pro-inflammatory cell signaling. Intravital microscopy was utilized for in vivo visualization of monocyte adhesion to postcapillary venules four hours after injection of LPS (1 mg/kg) or saline in SMPG KO and wild-type mice (Figure 3A and supplemental videos). The number of cells rolling on SMPG KO mouse venular endothelium per minute was greater compared with wild-type mouse venular endothelium in both LPS-injected and saline-injected studies (Figure 3B) (P < 0.05, ANOVA). In addition, LPS-stimulated SMPG KO mice demonstrated a greater increase in the number of monocytes adhering to the endothelial cell layer of postcapillary venules versus wild-type animals (Figure 3C) (P < 0.01, ANOVA). Taken together, these findings provide further evidence that VSMC PPARγ inhibits inflammatory signaling mechanisms involved in increasing monocyte homing and adhesion to the vessel wall.
Figure 3. VSMC PPARγ deletion increases monocyte adhesion in vivo.
Intravital microscopy was used for in vivo detection of monocytes labeled with rhodamine 6G. (A) Visualization of monocytes adhering to the venular endothelium of vascular smooth muscle cell PPARγ knockout (SMPG KO) and wild-type mice four hours after a 1 mg/kg lipopolysaccharide (LPS) injection. (B) Mice deficient in VSMC PPARγ displayed increases in the number of monocytes rolling on venular endothelium per minute in both saline- and LPS-treated studies. (C) LPS-injected SMPG KO mice demonstrated a significantly greater number of cells adhering to venular endothelium per minute versus wild-type mice. Data are representative of 6 separate experiments. Arrows represent rhodamine 6G-stained monocytes.
PPARγ affects VCAM-1 levels in VSMC
To gain a better understanding of the underlying mechanisms involving VSMC PPARγ-mediated anti-inflammatory signaling, we performed Western blot studies examining the effects of VSMC PPARγ on vascular cell adhesion molecule-1 (VCAM-1) expression. For this study, the smooth muscle cell layer from mouse thoracic aortas and primary cultured rat aortic smooth muscle cells (RASMC) were used for determining whether PPARγ-mediated signaling regulates VCAM-1 expression. Figure 4A is a representative Western blot showing that the vascular smooth muscle tissue of eight-week old SMPG KO mice has increased VCAM-1 expression compared with tissue of wild-type mice (P < 0.05, ANOVA). Next, we examined the expression of VCAM-1 in transplanted SMPG KO and wild-type carotid arteries by immunohistochemistry. SMPG KO allografts demonstrated a greater area positive for VCAM-1 at two and four weeks after transplantation (Figures 4B and 4C) (P < 0.05, ANOVA). Next, quantitative Western blot analysis, as shown in Figure 4D, indicated that hIL-1-stimulated RASMC overexpressed with PPARγ had significantly decreased VCAM-1 protein levels compared with hIL-1-stimulated cells treated with GFP (P < 0.05, ANOVA). In contrast, hIL-1-stimulated RASMC transfected with PPARγ RNAi showed evidence of increased VCAM-1 expression versus GFP-treated cells (P < 0.05, ANOVA). Next, pioglitazone reduced hIL-1-stimulated upregulation of VCAM-1 expression in RASMC (Figure 4E) (P < 0.05, ANOVA), further suggesting that VSMC PPARγ is critical for inhibiting VCAM-1 expression and pro-inflammatory signaling. In addition, we found that pioglitazone reduced cytokine-stimulated monocyte chemoattractant protein-1 (MCP-1) expression in VSMC from wild-type but not SMPG KO mice (Supplemental Figure V).
Figure 4. PPARγ affects VCAM-1 levels in VSMC.
(A) Western Blot analysis revealed elevated VCAM-1 expression in thoracic arteries of eight-week-old smooth muscle cell-selective PPARγ knockout (SMPG KO) mice. n=3 for each pooled sample. (B, C) SMPG KO allografts, as shown by immunohistochemical staining, demonstrated a greater area of positive for VCAM-1 at two and four weeks after transplantation. n=3 animals for each group in immunohistochemical study. Two slides per animal were analyzed for VCAM-1 expression. (D) PPARγ overexpression by adenovirus (Ad-PPARγ) resulted in decreased VCAM-1 expression in rat aortic smooth muscle cells (RASMC) stimulated with 10 ng/ml hIL-1. In contrast, adenoviral PPARγ knockdown elevated VCAM-1 expression in hIL-1-stimulated cells. Ad-GFP was utilized as a control. (E) The addition of pioglitazone decreased hIL-1-induced increases in RASMC VCAM-1 expression. Results are mean ± SEM. P-values < 0.05 were considered statistically significant. Data are representative of 3 separate experiments.
PPARγ knockdown increases NF-κB activation in VSMC
Previous work demonstrated that VCAM-1 is under the control of nuclear factor-kappa beta (NF-κB) signaling20. Importantly, in hIL-1-stimulated RASMC, we observed that PPARγ overexpression reduced (P < 0.05, ANOVA) while PPARγ knockdown increased (P < 0.05, ANOVA) nuclear protein levels of the NF-κB p65 subunit (Figure 5A). Neither PPARγ overexpression nor PPARγ knockdown significantly affected cytosolic p65 protein levels (Figure 5A). Furthermore, p65 mRNA expression did not significantly change in hIL-1-stimulated smooth muscle cells transfected with either PPARγ or PPARγ RNAi (Supplemental Figure VI).
Figure 5. PPARγ knockdown increases NF-κB activation in VSMC.
(A) Western Blot analysis of cytosolic and nuclear p65 levels in hIL-stimulated (10 ng/ml) rat aortic smooth muscle cells (RASMC). PPARγ overexpression reduced while PPARγ knockdown increased nuclear p65 levels. Neither PPARγ overexpression nor PPARγ RNAi had any significant effect on cytosolic p65 levels. (B) Western Blot analysis of RASMC nuclear fractions showed PPARγ knockdown increased phosphorylation of both IkappaB kinase (IKK) and IkappaBalpha (IκBα). Ad-GFP served as an adenovirus control. Results are mean ± SEM. P-values < 0.05 were considered statistically significant. Data are representative of 3 separate experiments.
Next, it has been reported that IκB kinase (IKK), upon activation, phosphorylates inhibitor kappa Balpha (IκBα), which consequently results in IκBα degradation21. These events eventually trigger shuttling of p65 from the cytoplasm to nucleus21. We found that PPARγ knockdown in hIL-1-stimulated RASMC increased both phosphorylated IKK and IκBα (Figure 5B) (P < 0.05, ANOVA). Thus, the findings presented in Figure 5 indicate that VSMC PPARγ is involved in abrogating p65 nuclear translocation. Collectively, our results in this study demonstrate the importance of VSMC PPARγ in attenuating the pro-inflammatory response involved in promoting vascular lesion formation following transplantation.
Discussion
There is accumulating evidence indicating PPARγ has many functions in the cardiovascular system. In particular, PPARγ-mediated signaling was first shown to reduce vascular injury-induced lesion formation by inhibiting VSMC proliferation and migration22. However, we provide compelling evidence suggesting that the contribution of PPARγ in protecting against transplantation-related vascular lesion formation involves anti-inflammatory signaling. Our study utilized VSMC PPARγ conditional knock-out animals and an allograft transplantation model to offer novel insight into the role of VSMC PPARγ during intragraft vascular lesion formation. The current study offers the following novel insights: 1) VSMC PPARγ signaling is critical for reducing vascular lesion formation seen in transplant arteriosclerosis (TA); 2) VSMC PPARγ plays an important role in preventing allograft rejection by reducing pro-inflammatory signaling; 3) the effects of VSMC PPARγ-mediated anti-inflammatory signaling are manifested, in part through attenuation of VCAM-1 expression; and 4) a critical contribution made by VSMC PPARγ in mediating pioglitazone-induced reduction of vascular lesion formation following carotid artery transplantation.
A role of PPARγ in attenuating vascular lesion formation from balloon-injury and carotid artery ligation has been suggested through the use of PPARγ gene delivery and a PPARγ mutant mouse model22, 23. However, the in vivo models mentioned in the previous reports did not address the role of VSMC-specific PPARγ in attenuating transplantation-associated vascular lesion formation. Also, it is important to mention that the experimental vascular lesion formation models reported above22, 23 focused on PPARγ attenuating the VSMC proliferative response as opposed to PPARγ promoting anti-inflammatory signaling. Furthermore, with respect to studying PPARγ mutations, it is unclear whether the dominant negative activity is specific for PPARγ-mediated signaling as the inhibitory functions of the PPARγ mutant have been shown to extend to PPARα and PPARδ24. Thus, dominant-negative PPARγ may exhibit alterations of PPARα and PPARδ signaling pathways24. Also, in the context of a targeted PPARγ dominant-negative mutation, absolute deletion of the functionality of PPARγ proteins is often difficult to achieve through mutations since a PPARγ mutant may still have the ability to interact with other co-factors to facilitate other signaling pathways25. In addition, it was reported that tyrosine agonists, high-affinity ligands of PPARγ, can reverse the molecular defects associated with PPARγ dominant-negative mutations26. The use of PPARγ cell-selective knockout animal models in the present study effectively eliminated both genomic and non-genomic PPARγ function. This was advantageous for directly studying the contribution of VSMC PPARγ in modulating vascular lesion formation in a carotid artery transplantation model.
Next, this investigation demonstrated that VSMC PPARγ-mediated reduction in transplant-associated vascular lesion formation occurs through anti-inflammatory signaling. The use of a transplant vasculopathy experimental model is advantageous for studying immunologic factors influencing vascular lesion progression27. Furthermore, the transplant model is ideal for addressing the contribution of VSMC PPARγ immunosuppressive signaling mechanisms that reduce vascular lesion development.
Our study is the first to provide direct in vivo evidence that VSMC PPARγ deletion results in elevated levels of VCAM-1 following transplantation. Also, our results show that vascular smooth muscle cells overexpressed with PPARγ have reduced VCAM-1 expression, and this effect is reversed when cells are transfected with PPARγ RNAi. One of the early hallmarks of transplant vasculopathy consists of inflammatory cells attaching to the endothelium and infiltrating the vessel wall27. Adhesion molecules, including VCAM-1 are important for inflammatory cell recruitment to the vessel wall and also the communication between inflammatory cells, endothelial cells and vascular smooth muscle cells during a normal immune-mediated response following transplantation27–30. Although several studies suggest VCAM-1 is produced mainly in endothelial cells31, 32, the functional significance of VSMC VCAM-1 should not be understated, particularly since several studies have confirmed VCAM-1 expression in vascular smooth muscle cells. For example, cytokine stimulation can dose- and time-dependently increase VCAM-1 mRNA and surface expression in cultured VSMC33. Also, VCAM-1 expressed in human vascular smooth muscle cells from atherosclerotic lesions is found to be associated with leukocyte recruitment34. Furthermore, of significant interest, experimental models for transplant vasculopathy demonstrate that VCAM-1 is upregulated in medial layer smooth muscle cells14, 29.
It has been well-documented that VCAM-1 expression is regulated by p65, a subunit of NF-κB20. In the current study, we found that PPARγ overexpression reduced and PPARγ knockdown increased nuclear p65 protein levels in cultured VSMC. In addition, we observed that loss of PPARγ signaling in vascular smooth muscle cells caused increased phosphorylation of IκB. This has important consequences as phosphorylation leads to proteolytic degradation of IκB and subsequent p65 nuclear translocation21. Our findings are consistent with previous studies demonstrating the involvement of PPAR family-dependent signaling in regulating expression of IκB35. Taken together, our results suggest VSMC PPARγ inhibition of NF-κB transcriptional activity can occur by attenuating p65 nuclear translocation. The elucidation of the precise mechanisms involving PPARγ-mediated sequestering of cytoplasmic p65 is beyond the scope of this investigation.
It is important to mention that PPARγ-induced inhibition of p65 nuclear translocation is not the sole mechanism by which PPARγ affects NF-κB gene activity. For example, there is clear evidence that PPARγ transrepresses NF-κB inflammatory gene expression in macrophages36. Next, PPARγ is an activator of antioxidant enzyme expression37. Reactive oxygen species are key initiators of NF-κB activity38 and PPARγ-induced upregulation of antioxidant genes may inhibit NF-κB activity by reducing the level and availability of cellular reactive oxygen species. Thus, we extend the previous work of others9, 36, 39 by introducing an alternative signaling pathway by which PPARγ may negatively regulate NF-κB activity. Moreover, it is quite possible that the mechanisms pertaining to PPARγ-induced inhibition of NF-κB transcriptional activity may also be cell-specific. Collectively, our in vivo and in vitro findings firmly establish that VSMC PPARγ plays an essential role in decreasing NF-κB-induced VCAM-1 expression.
The notion that VSMC PPARγ ablation results in elevated vascular TNF-α expression is further indicative of the importance of PPARγ during the observed early inflammatory response following allograft transplantation. In this study, we present evidence suggesting that abolishment of VSMC PPARγ-mediated inhibition of VCAM-1 will accentuate the number of inflammatory cells infiltrating the vessel wall and result in elevated TNF-α expression. Although smooth muscle cells may express TNF-α40, inflammatory cells infiltrating the vessel after transplantation are considered the major contributors of TNF-α expression41. This has important significance since TNF-α is known to further potentiate post-transplantation inflammatory responses41, 42. Thus, the increase in TNF-α expression found in transplanted SMPG KO vessels provides further support that VSMC PPARγ plays a protective role against the development of transplant vasculopathy by attenuating initial inflammatory-mediated signaling pathways involved in promoting vascular lesion formation. However, it is important to mention that loss of medial smooth muscle cells in allografts can occur following acute rejection19, 27 as the eventual increase in VSMC apoptosis can be attributed to infiltration of inflammatory cells27. Thus, VSMC PPARγ, in promoting early anti-inflammatory signaling following transplantation, not only decreases allograft vascular lesion formation, but may also prolong the survival and function of medial layer smooth muscle cells in donor vessels.
A role for pioglitazone has been demonstrated in attenuating experimental transplantation-associated vascular lesion formation17. Our results extend these findings by showing the effects of pioglitazone in reducing vascular lesion formation after transplantation are mediated by VSMC PPARγ, particularly since SMPG KO allografts do not show any evidence of a decreased lesional area when treated with pioglitazone. Furthermore, our study clearly suggests that the effects of pioglitazone in reducing VCAM-1 expression occur through PPARγ-dependent signaling in VSMC. However, in immune-mediated responses to transplanted tissue, we should consider that pioglitazone can promote cellular anti-inflammatory signaling mechanisms independent of PPARγ activation1, as the possibility exists whereby pioglitazone or other PPARγ ligands may repress transcription of pro-inflammatory genes by either directly binding to NF-κB or to the promoters of these genes. Nevertheless, our results have important clinical significance, particularly since pioglitazone administration has been shown to improve cardiovascular outcomes8.
In conclusion, our findings in this study indicate the importance of VSMC PPARγ in inhibiting the progression of transplant-associated vascular lesion formation. Given our results demonstrating that VSMC PPARγ deletion increases VCAM-1 expression, these studies identify that VSMC PPARγ exerts its anti-inflammatory properties during the early response to transplantation-induced vascular injury. Thus, we propose the increase in cellular adhesion molecule expression due to VSMC PPARγ deletion promotes increased inflammatory cell adherence to the vessel wall, contributing to greater vascular pro-inflammatory signaling and increased cytokine production. This is consistent with the literature demonstrating that enhanced cytokine signaling is a major contributor to transplant-associated vascular lesion formation15. Although our investigation establishes the critical importance of VSMC PPARγ signaling in attenuating lesional development associated with transplant arteriosclerosis, we must acknowledge the pathogeneses of lesion formation in TA, atherosclerosis, and vascular injury are distinct. Therefore, future studies must be conducted to further determine the contribution of VSMC PPARγ signaling cascades associated with reduced lesional progression in all experimental models of vascular lesion formation. In summary, our study provides evidence that VSMC PPARγ should be considered as a primary target for future pharmacological intervention in transplant arteriosclerosis and other diseases associated with vascular lesion formation.
Condensed Abstract.
Understanding of vascular smooth muscle cell (VSMC) PPARγ in attenuating vascular lesion formation is clinically relevant to the treatment of cardiovascular complications. In the present study, we used a VSMC-selective PPARγ knockout mouse model to demonstrate that VSMC PPARγ is a critical mediator of pioglitazone-reduced transplantation-associated vascular lesion formation.
Supplementary Material
Acknowledgements
This work was partially funded by Takeda Pharmaceuticals North America and National Institutes of Health (HL68878 and HL89544). M.H. is supported by a postdoctoral fellowship from the National Institutes of Health (T32 HL007853). L.C. is supported by the American Heart Association Scientist Development Grant (09SDG2230270). J.Z. was supported by the American Heart Association National Career Development Grant (0835237N). Y.E.C. is an established investigator of the American Heart Association (0840025N).
Footnotes
The authors have no conflict of interest to disclose.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hamblin M, Chang L, Fan Y, Zhang J, Chen YE. PPARs and the cardiovascular system. Antioxid Redox Signal. 2009;11:1415–1452. doi: 10.1089/ars.2008.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamblin M, Chang L, Zhang J, Chen YE. The role of peroxisome proliferator-activated receptor gamma in blood pressure regulation. Curr Hypertens Rep. 2009;11:239–245. doi: 10.1007/s11906-009-0041-6. [DOI] [PubMed] [Google Scholar]
- 3.Duan SZ, Ivashchenko CY, Russell MW, Milstone DS, Mortensen RM. Cardiomyocyte-specific knockout and agonist of peroxisome proliferator-activated receptor-gamma both induce cardiac hypertrophy in mice. Circ Res. 2005;97:372–379. doi: 10.1161/01.RES.0000179226.34112.6d. [DOI] [PubMed] [Google Scholar]
- 4.Ding G, Fu M, Qin Q, Lewis W, Kim HW, Fukai T, Bacanamwo M, Chen YE, Schneider MD, Mangelsdorf DJ, Evans RM, Yang Q. Cardiac peroxisome proliferator-activated receptor gamma is essential in protecting cardiomyocytes from oxidative damage. Cardiovasc Res. 2007;76:269–279. doi: 10.1016/j.cardiores.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 5.Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB, Liao D, Nagy L, Edwards PA, Curtiss LK, Evans RM, Tontonoz P. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161–171. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- 6.Babaev VR, Yancey PG, Ryzhov SV, Kon V, Breyer MD, Magnuson MA, Fazio S, Linton MF. Conditional knockout of macrophage PPARgamma increases atherosclerosis in C57BL/6 and low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:1647–1653. doi: 10.1161/01.ATV.0000173413.31789.1a. [DOI] [PubMed] [Google Scholar]
- 7.Villacorta L, Schopfer FJ, Zhang J, Freeman BA, Chen YE. PPARgamma and its ligands: therapeutic implications in cardiovascular disease. Clin Sci (Lond) 2009;116:205–218. doi: 10.1042/CS20080195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Koranyi L, Laakso M, Mokan M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 9.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 10.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 11.Wang N, Verna L, Chen NG, Chen J, Li H, Forman BM, Stemerman MB. Constitutive activation of peroxisome proliferator-activated receptor-gamma suppresses pro-inflammatory adhesion molecules in human vascular endothelial cells. J Biol Chem. 2002;277:34176–34181. doi: 10.1074/jbc.M203436200. [DOI] [PubMed] [Google Scholar]
- 12.Jackson SM, Parhami F, Xi XP, Berliner JA, Hsueh WA, Law RE, Demer LL. Peroxisome proliferator-activated receptor activators target human endothelial cells to inhibit leukocyte-endothelial cell interaction. Arterioscler Thromb Vasc Biol. 1999;19:2094–2104. doi: 10.1161/01.atv.19.9.2094. [DOI] [PubMed] [Google Scholar]
- 13.Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- 14.Tanaka H, Sukhova GK, Swanson SJ, Cybulsky MI, Schoen FJ, Libby P. Endothelial and smooth muscle cells express leukocyte adhesion molecules heterogeneously during acute rejection of rabbit cardiac allografts. Am J Pathol. 1994;144:938–951. [PMC free article] [PubMed] [Google Scholar]
- 15.Belperio JA, Ardehali A. Chemokines and transplant vasculopathy. Circ Res. 2008;103(5):454–466. doi: 10.1161/CIRCRESAHA.108.182865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmauss D, Weis M. Cardiac allograft vasculopathy: recent developments. Circulation. 2008;117:2131–2141. doi: 10.1161/CIRCULATIONAHA.107.711911. [DOI] [PubMed] [Google Scholar]
- 17.Kosuge H, Haraguchi G, Koga N, Maejima Y, Suzuki J, Isobe M. Pioglitazone prevents acute and chronic cardiac allograft rejection. Circulation. 2006;113:2613–2622. doi: 10.1161/CIRCULATIONAHA.105.594101. [DOI] [PubMed] [Google Scholar]
- 18.Chang L, Villacorta L, Zhang J, Garcia-Barrio MT, Yang K, Hamblin M, Whitesall SE, D'Alecy LG, Chen YE. Vascular smooth muscle cell-selective peroxisome proliferator-activated receptor-gamma deletion leads to hypotension. Circulation. 2009;119:2161–2169. doi: 10.1161/CIRCULATIONAHA.108.815803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hillebrands JL, Klatter FA, Rozing J. Origin of vascular smooth muscle cells and the role of circulating stem cells in transplant arteriosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:380–387. doi: 10.1161/01.ATV.0000059337.60393.64. [DOI] [PubMed] [Google Scholar]
- 20.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. Faseb J. 1995;9:899–909. [PubMed] [Google Scholar]
- 21.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF [kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 22.Lim S, Jin CJ, Kim M, Chung SS, Park HS, Lee IK, Lee CT, Cho YM, Lee HK, Park KS. PPARgamma gene transfer sustains apoptosis, inhibits vascular smooth muscle cell proliferation, and reduces neointima formation after balloon injury in rats. Arterioscler Thromb Vasc Biol. 2006;26:808–813. doi: 10.1161/01.ATV.0000204634.26163.a7. [DOI] [PubMed] [Google Scholar]
- 23.Meredith D, Panchatcharam M, Miriyala S, Tsai YS, Morris AJ, Maeda N, Stouffer GA, Smyth SS. Dominant-negative loss of PPARgamma function enhances smooth muscle cell proliferation, migration, and vascular remodeling. Arterioscler Thromb Vasc Biol. 2009;29:465–471. doi: 10.1161/ATVBAHA.109.184234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semple RK, Meirhaeghe A, Vidal-Puig AJ, Schwabe JW, Wiggins D, Gibbons GF, Gurnell M, Chatterjee VK, O'Rahilly S. A dominant negative human peroxisome proliferator-activated receptor (PPAR){alpha} is a constitutive transcriptional corepressor and inhibits signaling through all PPAR isoforms. Endocrinology. 2005;146:1871–1882. doi: 10.1210/en.2004-1405. [DOI] [PubMed] [Google Scholar]
- 25.Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O'Rahilly S. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 26.Agostini M, Gurnell M, Savage DB, Wood EM, Smith AG, Rajanayagam O, Garnes KT, Levinson SH, Xu HE, Schwabe JW, Willson TM, O'Rahilly S, Chatterjee VK. Tyrosine agonists reverse the molecular defects associated with dominant-negative mutations in human peroxisome proliferator-activated receptor gamma. Endocrinology. 2004;145:1527–1538. doi: 10.1210/en.2003-1271. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell RN, Libby P. Vascular remodeling in transplant vasculopathy. Circ Res. 2007;100:967–978. doi: 10.1161/01.RES.0000261982.76892.09. [DOI] [PubMed] [Google Scholar]
- 28.Weis M, von Scheidt W. Cardiac allograft vasculopathy: a review. Circulation. 1997;96:2069–2077. doi: 10.1161/01.cir.96.6.2069. [DOI] [PubMed] [Google Scholar]
- 29.Ardehali A, Laks H, Drinkwater DC, Ziv E, Drake TA. Vascular cell adhesion molecule-1 is induced on vascular endothelia and medial smooth muscle cells in experimental cardiac allograft vasculopathy. Circulation. 1995;92:450–456. doi: 10.1161/01.cir.92.3.450. [DOI] [PubMed] [Google Scholar]
- 30.Dietrich H, Hu Y, Zou Y, Dirnhofer S, Kleindienst R, Wick G, Xu Q. Mouse model of transplant arteriosclerosis: role of intercellular adhesion molecule-1. Arterioscler Thromb Vasc Biol. 2000;20:343–352. doi: 10.1161/01.atv.20.2.343. [DOI] [PubMed] [Google Scholar]
- 31.Muller WA. Mechanisms of transendothelial migration of leukocytes. Circ Res. 2009;105:223–230. doi: 10.1161/CIRCRESAHA.109.200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167:377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Couffinhal T, Duplaa C, Moreau C, Lamaziere JM, Bonnet J. Regulation of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 in human vascular smooth muscle cells. Circ Res. 1994;74:225–234. doi: 10.1161/01.res.74.2.225. [DOI] [PubMed] [Google Scholar]
- 34.O'Brien KD, Allen MD, McDonald TO, Chait A, Harlan JM, Fishbein D, McCarty J, Ferguson M, Hudkins K, Benjamin CD, et al. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest. 1993;92:945–951. doi: 10.1172/JCI116670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delerive P, Gervois P, Fruchart JC, Staels B. Induction of IkappaBalpha expression as a mechanism contributing to the anti-inflammatory activities of peroxisome proliferator-activated receptor-alpha activators. J Biol Chem. 2000;275:36703–36707. doi: 10.1074/jbc.M004045200. [DOI] [PubMed] [Google Scholar]
- 36.Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG, Glass CK. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Girnun GD, Domann FE, Moore SA, Robbins ME. Identification of a functional peroxisome proliferator-activated receptor response element in the rat catalase promoter. Mol Endocrinol. 2002;16:2793–2801. doi: 10.1210/me.2002-0020. [DOI] [PubMed] [Google Scholar]
- 38.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chinetti G, Griglio S, Antonucci M, Torra IP, Delerive P, Majd Z, Fruchart JC, Chapman J, Najib J, Staels B. Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J Biol Chem. 1998;273:25573–25580. doi: 10.1074/jbc.273.40.25573. [DOI] [PubMed] [Google Scholar]
- 40.Warner SJ, Libby P. Human vascular smooth muscle cells. Target for and source of tumor necrosis factor. J Immunol. 1989;142:100–109. [PubMed] [Google Scholar]
- 41.Arbustini E, Grasso M, Diegoli M, Bramerio M, Foglieni AS, Albertario M, Martinelli L, Gavazzi A, Goggi C, Campana C, et al. Expression of tumor necrosis factor in human acute cardiac rejection. An immunohistochemical and immunoblotting study. Am J Pathol. 1991;139:709–715. [PMC free article] [PubMed] [Google Scholar]
- 42.Imagawa DK, Millis JM, Seu P, Olthoff KM, Hart J, Wasef E, Dempsey RA, Stephens S, Busuttil RW. The role of tumor necrosis factor in allograft rejection. III. Evidence that anti-TNF antibody therapy prolongs allograft survival in rats with acute rejection. Transplantation. 1991;51:57–62. doi: 10.1097/00007890-199101000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.