Abstract
Background
The pathophysiology of dysphagia in patients with eosinophilic esophagitis (EoE) is unknown, but may be related to abnormal esophageal motor function. Symptoms rarely occur during stationary esophageal manometry so it has been difficult to establish an association between symptoms and motor events.
Aim
To evaluate esophageal motor function in children with EoE with the use of stationary manometry and ambulatory prolonged esophageal manometry and pH-metry (PEMP)
Methods
PEMP was performed in children with EoE, compared with controls and children with GERD. Effective peristalsis was considered when the esophageal contractions had a normal amplitude and propagation. Results expressed as mean ± S.E.
Results
Seventeen patients with EoE, 13 with GERD and 11 controls were studied. Values are expressed as mean ± se. Stationary manometry identified abnormal peristalsis in 41% of children with EoE. During PEMP, children with EoE had an increased number of isolated (16.7 ± 3.8 vs 9.5 ± 1.6 vs 6.5 ± 1.1 ; p< 0.03) and high amplitude contractions (4.1 ± 1.2 vs 1.8 ±0.8 vs 0.1 ± 0.1; p< 0.03), and more % ineffective peristalsis both during fasting (70.5% ± 2.5 vs 57.8% ± 3.0 vs 53.8% ± 1.9; p <0.05) and during meals (68.4 ± 3.4 vs 55.3 ± 2.8 vs 48.1 ± 2.8; p < 0.05) when compared with children with GERD and controls. Thirteen patients with EoE experienced 21 episodes of dysphagia and all correlated with simultaneous abnormal motor function.
Conclusions
PEMP allowed the detection of ineffective peristalsis in children with EoE. Symptoms observed in children with EoE may be related to esophageal motor dysfunction.
Dysphagia is a characteristic symptom of children and adults with eosinophilic esophagitis (EoE). While dysphagia is usually long standing, it is often intermittent and unpredictably interferes with activities of daily life (1, 2). While many studies have characterized clinical features of patients with EoE, the pathophysiology of dysphagia associated with EoE is uncertain. In some patients (1, 2) dysphagia may occur as a result of long segment luminal narrowing (1), isolated strictures, diffuse trachealization, or fixed rings, including Schatzki (3), features reflective of tissue remodeling and fibrosis (4). Yet most patients demonstrate no discernible anatomic lesion, raising the possibility that the dysphagia occurs as a result of motility disturbances (5, 6). Unfortunately, few studies have measured esophageal motor function in patients with EoE (6) and studies addressing this issue have utilized traditional methodologies.
In previous studies, stationary manometry in children revealed normal function (1, 2, 7), while in adults, findings ranged from normal motility to tertiary contractions, aperistalsis, simultaneous contractions, diffuse esophageal spasm and nutcracker esophagus (1, 5, 6). However, in these studies, it is not clear if these non-specific findings bear any relationship to symptoms. One of the limitations of stationary manometry is that the clinician only obtains 10 to 20 wet swallows in a laboratory setting, rather than during normal fasting and fed periods. Given the intermittent nature of dysphagia in patients with EoE, it is highly unlikely that stationary manometry accurately provides relevant assessment of esophageal physiology at the time of the dysphagia (6). The development of combined ambulatory prolonged esophageal manometry and pH-metry (PEMP) has contributed to more complete understanding of esophageal motor function and pathophysiology in both children and adults (8-10). PEMP has the advantage of providing a prolonged study of esophageal body contractions under normal physiologic conditions over a prolonged period of time that includes sleeping or eating (8-10). Therefore, PEMP has the capacity to identify subtle, episodic abnormal esophageal body contractions that will likely go unmeasured utilizing stationary manometry.
We hypothesize that patients with EoE have altered motility at the time of the dysphagia, alterations that cannot be captured on short term manometry monitoring. The aim of our study was to evaluate esophageal motor function in children with EoE with the use of ambulatory prolonged esophageal manometry and pH-metry.
Methods
This prospective study was conducted at Children's Hospital, Boston, MA. Patients with EoE and two comparison groups {gastroesophageal reflux disease (GERD) and controls} underwent prolonged (20-24 hours) esophageal manometry and pH-metry (9, 10)
Inclusion/exclusion criteria
We approached only children that were referred by their primary gastroenterologist to undergo 24 pH-metry followed by upper endoscopy as part of their clinical evaluation to r/o GERD reflux related atypical symptoms or esophageal disorders. All children needed to have a previous UGI series to exclude anatomic problems. Children with anatomic malformations, including hiatal hernia, congenital abnormalities, developmental delay, autism or previous gastrointestinal surgery, including tracheo-esophageal fistula repair, or fundoplication were excluded.. No children with a previous steroid therapy or elimination diet trial were included.
Recording technique and procedures
If the patients/parents agreed to participate, the only difference with their routine care was that instead of a regular pH probe catheter, they were nasally intubated with a specially designed catheter (see below) that besides the pH measurement transducer, had 4 strain gauge pressure transducers. Therefore with that catheter and only one nasal intubation, we could perform not only 24 hour pH measurements, but also a stationary esophageal manometry, followed by the PEMP,.
The PEMP and stationary esophageal manometry were performed as previously described with the use of a Microdiggitrapper recording device (Synectics, Inc., Stockholm, Sweden) (9, 10). All antacids, prokitnetics or any other medications that affects gastrointestinal motility were stopped 3 days prior to the test. PEMP was performed with a non-perfused solid state probe with 4 strain gauge pressure transducers separated by 5 cm from the distal end. The probe used had a pH transducer that was located 1 cm proximal to the distal port, with an external diameter of 5 mm (Konigsberg Instruments Inc., Pasadena, CA). Patients had probes placed either without sedation, or with sedation of oral midazolam (0.5 mg/kg to a maximum of 20 mg) after an overnight fast.
Initially a standard stationary esophageal manometry was performed. For this a special on line adaptor allowed the transmission of the pressure events from the microdiggitraper to a desktop computer. For the manometry the catheter was introduced into the stomach and a slow pull through done to determine the lower esophageal sphincter (LES) pressure and location. This was followed by the observation of peristalsis with at least 10 wet swallows of water.
After completion of standard esophageal manometry, the manometry probe was repositioned and taped with the pH transducer left 3-4 cm above the LES, and the PEMP initiated. During PEMP patients conducted normal activities and were fed at a minimal interval of 4 hours. All activities and symptoms that occurred during the study were recorded and analyzed.
The endoscopy was then performed 24 hours after the PEMP. In every subject, 2 biopsies were obtained in the distal esophagus, and 2 in the upper esophagus at least 10 cm above the GEJ, and just below the cricopharyngeous
Patient classification
a) Eosinophilic esophagitis (EoE)
According to recently published consensus criteria (1, 2) children with EoE demonstrated: a) greater than 15 eosinophils /HPF in the squamous epithelium with or without superficial layering or eosinophil microabscesses, b) histologic esophagitis unresponsive to a minimum of 4 weeks of treatment with a PPI given twice daily (2 mg/kg/day) c) normal pH monitoring of the distal esophagus (defined as pH <4 for less than 6% of the study) (11) and d) absence of eosinophilia in gastric and duodenal biopsies (1, 2). Patients who had esophageal narrowing, esophageal or Schatzki rings were excluded.
Controls
Considered normal controls if they fulfilled the following criteria: a) no dysphagia, b) normal upper gastrointestinal barium study without evidence of obstruction, malrotation, or hiatal hernia, c) normal gross appearance of the esophageal mucosa during endoscopy that had been performed within 2 weeks of the PEMP d) normal pH monitoring of the distal esophagus e) normal stationary esophageal manometry and e) normal histopathology after at least 1 year of follow up (9).
GERD
Patients with a reflux index of >6 % were considered to have GERD, independently of the presence or absence of esophagitis (11).
Histological Assessment
All esophageal mucosal biopsy specimens were formalin-fixed, routinely processed, paraffin-embedded, cut serially in 5-micron sections and stained with hematoxylin and eosin. Histological classification of the biopsies was done as previously described (3, 12). Quantification of intraepithelial eosinophil number was performed by counting the number of eosinophils in each biopsy in five separate high power fields (HPF) in areas with the densest inflammatory infiltrate as previously described (3, 12). Data were expressed as the mean number of eosinophils per HPF (Nikon Optiphot-2, Plan 40x lens-surface area=0.196 mm2). The distribution of eosinophils within the squamous epithelium was also assessed. Measurements of basal zone thickness were considered abnormal if there was basal zone hyperplasia greater than 20% of the total epithelial thickness or lengthening of the papillae to greater than, or equal to, 75% of the epithelial height (12). Superficial layering of eosinophils was defined as preferential concentration of eosinophils in the upper one third of the esophageal epithelium. Microabscesses of eosinophils were defined as clusters of 4 or more eosinophils, typically near the mucosal surface (3, 12)
All clinical and histopathological analyses were performed by investigators who were blinded to the patient's history and clinical diagnosis. Informed consent was obtained and this prospective study was approved by the investigational review board of Children's Hospital Boston.
Sample size
Based on our previous experience with PEMP on control children we expected to see ineffective peristalsis in 50 + 20 % of swallows during PEMP in the controls, and postulated an ineffective peristalsis in 70% in EoE patients. Using a power of 0.8 and alpha of 0.05 we calculated we would need 15 patients in each group. However as the study progressed it became apparent it was very difficult to enrole control and reflux patients, so we decided to stop the study after reaching 15 patients in the EoE group. By the time the 15th patient in the EoE had been diagnosed, we had already enrolled other patients, so the total final number of EoE patients was 17.
Data analysis
Data was analyzed as previously described (8-10) using a Synectics software package (Multigram Version 5.01 C2).
pH-metry
The pH parameters analyzed consisted of total % of time of acid exposure, total # of acid reflux episodes, duration of longest acid reflux episode, and # of prolonged (>5 min) acid reflux episode.
Stationary esophagel manometry
Upper esophageal sphincter (UES) pressure, upper esophageal contraction amplitude and duration, lower esophageal sphincter (LES) pressure, lower esophageal contraction duration and % of normal swallows during at least ten wet swallows were analyzed. Amplitude > 95% was defined as > 180 mmHg
PEMP
Motility variables for the PEMP were reported for the transducer in both the lower and upper esophageal body (8, 9). Variables studied included: # of esophageal contractions, frequency of esophageal body contractions per minute, median contraction amplitude and duration, % of multi-peak contractions, % of contractions >25 mm Hg, % of contractions >180 mm Hg, % of contractions >7 s in duration, % of peristaltic, simultaneous, and isolated contractions.
Peristalsis.- Analysis of the efficacy of the contractions and peristaltic sequences in all three transducers were also included (8, 9). Adequate amplitude was defined as amplitude of a wave > 40 mm Hg in the distal most channel. Contractions were defined as peristaltic between 2 adjacent recording sites if the onset of contraction at the distal site occurred 0.3-8.0 seconds after the onset of contraction at the proximal site. Intervals shorter than 0.3 sec between contractions at adjacent sites were considered to represent simultaneous contractions. Each peristaltic sequence was further subdivided in the following 3 groups: 1) Complete sequences .- contractions occurring in all channels, 2) Dropped sequences .- when a contraction was missing in the distal channel, or 3) Interrupted sequences.- when a contraction was missing in the proximal or middle channel (8-10).. Mechanical effectiveness of the peristaltic sequences was categorized as 1.) mechanically effective if both complete peristalsis and adequate amplitude were achieved, 2.) possibly effective if complete peristalsis without adequate amplitude was achieved, or 3.) ineffective if neither complete peristalsis nor adequate amplitude were achieved. (8-10). The peristaltic analysis was performed for total, meal, upright, and supine periods. (8-10).
Motility and pH measurements were analyzed at the time of each symptom. A positive correlation was classified if the motility abnormality occurred within a margin of 30 seconds before or after the symptom
A blinded investigator (SN) unaware of the patients’ diagnosis analyzed all manometric findings. Statistical analysis was performed using SPSS ® (Chicago, Il). Qualitative values are expressed as mean ± sd. Comparisons of proportions were made with Chi square. The Kruskal Wallace rank sum test was used to compare nonparametric data from the three groups. Significance was defined as a P <0.05. Bonferroni correction was used for multiple comparisons.
Results
Seventeen patients with EoE, 11 control patients and 13 patients with GERD were included. There were no statistically significant differences in clinical characteristics between patients with EoE and those with GERD (Table 1). Dysphagia was present in all patients with EoE; 7/17 (41.2%) had dysphagia while swallowing solids, 5/17 (31.3%) had dysphagia at every meal and 1/17 (5.9%) had dysphagia to both liquids and solids. Intermittent occasional dysphagia was also described in 54% of patients with GERD. All EoE had evidence of microscopic esophagitis. In the GERD group 5/13 had normal biopsies and (8/13) had esophagitis. All control patients had normal biopsies..
Table 1. Patient characteristics.
There were significant differences when comparing the controls with the EoE group, but no differences between EoE and GERD patients.
| Eosinophilic Esophagitis | GERD | Controls | |
|---|---|---|---|
| Number of patients | 17 | 13 | 11 |
| Gender (Female) (*) | 3/17 (18 %) | 4/13 (31%) | 7/11 (63%) |
| Age (years) | 9.7 ± 1.1 | 13.5 ± 1.3 | 12.7 ± 1.6 |
| Dysphagia (*) | 17/17 (100%) | 7/13 (54%) | 0/7 (0%) |
| Odynophagia | 0/17 (0%) | 1/13 (8%) | 0/11 (0%) |
| Food impaction | 5/17 (30%) | 3/13 (23%) | 0/7 (0%) |
| Chest pain | 4/17 (24 %) | 7/13 (54%) | 4/11 (36%) |
| Chest pain with Meals | 4/17 (24 %) | 3/13 (23%) | 1/11 (9%) |
| Regurgitation of food | 5/17 (30%) | 2/13 (15%) | 3/11 (27%) |
| Vomiting | 10/17 (60%) | 5/13 (36%) | 5/11 (46%) |
| Wheezing | 2/17 (12%) | 0/13 (0%) | 0/11 (0%) |
| Heartburn | 4/17 (23%) | 4/13 (31%) | 3/11 (27%) |
p<0.05. Values are expressed as mean + S.E.
1.- pH monitoring results
No differences were identified when comparing the pH values between control and EoE patients, whereas GERD patients showed significantly more reflux as compared with EoE and controls. (Table 2).
Table 2.
24 hour pH monitoring and stationary manometry results
| Eosinophilic Esophagitis | GERD | Controls | |
|---|---|---|---|
| Number of patients | 17 | 13 | 11 |
| Duration of prolonged study (hrs) | 21.6 ± 0.6 | 20.0 ± 1.7 | 21.3 ± 1.1 |
| Esophagitis in distal esophagus | 17 | 7/13 | 0 |
| Esophagits in proximal esophagus | 16/17 | 2/7 | 0 |
| pH-metry | |||
| % total time with pH < 4 (*) | 1.6 ± 0.4 | 12.4 ± 1.8 | 3.9 ± 1.4 |
| Total acid episodes > 5 min (*) | 0.7 ± 0.2 | 5.1 ± 0.7 | 0.6 ± 0.4 |
| Longest episode of acid reflux (*) | 5.4 ± 1.2 | 34.4 ± 10.5 | 3.8 ± 0.8 |
| Total number of reflux episodes (*) | 27.6 ± 6.1 | 139.6 ± 25.0 | 40.8 ± 8.6 |
| Stationary Esophageal manometry | |||
| UES pressure mmHg | 112 ± 8.0 | 133.0 ± 7.8 | 116.2 ± 9.6 |
| LES pressure mmHg | 23.7 ± 7.0 | 24.2 ± 2.11 | 19 ± 6.2 |
| Amplitude of lower esophageal contractions (mmHg) | 81 ± 7.0 | 96.7 ± 6.6 | 94.1 ± 3.3 |
| % normal swallows | 70.1 ± 6.5 | 97.3 ± 1.4 | 97.8 ± 2.2 |
| Abnormal peristalsis (>3 abnormal swallows) | 7/17 (41%) | 0/13 (0%) | 0/11 (0%)* |
| Abnormal amplitude (< 40 mmHg) | 1/17 (5.9%) | 0/13 (0%) | 0/11 (0%) |
| Number of patients with abnormal stationary motility | 7/17 (41%) | 0/13 (0%) | 0/13 (0%)* |
p < 0.01 between 3 groups. During subgroup comparison there were significant differences when comparing controls and EE , or GERD and EE. There were no differences when comparing GERD and controls. UES.- Upper esophageal sphincter; LES: Lower esophageal sphincter. Values are expressed as mean + S.E.
2.- Esophageal stationary manometry
Measurements were normal in all control and GERD patients (Table 2) All patients with EoE had normal LES pressure and function. (Table 2) but abnormal peristalsis was seen in 41%. The percentages of abnormal peristalsis, abnormal amplitude and overall abnormal stationary motility were significantly increased in children with EoE compared to those with GERD and controls. (p<0.01).
3.- Prolonged Esophageal Manometry with pH-metry
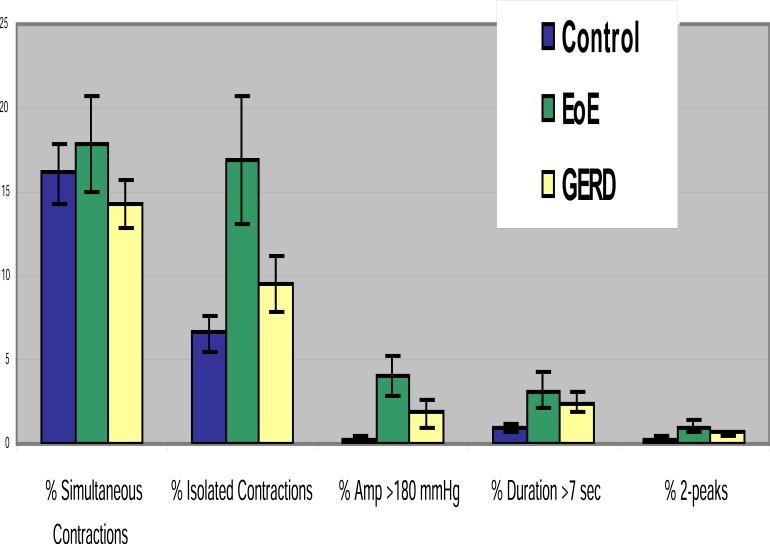
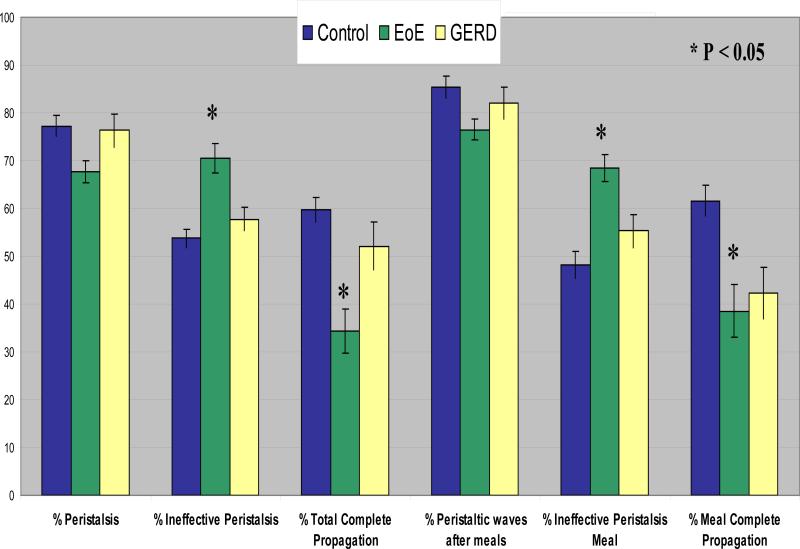
PEMP measurements were normal in all controls and GERD patients (1, 2, 4). Children with EoE showed nonspecific motility abnormalities compared to those with GERD and controls. In addition, children with EoE showed an increase number of isolated and high amplitude contractions in the distal esophagus, significantly fewer numbers of complete peristaltic waves, and more ineffective peristalsis both during fasting and during meals. (Figures 1-4). No other differences were observed. The % isolated contractions in the upper esophagus was 7.8 ± 2.3, in the controls, 9.9 ± 2.3 in GERD and 6.5 ± 1.2 in EoE. The mean amplitude of contractions in the upper esophagus was 44.8 ± 4.2 mmHg in controls, 45.2 ± 4.6 mmHg in GERD, and 43.9 ± 4.8 mmHg in EoE.
Figure 1. Major findings during the PEMP.
Patients with EoE had significantly higher number of isolated and high amplitude contractions. (* p< 0.03)
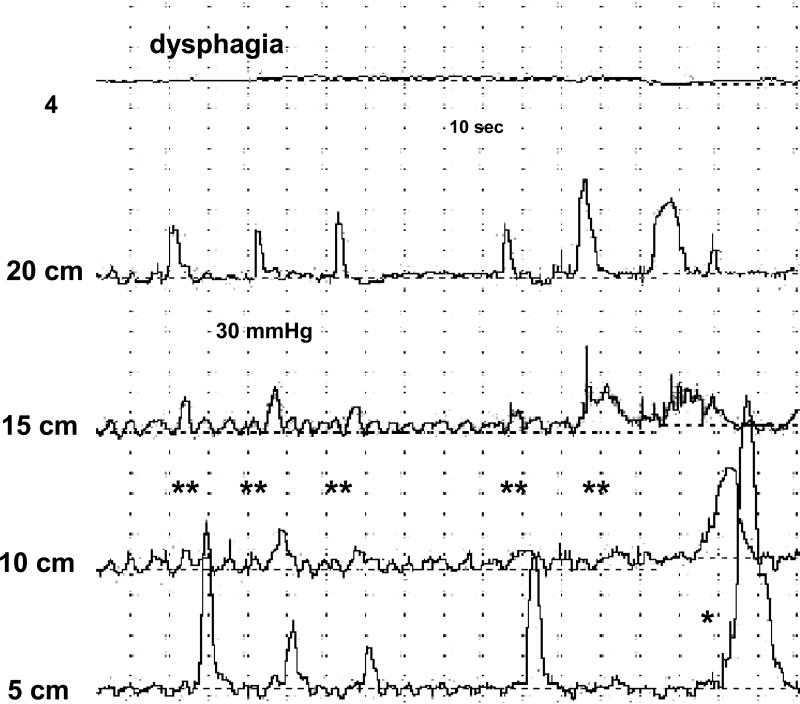
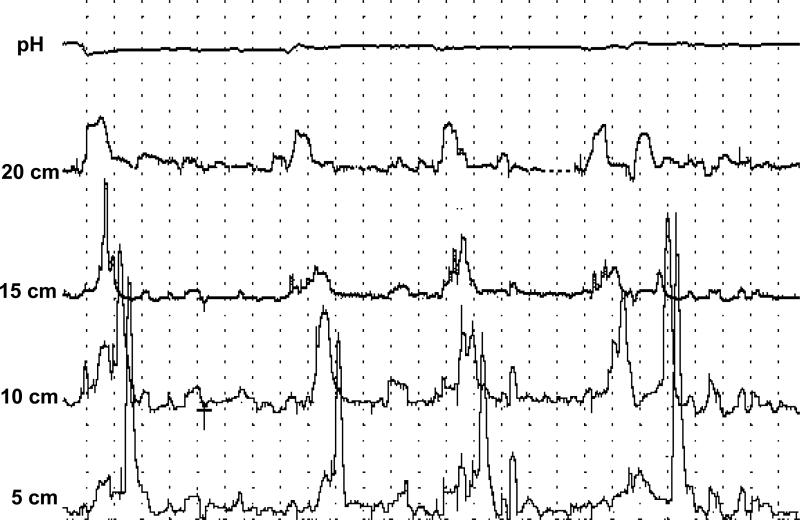
Figure 4.
Tracing from a PEMP in a patient with EoE showing ineffective peristalsis during an episode of dysphagia (**). A high amplitude contraction in the distal esophagus can also be appreciated (*). The first channel shows the distal intra-esophageal pH recording. The last 4 channels reflect esophageal pressure measurements at different levels above the lower esophageal sphincter.
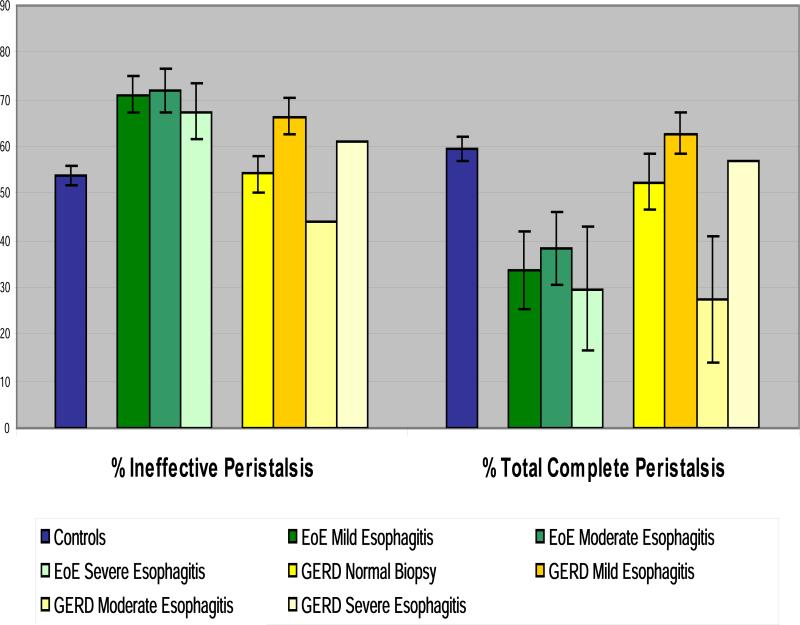
The manometric tracings were then analyzed comparing patients by group and by degree of esophagitis. As can be seen in Figure 5 there was no difference in the % of ineffective peristalsis when controlling for the degree of esophagitis.
Figure 5. Peristaltic abnormalities according to the patient group and degree of esophagitis.
Theree was no significant difference in the % of abnormalities within groups when comparing the different degrees of esophagitis.
Thirteen patients with EoE experienced 21 episodes of dysphagia during PEMP and all episodes correlated with abnormal motor function. Non-peristaltic contractions (Figure 4) occurred during 90% of the episodes, isolated and repetitive contractions in 90%, amplitude > 180 mmHg in 70%, abnormal peaked contractions in 41%, reflux events < 5 minutes in 29.5% and reflux events > 5 min in 7%. None of the GERD patients had dysphagia during testing.
All EoE patients with abnormal stationary manometry also had abnormalities in PEMP. The patients with EoE that had a normal stationary manometry also had abnormalities picked up during PEMP. No patients in the control or GERD group had abnormalities either in stationary manometry or the PEMP.
Discussion
Dysphagia accounts for the majority of complaints in adults and adolescents with EoE but its genesis and pathophysiology are not well understood (1, 2, 6, 13). In some patients, dysphagia may be explained by structural changes, such as strictures and rings, that result from tissue remodeling observed in diseases characterized by chronic eosinophilia (1, 2, 4). Yet in many patients no underlying anatomic lesions exist and the exact reason dysphagia develops is unclear. An alternative explanation is that some patients with EoE develop motor dysfunction as a result of eosinophilic inflammation, a hypothesis that has been addressed by a limited number of studies using mainly stationary manometry, a technique that is limited by the small number of wet swallows (1, 2, 6, 13). In patients with EoE, dysphagia is often intermittent, and rarely occurs during stationary manometry studies. As a result of this limited time of analysis, several features unique to EoE have likely been missed. Thus, information pertinent toward this relatively new disease may escape capture with stationary manometry and subtle motor abnormalities responsible for symptoms remain unrecorded (1, 2, 6, 13).
The first finding of our study is that PEMP was able to measure the temporal occurrence of children's symptoms, during meals and other daily activities, in association with esophageal dysfunction. With the use of PEMP, we have shown that children with EoE have ineffective peristalsis (particularly during meals), high amplitude contractions and increased number in isolated contractions, as compared with normal controls and GERD patients. Second, and most importantly, we documented that dysphagia correlated with these esophageal motor abnormalities. Taken together, these findings provide support for a role of esophageal motor dysfunction in the pathogenesis of dysphagia in children with EoE. Finally, stationary manometry measurements obtained in our study differ from those previously reported in children with EoE. Cheung et al showed that 11 children with EoE had normal esophageal manometry (7) whereas in our study, we found that 41% of children with EoE showed abnormal stationary manometry with non-specific abnormalities, suggesting that esophageal motor abnormalities may be more common than previously suspected. . Our data are more consistent with that seen in adults with EoE who showed abnormal stationary esophageal manometry in up to 35% to 53% of adults (1, 5, 6). Similar to most adult studies, all of our patients had normal LES function, but we did not identify any evidence of primary esophageal motor abnormalities (like achalasia or diffuse esophageal spasm) (1, 5, 6). This may reflect the fact that the duration of disease may influence in the type of motility abnormalities seen, and different phases in the development of esophageal motor abnormalities in EoE may exist (5, 6). While motility is initially normal, developing patterns consisting initially of hyperperistaltic or spastic abnormalities as seen here in children, may eventually evolve into abnormal peristalsis with low amplitude simultaneous contractions (5, 6, 13). This evolution has been observed in other disorders affecting esophageal motor function like GERD or achalasia (5, 6)
The exact genesis of the motility abnormalities seen in patients with EoE is not clear. It is possible that non-specific inflammation causes the motility abnormalities described here. To support this observation, two studies have shown that esophageal motor abnormalities associated with any form of esophagitis (GERD (14), EoE (6, 13)) disappeared after successful treatment. Alternatively, patients may have a primary motility disturbance, or abnormalities that occurred secondary to esophageal eosinophilic inflammation. We showed significant esophageal motor alterations in EoE patients as compared not only with controls, but also with GERD patients with esophagitis, and we did not find a relationship between the severity of the esophagitis and the manometric abnormalities, suggesting that the abnormal motility is not only related to mucosal inflammation, but may related to submucosal eosinophilic infiltration..Other reports suggest that motor abnormalities in EoE do not correlate with the degree of mucosal inflammation indicating that the problem may be related to deeper involvement of esophageal tissue (13). In fact, studies in which full thickness biopsies of adults with EoE documented eosinophilic infiltration in all esophageal layers (15, 16). In further support of this speculation, our previous study of children with EoE demonstrated significant esophageal wall expansion, with thickening of the total wall, mucosa, submucosa, and muscularis propria (16, 17). Alternatively, our findings may represent manifestations of tissue remodeling with associated fibrosis as shown in 2 previous studies that demonstrated increased trichrome staining of the esophageal subepithelial space of children with EoE. (4, 18) Future large prospective studies that control for the degree of inflammation and type of eosinophilic infiltration are needed to further establish if the motility abnormalities are a result of nonspecific inflammation, the degree and depth of the eosinophilic infiltration or are independent. (15, 16)
Mechanistic studies of esophageal motor function in EoE are lacking but in other systems, eosinophils affect motor function and nerve activation. For instance, eosinophil derived major basic protein (MBP) binds to muscarinic acetylcholine receptors (19) that are associated with smooth muscle contraction and subsequent dysmotility. Eosinophil derived TGF-beta leads to increased fibroblast contraction in vitro and eosinophil degranulation that has been associated with axonal necrosis which may produce abnormal motility patterns (16, 20). In contrast, deposition of extracellular MBP in esophageal epithelial surfaces did not correlate with the severity of the motility abnormalities in adults with EoE, (13), suggesting that other mechanisms may be important. Animal studies suggest other possible mechanisms. In a murine model of eosinophilic gastroenteritis, mucosal eosinophils lead to gastric dysmotility thru an eotaxin-1 dependent response (21). In animal models of esophagitis, the inflammatory cytokines IL1-beta and IL-6 inhibit acetylcholinesterase release and result in esophageal dysmotility (22). Finally, eosinophil deficient mice are protected from airway hyperreactivity, supporting a role for this cell in smooth muscle contraction. (23) Mast cells may also impact esophageal motor function. They are also present in higher numbers in patients with EoE and mast cell genes are upregulated in some patients with EoE (24) Activation of acetylcholine by histamine released from mast cells in the esophageal wall may therefore cause contraction of smooth muscle fibers in the muscularis mucosa resulting in uncoordinated contractions (25). Finally, several recent studies support the impact of eosinophil related inflammation on esophageal dysfunction. As mentioned above, Aceves et al and Chehade et al, demonstrated evidence of tissue remodeling with fibrosis in the esophageal tissues of children with EoE. (4, 18) In addition, Aceves et al, showed increases in TGF-beta and pSMAD activation supporting this pathway in the pathogenesis of fibrosis. (18)
When these basic findings are taken in the context of previous clinical studies, one could speculate that inflammation, particularly if it involves deeper esophageal layers, may lead to esophageal dysmotility and subsequent dysphagia or feeding difficulties. For instance, in well defined children with EoE, Sant'Anna et al reported dysphagia and food impaction were the most common associated feature. (26) In addition, a number of recent studies in adult and children with EoE have documented the presence of symptoms of dysphagia and feeding difficulties that may be associated with dysmotility. (27-33)
A limitation of the present study is that we did not repeat the PEMP after successful treatment to see if there was improvement on esophageal motor function. In a previous study, Lucendo et al described that in 7 patients with abnormal esophageal peristalsis on stationary manometry before treatment, the motility abnormalities and dysphagia improved after topical steroid treatment (13). Further prospective studies are needed to try to establish if the underlying manometric abnormalities are completely reversible after treatment. Another limitation is the fact we do not have any objective evidence that the motility abnormalities we found produce abnormal bolus transit. Studies in adults and children have shown that the manometric evidence of ineffective peristalsis may over state the clinical implication and that bolus clearance, as measured with impedance, may be a better indicator of esophageal motor function and may be a better tool to evaluate patients with dysphagia (6). Further studies to establish the relationship between esophageal transit and motor abnormalities in EoE patients are needed.
In conclusion, we have shown that children with EoE have esophageal motor abnormalities during both during stationary and prolonged esophageal manometry. Importantly, symptoms correlated with esophageal motor events, suggesting that the dysphagia present in children with EoE normal anatomy may be related to esophageal motior events.
Current knowledge.
Dysphagia is one of the main presenting symptoms in patents with eosinophilic esophagitis
The pathophysiology of the dysphagia is not understood
There is a lack of information on esophageal motor function in children with eosinophilic esophagitis
There is limited information about esophageal motor function in adults, and the studies are confined to standard esophageal manometry in which only 10-20 wet swallows are performed
What is new here.
We studied children with eosinophilic esophagitis using prolonged esophageal motility.
This technique allows the study of esophageal motor function over 24 hours, and during regular activities
We found manometric abnormalities in the eosinophilic esophagitis group that may explain the presence of dysphagia
Figure 2. Analysis of peristaltic waves during PEMP.
There were significant differences (p < 0.05 ) between EoE and the other two groups. There were no differences between controls and GERD.
Figure 3.
Tracing from a PEMP in a patient with EoE showing high amplitude contractions (*) in the distal esophagus. The first channel shows the distal intra-esophageal pH recording. The last 4 channels reflect esophageal pressure measurements at different levels above the lower esophageal sphincter.
Acknowledgments
This work was supported in part by the Pappas Foundation (SN, GF), grants DK77678-2 and DK082792-01 (SN) and the Campaign Urging Research for Eosinophilic Disorders (CURED) (GF).
Footnotes
There are no other financial disclosures
No conflict of interest
Dr Samuel Nurko is the guarantor of the submission
B. What is EACH AUTHOR'S contribution to the paper?
Conception, design and performance of the study: Samuel Nurko, Glenn T Furuta Monitoring of data acquisition, creation of data base and data cleaning: Samuel Nurko, Glenn T Furuta and Rachel Rosen
Analysis and interpretation: Samuel Nurko, Glenn T Furuta and Rachel Rosen
Critical revision of manuscript: Samuel Nurko, Glenn T Furuta and Rachel Rosen
All listed authors have seen and have approved the submitted manuscript. All authors take full responsibility for the content of the manuscript.
C. What financial support was received?
D. What may be the potential competing interests?
No competing interests
References
- 1.Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133(4):1342–63. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Liacouras CA, et al. Summary of the First International Gastrointestinal Eosinophil Research Symposium. Jour Pediatr Gastroenterol Nutr. 2007;45:370–91. doi: 10.1097/MPG.0b013e318142b4f8. [DOI] [PubMed] [Google Scholar]
- 3.Nurko S, Teitelbaum JE, Husain K, Buonomo C, Fox VL, Antonioli D, et al. Association of Schatzki Ring with Eosinophilic Esophagitis in Children. J Pediatr Gastroenterol Nutr. 2004;38:436–41. doi: 10.1097/00005176-200404000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Chehade M, Sampson HA, Morotti RA, Magid MS. Esophageal subepithelial fibrosis in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2007;45(3):319–28. doi: 10.1097/MPG.0b013e31806ab384. [DOI] [PubMed] [Google Scholar]
- 5.Lucendo AJ, Castillo P, Martin-Chavarri S, Carrion G, Pajares R, Pascual JM, et al. Manometric findings in adult eosinophilic oesophagitis: a study of 12 cases. Eur J Gastroenterol Hepatol. 2007;19(5):417–24. doi: 10.1097/MEG.0b013e328010bd69. [DOI] [PubMed] [Google Scholar]
- 6.Nurko S, Rosen R. Esophageal dysmotility in patients who have eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18(1):73–89. ix. doi: 10.1016/j.giec.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung KM, Oliver MR, Cameron DJ, Catto-Smith AG, Chow CW. Esophageal eosinophilia in children with dysphagia. J Pediatr Gastroenterol Nutr. 2003;37(4):498–503. doi: 10.1097/00005176-200310000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Stein HJ, DeMeester TR. Indications, technique, and clinical use of ambulatory 24-hour esophageal motility monitoring in a surgical practice. Annals of Surgery. 1993;217(2):128–37. doi: 10.1097/00000658-199302000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chitkara D, Fortunato C, Nurko S. Prolonged monitoring of esophageal motor function in healthy children. J Pediatr Gastroenterol Nutr. 2004;38:192–7. doi: 10.1097/00005176-200402000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Chitkara D, Fortunato C, Nurko S. Esophageal Motor Activity in Children with Gastroesophageal Reflux Disease and Esophagitis. J Pediatr Gastroenterol Nutr. 2005;40:70–5. doi: 10.1097/00005176-200501000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Rudolph CD, Mazur LJ, Liptak GS, et al. Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition. Jour Pediatr Gastroenterol Nutr. 2001;32(Suppl 2):s1–s31. doi: 10.1097/00005176-200100002-00001. [DOI] [PubMed] [Google Scholar]
- 12.Walsh SV, Antonioli DA, Goldman H, Fox VL, Bousvaros AB, Leichtner AM, et al. Allergic esophagitis in children; a clinicopathologic entitiy. Am J Surg Path. 1999;23:390–6. doi: 10.1097/00000478-199904000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Lucendo AJ, Pascual-Turrion JM, Navarro M, Comas C, Castillo P, Letran A, et al. Endoscopic, bioptic, and manometric findings in eosinophilic esophagitis before and after steroid therapy: a case series. Endoscopy. 2007;39(9):765–71. doi: 10.1055/s-2007-966738. [DOI] [PubMed] [Google Scholar]
- 14.Cucchiara S, Staiano A, Di Lorenzo C, D'Ambrosio R, Andreotti MR, Prato M, et al. Esopahgeal motor abnromalities in children with gastroesophageal relfux and peptic esophagitis. J Pediatr. 1986;198:907–10. doi: 10.1016/s0022-3476(86)80925-8. [DOI] [PubMed] [Google Scholar]
- 15.Evrard S, Louis H, Kahaleh M, Zalcman M, Nagy N, El Nakadi I, et al. Idiopathic eosinophilic oesophagitis: atypical presentation of a rare disease. Acta Gastroenterol Belg. 2004;67(2):232–5. [PubMed] [Google Scholar]
- 16.Stevoff C, Rao S, Parsons W, Kharilas P, Hirano I. EUS and histopathologic correlates in eosinophilic esophagits. Gastrintest Enosc. 2001;54:373–7. doi: 10.1067/mge.2001.116569. [DOI] [PubMed] [Google Scholar]
- 17.Fox VL, Nurko S, Teitelbaum JE, Badizadegan K, Furuta GT. High-resolution EUS in children with eosingophilic “allergic” esophagitis. Gastrointestinal Endoscopy. 2003;57(1):30–6. doi: 10.1067/mge.2003.33. [DOI] [PubMed] [Google Scholar]
- 18.Aceves SS, Newbury RO, Dohil R, Bastian JF, Broide DH. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119(1):206–12. doi: 10.1016/j.jaci.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Hogan SP, Mishra A, Brandt EB, et al. A pathological function for eotaxin and eosinophils in eosinophilic gastrointestinal inflammation. Nat Immunol. 2001;2:353–60. doi: 10.1038/86365. [DOI] [PubMed] [Google Scholar]
- 20.Dvorak AM, Onderdonk AB, McLeod RS, et al. Ultrastructural identification of exocytosis of granules from human gut eosinophils in vivo. Int Arch Allergy Immunol. 1993;102:33–45. doi: 10.1159/000236548. [DOI] [PubMed] [Google Scholar]
- 21.Martin ST, Collins CG, Fitzgibbon J, et al. Gastric motor dysfunction: is eosinophilic mural gastritis a causative factor?. Eur J Gastroenterol Hepatol. 2005;17:983–6. doi: 10.1097/00042737-200509000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Cao W, Cheng L, Behar J, et al. Proinflammatory cytokines alter/reduce esophageal circular muscle contraction in experimental cat esophagitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G–1131-9. doi: 10.1152/ajpgi.00216.2004. [DOI] [PubMed] [Google Scholar]
- 23.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O'Neill KR, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305(5691):1773–6. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 24.Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116(2):536–47. doi: 10.1172/JCI26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mann NS, Leung JW. Pathogenesis of esophageal rings in eosinophilic esophagitis. Med Hypotheses. 2005;64(3):520–3. doi: 10.1016/j.mehy.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 26.Sant'Anna AM, Rolland S, Fournet JC, Yazbeck S, Drouin E. Eosinophilic Esophagitis in Children: Symptoms, Histology and pH Probe Results. J Pediatr Gastroenterol Nutr. 2004;39(4):373–7. doi: 10.1097/00005176-200410000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Arora AS, Perrault J, Smyrk TC. Topical corticosteroid treatment of dysphagia due to eosinophilic esophagitis in adults. Mayo Clin Proc. 2003;78(7):830–5. doi: 10.4065/78.7.830. [DOI] [PubMed] [Google Scholar]
- 28.Attwood SE, Smyrk TC, DeMeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci. 1993;38:109–16. doi: 10.1007/BF01296781. [DOI] [PubMed] [Google Scholar]
- 29.Croese J, Fairley SK, Masson JW, Chong AK, Whitaker DA, Kanowski PA, et al. Clinical and endoscopic features of eosinophilic esophagitis in adults. Gastrointest Endosc. 2003;58:516–22. doi: 10.1067/s0016-5107(03)01870-4. [DOI] [PubMed] [Google Scholar]
- 30.Desai T, Stecevic V, Chang C, Goldstein N, Badizedegan K, Furuta G. Association of eosinophilic inflammation with esophageal food impaction in adults. Gastrointest Endosc. 2005;61:795–801. doi: 10.1016/s0016-5107(05)00313-5. [DOI] [PubMed] [Google Scholar]
- 31.Gonsalves N, Kahrilas PJ. Eosinophilic oesophagitis in adults. Neurogastroenterol Motil. 2009 doi: 10.1111/j.1365-2982.2009.01307.x. [DOI] [PubMed] [Google Scholar]
- 32.Pasha SF, DiBaise JK, Kim HJ, De Petris G, Crowell MD, Fleischer DE, et al. Patient characteristics, clinical, endoscopic, and histologic findings in adult eosinophilic esophagitis: a case series and systematic review of the medical literature. Dis Esophagus. 2007;20(4):311–9. doi: 10.1111/j.1442-2050.2007.00721.x. [DOI] [PubMed] [Google Scholar]
- 33.Spergel JM, Brown-Whitehorn TF, Beausoleil JL, Franciosi J, Shuker M, Verma R, et al. 14 years of eosinophilic esophagitis: clinical features and prognosis. J Pediatr Gastroenterol Nutr. 2009;48(1):30–6. doi: 10.1097/MPG.0b013e3181788282. [DOI] [PubMed] [Google Scholar]