Abstract
Maladaptive peripheral arterial remodeling, which leads to large arteries with low shear stress, may be associated with increased cardiovascular risk. We tested the hypothesis that arterial enlargement in severe obesity represents maladaptive remodeling and that weight reduction would reverse this process. We evaluated brachial arterial diameter and flow using ultrasound in 244 severely obese patients (age 44 ± 11 years, 80% female, body mass index (BMI) 46 ± 9 kg/m) at baseline and in a group of 67 subjects who experienced weight loss at 1 year. Higher BMI was associated with larger brachial artery diameter (p = 0.01) and lower shear stress (p = 0.008), indicating maladaptive remodeling. Significant (≥ 10%) weight reduction was associated with a decrease in resting arterial diameter (−0.19 ± 0.47 mm, p = 0.02) along with a trend toward increased shear stress. Decreased systemic inflammation was associated with weight loss-induced reverse remodeling of the brachial artery. Our findings demonstrate the presence of maladaptive arterial remodeling in advanced obesity that was ameliorated by significant weight loss.
Keywords: inflammation, obesity, remodeling, risk factors, ultrasonography, vascular diseases
Introduction
Obesity is associated with a pro-atherogenic vascular phenotype, premature atherosclerosis, and increased cardiovascular risk that is linked to the degree of excess fat mass burden.1,2 Significant weight loss reverses metabolic dysfunction and lowers the rate of cardiovascular events, in part, by improving vascular health.3
Arterial remodeling is an important mechanism for vascular adaptation in both normal and diseased states.4 Arterial structure changes in response to sustained alterations in blood flow.5 Experimental studies indicate that endothelial shear stress is the primary stimulus that initiates the remodeling process.6,7 Since vessel diameter relates inversely to shear stress, an adaptive increase in arterial dimension acts to conserve shear dynamics in the setting of increased flow. In contrast, maladaptive remodeling produces larger arteries with low shear stress, and chronic inflammation of the vascular wall may be associated with abnormal remodeling resulting in large arteries with reduced shear.8
Vascular remodeling in atherosclerosis may have components that are both adaptive and maladaptive. Luminal expansion at the location of a coronary plaque may initially represent a protective response to maintain distal perfusion. 9 However, growing evidence suggests that arterial enlargement may become disproportionate in disease conditions associated with excess inflammatory activation, leading to areas of low shear stress thereby promoting atherothrombosis. 10 In this regard, inflammatory burden and plaque vulnerability have been linked to excessive vessel expansion in coronary histopathological studies.11,12
Maladaptive arterial remodeling may also occur in the peripheral circulation. Risk factors including obesity have been associated with larger brachial and carotid diameters.13,14 Larger brachial arterial size is a marker for increased cardiovascular risk emphasizing the potential clinical relevance of peripheral arterial enlargement.15 We have shown previously that a number of cardiovascular risk factors including mild obesity are associated with adaptive changes.16 It remains unclear whether arterial remodeling remains adaptive into severe ranges of obesity. In addition, the effect of long-term intervention such as weight loss on arterial remodeling is not defined. Thus, the purpose of the present study was to characterize determinants of arterial remodeling in advanced obesity and the effect of weight loss on arterial characteristics.
Methods
Subjects
We prospectively enrolled 244 consecutive obese patients, body mass index (BMI) > 30 kg/m2, age > 18 years, from the Boston Medical Center Nutrition and Weight Management Center. Patients were excluded if they presented with active medical conditions including unstable coronary ischemia, heart failure, systemic infection, malignancy, or pregnancy. Subjects consisted of individuals receiving comprehensive dietary, medical, behavioral, or surgical treatments designed to promote weight loss. At the baseline visit, subjects had clinical, laboratory and vascular function assessments before any weight loss intervention. We have previously reported the vasodilator responses to hyperemia (flow-mediated dilation) and nitroglycerin in 204 of the current 244 subjects.17 Patients were offered to participate in a longitudinal study of vascular function. Of the 85 patients who completed 1 year of follow-up, 67 patients experienced weight loss. With the exception of LDL cholesterol level, there were no differences between the baseline clinical, metabolic or arterial characteristics of the overall study group and the 67 subjects who completed the 1-year longitudinal study. All subjects gave written, informed consent and the study was approved by the Boston Medical Center Institutional Review Board.
Vascular testing
Peripheral arterial structural parameters and flow were assessed using ultrasound imaging of the brachial artery in the fasting state as previously described.18 Subjects did not take vasoactive medications for at least 12 hours prior to the vascular study. Longitudinal B-mode images of the brachial artery and Doppler flow signals were acquired in the resting state using high-resolution ultrasonography (Toshiba Powervision 6000). Brachial artery diameter was also imaged 3 minutes after the administration of sublingual nitroglycerin (0.4 mg), a potent vasodilator agent. We omitted the nitroglycerin portion of the study if the subject declined, had a migraine headache history, systolic blood pressure < 100 mmHg, a previous adverse reaction to nitrates, or used phosphodiesterase type-5 medications. All images were digitized and the brachial artery diameter was measured using commercially available software (Medical Imaging Applications, LLC, Coralville, IA, USA).18 Baseline flow was expressed as flow velocity measured by Doppler ultrasound and as flow volume calculated from peak flow velocity and vessel cross-sectional area.19 Brachial artery shear stress was calculated as 8 μV/diameter, where μ is blood viscosity (assumed to be 0.035 dyne/sec/cm2) and V is brachial velocity at baseline.20
Metabolic testing
At each visit, subjects had a clinical assessment including measurement of blood pressure, heart rate, height, and weight and a fasting blood sample was collected. Total cholesterol, HDL cholesterol, triglycerides and glucose were measured using enzymatic methods. LDL cholesterol was calculated using the Friedewald formula. Insulin was measured using immunochemiluminometric methodology. In the subjects who participated in the longitudinal study, high sensitivity C-reactive protein was measured by enzyme linked immunoassay.
Statistical analysis
Analyses were completed using SPSS for Windows, version 12.1 (SPSS Inc.). Values are reported as mean ± SD, unless otherwise indicated. We assessed the relation of arterial dimension with degree of obesity. Clinical, biochemical and vascular measures were compared across tertiles of BMI using analysis of variance (ANOVA) evaluating for linear trend or chi-square test, as appropriate. We evaluated the correlates of resting brachial diameter and post-nitroglycerin brachial diameter and selected the clinical covariates related to either diameter measurement to include in a stepwise model. We examined stepwise selection (with age and sex forced in) to create multivariable models, with the criterion p < 0.05 for a variable to enter and stay in the model.
In the subjects with weight loss at the 1-year follow-up, linear regression was used to examine the correlation between the change in weight and the change in resting and post-nitroglycerin arterial diameter. To evaluate the effect of changes in weight on arterial dimensions, we classified the patients into two groups based on the degree of weight change at 1 year: significant weight loss defined as ≥ 10% weight loss (n = 40) or modest weight loss defined as < 10% weight loss (n = 27).21 The categorization using a cut point of 10% weight loss is consistent with guideline recommendations for initial target weight loss in obese adults.22 Clinical, biochemical and vascular measures were compared at baseline across the weight change categories by t-test or chi-square test, as appropriate. Paired t-tests were used to compare biochemical and vascular measures at the 12 months with the baseline visit. In the subjects with significant weight loss, we examined stepwise selection including covariates that changed significantly with weight loss to create multivariable models for resting brachial diameter, with the criterion p < 0.05 for a variable to enter and stay in the model.
Results
Cross-sectional study: relations of obesity to arterial dimensions
For the 244 patients (mean age 44 ± 11 years, 80% female, 48% ethnic/racial minority) who completed a baseline study visit, the average BMI was 46 ± 9 kg/m2 (range 30–86 kg/m2), total body weight 127 ± 30 kg (69–272 kg). Clinical characteristics displayed by tertiles of BMI are shown in Table 1. Subjects who were more obese were younger. Increasing obesity was associated with higher insulin levels.
Table 1.
Clinical and arterial characteristics by tertiles of BMI
| 1 | 2 | 3 | p | |
|---|---|---|---|---|
| BMI < 40.9 | 40.9 ≤ BMI ≤ 49.0 | BMI > 49.0 | ||
| n = 81 | n = 82 | n = 81 | ||
| Age, years | 46 ± 11 | 45 ± 11 | 42 ± 11 | 0.01 |
| Women, % | 83 | 84 | 73 | 0.14 |
| Race, % | 0.11 | |||
| White | 58 | 54 | 48 | – |
| Black | 25 | 38 | 28 | – |
| Hispanic | 17 | 8 | 24 | – |
| Diabetes, % | 28 | 33 | 32 | 0.77 |
| Hyperlipidemia, % | 44 | 39 | 32 | 0.32 |
| Hypertension, % | 36 | 48 | 54 | 0.07 |
| Smoking, % | 43 | 32 | 50 | 0.07 |
| Statin therapy, % | 25 | 21 | 17 | 0.39 |
| Systolic blood pressure, mmHg | 128 ± 14 | 134 ± 14 | 129 ± 15 | 0.89 |
| Diastolic blood pressure, mmHg | 73 ± 9 | 75 ± 11 | 70 ± 12 | 0.21 |
| Total cholesterol, mg/dl | 189 ± 38 | 194 ± 33 | 186 ± 39 | 0.61 |
| HDL cholesterol, mg/dl | 50 ± 12 | 47 ± 10 | 48 ± 16 | 0.44 |
| LDL cholesterol, mg/dl | 112 ± 31 | 116 ± 2 | 110 ± 3 | 0.70 |
| Triglycerides, mg/dl | 139 ± 79 | 150 ± 101 | 142 ± 88 | 0.85 |
| Glucose, mg/dl | 108 ± 39 | 102 ± 32 | 108 ± 31 | 0.96 |
| Insulin, μIU/ml | 16.0 ± 14.4 | 14.6 ± 9.9 | 20.9 ± 12.6 | 0.04 |
| Flow velocity, cm/sec | 23 ± 11 | 20 ± 8 | 20 ± 7 | 0.08 |
| Flow, ml/min | 172 ± 117 | 173 ± 94 | 179 ± 94 | 0.66 |
| Shear stress, dyne/cm2 | 16.4 ± 7.7 | 13.7 ± 5.6 | 13.6 ± 5.8 | 0.008 |
Mean ± SD; p for ANOVA linear trend or chi-square.
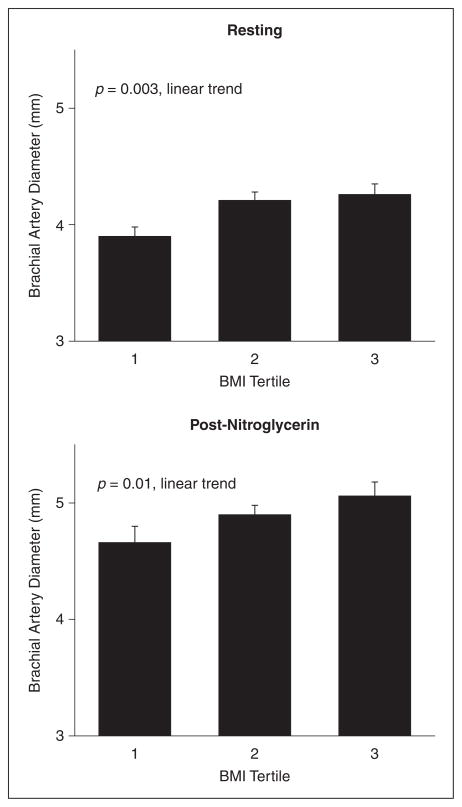
As shown in Figure 1, both resting and post-nitroglycerin arterial diameters were higher across the BMI categories. Resting and post-nitroglycerin arterial diameters were strongly associated with each other (r = 0.96, p < 0.0001). Additional brachial artery characteristics are displayed in Table 1. Shear stress was lower with a greater degree of obesity (r = −0.159, p = 0.01). An increasing severity of obesity remained associated with lower shear stress when adjusting for age, sex, and systolic blood pressure (data not shown).
Figure 1.
Relationship of brachial artery diameter and BMI. As shown, resting (n = 244) and post-nitroglycerin (n = 121) brachial artery diameters were larger with increasing BMI tertile. Values are mean ± standard error of the mean.
As displayed in Table 2, in unadjusted analyses, greater age, male sex, higher BMI, hyperlipidemia, hypertension, and higher systolic blood pressure were associated with a higher resting arterial diameter. Increasing HDL cholesterol was associated with smaller resting arterial diameter. In unadjusted analyses, male sex, higher BMI, and hypertension were associated with a higher post-nitroglycerin arterial diameter (Table 2). Higher total cholesterol, HDL cholesterol, and LDL cholesterol were associated with a smaller post-nitroglycerin arterial diameter.
Table 2.
Univariate correlates of brachial artery diameter Characteristic Baseline diameter Post-nitroglycerin diameter
| Characteristic | Baseline diameter
|
Post-nitroglycerin diameter
|
||
|---|---|---|---|---|
| r | p | r | p | |
| Age, years | 0.260 | < 0.001 | 0.176 | 0.05 |
| Sex, female vs male | −0.640 | < 0.001 | −0.659 | < 0.001 |
| Race, non-white vs white | 0.027 | 0.68 | −0.030 | 0.75 |
| BMI, kg/m2 | 0.137 | 0.03 | 0.183 | 0.04 |
| Diabetes | 0.017 | 0.80 | 0.023 | 0.81 |
| Hyperlipidemia | 0.165 | 0.01 | 0.022 | 0.81 |
| Hypertension | 0.294 | < 0.001 | 0.215 | 0.02 |
| Smoking | 0.074 | 0.26 | 0.141 | 0.13 |
| Statin use | 0.192 | < 0.01 | 0.138 | 0.13 |
| Systolic blood pressure, mmH | 0.132 | 0.04 | 0.001 | 0.99 |
| Diastolic blood pressure, mmHg | 0.12 | 0.05 | 0.098 | 0.29 |
| Total cholesterol, mg/dl | −0.044 | 0.51 | −0.218 | 0.02 |
| HDL cholesterol, mg/dl | −0.165 | 0.01 | −0.302 | 0.001 |
| LDL cholesterol, mg/dl | −0.032 | 0.63 | −0.241 | 0.009 |
| Triglycerides, mg/dl | 0.086 | 0.20 | 0.101 | 0.28 |
| Fasting glucose, mg/dl | 0.062 | 0.36 | 0.035 | 0.72 |
| Insulin | 0.052 | 0.50 | 0.110 | 0.30 |
Stepwise multivariable regression models for resting arterial diameter and post-nitroglycerin arterial diameter are shown in Table 3. In addition to age and male sex, higher BMI was associated with a higher resting arterial diameter in these multivariable models. In addition to age, male sex, lower HDL cholesterol, and lower LDL cholesterol, higher BMI was associated with a higher post-nitroglycerin arterial diameter. To evaluate a potential contribution of statin use to the observed relations to arterial diameter, we performed additional models for resting and post-nitroglycerin diameter with statin use as a covariate. Statin use was not associated with resting (partial r = 0.032, p = 0.62) or post-nitroglycerin diameter (partial r = −0.063, p = 0.52).
Table 3.
Multivariable models of determinants of brachial artery diameter
| Characteristic | Baseline diameter
|
Post-nitroglycerin diameter
|
||
|---|---|---|---|---|
| Partial r | p | Partial r | p | |
| Age, years | 0.302 | < 0.001 | 0.221 | 0.02 |
| Sex, female vs male | −0.638 | < 0.001 | −0.620 | < 0.001 |
| BMI, kg/m2 | 0.175 | 0.01 | 0.202 | 0.03 |
| HDL cholesterol | – | – | −0.210 | 0.03 |
| LDL cholesterol | – | – | −0.206 | 0.03 |
Age and sex were forced into all models. Variables included as covariates allowed to enter into stepwise regression models were: body mass index, hyperlipidemia, hypertension, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL cholesterol, LDL cholesterol.
Longitudinal study: effect of weight loss on arterial remodeling
Average weight loss over 1 year for this group was 24.5 ± 21.4 kg. At baseline, there were no significant differences in clinical, metabolic or arterial characteristics between the weight change groups (Table 4). All the patients who underwent gastric bypass surgery experienced significant weight loss (n = 24). Significant weight loss defined as ≥ 10% was associated with decreased triglyceride levels, glucose, insulin and C-reactive protein levels (Table 4).
Table 4.
Effect of weight loss on metabolic parameters and brachial artery characteristics
| Modest weight loss n = 27
|
Significant weight loss n = 40
|
|||
|---|---|---|---|---|
| Baseline | 1 year | Baseline | 1 year | |
| BMI, kg/m2 | 45 ± 9 | 43 ± 9a | 48 ± 9 | 34 ± 7a |
| Weight, kg | 127 ± 29 | 122 ± 29a | 131 ± 24 | 94 ± 17a |
| Systolic blood pressure, mmHg | 132 ± 18 | 127 ± 18 | 131 ± 14 | 130 ± 14 |
| Diastolic blood pressure, mmHg | 74 ± 13 | 74 ± 12 | 72 ± 11 | 74 ± 11 |
| Total cholesterol, mg/dl | 182 ± 28 | 181 ± 28 | 192 ± 34 | 182 ± 33 |
| HDL cholesterol, mg/dl | 46 ± 11 | 48 ± 11 | 52 ± 19 | 53 ± 14 |
| LDL cholesterol, mg/dl | 102 ± 30 | 107 ± 28 | 108 ± 28 | 106 ± 27 |
| Triglycerides, mg/dl | 158 ± 124 | 136 ± 86 | 161 ± 100 | 120 ± 70b |
| Glucose, mg/dl | 104 ± 36 | 104 ± 29 | 114 ± 43 | 99 ± 45b |
| Insulin, μIU/ml | 13.4 ± 10.1 | 13.5 ± 10.0 | 20.3 ± 16.9 | 7.5 ± 6.1a |
| hsCRP, mg/l | 13.6 ± 5.2 | 11.5 ± 5.2b | 10.3 ± 6.6 | 5.0 ± 5.5a |
| Baseline diameter, mm | 4.11 ± 0.86 | 4.19 ± 0.79 | 4.21 ± 0.71 | 4.02 ± 0.66c |
| Post-nitroglycerin diameter, mm | 4.76 ± 0.86 | 4.84 ± 0.92 | 5.14 ± 0.62 | 4.78 ± 0.60d |
| Baseline flow velocity, cm/sec | 19 ± 8 | 22 ± 9 | 21 ± 7 | 23 ± 12 |
| Baseline flow, ml/min | 166 ± 116 | 190 ± 117 | 174 ± 65 | 167 ± 81 |
| Baseline shear stress, dyne/cm2 | 13.5 ± 6.7 | 14.9 ± 6.2 | 14.4 ± 6.0 | 16.7 ± 11.1e |
Mean ± SD.
p < 0.001,
p < 0.05,
p = 0.01,
p < 0.01,
p = 0.08 compared to baseline. hsCRP; high-sensitivity C-reactive protein.
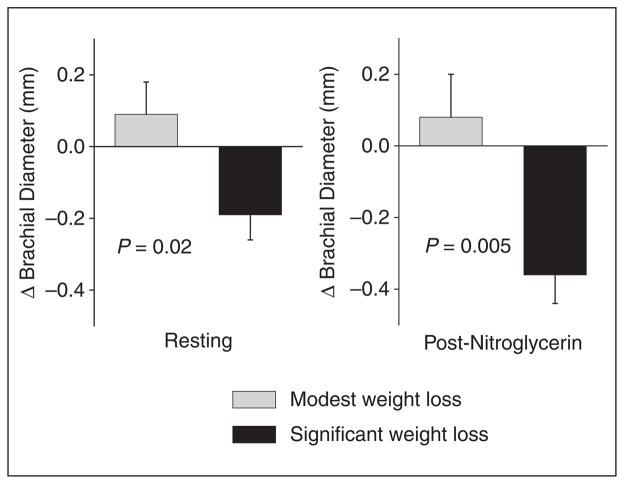
As shown in Table 4, subjects who had significant weight loss had a decrease in resting arterial diameter compared to baseline. Brachial artery blood flow did not change in these patients, and there was a trend for increased shear stress after 1 year of follow-up. The patients with only modest weight loss displayed no significant change in the brachial diameter, flow, or shear stress. As shown in Figure 2, the changes in resting arterial diameter and in post-nitroglycerin diameter compared to the baseline visit were significantly different between the two weight change groups.
Figure 2.
Effect of weight loss on brachial artery diameter. The change in brachial diameter is shown for subjects with modest weight loss and significant weight loss (n = 27 and 40 for resting diameter and n = 11 and 18 for post-nitroglycerin diameter, respectively). As shown, the change in brachial diameter in both the resting and post-nitroglycerin states differed between the categories of weight loss (p = 0.02, p = 0.005 by repeated measures ANOVA, respectively).
In the entire group, there was a significant correlation between weight loss and decreased resting arterial diameter (r = 0.399, p = 0.001) and decreased post-nitroglycerin arterial diameter change (r = 0.556, p = 0.002). In the subjects with significant weight loss, the relation between decrease in weight and decrease in resting arterial diameter persisted (r = 0.411, p = 0.008). In these subjects, the univariate correlates of resting artery diameter change among the metabolic parameters that changed with weight loss were: weight change (r = 0.411, p = 0.008), triglyceride change (r = 0.448, p = 0.005), insulin change (r = 0.398, p = 0.01), and C-reactive protein change (r = 0.592, p = 0.001). In a stepwise multivariable model selecting from these four covariates, the change in C-reactive protein was the only multivariable predictor of the change in resting artery diameter (partial r = 0.592, p = 0.001).
Discussion
In the present study of severely obese patients, we demonstrated expansive arterial remodeling with rising obesity when adjusting for clinical covariates. Larger arterial size was associated with lower shear stress, suggesting that maladaptive outward remodeling had occurred. In longitudinal follow-up, we observed that in subjects who lost > 10% initial body weight, these pathologic changes were reversed and correlated with reduced plasma C-reactive protein. Our findings suggest that structural arterial changes in severe obesity reflect a dysfunctional process that can be modulated by weight reduction strategies potentially through a decrease in systemic inflammation.
Prior work has suggested that obesity leads to systemic arterial enlargement. In community-based studies, higher BMI has been related to larger arterial diameter in the periphery.13,23 Overweight and modestly obese patients have been shown to have greater peripheral arterial size compared to lean controls.16,24,25 Since most of these prior studies examined structural changes in relatively mild degrees of obesity, the present study extends prior work by demonstrating that arterial enlargement continues into the extreme range of obesity. In addition, we demonstrated that the association between BMI and post-nitroglycerin arterial diameter persisted in multivariable models, providing evidence that the observed dimensional changes reflect structural remodeling and are not solely explained by fluctuations in vasomotor tone.
Growing evidence supports the notion that vascular morphology adapts to changing conditions in both healthy and diseased states. Adaptive remodeling facilitates coordination of blood flow with chronic tissue demands. A sustained increase in blood flow produces an increase in shear stress at the endothelial surface and arterial expansion in this setting tends to restore shear stress toward baseline level.4 We recently observed such a response when the ulnar artery is exposed to a chronic increase in blood flow following radial artery harvest in patients undergoing coronary artery bypass surgery.7 In the present study, the larger arteries observed in individuals with increasing obesity likely reflect, in part, a larger body size and higher tissue demands. While we did not directly measure arm volume, increasing arm size has been reported with increasing BMI.26 Thus, the brachial artery could enlarge in a purely adaptive manner to accommodate an increased requirement for blood flow to supply more tissue in the arm.
However, our finding of lower shear stress in individuals with the highest BMI in severely obese patients suggests that the extent of expansive remodeling is disproportionate to tissue demands and implies maladaptive remodeling. This result differs from our prior study that showed no difference in shear stress in moderately obese individuals compared to normal weight individuals.16 These apparently discrepant findings likely reflect differences in the study populations of the two studies. The present study included individuals with severe obesity (mean BMI 46 kg/m2) while our prior study involved a cohort of normal and moderately obese individuals (mean BMI 28 kg/m2). Studies in coronary arteries using intravascular ultrasound have demonstrated an association between regions of low shear stress and plaque progression.8,27,28 Endothelial cells in culture display an atheroprotective phenotype when exposed to levels of shear stress > 15 dyne/cm2 and become activated and pro-atherogenic when exposed to shear stress levels < 4 dyne/cm2.29 We observed a mean shear stress of 16.4 dyne/cm2 in the lowest BMI tertile and 13.6 dyne/cm2 in the highest BMI tertile. The variability of shear stress across the cardiac cycle makes it difficult to determine whether the mean level of shear stress in the most severely obese subjects is sufficiently low to induce endothelial activation. Consistent with the notion that a larger peripheral artery size represents a clinically relevant pathologic process, a higher brachial diameter is associated with increased risk for the development of cardiovascular disease and events.15,30 Our findings indicate the presence of maladaptive remodeling in patients with severe obesity. Further studies are needed to determine whether adverse arterial structural changes contribute to the premature development of vascular disease in severe obesity.
In the longitudinal part of our study, we observed that weight reduction was associated with a parallel decrease in arterial diameter. Previous studies with weight loss interventions have not reported a significant change in brachial artery diameter.31–33 Our findings may be explained by the longer follow-up period and the greater degree of weight loss experienced by many participants in the current study. Similarly, previous investigators have reported improvements in systemic inflammation and adipokines only with a 10% or greater reduction in body weight.21 Consistent with prior studies, we did not observe a significant change in arterial dimensions in patients who had more modest degrees of weight loss. Importantly, we observed that significant weight loss was associated with a trend toward increased shear stress. If increased tissue demand was the sole explanation for larger arterial diameter in the more obese subjects, we would have expected blood flow to decrease and shear stress to remain relatively constant. Thus, our findings suggest that interventions that produce significant weight loss reverse the pathologic remodeling and tend to restore shear stress levels. Taken together, our findings in subjects treated with weight loss intervention strengthen the pathophysiological link between severe obesity and a reversible process resulting in maladaptive arterial remodeling.
Our findings also support a connection between reduced inflammation and arterial remodeling during weight loss. In experimental studies, vessel inflammation is critical in initiating remodeling as flow alterations activate nuclear factor-κB leading to inflammatory cell infiltration and downstream changes in arterial artchitecture.10,34 In human coronary arteries, expansive remodeling occurs in regions with high levels of macrophage infiltration and in patients with higher C-reactive protein levels.12,35 In the present study, we observed that the change in C-reactive protein was a predictor of change in arterial size, after adjusting for change in weight, lipids, and insulin. These findings support the possibility that chronic inflammation in obesity contributes to adverse expansive arterial remodeling and that the remodeling process can be reversed with the lowering of inflammation induced by weight reduction.
Study limitations
The present study has several limitations. We measured artery diameters using ultrasound of the brachial artery. This technique permits assessment of luminal dimensions but not accurate arterial wall measurements. While prior studies in the coronary and carotid arteries have evaluated both lumen and wall thickness, this is less relevant in the non-atherosclerotic brachial artery. We determined shear stress based on a formula using assumed values for viscosity without measuring hematocrit levels or accounting for the pulsatility of arterial flow. Previous studies have suggested that this is a reasonable approach in the brachial artery.20 We did not measure arm volume at baseline or with weight loss. It remains possible that inward remodeling that occurred with weight loss partly reflects adaptive remodeling to match reduced tissue demands. We observed an unexpected relation between higher LDL cholesterol and lower brachial diameter that was not fully explained by statin usage. Further studies would be needed to understand the effects of cholesterol on arterial remodeling. Our study has several strengths that counterbalance these limitations, including a wide range of levels of obesity, a diverse patient sample, and longitudinal observation with weight change.
Conclusions
In conclusion, the present study demonstrates the occurrence of expansive peripheral arterial remodeling in severe obesity that can be reversed by marked weight reduction. Advanced obesity was associated with lower arterial shear, suggesting a maladaptive vascular response that may play a role in abnormal flow dynamics, endothelial activation and pro-atherosclerotic mechanisms in obese individuals. In addition, favorable reverse remodeling with weight loss occurred in association with reduced C-reactive protein, supporting a role for proinflammatory mechanisms in the arterial remodeling process associated with obesity.
Acknowledgments
This work was supported by NIH grants HL074097, HL084213, and HL083269. Dr Hamburg is supported by the Boston University Medical Center Leadership Program in Vascular Medicine (K12 HL083781). Dr Vita is supported by NIH grants (HL083801, HL081587, and HL75795). Dr Gokce is supported by NIH grants HL074097 and HL084213.
Footnotes
Reprints and permission: sagepub.co.uk/journalsPermissions.nav
References
- 1.Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case–control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 2.McTigue K, Larson JC, Valoski A, et al. Mortality and cardiac and vascular outcomes in extremely obese women. JAMA. 2006;296:79–86. doi: 10.1001/jama.296.1.79. [DOI] [PubMed] [Google Scholar]
- 3.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 4.Ward MR, Pasterkamp G, Yeung AC, Borst C. Arterial remodeling. Mechanisms and clinical implications. Circulation. 2000;102:1186–1191. doi: 10.1161/01.cir.102.10.1186. [DOI] [PubMed] [Google Scholar]
- 5.Korshunov VA, Schwartz SM, Berk BC. Vascular remodeling: hemodynamic and biochemical mechanisms underlying Glagov’s phenomenon. Arterioscler Thromb Vasc Biol. 2007;27:1722–1728. doi: 10.1161/ATVBAHA.106.129254. [DOI] [PubMed] [Google Scholar]
- 6.Silver AE, Vita JA. Shear-stress-mediated arterial remodeling in atherosclerosis: too much of a good thing? Circulation. 2006;113:2787–2789. doi: 10.1161/CIRCULATIONAHA.106.634378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vita JA, Holbrook M, Palmisano J, et al. Flow-induced arterial remodeling relates to endothelial function in the human forearm. Circulation. 2008;117:3126–3133. doi: 10.1161/CIRCULATIONAHA.108.778472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 9.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 10.Pasterkamp G, Galis ZS, de Kleijn DP. Expansive arterial remodeling: location, location, location. Arterioscler Thromb Vasc Biol. 2004;24:650–657. doi: 10.1161/01.ATV.0000120376.09047.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varnava AM, Mills PG, Davies MJ. Relationship between coronary artery remodeling and plaque vulnerability. Circulation. 2002;105:939–943. doi: 10.1161/hc0802.104327. [DOI] [PubMed] [Google Scholar]
- 12.Burke AP, Kolodgie FD, Farb A, Weber D, Virmani R. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation. 2002;105:297–303. doi: 10.1161/hc0302.102610. [DOI] [PubMed] [Google Scholar]
- 13.Benjamin EJ, Larson MG, Keyes MJ, et al. Clinical correlates and heritability of endothelial function in the community: The Framingham Heart Study. Circulation. 2004;109:613–619. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- 14.Crouse JR, Goldbourt U, Evans G, et al. Arterial enlargement in the atherosclerosis risk in communities (ARIC) cohort. In vivo quantification of carotid arterial enlargement. The ARIC Investigators. Stroke. 1994;25:1354–1359. doi: 10.1161/01.str.25.7.1354. [DOI] [PubMed] [Google Scholar]
- 15.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 16.Chung WB, Hamburg NM, Holbrook M, et al. The brachial artery remodels to maintain local shear stress despite the presence of cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2009;29:606–612. doi: 10.1161/ATVBAHA.108.181495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arkin JM, Alsdorf R, Bigornia S, et al. Relation of cumulative weight burden to vascular endothelial dysfunction in obesity. Am J Cardiol. 2008;101:98–101. doi: 10.1016/j.amjcard.2007.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vita JA. Nitric oxide-dependent vasodilation in human subjects. Methods Enzymol. 2002;359:186–200. doi: 10.1016/s0076-6879(02)59183-7. [DOI] [PubMed] [Google Scholar]
- 19.McMackin CJ, Vita JA. Update on nitric oxide-dependent vasodilation in human subjects. Methods Enzymol. 2005;396:541–553. doi: 10.1016/S0076-6879(05)960-16-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell GF, Parise H, Vita JA, et al. Local shear stress and brachial artery flow-mediated dilation: The Framingham Heart Study. Hypertension. 2004;44:134–139. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 21.Madsen EL, Rissanen A, Bruun JM, et al. Weight loss larger than 10% is needed for general improvement of levels of circulating adiponectin and markers of inflammation in obese subjects: a 3-year weight loss study. Eur J Endocrinol. 2008;158:179–187. doi: 10.1530/EJE-07-0721. [DOI] [PubMed] [Google Scholar]
- 22.NIH. [accessed December 2009];Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. 1998 doi: 10.1093/ajcn/68.4.899. www.nhlbi.nih.gov/guidelines/obesity/ob_gdlns.htm. [DOI] [PubMed]
- 23.Patel AS, Mackey RH, Wildman RP, et al. Cardiovascular risk factors associated with enlarged diameter of the abdominal aortic and iliac arteries in healthy women. Atherosclerosis. 2005;178:311–317. doi: 10.1016/j.atherosclerosis.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 24.Olson TP, Schmitz KH, Leon AS, Dengel DR. Vascular structure and function in women: relationship with body mass index. Am J Prev Med. 2006;30:487–492. doi: 10.1016/j.amepre.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Wildman RP, Mehta V, Thompson T, Brockwell S, Sutton-Tyrrell K. Obesity is associated with larger arterial diameters in Caucasian and African-American young adults. Diabetes Care. 2004;27:2997–2999. doi: 10.2337/diacare.27.12.2997. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Gallagher D, Thornton JC, et al. Regional body volumes, BMI, waist circumference, and percentage fat in severely obese adults. Obesity (Silver Spring) 2007;15:2688–2698. doi: 10.1038/oby.2007.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoenhagen P, Ziada KM, Kapadia SR, Crowe TD, Nissen SE, Tuzcu EM. Extent and direction of arterial remodeling in stable versus unstable coronary syndromes: an intravascular ultrasound study. Circulation. 2000;101:598–603. doi: 10.1161/01.cir.101.6.598. [DOI] [PubMed] [Google Scholar]
- 28.Stone PH, Coskun AU, Kinlay S, et al. Effect of endothelial shear stress on the progression of coronary artery disease, vascular remodeling, and in-stent restenosis in humans: in vivo 6-month follow-up study. Circulation. 2003;108:438–444. doi: 10.1161/01.CIR.0000080882.35274.AD. [DOI] [PubMed] [Google Scholar]
- 29.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 30.Holubkov R, Karas RH, Pepine CJ, et al. Large brachial artery diameter is associated with angiographic coronary artery disease in women. Am Heart J. 2002;143:802–807. doi: 10.1067/mhj.2002.121735. [DOI] [PubMed] [Google Scholar]
- 31.Skilton MR, Sieveking DP, Harmer JA, et al. The effects of obesity and non-pharmacological weight loss on vascular and ventricular function and structure. Diabetes Obes Metab. 2008;10:874–884. doi: 10.1111/j.1463-1326.2007.00817.x. [DOI] [PubMed] [Google Scholar]
- 32.Raitakari M, Ilvonen T, Ahotupa M, et al. Weight reduction with very-low-caloric diet and endothelial function in over-weight adults: role of plasma glucose. Arterioscler Thromb Vasc Biol. 2004;24:124–128. doi: 10.1161/01.ATV.0000109749.11042.7c. [DOI] [PubMed] [Google Scholar]
- 33.Williams IL, Chowienczyk PJ, Wheatcroft SB, et al. Endothelial function and weight loss in obese humans. Obes Surg. 2005;15:1055–1060. doi: 10.1381/0960892054621134. [DOI] [PubMed] [Google Scholar]
- 34.Ivan E, Khatri JJ, Johnson C, et al. Expansive arterial remodeling is associated with increased neointimal macrophage foam cell content: the murine model of macrophage-rich carotid artery lesions. Circulation. 2002;105:2686–2691. doi: 10.1161/01.cir.0000016825.17448.11. [DOI] [PubMed] [Google Scholar]
- 35.Schoenhagen P, Tuzcu EM, Apperson-Hansen C, et al. Determinants of arterial wall remodeling during lipid-lowering therapy: serial intravascular ultrasound observations from the Reversal of Atherosclerosis with Aggressive Lipid Lowering Therapy (REVERSAL) trial. Circulation. 2006;113:2826–2834. doi: 10.1161/CIRCULATIONAHA.105.585703. [DOI] [PubMed] [Google Scholar]