Abstract
After translocation through the pleuristratified epithelium of the lower female genital tract, HIV-1 encounters potential target mononuclear cells in the lamina propria of the vagina and ectocervix. Here we show that each major type of genital mononuclear cell, including dendritic cells (DCs), macrophages and lymphocytes, are susceptible to HIV-1 in vitro. Among suspensions of vaginal and ectocervical mononuclear cells, DCs were the first cells to take up virus, containing GFP-tagged virions as early as 15 minutes after exposure. At 2 hr after exposure, DCs still contained the largest proportion of infected cells compared to lamina propria macrophages and lymphocytes from the same mucosal compartment. By 4 days, however, lymphocytes from both vaginal and ectocervical mucosa supported the highest level of HIV-1 replication. Genital macrophages from the same mucosal tissues also were permissive to HIV-1, in sharp contrast to intestinal macrophages, which we previously have shown do not support HIV-1 replication. Thus, among human vaginal and ectocervical mononuclear target cells, DCs are the first to take up HIV-1 and T cells support the most robust viral replication. Further characterization of the parameters of HIV-1 infection in genital mononuclear cells will enlarge our understanding of HIV-1 infection in the female genital tract.
Keywords: Dendritic cells, entry, genital, lymphocytes, macrophages, replication
Introduction
Despite remarkable scientific advancements during the past three decades, the AIDS pandemic continues to claim millions of lives, particularly in sub-Saharan Africa. In this region of Africa, more than 22 million people, nearly 60% of whom are women, are infected with HIV-1, causing 1.5 million deaths in 2007 alone 1. In the U.S., more than 25% of new HIV-1 infections occur in women, particularly young black and Hispanic women 2. The latter is especially alarming, as the prevalence of HIV-1 infection in some U.S. populations is comparable to that of some sub-Saharan countries. As pointed out by El-Sadr 2, the HIV-1 prevalence of 1 in 30 adults in Washington, D.C. exceeds the country-wide prevalence in Ethiopia, Nigeria and Rwanda. Consequently, the development of an effective HIV-1 vaccine and novel agents to prevent HIV-1 transmission remains an urgent and pressing need for both resource-limited nations and the U.S.
Worldwide, the heterosexual route is the predominant mode of HIV-1 transmission 3, 4, underscoring the need for measures to prevent the sexual transmission of HIV-1. To be effective, such measures must interrupt one or more of the early events in HIV-1 mucosal transmission and infection, including HIV-1 translocation across the epithelial barrier, entry into subepithelial target mononuclear cells, and mucosal and systemic dissemination. Although some antibodies and topically applied microbicides are reportedly capable of preventing HIV-1 infection in model systems 5–9, including SIV infection in macaques 10–16, successful microbicide intervention in humans has been limited or incomplete 17. In this connection, the recent detection of potent anti-HIV-1 activity in the cervicovaginal fluids of women not infected with HIV-1 18 suggests the possibility that endogenous anti-viral defense mechanisms in the female genital tract could be exploited for protective purposes. However, critical issues pertaining to HIV-1 infection in the female genital mucosa remain unresolved, including characterization of the initial target cells, parameters of local viral replication, pathways of viral dissemination, and the mechanism of R5 selection. Thus, greater understanding of the mechanisms involved in the earliest stages of HIV-1 infection, especially the biological parameters of establishing infection in genital mucosa, must be achieved in order to devise effective strategies for prevention.
HIV-1 Entry in the Female Genital Tract
In female genital mucosa, HIV-1 infection involves three major events: (a) Entry through the mucosal epithelium; (b) Infection and subsequent replication in subepithelial mononuclear cells; and (c) Delivery to lymph nodes to initiate systemic infection. Entry may occur via the vagina, ectocervix or endocervix. The epithelial architecture is variable in these regions. The epithelium of the vagina and ectocervix is composed of multi-layered, pluristratified epithelial cells that do not have polarized plasma membranes or tight junctions. In contrast, the epithelium of the endocervix is a single layer of polarized, columnar epithelial cells with a plasma membrane that is separated by tight junctions, dividing the epithelium into apical and basolateral domains. Key features of these two architecturally distinct epitheliae relevant to HIV-1 entry include (a) the polarity of the endocervical columnar epithelium, which influences processing and sorting in endosomal transcytosis, and (b) the “leakiness” of ectocervical and vaginal epithelium, which likely allows CD4+ T cells to migrate into the vaginal epithelium, as well as dendritic cells (DCs), which migrate into and then extend their processes between the epithelial cells. Thus, distinct tnaslocation processes may participate in HIV-1 entry in different regions of the genital tract.
The extensive surface area of the vagina and ectocervix, estimated to be 15 times greater than that of the endocervix 19, is the likely region in which HIV-1 enters the lower female genital mucosa. Several routes of entry may be involved in the translocation of virus across the non-keratinized squamous epithelium that lines these tissues. Free virus may enter at sites of physical abrasion from microtrauma incurred during intercourse, inflammatory lesions associated with vaginosis and cervicitis, and mucosal disruption from ulcer-inducing infections. Free virus also could penetrate between squamous cells of the stratified epithelial barrier, at least in the upper layer. Although transcytosis of virions through squamous epithelial cells has been suggested 19, classic transcytosis occurs in polarized, columnar epithelial cells rather than non-polarized, pluristratified squamous epithelium 20. The identification of CD4+ T cells in the human vaginal epithelium and documentation of HIV-1 penetration into lymphocytes in the epithelial sheets obtained by suction blister 21 suggest that CD4+ T cells also may be involved in HIV-1 entry into vaginal mucosal. Using the macaque model, earlier investigations identified CD4+ T cells in genital mucosa as important, possibly the predominant, target cell for productive SIV infection 22. In addition to CD4+ T cells, Hladik and colleagues 21 showed that HVI-1 rapidly penetrates intraepithelial CD1a+ Langerhans cells, which reside in the genital tract epithelium. Although Langerhans cells do not express DC-SIGN or CCR5, they may participate in early HIV-1 uptake, as shown in macaques inoculated intravaginally with SIV 23. The location of Langerhans cells in the upper layer of the stratified epithelium positions these cells for the uptake of free virions that have penetrated this region of the squamous epithelial barrier.
Dendritic cells also likey contribute to the array of cells potentially involved in HIV-1 entry into the vaginal and ectocervical mucosae. Dendritic cells efficiently capture, disseminate and transmit virus to mononuclear target cells without productively infecting the DCs themselves, a process termed trans-infection 5, 23–33. Our understanding of DCs in HIV-1 transmission is derived from studies of monocyte-derived DCs, blood DCs, Langerhans cells, and DCs in the SIV/rhesus macaque non-human primate model 23,25–28. However, DCs are a heterogeneous population of cells that display distinct phenotypes and functional profiles in different tissues and mucosal compartments 34,35, precluding simple extrapolation from the fore-mentioned DCs to human mucosal DCs. Due to the limited availability of human vaginal, rectal and intestinal mucosae, the tissues through which HIV-1 is acquired in the vast majority of infections, and the difficulty in isolating mucosal DCs, the role of mucosal DCs in HIV-1 entry and mucosal spread is poorly understood. Vaginal DCs consist of myeloid DCs, plasmacytoid DCs, and Langerhans cells. Langerhans cells have been shown to take up HIV-1 in human vaginal epithelial sheets, as discussed above 21, 36, and in human skin explants 37 and epidermal cells isolated from human skin 38. In addition, DC-SIGN+ cells from human rectal mucosa have been shown to bind and transfer HIV-1 to peripheral blood CD4+ T cells 39, and human intestinal DCs also rapidly take up HIV-1, transport the virus through the mucosa, and transmit virions in trans to peripheral blood and intestinal lymphocytes, as we recently showed 40. In contrast, surprisingly little is known about the role of DCs in HIV-1 transmission in the human vagina and ectocervix, but the role of these cells in HIV-1 entry is currently under investigation in several laboratories.
Kinetics of HIV-1 Uptake by Vaginal and Ectocervical Mononuclear Cells
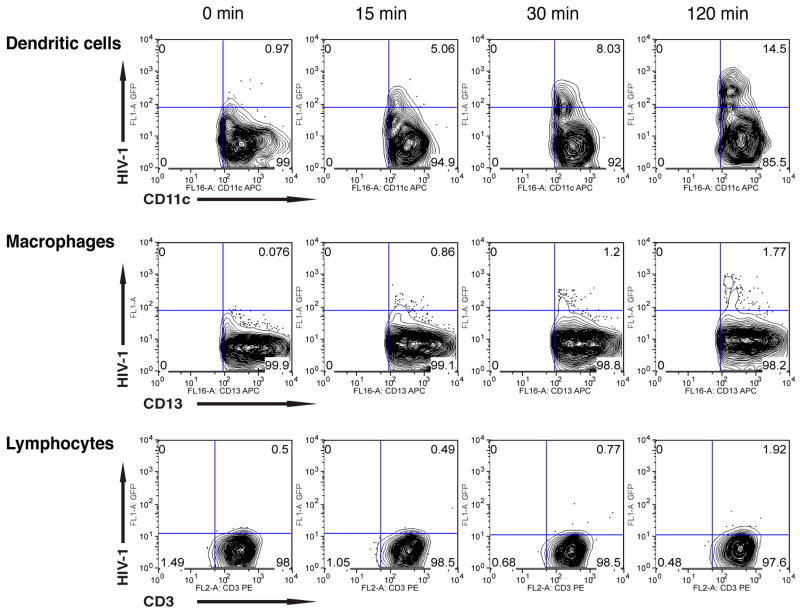
To begin to elucidate the early events in human cervicovaginal infection by HIV-1, we determined the kinetics of vaginal and ectocervical DC, macrophage and lymphocyte uptake of HIV-1. Vaginal and ectocervical mononuclear leukocytes (MNLs) were isolated using our previously described protocol 42 from human vaginal and ectocervical tissues provided by healthy women undergoing reconstructive pelvic surgery in accordance with Institutional Review Board approved protocols. To assure a high level of HIV-1 exposure, suspensions of 5×105 MNLs were inoculated with 3.75×108 GFP-tagged YU2 viral-like particles (VLPs) and cultured in RPMI plus 10% human AB serum at 37°C. YU2 envelope (Env)-pseudotyped GFP-Gag VLPs were produced by transfection of 293T cells, as previously described 42. The cultures were harvested 2 hr after inoculation and stained with CD13-APC, CD11c-APC or CD3-PE to identify DCs, macrophages and lymphocytes that contained HIV-1-GFP viral particles by flow cytometry. As shown in Figure 1, as early as 15 minutes after virus inoculation, 5.06% of the CD11c+ DCs contained HIV-1-GFP, which increased to 14.5% at 2 hr. In sharp contrast, vaginal macrophages first showed detectable virion uptake (1.2%) at 30 minutes post-inoculation, increasing to 1.77% at 2 hr, and vaginal lymphocytes first displayed detectable HIV-1 uptake at 2 hr, when 1.92% of the cells contained virions.
Figure 1.
Vaginal macrophages, dendritic cells and lymphocytes uptake HIV-1 in isolated mucosal mononuclear cells. Cultures of mononuclear cells isolated from normal human vaginal tissue were inoculated with GFP-tagged YU2 and incubated at 37°C for 2 hrs. Cells then were analyzed by flow cytometry using anti-CD13, anti-CD11c or anti-CD3 antibodies. Results are representative of cells isolated from three separate donors.
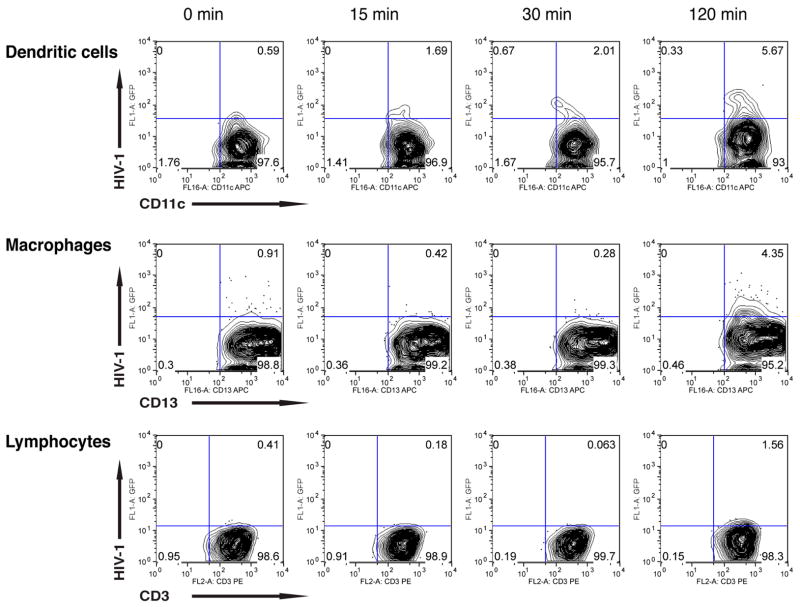
Cultures of ectocervical MNLs were inoculated in parallel to the vaginal MNLs and also examined for uptake of GFP-tagged viral particles by flow cytometry. Again, DCs took up virions at 15 minutes (1.69%), increasing to 5.67% at 2 hr, whereas macrophages and lymphocytes first displayed detectable HIV-1 uptake at 2 hr, the proportion of macrophages increasing from 0.91% to 4.35% and lymphocytes from 0.41% to 1.56%, respectively (Figure 2). Thus, among vaginal and ectocervical MNLs, DCs were the first cells to take up HIV-1, containing virus at 15 minutes. In sharp contrast, vaginal and ectocervical macrophages first contained detectable HIV-1 at 30 minutes and 2 hr, respectively. Vaginal macrophages and lymphocytes contained similar proportions of infected cells at 2 hr, whereas the proportion of infected ectocervical macrophages was two-fold more than that of infected lymphocytes.
Figure 2.
Ectocervical macrophages, dendritic cells and lymphocytes uptake HIV-1 in isolated mucosal mononuclear cells. Ectocervical mononuclear cells were isolated from normal human ectocervix tissue, exposed to GFP-tagged YU2, and analyzed at 2 hrs post-exposure by flow cytometry using anti-CD13, anti-CD11c or anti-CD3 antibodies. Results are representative of cells isolated from two separate donors.
Vaginal and Ectocervical Macrophages and Lymphocytes Support HIV-1 Replication
The female genital tract mucosa is a site of HIV-1 entry but replication in vaginal and ectocervical mucosal cells has been difficult to document. Zhang et al. 22 showed that in sexually transmitted SIV infection and in early and later stages of HIV-1 infection, the predominant target cells in the genital tract are resting and activated CD4+ T cells. Using an organ culture system, Gupta et al. 41 corroborated these results by showing that memory CD4+ T cells are the earliest HIV-1-infected cells in the cervix. In contrast to these findings, Greenhead et al. 42 identified macrophages by immunohistochemical analysis as the dominant HIV-1 target cell in the vaginal lamina propria. As noted above, CD4+ T cells may participate in HIV-1 transport across the epithelium. We have reported the presence of low numbers of CD3+ lymphocytes in the basal region and bordering the dermal papillae in the epithelium and scattered throughout the lamina propria of non-inflamed human vaginal mucosa 43. These cells supported HIV-1 replication, as did the vaginal macrophages 43. We next extended these findings by comparing HIV-1 replication in human vaginal and ectocervical macrophages and lymphocytes.
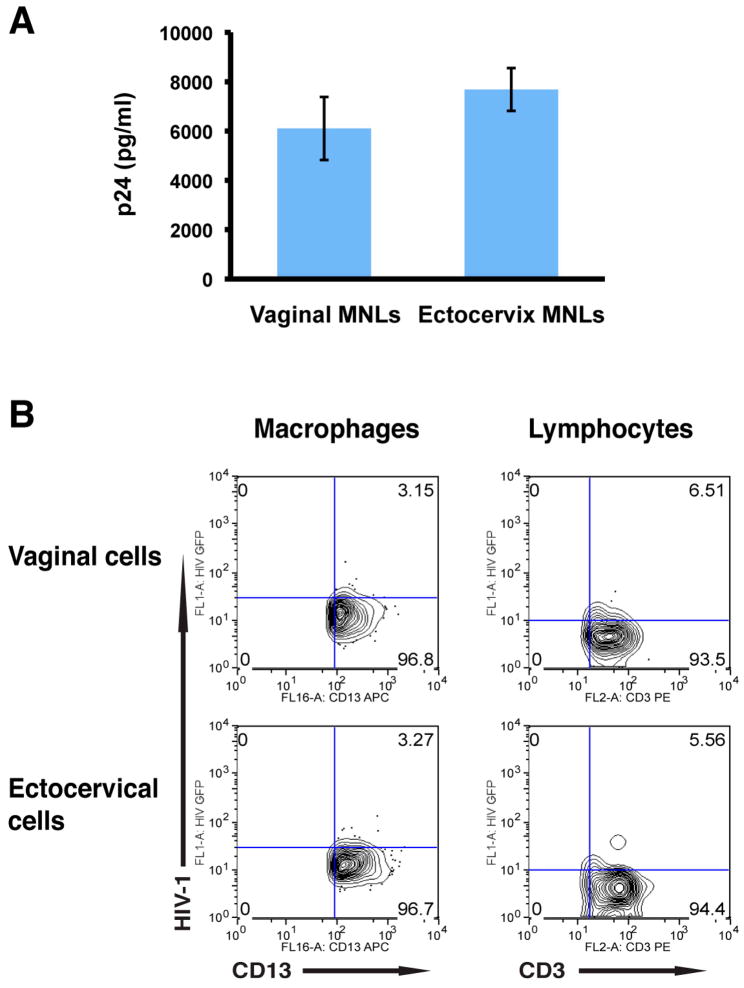
To determine whether HIV-1 uptake was associated with viral replication, suspensions of vaginal and ectocervical MNLs were inoculated with infectious R5 (YU2) HIV-1 (MOI=1), cultured under standard conditions, and on day 4 culture supernatants were harvested and analyzed for p24 by ELISA. As shown in Figure 3A, both vaginal and ectocervical MNLs released p24, confirming productive viral infection. To identify the cells that supported HIV-1 replication, suspensions of vaginal and ectocervical MNLs were inoculated with GFP-expressing YU2 (MOI=1), cultured for 4 days, and analyzed by FACS. As shown in Figure 3B, vaginal as well as ectocervical macrophages and lymphocytes supported HIV-1 replication. Macrophages from vaginal and ectocervical mucosa were similar in their capacity to support infection, as were lymphocytes from vaginal and ectocervical mucosa. However, when lymphocytes were compared to macrophages from the same mucosal compartment, lymphocytes supported more robust replication than the macrophages.
Figure 3.
Vaginal and ectocervical macrophages and lymphocytes support HIV-1 replication. (A) Vaginal and ectocervical MNLs were inoculated with YU2 and 4 days later culture supernatants were harvested and analyzed for p24. (B) Parallel cultures were inoculated with YU-2env pseudotyped GFP-expressing HIV-1, and 4 days post-infection cells were analyzed by flow cytometry to identify the cells that support HIV-1 replication.
Conclusions
HIV-1 infection of the female genital tract mucosa involves translocation of virus across the epithelium, infection and replication in subepithelial mononuclear cells, and local and systemic dissemination. After translocation across the epithelium by CD4+ T cells, Langerhans cells and/or DCs, virus encounters mononuclear target cells in the subepithelial tissue. Vaginal and ectocervical DCs appear to be the first cells to take up virus and do so more efficiently than macrophages or lymphocytes within the first 2 hr of virus exposure. Interestingly, by 4 days after infection, lymphocytes support more robust viral replication than macrophages in both mucosal compartments. Further characterization of the early events in HIV-1 infection of vaginal and ectocervical mucosa should provide important biological insights for devising effective strategies to prevent genital transmission of HIV-1.
Acknowledgments
This study was supported by the National Institutes of Health (DK-54495, DK-84063, AI-83027, AI-83539, DK-47322, RR-20136 and the Mucosal HIV and Immunobiology Center, DK-64400) and the Research Service of the Veterans Administration.
References
- 1.Joint United Nation’s program on HIV/AIDS (UNAIDS) 2006 report on the global AIDS epidemic. Geneva: UNAIDS; 2006. [Google Scholar]
- 2.El-Sadr WM, Mayer KH, Hodder SL. AIDS in America--forgotten but not gone. N Engl J Med. 2010;362:967–970. doi: 10.1056/NEJMp1000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Royce RA, Sena A, Cates W, Jr, Cohen MS. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 4.Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nature Reviews Microbiology. 2003;1:25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- 5.Hu Q, Frank I, Williams V, Santos JJ, Watts P, Griffin GE, Moore JP, Pope M, Shattock RJ. Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue. J Exp Med. 2004;199:1065–1075. doi: 10.1084/jem.20022212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawamura T, Cohen SS, Borris DL, Aquilino EA, Glushakova S, Margolis LB, Orenstein JM, Offord RE, Neurath AR, Blauvelt A. Candidate microbicides block HIV-1 infection of human immature Langerhans cells within epithelial tissue explants. J Exp Med. 2000;192:1491–1500. doi: 10.1084/jem.192.10.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bobardt MD, Chatterji U, Selvarajah S, Van der Schueren B, David G, Kahn B, Gallay PA. Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. J Virol. 2007;81:395–405. doi: 10.1128/JVI.01303-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fletcher PS, Elliott J, Grivel JC, Margolis L, Anton P, McGowan I, Shattock RJ. Ex vivo culture of human colorectal tissue for the evaluation of candidate microbicides. Aids. 2006;20:1237–1245. doi: 10.1097/01.aids.0000232230.96134.80. [DOI] [PubMed] [Google Scholar]
- 9.Shen R, Drelichman ER, Bimczok D, Ochsenbauer C, Kappes JC, Cannon JA, Tudor D, Bomsel M, Smythies LE, Smith PD. GP41-specific antibody blocks cell-free HIV type 1 transcytosis through human rectal mucosa and model colonic epithelium. J Immunol. 2010;184:3648–3655. doi: 10.4049/jimmunol.0903346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mascola JR, Frankel SS, Broliden K. HIV-1 entry at the mucosal surface: role of antibodies in protection. AIDS (London, England) 2000;14 (Suppl 3):S167–174. [PubMed] [Google Scholar]
- 11.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nature medicine. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 12.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho MW, Martin MA. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nature medicine. 1999;5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 13.Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini LA, Posner MR, Katinger H, Stiegler G, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Lu Y, Wright JE, Chou TC, Ruprecht RM. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nature medicine. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 14.Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, Ketas T, Marx PA, Klasse PJ, Burton DR, Moore JP. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nature medicine. 2003;9:343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 15.Ambrose Z, Compton L, Piatak M, Jr, Lu D, Alvord WG, Lubomirski MS, Hildreth JE, Lifson JD, Miller CJ, KewalRamani VN. Incomplete protection against simian immunodeficiency virus vaginal transmission in rhesus macaques by a topical antiviral agent revealed by repeat challenges. Journal of virology. 2008;82:6591–6599. doi: 10.1128/JVI.02730-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veazey RS, Klasse PJ, Schader SM, Hu Q, Ketas TJ, Lu M, Marx PA, Dufour J, Colonno RJ, Shattock RJ, Springer MS, Moore JP. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature. 2005;438:99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- 17.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh M, Fahey JV, Shen Z, Lahey T, Cu-Uvin S, Wu Z, Mayer K, Wright PF, Kappes JC, Ochsenbauer C, Wira CR. Anti-HIV activity in cervical-vaginal secretions from HIV-positive and -negative women correlate with innate antimicrobial levels and IgG antibodies. PLoS One. 2010;5:e11366. doi: 10.1371/journal.pone.0011366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hladik F, McElrath MJ. Setting the stage: host invasion by HIV. Nat Rev Immunol. 2008;8:447–457. doi: 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bomsel M, Alfsen A. Entry of viruses through the epithelial barrier: pathogenic trickery. Nature Reviews Molecular Cell Biology. 2003;4:57–68. doi: 10.1038/nrm1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hladik F, Sakchalathorn P, Ballweber L, Lentz G, Fialkow M, Eschenbach D, McElrath MJ. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26:257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, Reinhart TA, Rogan M, Cavert W, Miller CJ, Veazey RS, Notermans D, Little S, Danner SA, Richman DD, Havlir D, Wong J, Jordan HL, Schacker TW, Racz P, Tenner-Racz K, Letvin NL, Wolinsky S, Haase AT. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 23.Spira AI, Marx PA, Patterson BK, Mahoney J, Koup RA, Wolinsky SM, Ho DD. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med. 1996;183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pope M. Mucosal dendritic cells and immunodeficiency viruses. J Infect Dis. 1999;179:S427–S430. doi: 10.1086/314798. [DOI] [PubMed] [Google Scholar]
- 25.Teleshova N, Frank I, Pope M. Immunodeficiency virus exploitation of dendritic cells in the early steps of infection. Journal of leukocyte biology. 2003;74:683–690. doi: 10.1189/jlb.0403178. [DOI] [PubMed] [Google Scholar]
- 26.Piguet V, Steinman RM. The interaction of HIV with dendritic cells: outcomes and pathways. Trends in immunology. 2007;28:503–510. doi: 10.1016/j.it.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu L, KewalRamani VN. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol. 2006;6:859–868. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu J, Gardner MB, Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol. 2000;74:6087–6095. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cameron P, Pope M, Granelli-Piperno A, Steinman RM. Dendritic cells and the replication of HIV-1. Journal of Leukocyte Biology. 1996;59:158–171. doi: 10.1002/jlb.59.2.158. [DOI] [PubMed] [Google Scholar]
- 30.David SA, Smith MS, Lopez GJ, Adany I, Mukherjee S, Buch S, Goodenow MM, Narayan O. Selective transmission of R5-tropic HIV type 1 from dendritic cells to resting CD4+ T cells. AIDS Research and Human Retroviruses. 2001;17:59–68. doi: 10.1089/088922201750056799. [DOI] [PubMed] [Google Scholar]
- 31.Cameron PU, Freudenthal PS, Barker JM, Gezelter S, Inaba K, Steinman RM. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992;257:383–387. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- 32.Pope M, Betjes MGH, Romani N, Hirmand H, Cameron PU, Hoffman L, Gezelter S, Schuler G, Steinman RM. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 1994;78:389–398. doi: 10.1016/0092-8674(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 33.Hu J, Miller CJ, O’Doherty U, Marx PA, Pope M. The dendritic cell-T cell milieu of the lymphoid tissue of the tonsil provides a locale in which SIV can reside and propagate at chronic stages of infection. AIDS Research and Human Retroviruses. 1999;15:1305–1314. doi: 10.1089/088922299310205. [DOI] [PubMed] [Google Scholar]
- 34.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 35.Heath WR, Carbone FR. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat Immunol. 2009;10:1237–1244. doi: 10.1038/ni.1822. [DOI] [PubMed] [Google Scholar]
- 36.Kawamura T, Gulden FO, Sugaya M, McNamara DT, Borris DL, Lederman MM, Orenstein JM, Zimmerman PA, Blauvelt A. R5 HIV productively infects Langerhans cells, and infection levels are regulated by compound CCR5 polymorphisms. Proceedings of the National Academy of Science USA. 2003;100:8401–8406. doi: 10.1073/pnas.1432450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reece JC, Handley AJ, Anstee EJ, Morrison WA, Crowe SM, Cameron PU. HIV-1 selection by epidermal dendritic cells during transmission across human skin. J Exp Med. 1998;187:1623–1631. doi: 10.1084/jem.187.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blauvelt A, Glushakova S, Margolis LB. HIV-infected human Langerhans cells transmit infection to human lymphoid tissue ex vivo. AIDS (London, England) 2000;14:647–651. doi: 10.1097/00002030-200004140-00003. [DOI] [PubMed] [Google Scholar]
- 39.Gurney KB, Elliott J, Nassanian H, Song C, Soilleux E, McGowan I, Anton PA, Lee B. Binding and transfer of human immunodeficiency virus by DC-SIGN+ cells in human rectal mucosa. Journal of virology. 2005;79:5762–5773. doi: 10.1128/JVI.79.9.5762-5773.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen R, Smythies LE, Clements RH, Novak L, Smith PD. Dendritic cells transmit HIV-1 through human small intestinal mucosa. Journal of Leukocyte Biology. 2010;87:663–670. doi: 10.1189/jlb.0909605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta P, Collins KB, Ratner D, Watkins S, Naus GJ, Landers DV, Patterson BK. Memory CD4+ T cells are the earliest detectable human immunodeficiency virus type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. J Virol. 2002;76:9868–9876. doi: 10.1128/JVI.76.19.9868-9876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greenhead P, Hayes P, Watts PS, Laing KG, Griffin GE, Shattock RJ. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J Virol. 2000;74:5577–5586. doi: 10.1128/jvi.74.12.5577-5586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen R, Richter HE, Clements RH, Novak L, Huff K, Bimczok D, Sankaran-Walters S, Dandekar S, Clapham PR, Smythies LE, Smith PD. Macrophages in vaginal but not in intestinal mucosa are monocyte-like and permissive to HIV-1. J Virol. 2009;83:3258–3267. doi: 10.1128/JVI.01796-08. [DOI] [PMC free article] [PubMed] [Google Scholar]