Abstract
Purpose
The purpose of this study was to test the hypothesis that eating a meal reduces tongue strength and endurance in healthy old and young adults. It was predicted that older adults would show greater declines in tongue endurance, while demonstrating higher perceived effort, longer meal durations, and clinical signs of swallowing difficulty.
Methods
Twenty-two healthy adults were enrolled into two groups (ages 20-35 years & 65-82 years, each including 5M, 6F). Maximum tongue strength (Pmax) and endurance (duration 50% of Pmax could be maintained) were measured twice at baseline and once post-meal. Subjects consumed half of a bagel with peanut butter, carrot sticks and milk between measures.
Results
All subjects demonstrated reduced tongue strength and endurance post-meal. Young adults showed a greater decline in anterior tongue endurance compared with older adults (p=0.05). There was no evidence that changes in tongue strength, perceived effort or meal duration varied by age or gender. The three oldest subjects reported the highest effort and displayed signs of difficulty swallowing while dining.
Conclusions
Young and old adults demonstrated reduced tongue strength and endurance after dining, but younger subjects showed greater declines in anterior tongue endurance while older adults exhibited signs of swallowing difficulty.
Keywords: Deglutition, Tongue, Fatigue, Aging
Introduction
Fatigue is one of the most common chronic conditions reported by older adults (Poluri, Mores, Cook, Findley, & Cristian, 2005), with pervasive effects on activities of daily living including dining. Speech Language Pathologists who specialize in dysphagia treatment often clinically comment about the fatiguing effects of a meal on a patient’s ability to swallow safely. Frail adults at risk for or diagnosed with dysphagia regularly report that it takes them longer than others to eat and that swallowing is more difficult at the end of the day (Roy, Stemple, Merrill, & Thomas, 2007).
The tongue is the primary propulsive agent to accomplish oropharyngeal swallowing (McConnel, 1988). It is conceivable that an age-related reduction in tongue endurance increases the physiological demands of dining. Such demands may result in longer meal times that leave older individuals alone and unmonitored at the dining table, thereby causing frustration, social stigma and reduced safety and quality of life. Despite these reports, there is a paucity of empirical evidence supporting a relationship between tongue endurance and swallowing function. The purpose of this study was to test the hypothesis that eating a meal reduces tongue strength and endurance in healthy old and young adults.
Muscle endurance generally is described as the ability to maintain a required or expected force and is operationally defined as “the time to task failure for a sustained isometric contraction performed at a submaximal intensity” (Hunter, Critchlow, & Enoka, 2005). A reduction in muscle endurance can indicate fatigue. Muscle fatigue is characterized by an acute reduction in the ability to exert muscle force, independent of whether the force can be sustained (Gandevia, 2001). Fatigue can be influenced by a variety of task-dependent mechanisms (e.g., exercise intensity, type of contraction, muscle group and fiber distribution, environment, training). Furthermore, fatigue can result from peripheral factors at the level of the motor neuron and muscle, as well as central factors mediating supraspinal and spinal neural drive (McCloskey, Gandevia, Potter, & Colebatch, 1983).
Studies of the relationship between the strength of the head and neck musculature, particularly the tongue, and swallowing function throughout the age span are well documented (Clark, Henson, Barber, Stierwalt, & Sherrill, 2003; Logemann et al., 2000; Robbins, Gangnon, Theis, Kays, & Hind, 2005; Robbins, Levine, Wood, Roecker, & Luschei, 1995). In contrast, studies of oromotor endurance only recently are emerging and, with few exceptions (Lazarus et al., 2000), concentrate on the role of the tongue for speech (Goozee, Murdoch, & Theodoros, 2001; McAuliffe, Ward, Murdoch, & Farrell, 2005; Robin, Goel, Somodi, & Luschei, 1992; Solomon, 2000; Youmans & Stierwalt, 2006) or upper airway patency for sleep (Mortimore, Bennett, & Douglas, 2000; Scardella et al., 1993) as opposed to the critical function of swallowing. With implications for the diagnosis and treatment of speech disorders, Kent and colleagues stated (Kent, Kent, & Rosenbek, 1987, p. 377), “Few, if any, data have been published on …measures of endurance or fatigue although it could be important clinically to make these determinations, especially for clients with neurological disease.” Since swallowing is life-sustaining and its disorder can lead to aspiration and related dire consequences (pneumonia, malnutrition, dehydration and death), an understanding of the relationship between tongue endurance and swallowing function is essential.
Several studies of tongue endurance in healthy (Crow & Ship, 1996; Robin et al., 1992; Stierwalt & Youmans, 2007) and disordered (Chang, Chen, Ko, & Lin, 2008; Goozee et al., 2001; Lazarus et al., 2000; McAuliffe et al., 2005; Solomon, 2000) populations have been published, particularly with regard to speech intelligibility. The majority of these studies have measured tongue endurance by instructing participants to maintain 50% of their maximum isometric tongue pressure for as long as possible. Typically this is accomplished by elevating the tongue to compress a pressure sensor against the hard palate. All but one of these previous studies positioned the pressure sensor on the anterior portion of the tongue, “just posterior to the alveolar ridge,” representing the location where the tongue tip is anchored during swallowing in order to facilitate bolus transit through the oral cavity. Stierwalt and Youmans (2007) instructed subjects to “place the bulb on the center of the tongue.” No measures have been obtained from the posterior tongue muscles, which arguably provide critical forces necessary for safe and effective oropharyngeal swallowing.
Establishing measures of tongue strength and endurance relative to “within tongue location” is warranted, given known variations in the morphological structure, innervation and specific functions of the anterior and posterior segments of the tongue. Primate models suggest that the anterior tongue tends to have a greater proportion of fast-twitch and fatigable fibers than the rest of the tongue (DePaul & Abbs, 1996; Saigusa, Niimi, Yamashita, Gotoh, & Kumada, 2001; Stal, Marklund, Thornell, De Paul, & Eriksson, 2003), which likely relates to the role of the tongue tip in producing quick, precise movements needed for oral preparation. The anterior tongue is thus active during the cortically-mediated processes of bolus manipulation and mastication. In contrast, the posterior tongue has a larger proportion of slow, fatigable fibers and provides the major propulsive force for transferring food and liquid from the oral cavity into the pharynx repetitively over the course of a meal. The posterior tongue is intricately linked to the pharyngeal swallow response, as its retraction against the posterior pharyngeal wall triggers the remaining swallow sequence (Dodds, 1989), and its primary efferent control stems from the medullary swallowing center with suggested contributions from the nucleus ambiguous within the brainstem (Fujiu, Logemann, & Pauloski, 1995; Zemlin, 1988). Lingual swallowing pressures also have been shown to differ at anterior and posterior tongue locations, with the posterior tongue generating higher pressures during swallows of liquid and semisolid boluses (Nicosia, Hind, Roecker, Carnes, & Robbins, 2000; Robbins et al., 2005). Subsequent to bolus preparation and formation, anterior tongue pressures are necessary to anchor the tongue against the alveolar ridge in order to initiate bolus propulsion, but posterior pressures are essential for stimulating the pharyngeal swallow response and contributing to hyoid ascent (Dodds, 1989; Stone & Shawker, 1986).
In a study of ten young adults who ate a 1000-calorie meal, a total of 440 solid bolus swallows were observed suggesting an average of 44 solid bolus swallows per individual (Dua, Ren, Bardan, Xie, & Shaker, 1997). These data indicate that the consumption of an entire meal, which demands multiple swallows of various textures, volumes and consistencies, truly is an endurance task. In contrast, standard evaluation techniques for dysphagic patients are based on isolated, single swallows typically performed during an instrumental examination such as a videofluoroscopic swallow study. Judgments about the effectiveness of a treatment strategy, ideally designed to enhance swallowing safety with practical extensions to dining, are based on the immediate outcomes of only several swallows and fail to capture the potential for fatigue over the course of an entire meal. To simulate the demands of a meal experience when decreased endurance is suspected, Logemann suggested observing a patient instrumentally before and after eating a meal (1998). Although anecdotal clinical reports indicate that such methods are implemented frequently, objective measures of tongue endurance and its effects on swallowing performance over time have not been established for functional use. This study is the first to quantify the effects of consuming a meal on tongue strength and endurance in healthy young and old adults, with respect to factors such as meal duration, meal-related perceptions of effort, and clinical signs of swallowing difficulty.
Methods
Subjects
Twenty-two healthy young (ages 20-35 years, mean 25.7) and old (ages 65-82 years, mean 70.7) adults provided informed consent and were enrolled in this study. Each group included 5 men and 6 women. All subjects completed a baseline screening questionnaire to ensure that they had no symptoms of swallowing difficulty or history of any medical condition potentially associated with dysphagia (e.g., neurological or neuromuscular disease). Subjects were excluded if they reported a history of insulin-dependent diabetes; medically documented esophageal dysmotility; surgery to the head, neck, throat or esophagus; food allergies; eating disorder; history of tobacco or alcohol abuse; or suspected impairment in decision-making capacity. In addition, all subjects scored below the cutoff for fatigue on the Iowa Fatigue Scale, a clinical research screening questionnaire that measures four constructs of general fatigue (Hartz, Bentler, & Watson, 2003). This study was approved by the University of Wisconsin Health Sciences Institutional Review Board prior to subject enrollment.
Instrumentation
Tongue strength and endurance measures were assessed using the Iowa Oral Performance Instrument® (IOPI) (IOPI Northwest; Carnation, WA). The IOPI consists of a nickel-sized, air-filled bulb that senses pressure when squeezed between the tongue and the hard palate. Visual inspection of bulb position was completed with each trial; however, given the nature of the instrument (air-filled bulb attached to pliable tubing) and palatal variability, bulb location was approximate. Measures were taken with the bulb positioned on the anterior tongue (operationally defined as approximately 10 mm posterior to the tongue tip) and the posterior tongue (operationally defined as approximately 10 mm anterior to the most posterior circumvallate papilla). Once the bulb was positioned appropriately on the posterior tongue, a piece of tape was used to mark the point where the connective tubing running between the IOPI device and the intra-oral bulb met the lips. In this manner, reliable bulb placement relative to posterior distance into the oral cavity was achieved between trials. Visual feedback was provided to subjects by a vertical array of colored light-emitting diodes (LEDs) on the IOPI. These lights signify linear increments, such that the uppermost red light represents the maximum pressure boundary, and the middle green light represents 50 percent of the pressure scale. Pressure data from the IOPI were digitized at 240 Hz and displayed on a personal computer using data acquisition hardware and software (Model DI-158U, DATAQ® Instruments, Inc., Akron, OH).
Procedures
Double baseline measures of tongue strength and endurance
Each subject completed two baseline measures of tongue strength and endurance with a 20-minute rest period between measures (Table 1). The two baseline measures subsequently were averaged in order to obtain a single pre-meal tongue strength and endurance value for data analysis.
Table 1.
Order of Procedures
| Baseline Measure 1 | Rest | Baseline Measure 2 | Meal | Post-Meal Measure | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Tongue Strength | Tongue Endurance* | Baseline Effort | Tongue Strength | Tongue Endurance | Meal Effort | Meal Duration | Swallowing Difficulty | Tongue Strength | Tongue Endurance |
| Measure | Max Isometric Pressure (P1max) | Duration of Pressure ≥ 0.5 × P1max | Percent of Visual Analog Scale (VAS) | Max Isometric Pressure (P2max) | Duration of Pressure ≥ 0.5 × P2max | Percent of VAS | Time to Complete Meal | # of Coughs, Throat Clears, & Changes in Vocal Quality | Max Isometric Pressure (P3max) | Duration of Pressure ≥ |
| Approx Time | 10 Minutes | 20 Minutes | 10 Minutes | 30 Minutes | 10 Minutes | |||||
Refer to Figure 1 for tongue endurance measurement criteria
Tongue strength
Subjects were randomly assigned to begin with the IOPI bulb positioned on the anterior or posterior tongue and to press the tongue against the IOPI bulb as hard as possible. Two sets of three trials were conducted at one tongue location before repositioning the bulb at the alternate location and repeating the procedure. Approximately 30 seconds of rest were required between trials. The highest pressure generated was selected as the maximum tongue strength (Pmax) provided that the mean value of each of the two sets did not differ by more than five percent. If the variation between the sets was greater than five percent, a third set was conducted. No subject performed more than three sets for either tongue location.
Tongue endurance
After tongue strength was assessed, the bulb was repositioned to the initial tongue location for measuring tongue endurance. The target pressure for each of the baseline endurance measures was defined as 50% of the Pmax obtained during the preceding tongue strength assessment, and the middle LED on the IOPI was programmed to represent this target value. Tongue endurance was defined as the duration that 50% of the Pmax could be maintained. Subjects were instructed to press the tongue against the IOPI bulb in order to sustain illumination of the middle green LED on the IOPI lights array “for as long as possible”. The investigator provided consistent, enthusiastic verbal encouragement in order to motivate subjects to produce their maximal effort throughout each trial. Subjects were instructed to breathe through the nose and to attempt to suppress any spontaneous swallows during the endurance trials. Trials were terminated when one of the following occurred first: (a) 50% of the Pmax (represented by the middle green LED) could not be maintained for more than 2 seconds; (b) 40% of the Pmax (represented by the light just below the middle green LED) could not be maintained for more than 0.5 seconds, or; (c) the pressure dropped precipitously (Solomon, 2000) (Figure 1). One trial was performed with the anterior and posterior tongue for each set of measures and a three-minute rest period was required between trials.
Figure 1.
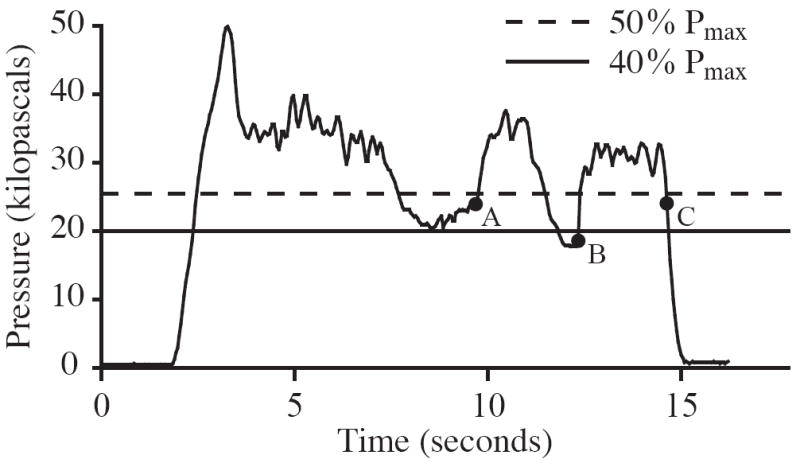
Tongue endurance trials were terminated when one of the following criteria were met (P = pressure): a) 40 % ≤ P < 50 % of Pmax for 2.0 seconds; b) P < 40 % of Pmax for 0.5 seconds; c) P dropped precipitously. The depicted pressure waveform is for illustration purposes and does not represent data collected during this study.
Baseline effort ratings
During the 20-minute rest period between the two baseline tongue strength and endurance measures, subjects completed a baseline rating of their perceived effort during a reference dining task. Subjects were instructed to self-administer a four-ounce serving of applesauce and an equal volume of water, after which they judged their perceived effort during this task by marking a visual-analog scale (VAS). The VAS was a 10-centimeter horizontal, undifferentiated line labeled “no effort” and “extreme effort” at the left and right extremes. This task was designed to provide a minimally challenging dining experience, which served as a reference point for subjects when they were asked to complete later perceived effort ratings during the standardized meal.
Meal consumption and effort ratings
The standardized meal consisted of half of a bagel spread with one tablespoon of creamy peanut butter, eight baby carrot sticks and eight ounces of chocolate milk (Babcock Dairy, Madison, WI; apparent viscosity 28.4 ± 1.5 centipoise). The meal was administered on a tray in two equal portions so that perceived effort ratings could be completed at the middle and end of the meal. Subjects were seated at a table and instructed to consume each portion as naturally as possible without talking during the meal. After each half of the meal, subjects judged their perceptions of dining-related effort by marking a VAS identical to the scale used to collect baseline perceived effort ratings. Subjects were video-recorded during the meal in order to obtain measures of meal duration (defined as the time the first bolus entered the oral cavity to the time the last swallow was visualized) and to document any signs of swallowing difficulty (defined as a cough, wet voice, or throat clear).
Post-meal measures of tongue strength and endurance
Immediately after completing the meal, each subject performed a post-meal measure of tongue strength and endurance. Tongue strength and endurance were assessed using the baseline procedures described earlier. The target pressure for the post-meal measure of tongue endurance was set to 50% of the mean of the two baseline maximum tongue strength values (Table 1).
Statistical Analysis
Repeated-measures analysis of variance models were used to assess the impact of meal consumption, age and sex on isometric tongue strength and endurance. Separate analyses were conducted for anterior and posterior tongue measurements. Tongue strength (isometric pressure) was analyzed on the original scale; tongue endurance (time) was analyzed after log transformation. A nominal p-value of 0.05 was regarded as statistically significant. The standardized mean difference statistic (d) was used to calculate effect size. The standard deviation of the change in pre-meal to post-meal pressure was used for tongue strength and endurance effect size estimates. The standard deviation of the change in effort from pre-meal to mid-meal, pre-meal to post-meal, or mid-meal to post-meal was used as appropriate for meal effort effect size estimates. Analyses were conducted using Proc Mixed in SAS Version 9.1 (SAS Institute Inc, Cary NC).
Results
Double Baseline Tongue Strength and Endurance
Tongue strength
Pre-meal tongue strength measures (derived from the mean of the two baseline tongue endurance measures) are reported in Table 2.
Table 2.
Summary of Pre-Meal Descriptive Statistics
| Tongue Strength (kilopascals) | Tongue Endurance (seconds) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Anterior Tongue | Posterior Tongue | Anterior Tongue | Posterior Tongue | ||||||
| Subgroup | N | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Young Female | 6 | 67.8 | 10.6 | 62.5 | 14.5 | 37.5 | 11.8 | 29.6 | 9.3 |
| Young Male | 5 | 59.2 | 5.2 | 50.0 | 7.9 | 40.2 | 14.0 | 26.0 | 19.5 |
| Old Female | 6 | 50.3 | 11.1 | 49.0 | 12.6 | 34.3 | 19.3 | 24.4 | 14.4 |
| Old Male | 5 | 62.6 | 8.8 | 61.4 | 7.5 | 29.6 | 12.5 | 24.2 | 13.6 |
| All | 22 | 59.9 | 11.3 | 55.7 | 12.6 | 35.4 | 14.8 | 26.1 | 14.1 |
Tongue endurance
Pre-meal tongue endurance measures (derived from the mean of the two baseline tongue endurance measures) are reported in Table 2. Mean pre-meal anterior tongue endurance times were 51% longer (95% confidence interval: 23 – 85%) than posterior endurance times (p = 0.0005). Intra-subject variance was 111.93 seconds (SD = 10.58 seconds) for anterior tongue endurance and 115.26 seconds (SD = 10.74 seconds) for posterior tongue endurance. Inter-subject variance was 120.93 seconds (SD = 11.00 seconds) for the anterior tongue and 102.90 seconds (SD = 10.14 seconds) for the posterior tongue. Similar measures of tongue endurance in the literature report an inter-subject variance ranging from 49 to 1664.64 seconds (Crow & Ship, 1996; Goozee et al., 2001; McAuliffe et al., 2005; Robin et al., 1992; Solomon, 2000).
Change in Tongue Strength and Endurance
Tongue strength
All twenty-two subjects demonstrated a significant decline in anterior and posterior isometric tongue strength post-meal compared with pre-meal (anterior: 2.0 kPa, 95% CI 0.5-3.5 kPa, p = 0.01, d = 0.33; posterior: 2.2 kPa, 95% CI 0.7-3.8 kPa, p = 0.01, d = 0.45). There was no evidence that the magnitude of change in either anterior or posterior isometric tongue strength differed by age or gender.
Tongue endurance
All subjects demonstrated a statistically significant decline in anterior and posterior tongue endurance post-meal compared with pre-meal (Figure 2). After eating a meal, subjects were able to achieve only 76% of their pre-meal anterior tongue endurance (95% CI 61-94%, p = 0.01, d = 0.41) and only 70% of their pre-meal posterior tongue endurance (95% CI 51-96%, p = 0.03, d = 0.43). There was marginal evidence that the percentage of pre-meal anterior tongue endurance generated post-meal varied with age (p = 0.05, d = 0.58) but not gender. That is, young subjects achieved a smaller percentage of their pre-meal anterior tongue endurance after dining compared with older subjects (61% in young versus 94% in old) (Figure 2).
Figure 2.
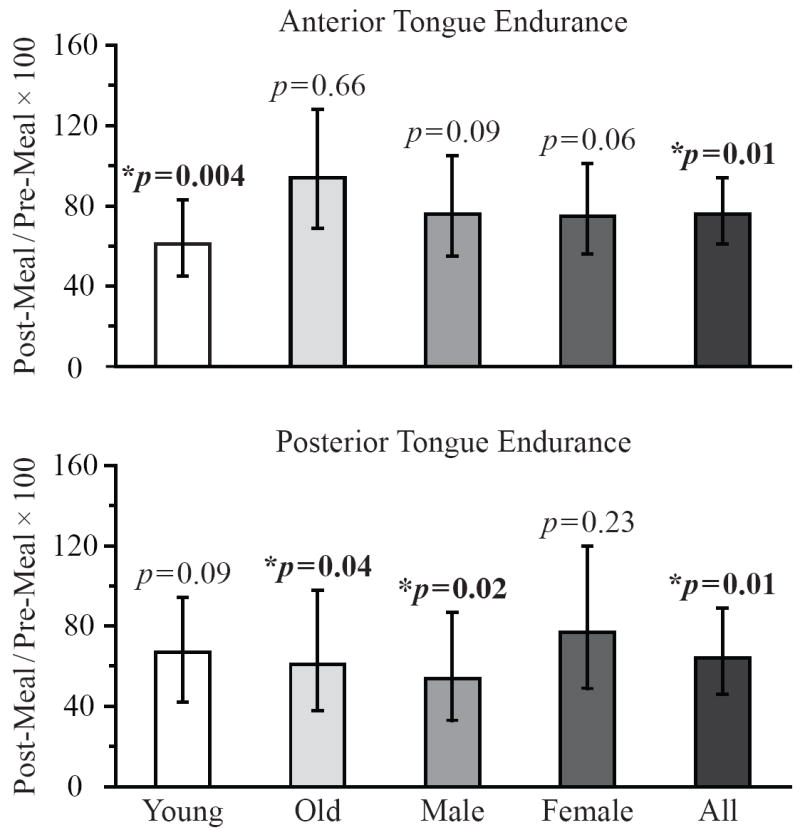
Data bars represent the percentage of pre-meal tongue endurance generated post-meal by each subject group. P-values represent the statistical significance of the change in tongue endurance from pre-meal to post-meal, and error bars represent ± 95% confidence intervals.
Effort Ratings
Perceptions of effort as indicated by visual analog scale ratings increased from pre-meal to post-meal (p < 0.0001, d = 1.13), pre-meal to mid-meal (p < 0.0001, d = 1.16), and mid-meal to post-meal (p = 0.02, d = .56). There were no differences in the overall levels of perceived effort or the patterns of change in perceived effort over time based on age or gender. Among the group of older adults, the oldest individuals reported the highest amount of perceived effort mid-meal (79 y.o. F = 38.8%; 78 y.o. M = 58.3%; 82 y.o. M = 53%). The same subjects maintained these elevated levels of perceived effort post-meal (38.8%, 68.3% and 53%, respectively).
Meal Consumption
There was a trend toward longer meal durations in older adults compared to young adults (p = 0.07; Table 3). There were no differences in meal duration based on gender. The three oldest individuals demonstrated signs of swallowing difficulty during the meal, including wet voice, throat clear and cough. The oldest female (79 years old) requested to skip the second portion of the meal due to reports of a diminished appetite.
Table 3.
Summary of Meal Duration Descriptive Statistics
| Meal Duration (seconds) | |||||
|---|---|---|---|---|---|
| First Half of Meal | Second Half of Meal | ||||
| Subgroup | N | Mean | SD | Mean | SD |
| Young Female | 6 | 275 | 76 | 275 | 65 |
| Young Male | 5 | 210 | 54 | 216 | 21 |
| Old Female | 6 | 335 | 179 | 313 | 210 |
| Old Male | 5 | 312 | 68 | 335 | 81 |
| All | 22 | 282 | 108 | 287 | 110 |
Discussion
This study is the first to examine the effects of meal consumption, representing functional swallowing, on tongue strength and endurance. The most notable finding was that all individuals demonstrated a moderate, yet significant decline in both tongue strength and endurance after eating a standardized meal, suggesting that the daily activity of dining may be sufficient to negatively impact lingual pressure generation. This central finding has implications for the development of swallowing evaluation techniques that better represent functional outcomes; that is, swallowing as it relates to the dining experience. A second intriguing finding was that young subjects demonstrated a significantly greater decline in anterior tongue endurance than older adults after eating a meal. Despite the marginal statistical significance of this finding (p = 0.05, d = 0.58), it stimulates a discussion of age-associated differences in central and peripheral factors that may affect measures of lingual function relative to tongue site.
There are several extant investigations of age effects on tongue endurance, but no previous study has investigated changes in tongue strength or endurance after a fatiguing swallowing task in adults of various ages. A comparison of healthy young (n = 15, mean age = 25) and old (n = 13, mean age = 67) controls and patients with Parkinson’s disease found that young subjects sustained a submaximal lingual pressure more than 50% longer than older controls (McAuliffe et al., 2005). However, two much larger studies comprising nearly 100 healthy subjects aged 19-96 years (Crow & Ship, 1996; McAuliffe et al., 2005) found no significant differences in tongue endurance related to age or gender. The current study emphasizes a need to examine not only baseline differences between young and old individuals, but also age-dependent variations in tongue pressure measures following functional endurance tasks.
Solomon (2000) designed such a study in her investigation of the effects of tongue fatigue on functional speech intelligibility. Her findings from young healthy individuals suggest that a strenuous isometric lingual resistance task (i.e., repeated submaximal sustained contractions for approximately 30 minutes) is necessary to fatigue the tongue substantially enough to cause perceptual changes in speech. Performing a tiring speech-specific task (i.e., rapid syllable repetition) was not sufficient for inducing perceptual changes in articulatory precision in healthy or neurologically disordered populations (Solomon, Makashay, & Cannard, 2006). Solomon’s findings suggest that tongue muscles are designed to endure the quick, submaximal lingual pressures exerted during functional speech. It is interesting that the results of this study indicate that simply eating a meal, a task requiring submaximal lingual pressures but different muscle movements and forces than speech, may be sufficient to cause a reduction in tongue strength and endurance.
The second major finding of this study is consistent with studies of limb muscles, which suggest that older adults tend to sustain submaximal contractions longer than young adults. The older subjects in this study demonstrated longer anterior tongue endurance times compared to young individuals after a fatigue-inducing task. Allman and Rice (2002) conducted a review of the evidence on aging and muscle fatigue, and identified six studies examining age effects on time to task failure during a sustained limb, muscle contraction. All of the studies reported a trend toward longer endurance times in older adults and the results of one study reached statistical significance. These observed age-related differences in muscle endurance may relate to changes in peripheral and central factors associated with normal aging.
Age-related adaptations in both peripheral and central mechanisms likely affected the measures of tongue endurance observed in this study, although the specific underpinnings cannot be elucidated by the current design. In the periphery, a natural age-related reduction in muscle mass known as sarcopenia is characterized by a preferential loss of type II fibers and adaptations in neural wiring that increase the number of fibers innervated by slow-twitch motor units (Roos, Rice, & Vandervoort, 1997). As a result, older adults naturally develop a greater amount of muscle mass designed for slow, fatigue-resistant activities. Age-associated increases in adipose and connective tissue (Bassler, 1987; Yamaguchi, Nasu, Esali, & et al., 1982), along with a decline in muscle fiber diameter (Nakayama, 1991), have been documented in lingual muscles. However, further studies are needed to demonstrate whether a transformation of fast-twitch muscle fibers to more slowly innervated fibers occurs within aging tongue muscles.
Insufficient neural drive descending from the central nervous system to motor neurons also can account for age-related differences in muscle endurance. One factor influencing central drive is the type of muscle contraction. Several studies suggest that contractions applied through a range of motion or with a specified velocity may reduce age-related enhancements in muscle endurance (Jasperse, Seals, & Callister, 1994; Lanza, Towse, Caldwell, Wigmore, & Kent-Braun, 2003). This concept suggests that the addition of dynamic measures of tongue endurance to future studies may further clarify age-related effects of dining on tongue function.
Central neural drive also can be affected by the physical and motivational status of the subjects (Enoka & Stuart, 1992). It is possible that the young adults in this study were more competitive than the older subjects and therefore able to put forth greater central effort during the pre-meal measures. High effort during early measures could have a subsequent negative fatigue effect on later post-meal measures. Training effects or familiarity with the task also can impact central drive. Many subjects qualitatively report that it is more difficult and less natural to elevate the posterior tongue toward the palate to perform measures of posterior tongue strength and endurance. These reports likely reflect the fact that anterior tongue movements are under greater volitional neural control (Pouderoux & Kahrilas, 1995), and may explain the findings that baseline anterior tongue endurance times were more than twice as long as posterior tongue endurance times in this study.
Sense of effort is often described as an indicator of central fatigue and is defined as an awareness of descending motor drive via central feedback (Gandevia, 1992; McCloskey et al., 1983). In this study, subjects rated their perception of effort before, during and after the meal to determine if sense of effort increased while endurance decreased. A relationship between higher perceived effort ratings and reduced endurance times was not confirmed. However, the oldest subjects in this study reported the highest levels of effort and demonstrated symptoms of swallowing difficulty during the meal, suggesting that the oldest adults may experience increased central processing demands with possible effects on functional performance. These findings begin to illustrate the importance of self-monitoring dining effort as a critical component to safe swallowing, as well as the importance of behavioral interventions for elders, such as meal pacing or the provision of high-caloric, small frequent meals. Future studies also should examine age-related differences in the recovery of tongue muscle strength and endurance with respect to perceptions of effort after dining.
The fact that age-related differences were found in comparisons of pre-meal and post-meal endurance at the anterior, but not the posterior tongue site, is not surprising. The anterior tongue in young adults predominantly comprises fatigue-susceptible type II fibers that respond to fast, frequent bursts of neural activity (Saigusa et al., 2001; Stal et al., 2003) but are poorly designed for endurance tasks. In contrast, the posterior tongue comprises a higher percentage of slow-twitch muscle fibers, a pattern that may expand to the entire tongue with aging in a process similar to that documented in limb muscles (Roos et al., 1997). Thus, age-related differences in muscle fiber type may be more pronounced at the anterior tongue location. Furthermore, central innervation of the anterior and posterior tongue segments differs. The anterior tongue, which is activated during the earliest stage of swallowing, is under cortical, and hence primarily voluntary, control (Mosier, Liu, Maldjian, Shah, & Modi, 1999). Although it can be placed under a certain degree of volitional control, the posterior tongue is a major component of the pharyngeal swallow response, primarily controlled by the medullary reticular formation, or swallowing center, with potential contributions from the nucleus ambiguous within the brainstem (Logemann, 1998).
Variations in both neural input and peripheral muscle structures must be considered when interpreting these results, as it is likely the interaction of central and peripheral neural mechanisms with the muscles of the end organ that underlies differential findings in anterior and posterior tongue endurance. When coupled with natural age-related changes, this interrelation may result in noticeable effects on lingual pressure generation and functional swallowing. For example, Levine et al. (1992) found that older adults with a greater degree of cortical periventricular white matter lesions had slower swallowing durations and lower isometric tongue pressures compared with young adults. Future studies describing the composition and organization of intrinsic and extrinsic lingual muscle fibers as well as medullary and supertentorial integrity are necessary to facilitate an understanding of the observed differences in tongue function relative to age, gender and location.
Several confounding variables are acknowledged in this preliminary study of the effects of dining on tongue strength and endurance. First, it is likely that the standardized meal, which required mastication of a dense, chewy bagel, induced fatigue in other oropharyngeal muscles such as the jaw. However, the role of the tongue during mastication cannot be ignored. Rotational, forward and backward lingual movements are necessary for bolus formation (Hiiemae & Palmer, 2003). Furthermore, a study of the coordination of tongue pressure and jaw movement (Hori, Ono, & Nokubi, 2006) reported significant lingual pressure was applied to a 7-sensory array attached to the hard palate during mastication. Future studies could consider using a bite block during measures of lingual pressure in order to isolate the muscles of the tongue. Second, it is possible that fatigue was induced by performing double baseline lingual pressure measures before the meal, and that these fatigue effects were still present post-meal. Subjects were provided with a 20-minute rest period between endurance measures, which was considered liberal based on a review of the literature. Previous studies of tongue endurance permitted a range of one to fifteen minutes between tongue endurance measures (Lazarus et al., 2000; Scardella et al., 1993; Solomon, Robin, Mitchinson, VanDaele, & Luschei, 1996), and a study of intermittent limb contractions in old and young men found that muscle strength returned to 83% of baseline in all subjects within 3 minutes of recovery (Allman & Rice, 2001). Collecting pre-meal measures of tongue strength and endurance across several days would help diminish these potential fatigue effects and provide a better understanding of intrasubject variability.
In summary, meal consumption was shown to diminish tongue strength and endurance in both young and old adults, confirming that the universal act of eating a meal can negatively impact lingual pressure generation. These findings have implications for frail adults, who may possess less functional lingual muscle reserve than the healthy community-dwelling adults enrolled in this study, which could place them at risk for increased swallowing complications during a challenging meal. Future work is needed to clarify the effects of the observed decline in tongue endurance on physiological swallowing measures, including airway invasion, through the use of pre- and post-meal instrumental measures of swallowing function. Additionally, it is strongly encouraged that the range of normal variation in tongue endurance continues to be defined prior to extending this work to disordered populations.
Acknowledgments
Special thanks to Erich Luschei, Ph.D., and Nancy Solomon, Ph.D., for their consultation on study equipment and design, Nadine Connor, Ph.D., for manuscript review, and Abby Duane, B.S., for manuscript preparation.
This study was conducted in the Geriatric Research, Education and Clinical Center (GRECC) of the William S. Middleton Memorial VA Hospital in Madison, Wisconsin. This is GRECC Manuscript #2008-22.
Contributor Information
Stephanie A. Kays, Geriatric Research, Education and Clinical Center, William S. Middleton Memorial VA Hospital and Department of Medicine, University of Wisconsin-Madison
Jacqueline A. Hind, Geriatric Research, Education and Clinical Center, William S. Middleton Memorial VA Hospital and Department of Medicine, University of Wisconsin-Madison
Ronald E. Gangnon, Department of Biostatistics and Medical Informatics, University of Wisconsin-Madison
JoAnne Robbins, Geriatric Research, Education and Clinical Center, William S. Middleton Memorial VA Hospital and Department of Medicine, University of Wisconsin-Madison
References
- Allman BL, Rice CL. Incomplete recovery of voluntary isometric force after fatigue is not affected by old age. Muscle Nerve. 2001;24(9):1156–1167. doi: 10.1002/mus.1127. [DOI] [PubMed] [Google Scholar]
- Allman BL, Rice CL. Neuromuscular fatigue and aging: central and peripheral factors. Muscle Nerve. 2002;25(6):785–796. doi: 10.1002/mus.10116. [DOI] [PubMed] [Google Scholar]
- Bassler R. Histopathology of different types of atrophy of the human tongue. Pathology, research and practice. 1987;182(1):87–97. doi: 10.1016/S0344-0338(87)80147-4. [DOI] [PubMed] [Google Scholar]
- Chang CW, Chen SH, Ko JY, Lin YH. Early radiation effects on tongue function for patients with nasopharyngeal carcinoma: a preliminary study. Dysphagia. 2008;23(2):193–198. doi: 10.1007/s00455-007-9128-x. [DOI] [PubMed] [Google Scholar]
- Clark HM, Henson PA, Barber WD, Stierwalt JA, Sherrill M. Relationships among subjective and objective measures of tongue strength and oral phase swallowing impairments. American journal of speech-language pathology. 2003;12(1):40–50. doi: 10.1044/1058-0360(2003/051). [DOI] [PubMed] [Google Scholar]
- Crow HC, Ship JA. Tongue strength and endurance in different aged individuals. The journals of gerontology Series A, Biological sciences and medical sciences. 1996;51(5):M247–250. doi: 10.1093/gerona/51a.5.m247. [DOI] [PubMed] [Google Scholar]
- DePaul R, Abbs JH. Quantitative morphology and histochemistry of intrinsic lingual muscle fibers in Macaca fascicularis. Acta anatomica. 1996;155(1):29–40. doi: 10.1159/000147787. [DOI] [PubMed] [Google Scholar]
- Dodds WJ. The physiology of swallowing. Dysphagia. 1989;3:171–178. doi: 10.1007/BF02407219. [DOI] [PubMed] [Google Scholar]
- Dua KS, Ren J, Bardan E, Xie P, Shaker R. Coordination of deglutitive glottal function and pharyngeal bolus transit during normal eating. Gastroenterology. 1997;112(1):73–83. doi: 10.1016/s0016-5085(97)70221-x. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Stuart DG. Neurobiology of muscle fatigue. Journal of applied physiology. 1992;72(5):1631–1648. doi: 10.1152/jappl.1992.72.5.1631. [DOI] [PubMed] [Google Scholar]
- Fujiu M, Logemann J, Pauloski BR. Increased postoperative posterior pharyngeal wall movement in patients with anterior oral cancer: Preliminary findings and possible implications for treatment. American journal of speech-language pathology. 1995;4:24–30. [Google Scholar]
- Gandevia SC. Some central and peripheral factors affecting human motoneuronal output in neuromuscular fatigue. Sports medicine (Auckland, N Z) 1992;13(2):93–98. doi: 10.2165/00007256-199213020-00004. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiological reviews. 2001;81(4):1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Goozee JV, Murdoch BE, Theodoros DG. Physiological assessment of tongue function in dysarthria following traumatic brain injury. Logopedics, phoniatrics, vocology. 2001;26(2):51–65. doi: 10.1080/140154301753207421. [DOI] [PubMed] [Google Scholar]
- Hartz A, Bentler S, Watson D. Measuring fatigue severity in primary care patients. Journal of psychosomatic research. 2003;54(6):515–521. doi: 10.1016/s0022-3999(02)00600-1. [DOI] [PubMed] [Google Scholar]
- Hiiemae KM, Palmer JB. Tongue movements in feeding and speech. Critical reviews in oral biology and medicine. 2003;14(6):413–429. doi: 10.1177/154411130301400604. [DOI] [PubMed] [Google Scholar]
- Hori K, Ono T, Nokubi T. Coordination of tongue pressure and jaw movement in mastication. Journal of dental research. 2006;85(2):187–191. doi: 10.1177/154405910608500214. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Critchlow A, Enoka RM. Muscle endurance is greater for old men compared with strength-matched young men. Journal of applied physiology. 2005;99(3):890–897. doi: 10.1152/japplphysiol.00243.2005. [DOI] [PubMed] [Google Scholar]
- Jasperse JL, Seals DR, Callister R. Active forearm blood flow adjustments to handgrip exercise in young and older healthy men. The Journal of physiology. 1994;474(2):353–360. doi: 10.1113/jphysiol.1994.sp020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent RD, Kent JF, Rosenbek JC. Maximum performance tests of speech production. The Journal of speech and hearing disorders. 1987;52(4):367–387. doi: 10.1044/jshd.5204.367. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Towse TF, Caldwell GE, Wigmore DM, Kent-Braun JA. Effects of age on human muscle torque, velocity, and power in two muscle groups. Journal of applied physiology. 2003;95(6):2361–2369. doi: 10.1152/japplphysiol.00724.2002. [DOI] [PubMed] [Google Scholar]
- Lazarus CL, Logemann JA, Pauloski BR, Rademaker AW, Larson CR, Mittal BB, et al. Swallowing and tongue function following treatment for oral and oropharyngeal cancer. Journal of speech, language, and hearing research : JSLHR. 2000;43(4):1011–1023. doi: 10.1044/jslhr.4304.1011. [DOI] [PubMed] [Google Scholar]
- Levine R, Robbins JA, Maser A. Periventricular white matter changes and oropharyngeal swallowing in normal individuals. Dysphagia. 1992;7(3):142–147. doi: 10.1007/BF02493446. [DOI] [PubMed] [Google Scholar]
- Logemann J. Evaluation and Treatment of Swallowing Disorders. Second Edition. Pro-Ed, Inc; 1998. [Google Scholar]
- Logemann JA, Pauloski BR, Rademaker AW, Colangelo LA, Kahrilas PJ, Smith CH. Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. Journal of speech, language, and hearing research : JSLHR. 2000;43(5):1264–1274. doi: 10.1044/jslhr.4305.1264. [DOI] [PubMed] [Google Scholar]
- McAuliffe MJ, Ward EC, Murdoch BE, Farrell AM. A nonspeech investigation of tongue function in Parkinson’s disease. The journals of gerontology Series A, Biological sciences and medical sciences. 2005;60(5):667–674. doi: 10.1093/gerona/60.5.667. [DOI] [PubMed] [Google Scholar]
- McCloskey DI, Gandevia S, Potter EK, Colebatch JG. Muscle sense and effort: motor commands and judgments about muscular contractions. Advances in neurology. 1983;39:151–167. [PubMed] [Google Scholar]
- McConnel FM. Analysis of pressure generation and bolus transit during pharyngeal swallowing. Laryngoscope. 1988;98(1):71–78. doi: 10.1288/00005537-198801000-00015. [DOI] [PubMed] [Google Scholar]
- Mortimore IL, Bennett SP, Douglas NJ. Tongue protrusion strength and fatiguability: relationship to apnoea/hypopnoea index and age. Journal of sleep research. 2000;9(4):389–393. doi: 10.1046/j.1365-2869.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- Mosier KM, Liu WC, Maldjian JA, Shah R, Modi B. Lateralization of cortical function in swallowing: a functional MR imaging study. AJNR American journal of neuroradiology. 1999;20(8):1520–1526. [PMC free article] [PubMed] [Google Scholar]
- Nakayama M. Histological study on aging changes in the human tongue. Journal of otolaryngology Japan. 1991;94:541–555. doi: 10.3950/jibiinkoka.94.541. [DOI] [PubMed] [Google Scholar]
- Nicosia MA, Hind JA, Roecker EB, Carnes M, Robbins JA. Age effects on the temporal evolution of isometric and swallowing pressure. The journals of gerontology Series A, Biological sciences and medical sciences. 2000;55A(11):M634–M640. doi: 10.1093/gerona/55.11.m634. [DOI] [PubMed] [Google Scholar]
- Poluri A, Mores J, Cook DB, Findley TW, Cristian A. Fatigue in the elderly population. Physical medicine and rehabilitation clinics of North America. 2005;16(1):91–108. doi: 10.1016/j.pmr.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Pouderoux P, Kahrilas PJ. Deglutitive tongue force modulation by volition, volume, and viscosity in humans. Gastroenterology. 1995;108(5):1418–1426. doi: 10.1016/0016-5085(95)90690-8. [DOI] [PubMed] [Google Scholar]
- Robbins J, Gangnon R, Theis S, Kays SA, Hind J. The effects of lingual exercise on swallowing in older adults. Journal of the American Geriatrics Society. 2005;53(9):1483–1489. doi: 10.1111/j.1532-5415.2005.53467.x. [DOI] [PubMed] [Google Scholar]
- Robbins J, Levine R, Wood J, Roecker EB, Luschei E. Age effects on lingual pressure generation as a risk factor for dysphagia. The journals of gerontology Series A, Biological sciences and medical sciences. 1995;50(5):M257–262. doi: 10.1093/gerona/50a.5.m257. [DOI] [PubMed] [Google Scholar]
- Robin DA, Goel A, Somodi LB, Luschei ES. Tongue strength and endurance: relation to highly skilled movements. Journal of speech, language, and hearing research : JSLHR. 1992;35(6):1239–1245. doi: 10.1044/jshr.3506.1239. [DOI] [PubMed] [Google Scholar]
- Roos MR, Rice CL, Vandervoort AA. Age-related changes in motor unit function. Muscle Nerve. 1997;20(6):679–690. doi: 10.1002/(sici)1097-4598(199706)20:6<679::aid-mus4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Roy N, Stemple J, Merrill RM, Thomas L. Dysphagia in the elderly: preliminary evidence of prevalence, risk factors, and socioemotional effects. The Annals of otology, rhinology, and laryngology. 2007;116(11):858–865. doi: 10.1177/000348940711601112. [DOI] [PubMed] [Google Scholar]
- Saigusa H, Niimi S, Yamashita K, Gotoh T, Kumada M. Morphological and histochemical studies of the genioglossus muscle. The Annals of otology, rhinology, and laryngology. 2001;110(8):779–784. doi: 10.1177/000348940111000815. [DOI] [PubMed] [Google Scholar]
- Scardella AT, Krawciw N, Petrozzino JJ, Co MA, Santiago TV, Edelman NH. Strength and endurance characteristics of the normal human genioglossus. The American review of respiratory disease. 1993;148(1):179–184. doi: 10.1164/ajrccm/148.1.179. [DOI] [PubMed] [Google Scholar]
- Solomon NP. Changes in normal speech after fatiguing the tongue. Journal of speech, language, and hearing research : JSLHR. 2000;43(6):1416–1428. doi: 10.1044/jslhr.4306.1416. [DOI] [PubMed] [Google Scholar]
- Solomon NP, Makashay M, Cannard K. Does a prolonged speech-like task affect sense of effort in Parkinson disease?. Presented at the Biennial Conference on Motor Speech; Austin, TX. 2006. [Google Scholar]
- Solomon NP, Robin DA, Mitchinson SI, VanDaele DJ, Luschei ES. Sense of effort and the effects of fatigue in the tongue and hand. Journal of speech, language, and hearing research : JSLHR. 1996;39(1):114–125. doi: 10.1044/jshr.3901.114. [DOI] [PubMed] [Google Scholar]
- Stal P, Marklund S, Thornell LE, De Paul R, Eriksson PO. Fibre composition of human intrinsic tongue muscles. Cells Tissues Organs. 2003;173(3):147–161. doi: 10.1159/000069470. [DOI] [PubMed] [Google Scholar]
- Stierwalt JA, Youmans SR. Tongue measures in individuals with normal and impaired swallowing. American journal of speech-language pathology. 2007;16(2):148–156. doi: 10.1044/1058-0360(2007/019). [DOI] [PubMed] [Google Scholar]
- Stone M, Shawker TH. An ultrasound examination of tongue movement during swallowing. Dysphagia. 1986;1(2):78–83. doi: 10.1007/BF02407118. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A, Nasu M, Esali Y, et al. Amyloid deposits in the aged tongue. A post mortem study of 107 individuals over 67 years of age. Journal of oral pathology. 1982;11:237–244. doi: 10.1111/j.1600-0714.1982.tb00161.x. [DOI] [PubMed] [Google Scholar]
- Youmans SR, Stierwalt JA. Measures of tongue function related to normal swallowing. Dysphagia. 2006;21(2):102–111. doi: 10.1007/s00455-006-9013-z. [DOI] [PubMed] [Google Scholar]
- Zemlin W. Speech and hearing science: Anatomy and physiology. 3. Englewood Cliffs, NJ: Prentice Hall; 1988. [Google Scholar]


