Abstract
Background
Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) is an inherited disease causing structural and functional abnormalities of the right ventricle (RV). The presence of late potentials as assessed by the signal averaged electrocardiogram (SAECG) is a minor Task Force criterion.
Objective
The purpose of this study was to examine the diagnostic and clinical value of the SAECG in a large population of genotyped ARVC/D probands.
Methods
We compared the SAECGs of 87 ARVC/D probands (age 37 ± 13 years, 47 males) diagnosed as affected or borderline by Task Force criteria without using the SAECG criterion with 103 control subjects. The association of SAECG abnormalities was also correlated with clinical presentation; surface ECG; VT inducibility at electrophysiologic testing; ICD therapy for VT; and RV abnormalities as assessed by cardiac magnetic resonance imaging (cMRI).
Results
When compared with controls, all 3 components of the SAECG were highly associated with the diagnosis of ARVC/D (p<0.001). These include the filtered QRS duration (fQRSD) (97.8 ± 8.7 msec vs. 119.6 ± 23.8 msec), low amplitude signal (LAS) (24.4 ± 9.2 msec vs. 46.2 ± 23.7 msec) and root mean square amplitude of the last 40 msec of late potentials (RMS-40) (50.4 ± 26.9 µV vs. 27.9 ± 36.3 µV). The sensitivity of using SAECG for diagnosis of ARVC/D was increased from 47% using the established 2 of 3 criteria (i.e. late potentials) to 69% by using a modified criterion of any 1 of the 3 criteria, while maintaining a high specificity of 95%. Abnormal SAECG as defined by this modified criteria was associated with a dilated RV volume and decreased RV ejection fraction detected by cMRI (p<0.05). SAECG abnormalities did not vary with clinical presentation or reliably predict spontaneous or inducible VT, and had limited correlation with ECG findings.
Conclusion
Using 1 of 3 SAECG criteria contributed to increased sensitivity and specificity for the diagnosis of ARVC/D. This finding is incorporated in the recent modification of the Task Force criteria.
Keywords: Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D), signal-averaged electrocardiogram (SAECG)
INTRODUCTION
Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) is an inherited myocardial disease characterized pathologically by fibrofatty replacement that causes structural and functional abnormalities of the right ventricle (RV). Clinical manifestations of the disease include ventricular arrhythmias, congestive heart failure, and sudden death. The diagnosis of ARVC/D is based on Task Force criteria established in 1994 that are divided into major and minor components (1). Localized conduction delay by a variety of ECG measurements is seen in a large number of ARVC/D patients (2–4). The high resolution signal-averaged electrocardiogram (SAECG) is particularly well suited to detect localized slowed conduction and has been viewed as an adjunct for the diagnosis of ARVC/D. Currently, the presence of late potentials on SAECG is a minor Task Force criterion, however data corroborating its diagnostic value are limited.
The purpose of this study was to reexamine the value of SAECG in a large population of genotyped ARVC/D probands enrolled in the Multidisciplinary Study of ARVC/D (5, 6). The specific aims of the present report are as follows: (1) to test the contribution of SAECG for the diagnosis of ARVC/D, (2) to determine the optimal cut-off values for the SAECG for the diagnosis of ARVC/D and (3) to compare the diagnostic sensitivity and specificity of 1, 2 and 3 standard individual SAECG components. Additional sub-studies evaluated the association of the abnormalities of SAECG using the optimal criteria with (1) clinical presentation of ARVC/D; (2) abnormalities on the surface ECG; (3) ventricular tachycardia (VT) inducibility at electrophysiologic testing; (4) implantable cardioverter-defibrillator (ICD) therapy for sustained VT; and (5) volume/morphologic features as defined by cardiac magnetic resonance imaging (cMRI).
METHODS
Clinical Characteristics of Study Subjects
The study included 87 ARVC/D probands (age 37 ± 13 years, 47 males), diagnosed as affected (n=62) or borderline (n=25) by Task Force criteria (1) without using the SAECG criterion (Table 1). Probands were excluded if that test was crucial for the diagnosis of the individual patient. This was done to eliminate bias in estimating the sensitivity and specificity of that particular test. In general, when determining the sensitivity and specificity of a new screening test, it is recommended that none of the screening test elements be used in making the primary diagnosis; this principle also holds when establishing diagnostic criteria. At enrollment, 20 (23%) of the ARVC/D probands were asymptomatic. The most frequent presentation included palpitations in 52 (60%), syncope/pre-syncope in 43 (49%) and chest pain in 11 (13%). History of VT/ventricular fibrillation (VF) prior to enrollment was documented in 61 (70%) of probands. All patients underwent genotyping for known mutations associated with ARVC/D and 30 patients (34%) had desmosome gene mutations. The controls were age and gender matched, and included 83 genotype negative normal family members (age 30 ± 14 years, 34 males) who had undergone comprehensive genotypic and phenotypic analysis and 20 unaffected unrelated volunteers (age 37 ± 13 years, 10 males) for a total of 103 control subjects.
Table 1.
Baseline characteristics of ARVC/D probands according to Task Force criteria (n=87)*
| Family history | |
| Familial disease confirmed at necropsy or surgery | 13 (15%) |
| Family history (clinical diagnosis based on present criteria) | 4 (5%) |
| ECG depolarization/conduction abnormalities | |
| Epsilon waves or localized prolongation (>110 msec) of the QRS complex in the right precordial leads (V1 to V3) | 3 (3%) |
| Late potentials seen on signal averaged ECG | 42 (48%) |
| Repolarization abnormalities | |
| Inverted T waves in right precordial leads (V2 and V3) in people above age 12 years and in the absence of RBBB | 61 (70%) |
| Tissue characterization of walls | |
| Fibrofatty replacement of myocardium on endomyocardial biopsy | 9 (10%) |
| Global and/or regional dysfunction and structural alterations | |
| Severe dilatation and reduction of RV ejection fraction with no (or only mild) LV impairment | 14 (16%) |
| Localized RV aneurysms (akinetic or dyskinetic areas with diastolic bulging) | 43 (49%) |
| Severe segmental dilatation of the RV | 2 (2%) |
| Mild global RV dilatation and/or ejection fraction reduction with normal LV | 19 (22%) |
| Mild segmental dilatation of the RV/ regional RV hypokinesis | 5 (6%) |
| Arrhythmias | |
| Left bundle branch block type VT (sustained and non-sustained) | 62 (71%) |
| Frequent ventricular extrasystoles (more than 1000/24 h) on Holter | 19 (22%) |
Excluding 11 number of patients in whom SAECG was essential to meet 1994 Task Force Criteria
SAECG Acquisition and Analysis
The QRS signals were acquired from standard X, Y and Z orthogonal leads and recorded until low noise was achieved. There were several commercial devices used for recording the SAECG. The individual leads were then combined into a vector magnitude, using the square root of the sum of the square signals of each of the 3 leads. The conventional time-domain analysis was obtained using a high pass filter (f) at 40 Hz. Vector magnitude composite was analyzed for filtered QRS duration (fQRSD), low amplitude signal duration below 40 µV (LAS) and root mean square voltage in last 40 msec of the QRS (RMS-40). It has been suggested that the normal values for fQRSD are <114 msec, for LAS 40 <38 msec and for RMS-40 >20 µV (4). Although, there are no guidelines for abnormal SAECG in patients with ARVC/D, it is a common practice to categorize the SAECG as abnormal if two or more of these parameters are abnormal (7,8).
Electrocardiography
In addition to standard ECG measurements, the following parameters were evaluated: (1) the extent of T wave inversion in the precordial leads, (2) QRS duration in leads V1–V3 (normal < 110msec), (3) prolonged S wave in leads V1–V3 (normal ≤ 55 msec), (4) ratio of QRS duration in leads V2/V5 (normal < 1.2), (5) and presence of epsilon waves, defined as notches/deflections occurring after QRS complex (in ST segment) in at least one of the leads V1–V6 leads that occurred after the end of the QRS complex (9,10).
Cardiac Magnetic Resonance Imaging (cMRI)
cMRI was performed in 77 of the 87 ARVC/D probands according to a protocol described previously (11). Major morphologic abnormalities by cMRI were defined using 1994 Task Force Criteria as a) severe dilatation and reduction of RV ejection fraction with minimal or absent LV impairment; b) localized RV aneurysms (akinetic or dyskinetic areas with diastolic bulging) and/or c) severe segmental dilatation of the right ventricle. Minor morphologic abnormalities by cMRI were defined as a) mild global RV dilatation b) depressed RV ejection in the presence of normal LV function, and/or c) mild segmental dilatation of the RV and regional RV hypokinesia.
Statistical Analysis
All continuous variables are expressed as mean ± SD. Categorical variables are summarized as absolute number and relative frequencies. Continuous variables were compared using the Student’s t-test, and chi-square test was used for categorical variables. Receiver operator characteristic (ROC) curves were plotted to determine the value of using SAECG parameters for the diagnosis of ARVC/D. Optimal cut-off points for individual or combination of SAECG parameters were evaluated at 90–95% specificity. Statistical comparisons with p< 0.05 were considered statistically significant.
RESULTS
SAECG Findings
Table 2 compares the individual SAECG parameters of 87 ARVC/D patients and 103 matched controls. All 3 SAECG parameters, fQRSD, LAS and RMS-40, were significantly more abnormal in ARVC/D probands versus controls. The difference for each parameter was highly significant (P <.001). SAECG parameters, fQRSD (124.2 ± 27.4 msec vs. 118.0 ± 22.7 msec; p=0.28), LAS (52.4 ± 26.9 msec vs. 44.5 ± 22.4 msec; p=0.16) and RMS-40 (19.3 ± 15.4 µV vs. 27.4 ± 30.1 µV; p=0.18) were not significantly different in ARVC/D probands with and without desmosome gene mutations respectively.
Table 2.
SAECG parameters for ARVC/D probands and controls (mean ± SD)
| ARVC/D Probands (n=87) |
Controls (n=103) |
P-Value | |
|---|---|---|---|
| fQRSD (msec) | 119.6 ± 23.8 | 97.8 ± 8.7 | P<0.001 |
| LAS (msec) | 46.2 ± 23.7 | 24.4 ± 9.2 | P<0.001 |
| RMS-40 (µV) | 27.9 ± 36.3 | 50.4 ± 26.9 | P<0.001 |
Normal values: fQRSD<114 msec; LAS<38 msec; RMS-40>20 µV
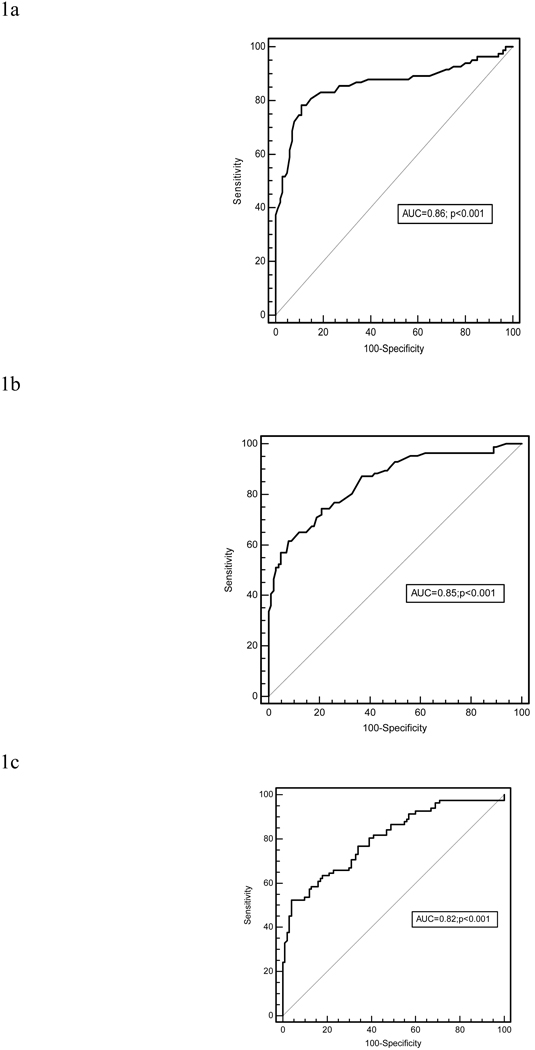
Figure 1 shows the ROC curve for the diagnosis of ARVC/D using the individual SAECG parameters. The diagnostic accuracy of fQRSD to identify those with and without ARVC/D is represented by the area under curve (AUC) and was 0.86. Similarly, the ROC curves for LAS (AUC=0.85) and RMS-40 (AUC=0.82) demonstrated high diagnostic accuracy. All were statistically significant (p<0.001) (Figures 1a–c).
Figure 1.
ROC curve for the diagnosis of ARVC/D using (a) fQRSD (msec), (b) LAS (msec), (c) RMS-40 (µV). (ARVC/D probands n=87; Controls n=103)
On the basis of the ROC curves, various cut-points for fQRSD and for the other parameters were evaluated to obtain the optimal sensitivity and specificity. In accordance with the Task Force criteria, we aimed to maximize specificity at 90–95%. All 3 individual SAECG parameters had high specificity (95%) for the diagnosis of ARVC/D. The sensitivity of fQRSD > 114 msec was 53%, LAS > 38 msec was 52% and RMS-40 < 20 µV was 52%.
The utility of using a combination of the traditional criteria (fQRSD>114msec, LAS>38msec, and RMS-40<20µV) was examined by using any 1 of the 3, 2 of the 3 or 3 of the 3 for diagnosis of ARVC/D. The sensitivity of using 1 of the 3 was 69% and the specificity was 92%; using 2 of the 3 had a sensitivity of 47% and a specificity of 95%; and using all 3 had a sensitivity of 33% and specificity of 100%.
Using 1 of the 3 criteria modified the diagnosis of the 25 borderline probands to affected in 9 (36%); 2 of the 3 criteria in 6 (24%) and 3 of the 3 criteria in 5 (20%). Several new cut-off points were examined based on the ROC curves. None of these alternative cut-off values had a superior sensitivity and specificity (95%) when compared to using traditional values of the SAECG. We therefore elected to utilize modified SAECG optimal criteria as defined by abnormalities of any 1 of the 3 traditional criteria (fQRSD> 114 msec, LAS> 38 msec, RMS-40<20 µV).
Clinical Presentation of ARVC/D and SAECG Findings
The presence of palpitations was not significantly different in the normal and abnormal SAECG groups (45% vs. 67%; p=0.4). Similarly, the frequency of abnormal SAECG was not significantly different with other clinical presentations (Table 3).
Table 3.
Association of clinical presentation by abnormal SAECG (using 1 of 3 criteria: fQRSD > 114msec, LAS > 38msec, RMS-40 < 20 µV)
| Clinical symptoms at presentation |
Normal SAECG (n=29) |
Abnormal SAECG (n=58) |
P-value |
|---|---|---|---|
| Asymptomatic | 9 (31%) | 11 (19%) | 0.2 |
| Syncope | 6 (21%) | 15 (26%) | 1.0 |
| Pre-syncope | 5 (17%) | 17 (29%) | 0.6 |
| Palpitations | 13 (45%) | 39 (67%) | 0.4 |
| Chest Pain | 3 (10%) | 8 (14%) | 1.0 |
| Other | 3 (10%) | 4 (7%) | 0.4 |
Association of Abnormal SAECG With Surface ECG Abnormalities
The presence of T wave inversions limited to V1–V3 (59% vs. 36%; p= 0.05) and beyond V3 (17% vs. 41%; p= 0.02) was significantly associated with abnormal SAECG. The presence of other ECG abnormalities was not associated with greater frequency relative to SAECG abnormality (Table 4).
Table 4.
Association of ECG abnormalities by abnormal SAECG (using 1 of 3 criteria: fQRSD > 114msec, LAS > 38msec, RMS-40 < 20 µV)
| ECG abnormalities | Normal SAECG (n=29) |
Abnormal SAECG (n=58) |
P-value |
|---|---|---|---|
| Negative T wave in V1 only* | 7 (24%) | 10 (17%) | 0.4 |
| Negative T wave in V1–V2 only* | 11 (38%) | 14 (24%) | 0.2 |
| Negative T wave in V1–V3 only* | 17 (59%) | 21 (36%) | 0.05 |
| Negative T wave in V1–V3† | 22 (76%) | 42 (72%) | 0.7 |
| Negative T wave beyond V3 | 5 (17%) | 24 (41%) | 0.02 |
| Negative T in V4–V6 only* | 0 (0%) | 3 (5%) | 0.5 |
| QRS in V1–V3 > 110 msec | 4 (14%) | 11 (19%) | 0.5 |
| QRS in V2 > 110 msec | 4 (14%) | 18 (31%) | 0.1 |
| V2/V5 ≥ 1.2 | 10 (34%) | 13 (22%) | 0.2 |
| S wave in V1–V3 ≥ 55 msec | 13 (45%) | 19 (33%) | 0.2 |
| QRS dispersion > 40 msec | 0 (0%) | 4 (7%) | 0.3 |
| QT dispersion > 65 msec | 2 (8%) | 6 (10%) | 1.0 |
Patients have negative T wave in the designated lead(s) and do not have negative T wave in the other precordial leads
Patients have negative T wave in V1–V3 and may or may not have negative T wave in V4–V6
VT Inducibility at Electrophysiologic Study and the SAECG
87 patients in the cohort underwent both programmed electrical stimulation and SAECG. At EPS, 50 (57%) were found to have inducible VT. There were no significant differences in each of the 3 SAECG parameters between those with and without VT inducibility (Table 5). For 1 of 3 abnormal SAECG parameters, the sensitivity was 76% and the specificity was 40%. These values resulted in a positive predictive value of 63% and a negative predictive value of 56%. When examining for 2 of 3 or 3 of 3 parameters, the positive and negative predictive values remained essentially unchanged.
Table 5.
SAECG parameters for ARVC/D probands with inducible VT and appropriate ICD therapy
| fQRSD (msec) | LAS (msec) | RMS-40 (µV) | |
|---|---|---|---|
| Induced VT | |||
| No (n=37) | 117.5 ± 25.5 | 43.4 ± 24.1 | 29.4 ± 17.6 |
| Yes (n=50) | 121.2 ± 22.5 | 48.3 ± 23.4 | 26.9 ± 45.3 |
| P=0.5 | P=0.3 | P=0.8 | |
| Appropriate ICD therapy | |||
| No (n=60) | 119.1 ± 28.8 | 49.0 ± 27.3 | 26.2 ± 40.5 |
| Yes (n=27) | 120.6 ± 20.2 | 43.5 ± 21.4 | 33.4 ± 37.8 |
| P=0.8 | P=0.4 | P=0.5 | |
Implantable Cardioverter-Defibrillator (ICD) Therapy for Sustained VT and the SAECG
Appropriate ICD therapy for sustained VT during follow-up (mean 3.6 ± 1.3 yrs) was documented in 27 patients (31%). The individual SAECG parameters did not significantly differ between those with and without appropriate ICD therapy (Table 5).
cMRI and SAECG
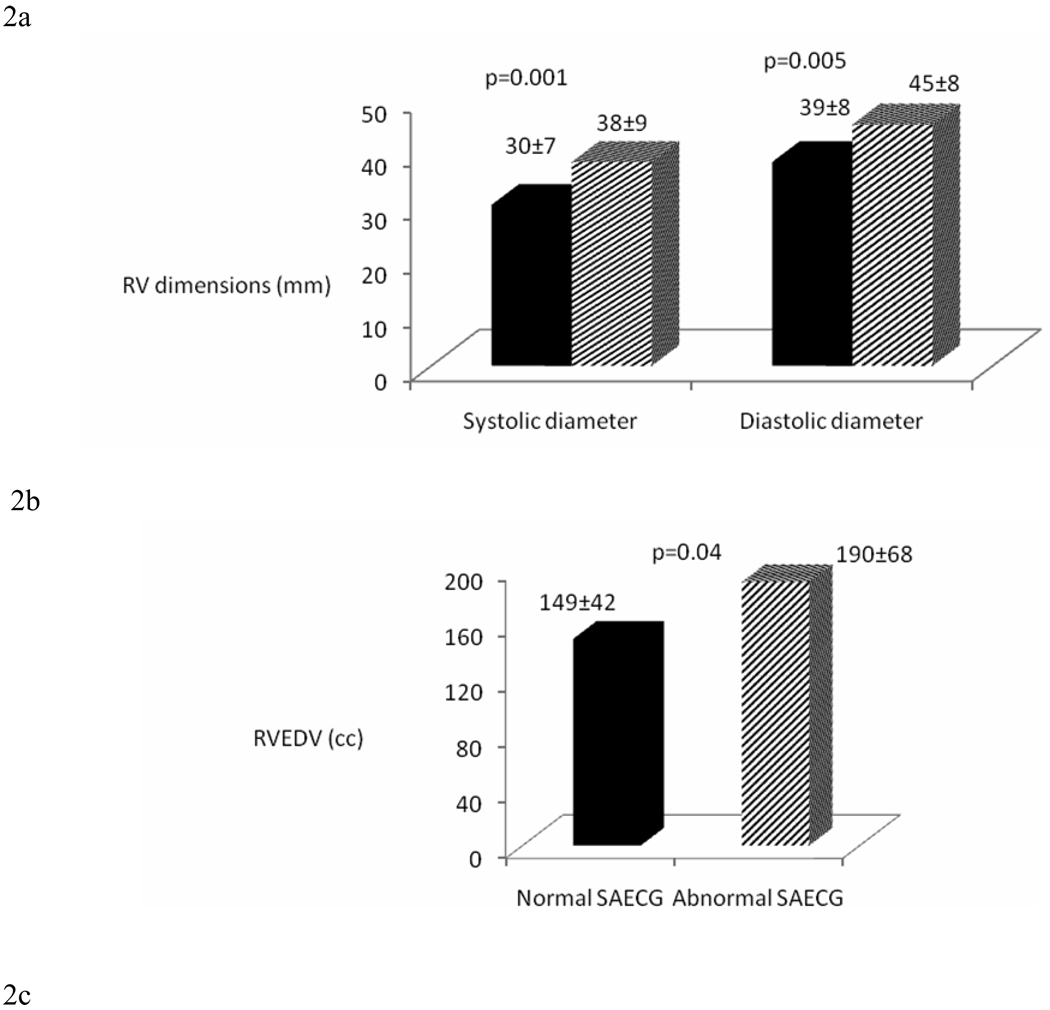
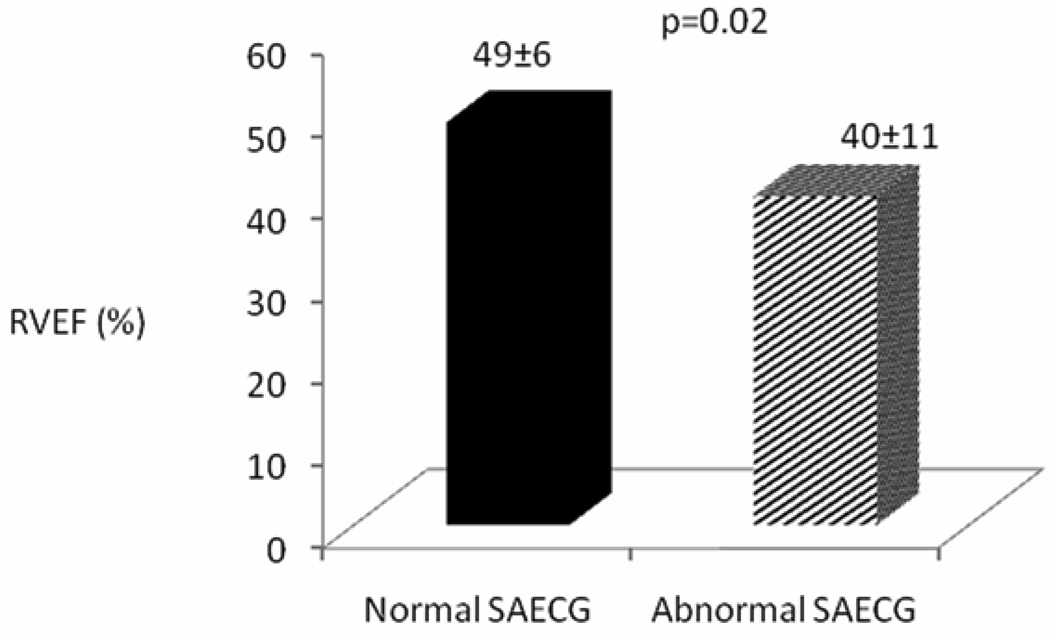
This sub study included 77 ARVC/D probands who had cardiac MRI. Based on cMRI results, major morphological abnormalities of the RV were present in 50/77 (65%) and minor abnormalities were present in 31/77 (40%) ARVC/D probands. ARVC/D probands with abnormal SAECG had significantly larger RV systolic and diastolic diameters (38 ± 9 mm vs. 30 ± 7 mm, p= 0.001; and 45 mm ± 8 vs. 39 ± 8 mm, p= 0.005, respectively), larger RV end-diastolic volumes (190 ± 68 cc vs. 149 ± 42 cc; p=0.04), and lower RV ejection fraction (40 ± 11% vs. 49 ± 6%; p=0.02) (Figure 2a–c).
Figure 2.
Comparison of (a) RV diastolic and systolic diameter (mm), (b) RV end-diastolic volume (ml) and RV ejection fraction (%) (c) in normal and abnormal SAECG (using 1 of 3 criteria: fQRSD > 114msec, LAS > 38msec, RMS-40 < 20 µV) by cMRI. (ARVC/D probands n=77)
DISCUSSION
This study is the largest study to date to examine the diagnostic value of SAECG in ARVC/D and the association of clinical parameters with abnormal SAECG. In the present study, the SAECG and its components, fQRSD, LAS and RMS-40, were highly associated with the diagnosis of ARVC/D. The sensitivity of using SAECG for diagnosis of ARVC/D was increased from 47% using 2 of the 3 criteria to 69% by using any 1 of the 3 criteria while maintaining high specificity of 90–95%. Abnormal SAECG as defined by this modified criteria was strongly associated with dilated RV volumes and decreased RV ejection fraction detected by cMRI. SAECG abnormalities did not vary with clinical presentation or reliably predict spontaneous or inducible VT, and had limited correlation with ECG findings.
SAECG detects delayed ventricular activation signals on the body surface that are referred to as late potentials (LP). LPs reflect the slow conduction in the ventricular myocardium and electrical potentials that extend beyond the activation time of normal myocardium (12, 13), a potential substrate for re-entrant arrhythmias. It has been reported that LPs in patients with ARVC/D has ranged from 50–100% (4, 14–15) with higher prevalence of LPs in patients with sustained VT. The cutoff values for LPs have been derived from studies in post-infarct patients with ischemic cardiomyopathy. These cut-off values have not been tested specifically in ARVC/D. In this study, SAECG data on ARVC/D probands provided detailed information to examine cutoff points to best define how the SAECG can be used for diagnosis.
In our cohort of patients with ARVC, n=38 probands (44 %) had T wave inversion in V1–V3 only and n=29 beyond V3 (33%), observations that significantly correlated with the presence of abnormal SAECG. The epsilon wave is specific but less sensitive and has been described 9–36% of ARVC/D (3, 16). The presence of epsilon wave (3% of ARVC/D probands) was present at a lower rate to those reported in other studies (3, 17–19). Nasir et al. proposed prolonged S-wave upstroke to baseline in V1–V3 ≥ 55 msec as the most prevalent ECG feature and this finding correlated with disease severity and induction of VT at electrophysiologic study. In that study, measurement of ECG intervals was done using digital calipers capable of measuring to within 1 msec after enlarging the ECG two times. The cutoff of >55 msec was based on the best value that differentiated ARVD/C from patients with idiopathic VT and normal controls (4). The presence of prolonged S wave upstroke (37% of genotyped ARVC/D probands) was lower than the previously reported rates of 60–95% (4, 18). The heterogeneity of the clinical presentations (all patients in study by Nasir et al. were symptomatic at presentation and 67% had inducible VT) and differences in the definition of the S wave may explain this difference.
cMRI an important imaging modality for the diagnosis of patients of ARVC/D. RV quantitative analysis as assessed by cMRI is useful in the diagnosis as well as the follow-up of ARVC/D patients (20–24). In this study, probands with SAECG abnormalities had increased RV end-systolic and end-diastolic diameters, RV end-diastolic volume and depressed right ventricular ejection fraction compared to those with normal SAECG. Thus abnormalities in structural and functional indices were accompanied by abnormal electrical substrate in our cohort.
The most common presentations of ARVC/D in studies have been palpitations, syncope and atypical chest pain (25). We studied the presenting symptoms of all ARVC/D probands and tabulated the frequency of abnormal SAECG using the modified criteria and hypothesized that certain clinical presentations, i.e. arrhythmia, may be more common with SAECG abnormalities. However, no clinical symptoms were associated with the presence of abnormal SAECG. This may be related to the fact that our cohort included newly diagnosed patients. Patients included in the other studies have included patients with long standing diagnosis
The utility of SAECG for predicting inducible VT in ARVC/D is uncertain. Nasir et al. reported that fQRSD of >110 msec was found to be predictive of inducible VT in ARVC/D with a positive predictive value of 95% and a negative predictive value of 82% (26). The presence of abnormal late potentials yielded a sensitivity of 62% and a specificity of 90% (26). In a small cohort of 34 patients, VT was inducible in 68% of cases with LPs as compared to 32% in non-inducible patients, yielding a PPV of 63% and a NPV of 93% (p<0.001) (27). Using the modified or traditional SAECG criteria in our study, the values for fQRSD, RMS, and LAS did not differ between the inducible and non-inducible patients yielding a low sensitivity and specificity. The rates of inducible VT in published reports were higher than in our cohort, 57%, which may explain the variations in sensitivity and specificity when using the SAECG.
Data are sparse in regard to whether ARVC/D with LPs on SAECG correlates with sustained VT. In the study by Pezawas et al, LPs on SAECG were highly correlated with spontaneous VT events (27). Folino et al. found that those with sustained VT events had a greater prolongation of fQRSD (130.3 msec vs. 116.9 msec, p<0.05) over an 8-year follow-up, however the presence of LPs was unable to predict arrhythmic events (28). Nava et al found that only a reduced RMS-40 correlated with VT events (15, 29). In our cohort, there was no association between an abnormal SAECG and appropriate ICD therapy for sustained VT. The individual SAECG parameters also did not differ between the two groups.
From our cMRI data and that reported by Nava et al, it appears that SAECG is related to severity of disease (15, 30). The SAECG did not correlate with induced VT. However the risk of VT is not entirely dependent on disease severity as shown by an autopsy study. Dalal et al found that among patients whose first presentation was sudden cardiac death, the RV was only mildly involved in 65% of cases (31). Among studies examining risk factors for appropriate ICD therapy in patients with ARVC/D, the results are discordant and disease severity is not consistently related to arrhythmic events (32–36). Many patients with ARVC/D have an early risk of VT/VF events, as documented by the patients who initially present with sudden cardiac death. The arrhythmic risk in ARVC/D is likely multifactorial, a combination of both deranged gap junctions and scar-induced micro and macro reentrant circuits (37, 38). For those patients with a low scar burden, the malfunctioning gap junctions could explain the arrhythmic risk. Another possible explanation for the discordance between SAECG and risk of arrhythmic events may be related to the location and the heterogeneity of VT observed in ARVC/D patients. Although the VT in ARVC/D is most often a macroreentrant mechanism, it is possible that microreentrant or focal mechanisms may not be associated with an abnormal SAECG (39–41).
Conclusions
The SAECG was shown to contribute to the diagnosis of ARVC/D in a well characterized population of ARVC/D patients with diagnosis within 3 years of onset. Using any 1 of the 3 SAECG criteria provided optimal sensitivity and specificity. This finding is incorporated in the current recent modification of the Task Force criteria (42, 43). The evidence that abnormal SAECG reflects functional abnormalities of the RV and RV enlargement suggests that the SAECG correlates with disease severity; however VT events were not more prevalent when the SAECG was more normal.
Acknowledgement
Dr. Steinberg is the Al-Sabah Endowed Director of the Arrhythmia Institute at St. Luke's and Roosevelt Hospitals
Funded by National Heart, Lung, and Blood Institute (NHLBI) U01 HL65594, HL65652 and HL65691
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors do not have any conflicts of interest to disclose.
References
- 1.McKenna WJ, Thiene G, Nava A, et al. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J. 1994;71:215–218. doi: 10.1136/hrt.71.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turrini P, Corrado D, Basso C, Nava A, Bauce B, Thiene G. Dispersion of ventricular depolarization-repolarization: a noninvasive marker for risk stratification in arrhythmogenic right ventricular cardiomyopathy. Circulation. 2001;103:3075–3080. doi: 10.1161/01.cir.103.25.3075. [DOI] [PubMed] [Google Scholar]
- 3.Peters STM. Diagnosis of arrhythmogenic right ventricular dysplasia-cardiomyopathy: value of standard ECG revisited. Ann Noninvasive Electrocardiol. 2003;8:238–245. doi: 10.1046/j.1542-474X.2003.08312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nasir K, Bomma C, Tandri H, et al. Electrocardiographic features of arrhythmogenic right ventricular dysplasia/cardiomyopathy according to disease severity: a need to broaden diagnostic criteria. Circulation. 2004;110:1527–1534. doi: 10.1161/01.CIR.0000142293.60725.18. [DOI] [PubMed] [Google Scholar]
- 5.Marcus FI, Towbin J, Zareba W, et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) a multidisciplinary study: Design and protocol. Circulation. 2003;107:2975–2978. doi: 10.1161/01.CIR.0000071380.43086.29. [DOI] [PubMed] [Google Scholar]
- 6.Marcus FI, Zareba W, Calkins H, et al. Arrhythmogenic right ventricular Cardiomyopathy/Dysplasia: Clinical Presentation and Diagnostic Evaluation: Results from the North American Multidisciplinary Study. Heart Rhythm. 2009;6:984–992. doi: 10.1016/j.hrthm.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cain ME, Arnsdorf MF. Signal-averaged electrocardiography. J Am Coll Cardiol. 1996;27:238–249. [PubMed] [Google Scholar]
- 8.Marcus FI, Zareba W, Sherrill D. Evaluation of Normal Values for Signal Averaged Electrocardiogram. J Cardiovasc Electrophysiol. 2007;18:231–233. doi: 10.1111/j.1540-8167.2006.00685.x. [DOI] [PubMed] [Google Scholar]
- 9.Zareba W, Turini P. ECG Manifestation of ARVC/D. In: Marcus FI, Nava A, Thiene G, editors. Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia – Recent Advances. Springer; 2007. pp. 121–128. [Google Scholar]
- 10.Wu S, Wang P, Hou Y, Yang P, Xiao Y, Zhan X. Epsilon wave in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Pacing Clin Electrophysiol. 2009;32:59–63. doi: 10.1111/j.1540-8159.2009.02176.x. [DOI] [PubMed] [Google Scholar]
- 11.Tandri H, Bomma C, Calkins H, Bluemke DA. Magnetic resonance and computed tomography imaging of arrhythmogenic right ventricular dysplasia. J Magn Reson Imaging. 2004;19:848–858. doi: 10.1002/jmri.20078. [DOI] [PubMed] [Google Scholar]
- 12.Breithardt G, Borggrefe M. Pathophysiological mechanisms and clinical significance of ventricular late potentials. Eur Heart J. 1986;7:364–385. doi: 10.1093/oxfordjournals.eurheartj.a062078. [DOI] [PubMed] [Google Scholar]
- 13.Steinberg JS, Regan A, Sciacca RR, Bigger JT, Jr, Fleiss JL. Predicting arrhythmic events after acute myocardial infarction using the signal-averaged electrocardiogram. Am J Cardiol. 1992;69:13–21. doi: 10.1016/0002-9149(92)90669-p. [DOI] [PubMed] [Google Scholar]
- 14.Nasir K, Rutberg J, Tandri H, Berger R, Tomaselli G, Calkins H. Utility of SAECG in arrhythmogenic right ventricle dysplasia. Ann Noninvasive Electrocardiol. 2003;8:112–120. doi: 10.1046/j.1542-474X.2003.08204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nava A, Folino AF, Bauce B, et al. Signal-averaged electrocardiogram in patients with arrhythmogenic right ventricular cardiomyopathy and ventricular arrhythmias. Eur Heart J. 2000;21:58–65. doi: 10.1053/euhj.1999.1733. [DOI] [PubMed] [Google Scholar]
- 16.Turrini P, Corrado D, Basso C, Nava A, Thiene G. Noninvasive risk stratification in arrhythmogenic right ventricular cardiomyopathy. Ann Noninvasive Electrocardiol. 2003;8:161–169. doi: 10.1046/j.1542-474X.2003.08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piccini JP, Nasir K, Bomma C, et al. Electrocardiographic findings over time in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am J Cardiol. 2005;96:122–126. doi: 10.1016/j.amjcard.2005.02.057. [DOI] [PubMed] [Google Scholar]
- 18.Peters S, Trummel M, Koehler B, Westermann KU. The value of different electrocardiographic depolarization criteria in the diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Electrocardiol. 2007;40:34–37. doi: 10.1016/j.jelectrocard.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Steriotis AK, Bauce B, Daliento L, et al. Electrocardiographic pattern in arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol. 2009;103:1302–1308. doi: 10.1016/j.amjcard.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Van der Wall EE, Kayser HW, Bootsma MM, de Roos A, Schalij MJ. Arrhythmogenic right ventricular dysplasia: MRI findings. Herz. 2000;25:356–364. doi: 10.1007/s000590050028. [DOI] [PubMed] [Google Scholar]
- 21.Pennell D, Casolo G. Right ventricular arrhythmia: emergence of magnetic resonance imaging as an investigative tool. Eur Heart J. 1997;18:1843–1845. doi: 10.1093/oxfordjournals.eurheartj.a015187. [DOI] [PubMed] [Google Scholar]
- 22.Prakasa KR, Dalal D, Wang J, et al. Feasibility and variability of three dimensional echocardiography in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am J Cardiol. 2006;97:703–709. doi: 10.1016/j.amjcard.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 23.Tandri H, Castillo E, Ferrari VA, et al. Magnetic resonance imaging of arrhythmogenic right ventricular dysplasia: sensitivity, specificity, and observer variability of fat detection versus functional analysis of the right ventricle. J Am Coll Cardiol. 2006;48:2277–2284. doi: 10.1016/j.jacc.2006.07.051. [DOI] [PubMed] [Google Scholar]
- 24.Bluemke DA, Krupinski EA, Ovitt T, et al. MR Imaging of arrhythmogenic right ventricular cardiomyopathy: morphologic findings and interobserver reliability. Cardiology. 2003;99:153–162. doi: 10.1159/000070672. [DOI] [PubMed] [Google Scholar]
- 25.Hulot J-S, Jouven X, Empana J-P, Frank R, Fontaine G. Natural history and risk stratification of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation. 2004;110:1879–1884. doi: 10.1161/01.CIR.0000143375.93288.82. [DOI] [PubMed] [Google Scholar]
- 26.Nasir K, Tandri H, Rutberg J, et al. Filtered QRS duration on signal-averaged electrocardiography predicts inducibility of ventricular tachycardia in arrhythmogenic right ventricle dysplasia. Pacing Clin Electrophysiol. 2003;26:1955–1960. doi: 10.1046/j.1460-9592.2003.00302.x. [DOI] [PubMed] [Google Scholar]
- 27.Pezawas T, Stix G, Kastner J, Schneider B, Wolzt M, Schmidinger H. Ventricular tachycardia in arrhythmogenic right ventricular dysplasia/cardiomyopathy: clinical presentation, risk stratification and results of long-term follow-up. Int J Cardiol. 2006;107:360–368. doi: 10.1016/j.ijcard.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 28.Folino AF, Bauce B, Frigo G, Nava A. Long-term follow-up of the signal-averaged ECG in arrhythmogenic right ventricular cardiomyopathy: correlation with arrhythmic events and echocardiographic findings. Europace. 2006;8:423–429. doi: 10.1093/europace/eul035. [DOI] [PubMed] [Google Scholar]
- 29.Turrini P, Angelini A, Thiene G, et al. Late potentials and ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol. 1999;83:1214–1219. doi: 10.1016/s0002-9149(99)00062-4. [DOI] [PubMed] [Google Scholar]
- 30.Bauce B, Basso C, Nava A. Signal-averaged electrocardiographic parameter progression as a marker of increased electrical instability in two cases with an overt form of arrhythmogenic right ventricular cardiomyopathy. Pacing Clin Electrophysiol. 2002;25:362–364. doi: 10.1046/j.1460-9592.2002.00362.x. [DOI] [PubMed] [Google Scholar]
- 31.Dalal D, Nasir K, Bomma C, et al. Arrhythmogenic right ventricular dysplasia: a United States experience. Circulation. 2005;112:3823–3832. doi: 10.1161/CIRCULATIONAHA.105.542266. [DOI] [PubMed] [Google Scholar]
- 32.Corrado D, Leoni L, Link MS, et al. Implantable cardioverter-defibrillator therapy for prevention of sudden death in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2003;108:3084–3091. doi: 10.1161/01.CIR.0000103130.33451.D2. [DOI] [PubMed] [Google Scholar]
- 33.Wichter T, Paul M, Wollmann C, et al. Implantable cardioverter/defibrillator therapy in arrhythmogenic right ventricular cardiomyopathy: single-center experience of long-term follow-up and complications in 60 patients. Circulation. 2004;109:1503–1508. doi: 10.1161/01.CIR.0000121738.88273.43. [DOI] [PubMed] [Google Scholar]
- 34.Roguin A, Bomma CS, Nasir K, et al. Implantable cardioverter-defibrillators in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol. 2004;43:1843–1852. doi: 10.1016/j.jacc.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 35.Piccini JP, Dalal D, Roguin A, et al. Predictors of appropriate implantable defibrillator therapies in patients with arrhythmogenic right ventricular dysplasia. Heart Rhythm. 2005;2:1188–1194. doi: 10.1016/j.hrthm.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 36.Woźniak O, Włodarska EK. Prevention of sudden cardiac deaths in arrhythmogenic right ventricular cardiomyopathy: how to evaluate risk and when to implant a cardioverter-defibrillator? Cardiol J. 2009;16:588–591. [PubMed] [Google Scholar]
- 37.Saffitz JE, Hames KY, Kanno S. Remodeling of gap junctions in ischemic and nonischemic forms of heart disease. J Membr Biol. 2007;218:65–71. doi: 10.1007/s00232-007-9031-2. [DOI] [PubMed] [Google Scholar]
- 38.Oxford EM, Musa H, Maass K, Coombs W, Taffet SM, Delmar M. Connexin43 remodeling caused by inhibition of plakophilin-2 expression in cardiac cells. Circ Res. 2007;101:703–711. doi: 10.1161/CIRCRESAHA.107.154252. [DOI] [PubMed] [Google Scholar]
- 39.Blomstrom-Lundqvist C, Hirsch I, Olsson SB, Edvardsson N. Quantitative analysis of the signal-averaged QRS in patients with arrhythmogenic right ventricular dysplasia. Eur Heart J. 1988;9:301–312. doi: 10.1093/oxfordjournals.eurheartj.a062501. [DOI] [PubMed] [Google Scholar]
- 40.Miljoen H, State S, de Chillou C, et al. Electroanatomic mapping characteristics of ventricular tachycardia in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Europace. 2005;7:516–524. doi: 10.1016/j.eupc.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Reithmann C, Hahnefeld A, Remp T, et al. Electroanatomic mapping of endocardial right ventricular activation as a guide for catheter ablation in patients with arrhythmogenic right ventricular dysplasia. Pacing Clin Electrophysiol. 2003;26:1308–1316. doi: 10.1046/j.1460-9592.2003.t01-1-00188.x. [DOI] [PubMed] [Google Scholar]
- 42.Marcus FI, McKenna WJ, Sherrill D, Basso C, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marcus FI, McKenna WJ, Sherrill D, Basso C, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force criteria. Eur Heart J. 2010;31:806–814. doi: 10.1093/eurheartj/ehq025. [DOI] [PMC free article] [PubMed] [Google Scholar]