Abstract
Previous emotion regulation research has been successful in altering aversive emotional reactions. It is unclear, however, whether such strategies can also efficiently regulate expectations of reward arising from conditioned stimuli, which can at times be maladaptive (for example, drug cravings). Using a monetary reward-conditioning procedure with cognitive strategies, we observed attenuation in both the physiological (skin conductance) and neural correlates (striatum) of reward expectation as participants engaged in emotion regulation.
The expectation of a potential reward elicits positive feelings and aids in the learning of environmental cues that predict future rewards. Central to this process is the role of the striatum, a multifaceted structure that is involved in affective learning and general reward processing across species1–3, which is particularly engaged when potential rewards are predicted or anticipated4–6. However, this striatum signal can also be maladaptive and correlates with drug specific cravings7, potentially increasing urges to partake in risk-seeking behavior8. Given this, it is important to understand how to regulate or control the positive feelings associated with reward expectation. One promising method for examining this is the utilization of cognitive strategies commonly used in both social9 and clinical8 disciplines. Emotion regulation strategies, for example, have been successful in attenuating aversive emotional reactions that are elicited by various types of negative stimuli10, a pattern that is also reflected in neural regions involved in emotion, such as the amygdala, with both behavioral and subcortical neural modulations possibly mediated by prefrontal cortical regions11,12. Less is known, however, about the efficacy of such strategies with positive, anticipatory feelings that are elicited by a conditioned appetitive stimulus. The goal of our study was to investigate the influence of emotion regulation strategies on the physiological and neural correlates underlying expectations of reward. We hypothesized that cognitive strategies should successfully decrease arousal elicited by reward-conditioned cues while attenuating reward-related activity in the striatum.
Fifteen participants who gave written consent were presented with an adapted version of a classical conditioning procedure that has been previously used to study aversive learning13. Specifically, participants were presented for 4 s with two conditioned stimuli, a blue and a yellow square, that either predicted (CS+) or did not predict (CS−) a potential monetary reward ($4.00; Fig. 1a). Prior to each trial, participants were also given a written cue for 2 s that instructed them to either attend to the stimulus (that is, “think of the meaning of the blue square, such as a potential reward”) or regulate their emotional response to the stimulus (that is, “think of something blue in nature that calms you down, such as the ocean”). These antecedent-focused emotion regulation strategies are postulated to work early in the emotional process to influence the final emotional output9. Notably, there are a variety of emotion regulation strategies, ranging from active reinterpretation to more diversion-based approaches, which share similar and distinctive neural mechanisms (for a review, see ref. 10). The particular instructions used in the current procedure were adapted from a previous emotion regulation study11 but involve more general processes of imagery given the nature of the conditioned stimuli (neutral squares versus detailed photos). Therefore, participants were exposed to two types of conditioned stimuli (CS+ and CS−) and two types of instruction (attend and regulate). Participants were aware of the contingencies and were well-practiced in the instructions before commencing a scanning session. Skin conductance responses (SCRs) were acquired at the onset of each conditioned stimulus as a behavioral measure of physiological arousal that may relate to reward anticipation (see Supplementary Methods online for further methodological details).
Figure 1.
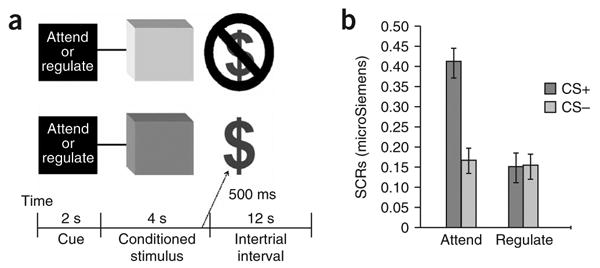
Depiction of task-related events and behavioral results. (a) Participants were presented with two conditioned stimuli (CS, colored squares depicted in figure as dark and light gray squares). The CS+ trial (dark gray) predicted a potential monetary reward ($4.00), whereas the CS− trial (light gray) predicted no monetary reward ($0). Prior to conditioned stimuli onset, the cues ‘Attend’ or ‘Regulate’ served as instructions for that trial. (b) SCRs from 15 participants showing an interaction between type of conditioned stimulus (CS+, CS−) and type of instruction (attend, regulate; ± s.e.m.).
We obtained written informed consent from 15 participants before the experiment. A repeated-measures ANOVA with the SCRs revealed a main effect of type of conditioned stimuli (CS+, CS−; F1,14 = 15.48, P < 0.001), a main effect of type of instruction (attend, regulate; F1,14 = 14.75, P < 0.002) and an interaction between the two factors (F1,14 = 23.51, P < 0.0001; Fig. 1b). This behavioral measure suggests that emotion regulation strategies effectively decreased arousal that was linked to the anticipation of a potential reward typically elicited by a conditioned stimulus.
On the basis of previous studies of reward processing6 and emotion regulation11, we sought to identify a priori regions of interest (ROIs) that were involved in general expectation of reward (CS+ versus CS− attend trials) and potential regulation sites in the prefrontal cortex (regulate versus attend trials). The first contrast (CS+ versus CS− attend trials) yielded regions that are typically observed in classical conditioning procedures14 and reward expectation6, including activation in the striatum bilaterally (P < 0.005; see Fig. 2a and Supplementary Table 1 online for specific regions and values). For each striatum ROI, mean beta weights were then extracted from each participant and input into repeated-measures ANOVAs for further analysis. We observed interactions between type of conditioned stimuli and type of instruction in both left (F1,14 = 16.70, P < 0.001) and right (F1,14 = 8.97, P < 0.01) striatum ROIs. In addition, post hoc t tests in the left striatum ROI (similar in the right) showed a differential response between attend and regulate CS+ trials (t(14) = 2.35, P < 0.05), but not CS− trials (t(14) = 1.42, P = 0.18), suggesting that emotion regulation strategies effectively attenuated increases in BOLD response typically observed by reward-predicting conditioned stimuli (see Supplementary Results online for additional discussion and analysis).
Figure 2.
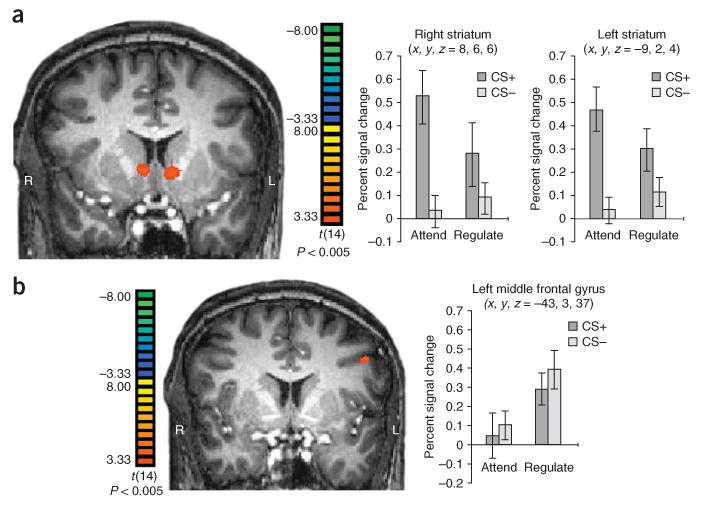
Neuroimaging results. (a) Activation of the striatum bilaterally identified by a contrast of attend CS+ versus CS− trials (expectation of reward). Mean beta weights from both ROIs showed an interaction between type of condition stimulus (CS+, CS−) and instruction (attend, regulate; ± s.e.m.). (b) Mean beta weights for left middle frontal gyrus ROI showing elevated responses during the regulate CS+ compared with the attend CS+ condition (± s.e.m.).
The second contrast (regulate versus attend trials) yielded a variety of cortical regions that have been previously implicated in emotion regulation10,11,15 (Supplementary Table 2 online), although the precise foci of activation in these cortical regions differs slightly between studies as a result of factors such as differences in stimuli or techniques used10. We observed activation in the left middle frontal gyrus (BA 6/9; Fig. 2b), left inferior frontal gyrus (BA 6/44) and left inferior parietal cortex (BA 40). Notably, activation also occurred in the left subgenual cingulate cortex (BA 25), a region previously linked to fear extinction and regulation14.
Our finding that emotion regulation strategies can successfully modulate physiological and neural correlates underlying the expectation of reward in a conditioning procedure is a first step to understanding how top-down modulation may effectively control positive emotions and eventual urges that may arise (for example, drug craving). This is consistent with recent neuroimaging studies suggesting that cognitive strategies modulate subcortical regions involved in aversive emotional processing10–12, further extending our results to the domain of emotional responses elicited by conditioned stimuli that predict potential rewards. Often, such reward expectations lead to impulsive decisions that are detrimental to an individual (for example, drug seeking behavior). Future investigations will target the influence of emotion regulation on subsequent decision-making.
Supplementary Material
Acknowledgments
The authors wish to acknowledge R. Jou for assistance. This work was supported by a James S. McDonnell foundation grant to E.A.P., the Beatrice and Samuel A. Seaver Foundation, and a US National Institute on Drug Abuse grant to M.R.D. (DA022998).
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
Author Contributions: M.R.D. and E.A.P. designed the fMRI experiment. M.M.G. and M.R.D. collected the data. M.R.D. and M.M.G. analyzed the physiological and neuroimaging data. M.R.D. and E.A.P. wrote the manuscript.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/
References
- 1.Balleine BW. Physiol Behav. 2005;86:717–730. doi: 10.1016/j.physbeh.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 2.Delgado MR. Ann NY Acad Sci. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- 3.Robbins TW, Everitt BJ. Semin Neurosci. 1992;4:119–127. [Google Scholar]
- 4.Kawagoe R, Takikawa Y, Hikosaka O. Nat Neurosci. 1998;1:411–416. doi: 10.1038/1625. [DOI] [PubMed] [Google Scholar]
- 5.Kirsch P, et al. Neuroimage. 2003;20:1086–1095. doi: 10.1016/S1053-8119(03)00381-1. [DOI] [PubMed] [Google Scholar]
- 6.Knutson B, Adams CM, Fong GW, Hommer D. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinha R, et al. Psychopharmacology (Berl) 2005;183:171–180. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- 8.Potenza MN, Winters KC. J Gambl Stud. 2003;19:7–10. doi: 10.1023/a:1021219012324. [DOI] [PubMed] [Google Scholar]
- 9.Gross JJ. J Pers Soc Psychol. 1998;74:224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- 10.Ochsner KN, Gross JJ. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 12.Goldin PR, McRae K, Ramel W, Gross JJ. Biol Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delgado MR, Labouliere CD, Phelps EA. Soc Cogn Affect Neurosci. 2006;1:250–259. doi: 10.1093/scan/nsl025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Hamann S. J Cogn Neurosci. 2007;19:776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


