Abstract
PURPOSE
To investigate in a longitudinal study whether foveolar choroidal blood flow changes are associated with the development of choroidal neovascularization (CNV) in AMD.
METHODS
Relative foveolar choroidal blood velocity (ChBVel), volume (ChBVol), and flow (ChBFlow) were assessed in 135 patients with AMD, at baseline and then annually with laser Doppler flowmetry. All study eyes had visual acuity of 20/40 or better and no CNV at the time of enrollment. Comparison of foveolar choroidal circulatory measurements at baseline and their change before the development of CNV was made between eyes that had CNV and those that did not.
RESULTS
CNV developed in 28 eyes during the study. Baseline average foveolar ChBVol and ChBFlow in these eyes were 24% (P < 0.0001) and 20% (P = 0.0007) lower than that observed in the 165 eyes in which CNV did not develop. In the eyes with CNV, foveolar ChBVol and ChBFlow decreased by 9.6% and 11.5% before the formation of CNV, whereas in the eyes that did not, they increased by 6.7% (P = 0.006) and 2.8% (P = 0.004), respectively. Eyes with lower baseline foveolar ChBFlow were more likely (risk ratio = 3.47, 95% CI: 1.24 – 8.70) to show visual loss of three or more lines than were eyes with a higher baseline ChBFlow (P = 0.005).
CONCLUSIONS
The development of CNV and visual loss are associated with lower choroidal circulatory parameters at baseline. In addition, the results suggest that decreases in the foveolar choroidal circulation precede the development of CNV in AMD and may play some role in its development.
The development of choroidal neovascularization (CNV) in patients with age-related macular degeneration (AMD) often leads to a significant decrease in visual function. Several mechanisms have been proposed to explain the pathogenesis of CNV, and they include, among others, the presence of senescence changes in the RPE–Bruch’s membrane–photoreceptor complex, the accumulation of oxidative damage, and the development of ischemia of the outer retina.
The purpose of our study was to investigate whether abnormalities of the foveolar choroidal circulation are associated with the development of CNV in AMD. Throughout the body, angiogenesis is often triggered by ischemia and hypoxia.1,2 Similarly, several ocular diseases, such as diabetic retinopathy, neovascular glaucoma, and vein occlusions, are associated with ocular ischemia and the development of neovascularization. A similar mechanism may also play a role in the development of CNV in AMD.
Previous cross-sectional studies of patients with AMD performed in our laboratory3,4 have suggested that foveolar choroidal blood flow is decreased in patients with dry AMD and that eyes with more severe AMD features have lower foveolar choroidal blood flow, giving some support to the ischemia theory.
We report the results of a prospective study in a cohort of patients with AMD at risk of development of CNV. Our purpose was to investigate in a longitudinal fashion whether foveolar choroidal blood flow decreases before the development of CNV and whether changes in blood flow precede the formation of CNV.
METHODS
This was a longitudinal, observational, prospective study in which choroidal circulatory measurements in the fovea were performed yearly in a cohort of 135 patients with AMD (193 study eyes). One hundred one patients had a year-1 follow-up visit, 89 had a year-2 visit, 87 had a year-3 visit, 59 had a year-4 visit, and 27 had a year-5 visit. The decrease in the number of patients with increasing years of follow-up was mainly due to a staggered, gradual recruitment and not to loss to follow-up.
At the beginning of the study, all 193 study eyes of the enrolled patients with AMD had visual acuity of 20/40 or better, steady fixation, pupillary dilatation of 5 mm or more, absence of other intraocular diseases, refraction error less than ± 7 D, and intraocular pressure (IOP) ≤ than 21 mm Hg. At baseline, all study eyes had typical ophthalmoscopic features of AMD and no signs of CNV. According to the inclusion and exclusion criteria, one or both eyes of the same patient were considered study eyes. All patients with AMD were older than 50 years (mean, 70 ± 9.2 [SD]; range, 50–86).
Detailed explanations of the study purposes and protocol were given to each study participant. All subjects were asked to sign appropriate informed and HIPAA (Health Insurance Portability and Accountability Act) consent forms approved by the human experimental committee of our institution. The tenets of the Declaration of Helsinki were observed.
Ocular foveolar choroidal blood flow (ChBFlow) was determined, and slit lamp and fundus ophthalmic examinations were performed at baseline and then at all subsequent annual visits. Color fundus photographs were also obtained at baseline and then every 2 years. Photographs were graded in a masked fashion by trained readers in the Fundus Photography Reading Center of the University of Pennsylvania. Grading of drusen characteristics and RPE changes in the study eyes was performed according to the Complications of Age Related Macular Degeneration Trial protocol. The presence of CNV was determined by the reading center in a masked fashion.
After visual acuity determination and slit lamp examination were performed, pupils were dilated with tropicamide 1% (Alcon, Fort Worth, TX) and phenylephrine hydrochloride 10% (Sanofi Winthrop, New York, NY). An ophthalmic examination of the fundus was performed, and then, measurements of relative foveolar choroidal blood velocity (ChBVel), volume (ChBVol), and flow (ChBFlow) were obtained with the laser Doppler flowmetry (LDF) technique (Oculix Instrument, Huntingdon Valley, PA). Detailed descriptions of the method have been published.5–8 A diode laser beam (670 nm) with an intensity of 20 mW was delivered through a fundus camera (model TRC; Topcon, Tokyo, Japan). Diameter of the probing laser beam was approximately 200 µm.
ChBVel and ChBVol are independent parameters obtained by instrument. ChBVel is proportional to the mean velocity of the RBCs within the volume sampled by the laser light and ChBVol is proportional to the number of RBCs. ChBFlow is calculated by the instrument from these two parameters, according to the following formula: ChBFlow = constant × ChBVel × ChBVol.9
Subjects were asked to fixate on the probing laser beam to determine foveolar ChBFlow. These measurements mainly represent choriocapillaris flow, as discussed previously by Riva et al.5 To monitor the position of the laser on the foveola, an area of the posterior retina (30° in diameter) was constantly illuminated at a wavelength of 570 nm, with a retinal irradiance of approximately 0.03 mW/cm2. Proper fixation was confirmed by constant observation through the fundus camera. In each subject, three continuous 30-second measurements of the choroidal circulation in the fovea were obtained while subjects were seated in the darkened room. In patients with newly developing CNV in the study eye during the course of the study, all subsequent yearly measurements were discontinued. This was due to the decrease in vision that usually accompanies the development of CNV.
Brachial artery systolic and diastolic blood pressures (BPs and BPd, respectively) were determined by sphygmomanometry (Accutorr 1A; Datascope, Paramus, NJ) immediately after blood flow measurements, while subjects were still seated. IOP was then measured by applanation tonometry. The mean brachial artery pressure (BPm) was calculated according to the following formula:
| (1) |
Perfusion pressure (PP) for the study eye was estimated according to the following formula
| (2) |
Analysis of the blood flow data was performed by a masked observer using a NeXT computer (no longer manufactured) with software specifically developed for the analysis of Doppler signals from ocular tissues.6 Only those parts of the recordings that showed stable circulatory parameters were selected for analysis. Approximately 12 seconds of measurements were included in the analysis of each study eye, and their average was used for the data analysis.
To assess the reproducibility of the blood flow data, we calculated a coefficient of variability (CV) for each study eye derived from three subsequent measurements. The CV was calculated using the formula CV = (SD/mean) × 100. CV was 10.3% ± 7.2% for ChBFlow.
Comparisons of baseline patient and eye characteristics including baseline choroidal circulatory measurements in the fovea were performed between patients in whom CNV developed versus those in whom it did not, using the two-group, nonpaired t-test for the comparison of means and the χ2 test for the comparison of proportions. To estimate a change in foveolar choroidal circulatory parameters right before CNV formation, we calculated the percentage of change as the percent difference between two measurements from the two most recent visits preceding CNV formation in the CNV eyes. The time elapsed between these two measurements was 1 year. For the eyes with no CNV, we calculated percentage of change from all pairs of measurements obtained during two adjacent visits separated by 1 year interval and used their average percentage of change to compare with that of CNV eyes. This ensured that the time elapsed between both measurements was similar in the two groups of eyes.
The association of baseline foveolar choroidal circulatory parameters with three or more lines of visual acuity loss was evaluated by using repeated logistic regression analysis. The correlation from paired eyes of the same patient was adjusted by using generalized estimating equations (as executed by PROC GENMOD in SAS ver. 9.1; SAS Inc., Cary, NC). A two-sided P < 0.05 was considered to be statistically significant.
RESULTS
During the course of our study, CNV developed in 28 eyes of 18 patients and 165 eyes of 117 patients showed no evidence of CNV. Of the 135 patients included in the study, both eyes were studied in 58 and one eye in 77. The fellow eyes of these 77 patients were not included mainly because their low visual acuity. Of the 77 eyes ineligible for the study, only 14 (18.2%) were ineligible due to CNV. Of 117 subjects who did not have CNV in the study eye, 12 (10.3%) had it in the fellow eye at baseline. Of 18 patients with CNV in the study eye, 2 (11.1%) subjects had CNV in the fellow eye at baseline. The difference between these two groups was not statistically significant (P = 0.91).
Comparisons of patient and eye characteristics between those in whom CNV developed and those who did not are summarized in Tables 1 and 2. Eyes in which CNV developed had higher perfusion pressure (P = 0.04) and were more likely to have drusen size ≥250 µm (P = 0.004) compared with eyes in which CNV did not develop.
TABLE 1.
Comparison of Patient and Eye Characteristics between Those with and without Newly Developed CNV
| Non-CNV (n = 117 Subjects) |
CNV (n = 18 Subjects) |
P | |
|---|---|---|---|
| Subject characteristics | |||
| Mean age ± SE (y) | 70.2 ± 0.83 | 73.3 ± 2.04 | 0.18† |
| Mean BP ± SE (mm Hg) | 94.8 ± 1.23 | 99.6 ± 2.67 | 0.15† |
| Male (%) | 57 (47.9) | 6 (31.6) | 0.32‡ |
| Current smoking (%) | 6 (5.04) | 1 (5.26) | 1.00‡ |
| Hypertension (%)* | 58 (48.7) | 12 (63.2) | 0.21‡ |
| (n = 165 Eyes) | (n = 28 Eyes) | ||
| Eye characteristics | |||
| Mean IOP ± SE (mm Hg) | 15.1 ± 0.32 | 15.4 ± 0.61 | 0.70§ |
| Mean PP ± SE (mm Hg) | 47.8 ± 0.85 | 52.0 ± 1.86 | 0.04§ |
| Mean Spherical equivalent ± SE (D) | 0.27 ± 0.22 | 1.26 ± 1.07 | 0.36§ |
PP, perfusion pressure.
Defined as having a history of systemic hypertension or of receiving long-term antihypertensiion therapy.
From two-group t-test.
Fisher exact test.
From generalized estimating equation to adjust the correlation from paired eyes.
TABLE 2.
Comparison of Baseline Fundus Characteristics between Eyes with Later-Developing CNV and Non-CNV Eyes
| Drusen Characteristics | Non-CNV (n =165 Eyes) Yes (%) |
CNV (n =28 Eyes) Yes (%) |
P* |
|---|---|---|---|
| Largest drusen size ≥250 µm | 57 (38.3) | 20 (71.4) | 0.004 |
| Predominant drusen size ≥125 µm | 54 (36.7) | 15 (55.6) | 0.10 |
| ≥10% Area covered by drusen | 20 (13.6) | 7 (25.0) | 0.18 |
| Drusen confluence ≥ 10 pairs | 46 (31.1) | 11 (40.7) | 0.36 |
| Focal hyperpigmentation ≥250 µm | 14 (9.3) | 3 (10.7) | 0.81 |
| RPE depigmentation | 8 (5.4) | 2 (7.1) | 0.71 |
From generalized estimating equation to adjust the correlation from paired eyes.
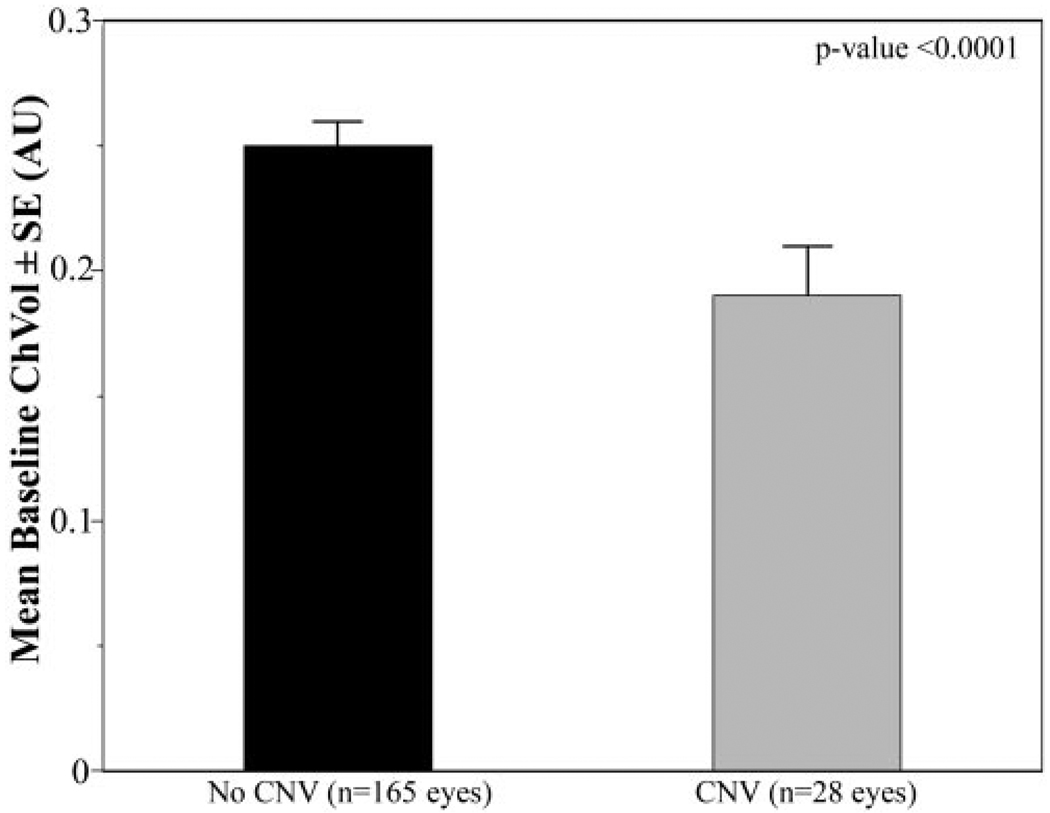
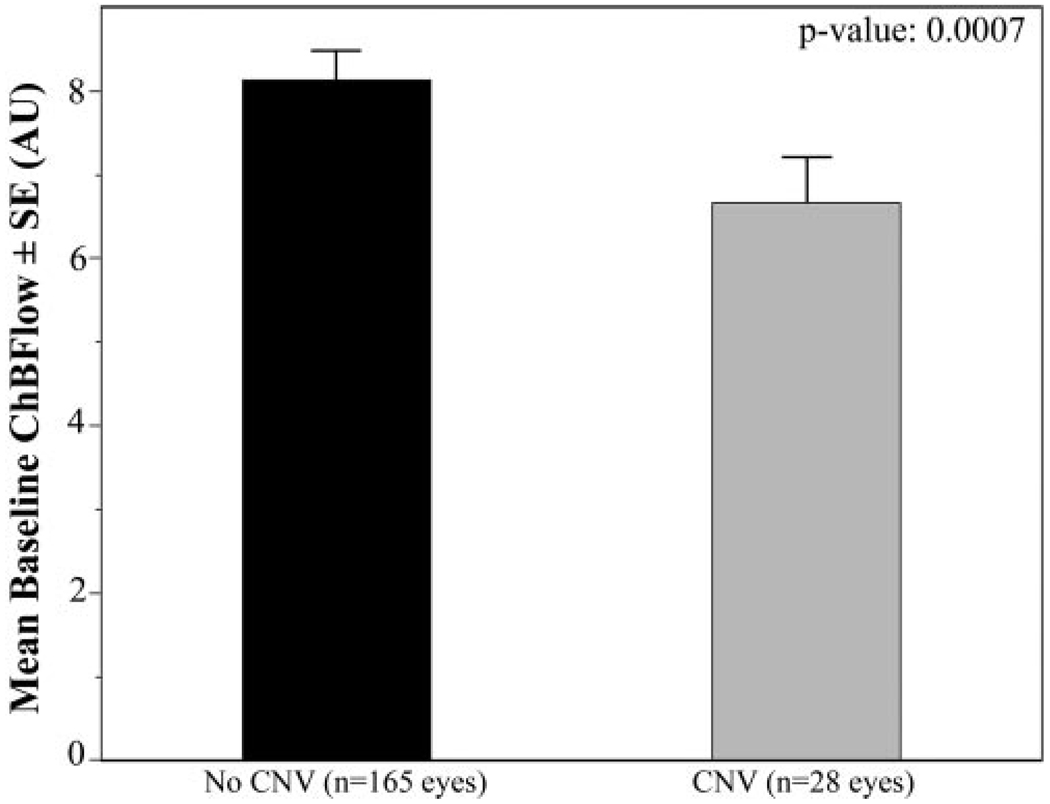
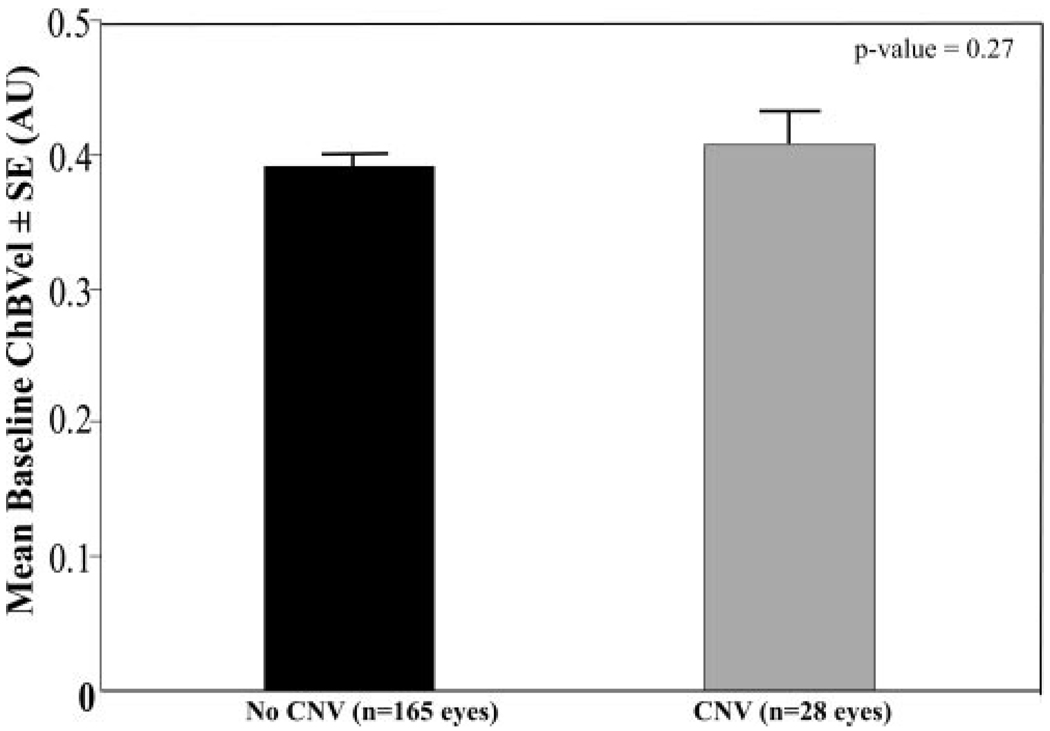
Comparison of baseline circulatory measurements for the eyes that had later development of CNV and eyes in which it did not develop during the study are shown in Table 3. At baseline, eyes in which CNV developed later had a 24% lower foveolar ChBVol (P < 0.0001; Fig. 1) and 20% lower foveolar ChBFlow (P = 0.0007; Fig. 2) than eyes in which it did not develop. There was no statistically significant difference in ChBVel between these two groups of eyes (Fig. 3).
TABLE 3.
Comparison of Baseline Circulatory Parameters between Eyes with CNV Formation and Non-CNV Eyes
| Non- CNV (n = 165 Eyes) |
CNV (n = 28 Eyes) |
P* | |
|---|---|---|---|
| ChBVol | 0.25 (0.01) | 0.19 (0.01) | <0.0001 |
| ChBVel | 0.39 (0.01) | 0.41 (0.02) | 0.27 |
| ChBFlow | 8.07 (0.33) | 6.39 (0.39) | 0.0007 |
Data are the mean (SE).
From two group t-test with correlation from paired eyes adjusted using the generalized estimating equation.
FIGURE 1.
Comparison of baseline foveolar ChBVol between eyes with or without newly developing CNV. Results were shown in arbitrary units (AU). Error bar, SE. Student’s t-test showed a statistically significant difference between the two groups (P < 0.0001).
FIGURE 2.
Comparison of baseline foveolar ChBFlow between eyes with and without newly developing CNV. Results are shown in arbitrary units (AU). Error bars, SE. Student’s t-test showed a statistically significant difference between the two groups (P = 0.0007).
FIGURE 3.
Comparison of baseline foveolar ChBVel between eyes with and without newly developing CNV. Results are shown in arbitrary units (AU). Error bars, SE. Student’s t-test showed a statistically significant difference between the two groups (P = 0.27).
In 19 of the 28 eyes with newly developed CNV, we had two or more choroidal circulatory measurements before the development of CNV. For these 19 eyes, we calculated the change in circulatory parameters before the appearance of CNV as the difference in measurements between the two most recent preceding visits. In the other nine eyes, we only had one measurement before the appearance of CNV, and therefore we could not calculate such a change. Of note, measurements obtained in eyes that already had subfoveal CNV were not included in the data analysis, because such measurements are usually not reliable due to the patient’s poor fixation. Furthermore, the flow measurements obtained in the eyes with subfoveal CNV represent mostly the blood flowing through the neovascular net, and therefore may not shed light on the choriocapillaris circulatory changes that led to the formation of CNV.
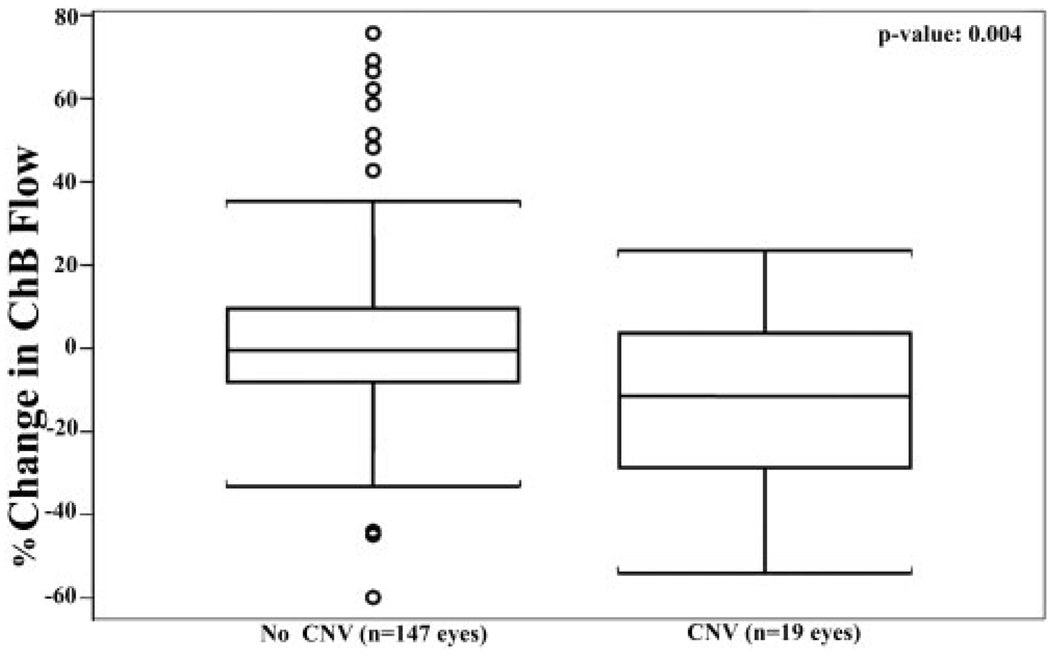
In 14 (74%) of these 19 eyes we observed yearly decreases in foveolar ChBFlow that ranged from 3% to 54%. Furthermore, in the 19 eyes with new CNV, ChBVol and ChBFlow decreased in a year by an average of 9.6% and 11.5%, respectively, before the formation of CNV, whereas in the 147 eyes with no CNV that had at least two choroidal circulatory measurements in the fovea, flow and volume increased in a year by an average of 6.7% and 2.8%, respectively (Fig. 4). In comparison to the average change observed in a year in eyes without new CNV, a significant decrease in foveolar ChBFlow and ChBVol was observed before its formation (univariate analysis, P = 0.006 for ChBVol and P = 0.004, for ChBFlow, respectively).
FIGURE 4.
Comparison of percentage of change in foveolar ChBFlow during two annual visits before CNV appeared in AMD eyes and yearly percentage of change in ChBFlow in eyes in which CNV it did not develop. Student’s t-test shows a statistically significant difference between the two groups (P = 0.004). Each box plot includes the upper extreme (whisker, excluding outliers indicated as circles outside the box), upper quartile (top portion of box), median (horizontal line in box), lower quartile (bottom portion of box), and lower extreme (whisker).
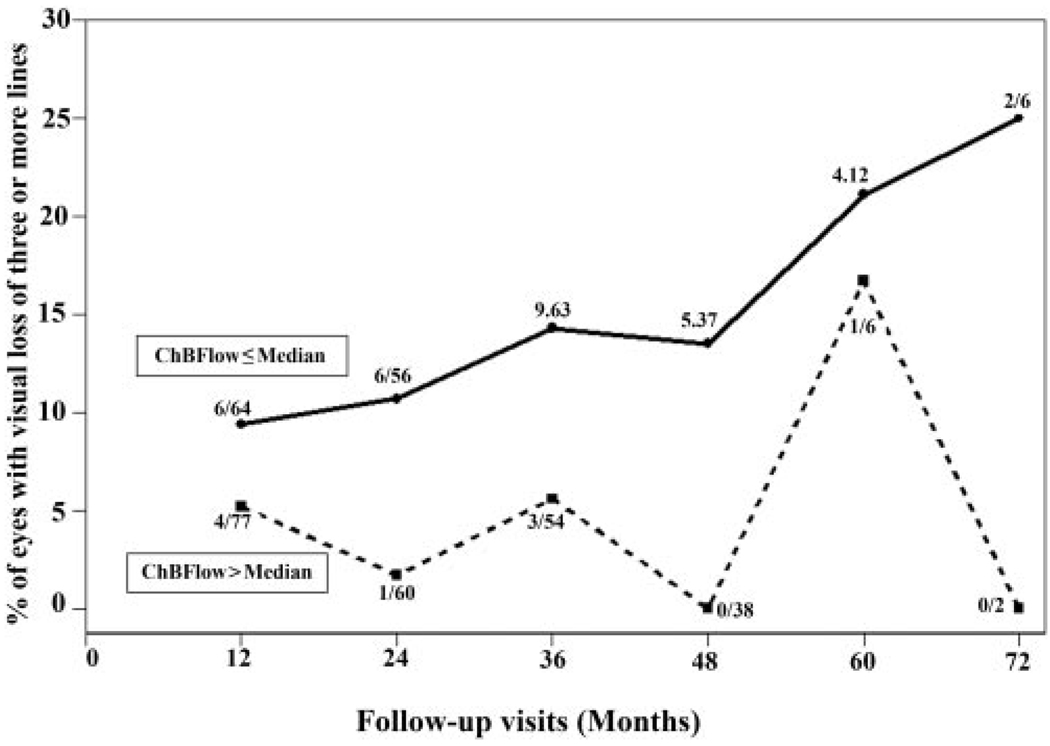
Although we have little data on patients who have lost at least three lines of visual acuity in our study, our limited data suggest that eyes with lower baseline foveolar ChBFlow (≤ median) are more likely (risk ratio = 3.47, 95% CI: 1.24 – 8.70) to have visual loss of three or more lines than are eyes with higher (> median) baseline foveolar ChBFlow (P = 0.005; Fig. 5), and eyes with lower baseline foveolar ChBVol (≤ median) are more likely (risk ratio = 2.31, 95% CI: 1.02–4.64) to have visual loss of three or more lines than are eyes with higher (> median) baseline foveolar ChBVol (univariate analysis, P = 0.03).
FIGURE 5.
All eyes in the study were divided into two groups according to the baseline foveolar ChBFlow. One group included all eyes with ChBFlow below or equal to the median (n = 92 eyes) and the other group included all patients with ChBFlow above the median (n = 91 eyes). The graph shows for each follow-up visit a comparison of the percentage of eyes with loss of visual acuity of three or more lines between the eyes with baseline ChBFlow below (solid line) and above (dotted line) the median. The numerator represents the number of eyes with visual acuity loss of three or more lines, and the denominator represents the number of eyes that completed the specific follow-up visit. Patients with ChBFlow below or equal to the median were three times more likely to have three or more lines of visual acuity loss within the study follow-up.
DISCUSSION
Our results suggest that, at the beginning of our longitudinal study, foveolar ChBVol and ChBFlow were significantly lower in AMD eyes with later-developing CNV than in those eyes in which CNV did not develop CNV during the length of our study. Furthermore, before the appearance of CNV, the affected eyes show marked choroidal circulatory decreases in the fovea. These decreases are significantly larger than the changes observed during a similar length of time in eyes without formation of CNV.
These data support our previous cross-sectional studies3,4 showing decreased foveolar choroidal circulatory parameters in AMD eyes and an association between more advanced nonexudative AMD and lower choroidal circulatory parameters in the fovea.
Of interest, our results also show that eyes with lower ChBFlow in the fovea at baseline have about three times higher risk of three or more lines of visual loss than eyes with higher circulatory parameters (P = 0.005), suggesting again that a decreased foveolar choroidal circulation may be an independent risk factor for the development of visual impairment in AMD.
Our current longitudinal results increase our understanding of the mechanisms involved in CNV formation by showing that foveolar choroidal circulatory decreases precede the development of CNV. These decreases were not observed in eyes without formation of CNV during the study period. Although our results do not show direct evidence that ischemia has a role in the pathogenesis of CNV, they strongly suggest that this may be the case.
The results from our previous cross-sectional study,4 which showed an association between worse AMD and decreased blood flow, did not allow us to establish whether the decrease in flow caused the deterioration of AMD or whether it was a result of the AMD progression. The results from our current longitudinal study show that the decreases in flow preceded the formation of CNV, strongly suggesting that these changes may have a role in the development of CNV.
Because we used noninvasive technology to assess this hypothesis in human subjects, we cannot answer with certainty the question of whether circulatory abnormalities are the cause or the effect of the disease process. However, our prospective study in which we assessed changes in foveolar ChBFlow that occurred before the development of CNV suggests a potentially causative role. In this sense, our study may support the ischemic theory, which suggests that a decrease in the delivery and diffusion of oxygen or metabolites may have a role in the formation of CNV, in a similar way to what occurs in other diseases leading to the formation of neovascularization throughout the body.
The choroidal circulation has a very high rate of blood flow and a relatively low oxygen extraction and is the only source of nourishment and waste removal for the outer retina. These conditions are necessary because the choroid must provide a large amount of oxygen and other nutrients across a relatively long distance to the highly metabolic inner portion of the photoreceptors. Thus, it seems logical to assume that the marked decrease in ChBFlow that we observed in the study eyes before CNV formation may create conditions of ischemia and hypoxia in the outer retina, triggering the formation of new vessels through activation of hypoxia-inducible genes such as vascular endothelial growth factor (VEGF).10–12
Several reports have suggested a role for decreased ChBFlow in the formation of CNV. Preliminary work of Ross et al.13 has shown an association between the location of macular choroidal watershed vascular filling zones detected by fluorescein angiography and choroidal neovascular membranes. During angiography, watershed-filling zones correspond to the last areas of the choroid that fill with the dye. These areas are most probably the boundaries between adjacent choroidal lobules. The presence of CNV in close proximity to these areas, which are the most prone to ischemia and hypoxia in conditions of decreased ChBFlow, suggests that ischemia may have a role in the development of AMD-related CNV. Similarly, Guagnini et al.14 in a recent case report study have shown an association between areas of early choroidal hypofluorescence on angiography, which presumably correspond to areas of acute choroidal ischemia, and later development of large neovascular membranes.
Because we compared the baseline circulatory parameters of a group of eyes with newly developed CNV with a group with no CNV formation it was important to ascertain whether the two groups were similar at baseline. As can be seen in Table 2, although age and systemic blood pressure were slightly higher in the CNV eyes, the differences were not statistically significant. A significantly higher perfusion pressure was observed, however, in the group in which CNV developed. This finding is in accordance with our previous study in which we reported that patients with AMD with higher systemic blood pressure had decreased foveolar choroidal circulatory parameters.15 This decreased foveolar ChBFlow in patients with systemic hypertension may explain the higher risk of development of CNV in patients with systemic hypertension and also the results of the Macular Photocoagulation Study, which reported a reduced beneficial effect of photocoagulation therapy for CNV in this condition.16
At baseline, we found no significant difference in the proportion of patients who had CNV in the fellow eye between patients who did and did not have CNV develop in the study eye. However, we detected a significantly higher proportion of eyes that had drusen >250 µm in the group with developing CNV (71%) than in the non-CNV group (38%). Similarly, predominant drusen ≥125 µm were observed in 56% of eyes with CNV formation versus 37% in the non-CNV group. Finally, drusen confluence and focal hyperpigmentation also seemed to be more prominent in the group of eyes with CNV formation.
More severe expression of AMD increases the accumulation of waste materials and debris in the form of basal laminar deposits. This process is probably associated with a thickening of Bruch’s membrane, leading to an increase in the diffusion distance across the RPE-Bruch’s membrane complex. A combination of decreased choroidal perfusion and increased distance between the choriocapillaries and the photoreceptors could result in a situation in which the choroid cannot supply an adequate amount of oxygen to the outer retina. This hypothesis is strongly supported by the work of Linsenmeier et al.17 and our previous work showing that ChBFlow decreases with age, even in normal subjects.18
Possibly, the increased accumulation of debris and thickening of the Bruch’s complex could also impede the passage of trophic substances that may be important in maintaining the viability of the choriocapillaries. A reduction in the diffusion of such trophic substances could have a role in causing the decrease in choriocapillaries flow observed in our study in eyes with AMD.
Several other studies have shown evidence suggesting decreases in ChBFlow in AMD eyes with CNV that is in agreement with our findings: Mori et al.19 reported that pulse amplitude is lower in patients with exudative AMD than in patients with nonexudative AMD and age-matched control subjects; Chen et al.20 showed that pulse amplitude was significantly decreased in eyes with disciform scars in comparison to the contralateral eyes with drusen; Uretmen et al.21 used color Doppler imaging and reported decreased ophthalmic artery and temporal posterior ciliary artery velocities in eyes with CNV in comparison to eyes with nonexudative AMD; and finally, Rigas et al. (IOVS 2004;45:ARVO E-Abstract 3110) found an increased resistance to blood flow in eyes with neovascular AMD.
The concept of decreased choroidal circulation in patients with AMD has been suggested by additional studies that further support our results. Histopathology conducted in AMD has shown reduction in the cross-sectional area of the choriocapillaris, 22,23 reduction of density and diameter of the macular choriocapillaris,24 and narrowing of the lumen and loss in the cellularity of the choriocapillaris.25 Choroidal perfusion abnormalities have been detected by Pauleikhoff et al.26,27 and Boker et al.28 using fluorescein angiography. Friedman et al.,29 using Doppler imaging reported that blood velocity was decreased and blood velocity pulsatility was increased in the central retinal artery and short posterior ciliary arteries in AMD. This finding led them to conclude that an increase in the resistance of the choroidal vasculature caused by a decrease in the compliance of the sclera and the choroidal vessels may play an important role in development of AMD. Using the same technique, Ciulla et al.30 showed decreased blood velocities in the retrobulbar vasculature, and by using scanning laser ophthalmoscopy and indocyanine green angiography, they also reported increased heterogeneity of choroidal filling time in patients with nonexudative AMD.31 All these studies strongly point toward a role for vascular impairment and ischemia in the etiology of AMD.
The laser Doppler flowmetry technique provides assessments of relative blood circulatory measurements. Because changes in the optical properties of the measured tissues may affect the hemodynamic measurements, the comparison of relative measurements between normal subjects and patients with AMD has some limitations.3 The concordance between our results and previous histopathologic13–25 and hemodynamic studies26–31 further supports our findings and our conclusions. In addition, these limitations are probably of lesser importance in our current prospective longitudinal study in which we measure changes occurring in time in the same eye.
In summary, our study suggests that development of CNV and visual loss are associated with lower choroidal circulatory parameters at baseline and also that decreases in the foveolar choroidal circulation precede the development of CNV in AMD and may play some role in its development. These findings strongly support the role of hypoperfusion and possibly ischemia as an inciting factor for CNV formation and deterioration of visual function. This mechanism probably acts in conjunction with the other etiological mechanisms that have been proposed such as senescence, oxidative damage, and chronic low-grade inflammatory response.32
Acknowledgments
Supported by National Eye Institute Grants EY12769 and 5 P30 EY01583, the Vivian Simkins Lasko Research Fund, the Nina C. Mackall Trust, and an unrestricted grant from Research to Prevent Blindness.
Footnotes
Disclosure: T.I. Metelitsina, None; J.E. Grunwald, None; J.C. DuPont, None; G.-S. Ying, None; A.J. Brucker, None; J.L. Dunaief, None
Presented at the annual meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, May 2007.
References
- 1.Shima DT, Adams AP, Ferrara N, et al. Hypoxic induction of endothelial cell growth factors in retinal cells: identification and characterization of vascular endothelial growth factor (VEGF) as the mitogen. Mol Med. 1995;1:182–193. [PMC free article] [PubMed] [Google Scholar]
- 2.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 3.Grunwald JE, Hariprasad SM, DuPont J, et al. Foveolar choroidal blood flow in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1998;39:385–390. [PubMed] [Google Scholar]
- 4.Grunwald JE, Metelitsina TI, Dupont JC, Ying GS, Maguire MG. Reduced foveolar choroidal blood flow in eyes with increasing AMD severity. Invest Ophthalmol Vis Sci. 2005;46:1033–1038. doi: 10.1167/iovs.04-1050. [DOI] [PubMed] [Google Scholar]
- 5.Riva CE, Cranstoun SD, Grunwald JE, Petrig BL. Choroidal blood flow in the foveal region of the human ocular fundus. Invest Ophthalmol Vis Sci. 1994;35:4273–4281. [PubMed] [Google Scholar]
- 6.Riva CE, Harino S, Petrig BL, Shonat RD. Laser-Doppler flowmetry in the optic nerve. Exp Eye Res. 1992;55:499–506. doi: 10.1016/0014-4835(92)90123-a. [DOI] [PubMed] [Google Scholar]
- 7.Petrig BL, Riva CE. Optic nerve head laser Doppler flowmetry: principles and computer analysis. In: Kaiser HJ, Flammer J, Hendrickson P, editors. Ocular Blood Flow. Basel, Switzerland: Karger; 1996. pp. 120–127. [Google Scholar]
- 8.Riva CE, Mendel M, Petrig BL. Flicker-induced optic nerve blood flow change. In: Kaiser HJ, Flammer J, Hendrickson P, editors. Ocular Blood Flow. Basel, Switzerland: Karger; 1996. pp. 128–137. [Google Scholar]
- 9.Riva CE. Basic principles of laser Doppler flowmetry and application to the ocular circulation. Int Ophthalmol. 2001;23:183–189. doi: 10.1023/a:1014433913240. [DOI] [PubMed] [Google Scholar]
- 10.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 12.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 13.Ross RD, Barofsky JM, Cohen G, Baber WB, Palao SW, Gitter KA. Presumed macular choroidal watershed vascular filling, choroidal neovascularization, and systemic vascular disease in patients with age-related macular degeneration. Am J Ophthalmol. 1998;125:71–80. doi: 10.1016/s0002-9394(99)80237-2. [DOI] [PubMed] [Google Scholar]
- 14.Guagnini AP, Snyers B, Kozyreff A, Levecq L, De Potter P. Choroidal neovascularization correlated with choroidal ischemia. Arch Ophthalmol. 2006;124:1063. doi: 10.1001/archopht.124.7.1063. [DOI] [PubMed] [Google Scholar]
- 15.Metelitsina TI, Grunwald JE, DuPont JC, Ying GS. Effect of systemic hypertension on foveolar choroidal blood flow in age related macular degeneration. Br J Ophthalmol. 2006;90:342–346. doi: 10.1136/bjo.2005.082974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macular Photocoagulation Study Group. Laser photocoagulation for juxtafoveal choroidal neovascularization: five-year results from randomized clinical trials. Arch Ophthalmol. 1994;112:500–509. [PubMed] [Google Scholar]
- 17.Linsenmeier RA, Padnick-Silver L. Metabolic dependence of photoreceptors on the choroid in the normal and detached retina. Invest Ophthalmol Vis Sci. 2000;41:3117–3123. [PubMed] [Google Scholar]
- 18.Grunwald JE, Hariprasad SM, DuPont J. Effect of aging on foveolar choroidal circulation. Arch Ophthalmol. 1998;116:150–154. doi: 10.1001/archopht.116.2.150. [DOI] [PubMed] [Google Scholar]
- 19.Mori F, Konno S, Hikichi T, Yamaguchi Y, Ishiko S, Yoshida A. Pulsatile ocular blood flow study: decreases in exudative age related macular degeneration. Br J Ophthalmol. 2001;85:531–533. doi: 10.1136/bjo.85.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen SJ, Cheng CY, Lee AF, et al. Pulsatile ocular blood flow in asymmetric exudative age related macular degeneration. Br J Ophthalmol. 2001;85:1411–1415. doi: 10.1136/bjo.85.12.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uretmen O, Akkin C, Erakgun T, Killi R. Color Doppler imaging of choroidal circulation in patients with asymmetric age-related macular degeneration. Ophthalmologica. 2003;217:137–142. doi: 10.1159/000068559. [DOI] [PubMed] [Google Scholar]
- 22.Sarks SH. XXIII Concilium Ophthalmologicum, Kyoto, 1978. Amsterdam: Excerpta Medica; 1978. Changes in the region of the choriocapillaries in aging and degeneration; pp. 228–238. [Google Scholar]
- 23.Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye. 1988;2:552–577. doi: 10.1038/eye.1988.106. [DOI] [PubMed] [Google Scholar]
- 24.Ramrattan RS, van der Schaft TL, Mooy CM, de Bruijn WC, Mulder PG, de Jong PT. Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994;35:2857–2864. [PubMed] [Google Scholar]
- 25.Korenzweig AB. Changes in the choriocapillaries associated with senile macular degeneration. Ann Ophthalmol. 1977;9:753–764. [PubMed] [Google Scholar]
- 26.Pauleikhoff D, Chen JC, Chisholm IH, Bird AC. Choroidal perfusion abnormality with age-related Bruch’s membrane change. Am J Ophthalmol. 1990;109:211–217. doi: 10.1016/s0002-9394(14)75989-6. [DOI] [PubMed] [Google Scholar]
- 27.Pauleikhoff D, Spital G, Radermacher M, Brumm GA, Lommatzsch A, Bird AC. A fluorescein and indocyanine green angiographic study of choriocapillaris in age-related macular disease. Arch Ophthalmol. 1999;117:1353–1358. doi: 10.1001/archopht.117.10.1353. [DOI] [PubMed] [Google Scholar]
- 28.Boker T, Fang T, Steinmetz R. Refractive error and choroidal perfusion characteristics in patients with choroidal neovascularization and age-related macular degeneration. Ger J Ophthalmol. 1993;2:10–13. [PubMed] [Google Scholar]
- 29.Friedman E, Krupsky S, Lane AM, et al. Ocular blood flow velocity in age-related macular degeneration. Ophthalmology. 1995;102:640–646. doi: 10.1016/s0161-6420(95)30974-8. [DOI] [PubMed] [Google Scholar]
- 30.Ciulla TA, Harris A, Chung HS, et al. Color Doppler imaging discloses reduced ocular blood flow velocities in nonexudative age-related macular degeneration. Am J Ophthalmol. 1999;128:75–80. doi: 10.1016/s0002-9394(99)00061-6. [DOI] [PubMed] [Google Scholar]
- 31.Ciulla TA, Harris A, Kagemann L, et al. Choroidal perfusion perturbations in non-neovascular age related macular degeneration. Br J Ophthalmol. 2002;86:209–213. doi: 10.1136/bjo.86.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spaide RF, Armstrong D, Browne R. Continuing medical education review: choroidal neovascularization in age-related macular degeneration: what is the cause? Retina. 2003;23:595–614. doi: 10.1097/00006982-200310000-00001. [DOI] [PubMed] [Google Scholar]