Abstract
Laparoscopic cholecystectomy remains the standard treatment for cholelithiasis. Ever increasing number of patients with myriad of medical illness is being treated by this technique. However, significant concern prevails among the surgical community regarding its safety in patients with cardiac co-morbidity. Patients with significant cardiac dysfunction and multiple co-morbidities were prospectively evaluated. Patients were assessed by cardiologists and anesthesiologists and laparoscopic cholecystectomy was performed. Patient demographics, details of peri-operative management and post-operative complications were studied. Between March 2005 and January 2009, 28 patients (M:F = 21:7) with mean age of 60 years (range 26–78) and having significant cardiac dysfunction had undergone laparoscopic cholecystectomy. Of these, 24 patients were in NYHA class-II, while 4 belonged to class-III. Left ventricular ejection fraction, as recorded by transthoracic echocardiography, was 20–30% in 13 (46%) patients and 30–40% in the rest 15 (54%). In addition, 13 (46%) patients had regional wall motion abnormalities, 11 (39%) patients had cardiomyopathy, 2 (7%) patients had valvular heart disease while 12 (43%) patients had prior cardiac interventions. Following laparoscopic cholecystectomy, hypertension (3), tachyarrhythmia (4) and bradycardia (1) were the commonest events encountered. One patient required laparotomy to deal with peritonitis in the immediate postoperative period and succumbed to myocardial infarction, but all other patients made an uneventful recovery. With appropriate cardiological support, laparoscopic cholecystectomy may be safely performed in patients with significant cardiac dysfunction.
Keywords: Laparoscopy, Cholecystectomy, Cardiac dysfunction
Introduction
Since the introduction of laparoscopic cholecystectomy by Philip Mouret in 1987, the technique was rapidly accepted by the surgical community [1]. The appeal of diminished pain and fatigue, early return to normal activities and superior cosmesis has made it a popular surgery [2]. Previous abdominal surgery, acute cholecystitis [3], morbid obesity [4], old age [5] and pregnancy [6] were traditional contraindications for laparoscopic cholecystectomy. However, in the recent years, development of surgical skills and better understanding of the pathophysiology of pneumoperitoneum have made it possible to offer laparoscopic cholecystectomy to patients suffering from myriad of medical illness. Nonetheless, concerns remain about the safety of this technique in patients with cardiac co-morbidity. During laparoscopy, positive pressure pneumoperitoneum using carbon dioxide could have deleterious effects on the cardiovascular system [7]. Therefore, standard surgical text books often cite patients with cardiac dysfunction a relative contraindication to laparoscopic cholecystectomy [3]. Similar anxiety also prevails amongst the surgical and anaesthetist community and laparoscopic cholecystectomy is often discouraged in patients with significant cardiac diseases. On the contrary, the physiological stress following minimally invasive surgery is lesser as compared to patients undergoing open cholecystectomy [8–10]. This begs the question whether the purported risk of pneumoperitoneum could be offset by the diminished stress following minimally invasive surgery, thereby bringing the patients with cardiac co-morbidity within the ambit of laparoscopic cholecystectomy. This prospective study was undertaken in a tertiary care cardiac hospital to evaluate the safety of laparoscopic cholecystectomy in patients with ischemic heart disease and significant cardiac dysfunction.
Materials and Methods
Data Collection
The minimally invasive unit was started in March 2005. Since inception, patient demographics, operative data and post-operative course of all patients were prospectively recorded in a computerised database.
Pre-operative Assessment
Patients with significant ischemic heart disease were evaluated by resting ECG and transthoracic echocardiography. They were subsequently evaluated by cardiologists and anaesthesiologists. The patients were grouped according to the New York Heart Association (NYHA) functional classification system [11]. For this study, patients belonging to NYHA II and III were included. Associated systemic diseases if present were optimized during out patient visits. Patients on antiplatelet drugs were asked to stop these medicines 5 days before surgery. Beta-blockers were started preoperatively in those who were not receiving such drugs previously. Patients on anti-coagulants were switched over to some form of heparin according to standard dosage schedule and were taken up for surgery when international normalized ratio (INR) fell below 1.5. All patients were premedicated with Nitrazepam (10 mg), Ondansetron (4 mg) and Omeprazole (20 mg) in the night before surgery and cardiac medications, except angiotensin converting enzyme inhibitors, were continued till the morning of surgery.
Operative Management
Laparoscopic cholecystectomy was performed early in the morning after a minimum of 4 hours of fasting. Venous access was obtained with an 18 G peripheral venous cannula. High risk patients also underwent radial artery and central venous cannulation for invasive haemodynamic monitoring. In patients who have had permanent pacemaker implanted, the mode was changed to fixed mode prior to surgery. Arrangement of temporary pacing, defibrillator and syringes with pre-loaded life saving drugs were kept ready. After pre-oxygenation with 100% oxygen, anaesthesia was induced with Fentanyl (2–3 mcg/kg) and Sevoflurane. Vecuronium (0.1 mg/kg) was used for intubation while anaesthesia was maintained with Oxygen, Isoflurane, intermittent Vecuronium and Fentanyl. Heart rate, blood pressure, oxygen saturation, ECG and end-tidal CO2 (ETCO2) were monitored continuously (Philips®, V24E, Boeblingen, Germany). Intravenous crystalloid solution administered cautiously. All patients were mechanically ventilated and ETCO2 was maintained between 30–35 mmHg (Datex-Ohmeda®, Madison, USA). Standard 4-port laparoscopic cholecystectomy was performed. In one patient with valve replacement and tricuspid regurgitation (Fig. 1), the presence of an enlarged liver till the umbilicus required the camera port to be placed low in the left iliac fossa. The initial infra-umbilical camera port was introduced using the open technique [12]. Carbon dioxide insufflation was initiated and maintained at 5 litres/min and other ports were introduced under vision. The higher limit of intra-abdominal pressure was kept at 8 mmHg and surgery was performed in 15°–20° reverse Trendelenburg position with right tilt. Upon completion of surgery, peritoneal carbon dioxide was released very slowly.
Fig. 1.
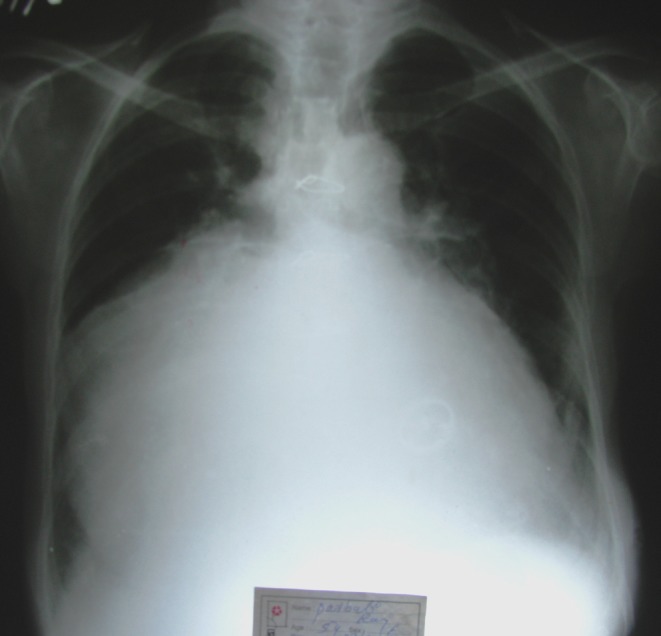
Chest X-Ray showing massive cardiomegaly in a patient with tricuspid regurgitation
Post-operative Management
After the procedure, patients were closely monitored in the post-anaesthetic care unit for 4 to 5 hrs. Majority of the patients were shifted to the ward, while those with troubled intra-operative course were shifted to ITU for overnight observation. Adequate post-operative analgesia was ensured by intravenous Fentanyl (1 mcg/kg/dose) in the immediate post-operative period and oral Diclofenac Sodium (1 mg/kg/dose) was administered subsequently. Intravenous fluid was continued till the evening while oral intake was commenced after 4–5 hours. Anticoagulants were started according to well described guidelines.
Results
Between March 2005 and January 2009, a total of 1248 patients underwent laparoscopic cholecystectomy. Of these, 77 patients gave history of ischaemic heart disease, 11 patients had dilated cardiomyopathy, well maintained on cardiac medicines, 38 had undergone coronary artery bypass grafting while 22 patients had angioplasty and stenting in the past. Amongst this heterogenous group, 28 patients belonging to NYHA grade II (24 patients) and grade III (4 patients) were included in this study (Table 1). The mean age was 60 years (range 26–78) with a striking male preponderance. Twelve (43%) patients had some kind of cardiac intervention prior to surgical referral while the rest were under medical management. Two patients had bifascicular block but did not require temporary pacemaker during the perioperative period (Table 2). Transthoracic echocardiography showed a left ventricular ejection fraction of 20–30% in 13 patients, while it was between 30–40% in the rest 15 patients. In addition, echocardiography also picked up regional wall motion abnormalities in 13 (46.5%), cardiomyopathy in 11 (39.3%) and insignificant associated valvular diseases in 2 patients.
Table 1.
Patient profile
| Parameters | Numbers of patients (%) |
|---|---|
| Mean age (range) | 60 years (26–78) |
| Male: Female ratio | 21:7 |
| Presence of medical co-morbidity | 20 patients (71.4) |
| Hypertension | 16 |
| Diabetes mellitus | 8 |
| Hypothyroidism | 4 |
| Asthma/COPD | 2 |
| CRF | 1 |
| Previous history of cardiac intervention | 12 patients (43) |
| CABG | 7 |
| PTCA | 2 |
| Pacemaker | 2 |
| Aortic valve replacement | 1 |
| NYHA: | |
| Class II | 24 (86) |
| Class III | 4 (14) |
COPD: chronic obstructive pulmonary disease, CRF: chronic renal failure, CABG: coronary artery bypass grafting, PTCA: percutaneous transcoronary angioplasty, NYHA: New York Heart Association
Table 2.
Pre-operative cardiac status of 28 patients as per Echocardiography and ECG findings
| Parameters | Number of patients (%) |
|---|---|
| 1. LVEF between 20–30% | 13 (46.5) |
| 2. LVEF between 30–40% | 15 (53.6) |
| 3. Regional wall motion abnormality | 13 (46.5) |
| 4. Dilated cardiomyopathy | 11 (39.3) |
| 5. Bifascicular block | 2 (7) |
| 6. Valvular disease | 2 (7) |
LVEF: left ventricular ejection fraction
Laparoscopic cholecystectomy was performed in all patients (Table 3). The commonest intra-operative problem was episodes of hypertension (3 patients), which always started during peritoneal insufflation, and was controlled by GTN infusion. On the other hand, 1 patient, who had previously undergone mitral valve replacement, developed hypotension necessitating immediate desufflation and intravenous infusion of vesopressor agents (noradrenaline and adrenaline). One patient developed tachyarrhythmia during surgery, requiring Amiodarone infusion. These intra-operative situations did not require conversion to laparotomy and all the patients were extubated in the operating room. In the post-operative period, 3 patients developed tachyarrhythmia, which required defibrillation in 1 patient. In the rest 2 patients, there was no haemodynamic compromise. These patients were monitored and the tachycardia subsided spontaneously. One patient developed subtle signs of peritonitis on the 3rd post-operative day requiring laparotomy. This revealed purulent peritonitis suggestive of hollow viscous perforation but the exact site could not be located. The adhesions were most marked around the duodenum and appeared as a sealed off perforation. However, despite thorough peritoneal lavage the patient succumbed to acute myocardial infarction few days after laparotomy.
Table 3.
Results of laparoscopic cholecystectomy in 28 patients with cardiac co-morbidities
| Intra-operative course and complications | Number (%) | Treatment |
|---|---|---|
| Intra-operative complications | 5 (17.9) | |
| • Persistent HTN | 3 | GTN |
| • Hypotension and | 1 | Desufflation and iv |
| • bradycardia | pressor support | |
| Tachyarrhythmia | 1 | Amiodarone |
| Post-operative complication | 4 (14.3) | |
| • Tachyarrhythmia | 3 | 1: defibrillator |
| • Acute myocardial infarction | 1 | |
| 30 day mortality | 1 |
HTN: Hypertension, GTN: Glycerin tri-nitrate
Discussion
This multispecialty hospital is the busiest cardiac unit in Eastern India performing approximately 4500 coronary angiograms and close to 2000 open heart surgery per year. Amongst these a large number of patients are referred for laparoscopic cholecystectomy. Many of these patients suffer from cardiac illness or have had some kind of cardiac intervention. Such a situation not only poses unique challenge to the general surgeons, but also offers exposure to a subset of high-risk patients. This experience is not obtained during routine training and clinical practice and is worth sharing with the surgical community at large.
Haemodynamic and cardiovascular changes of positive pressure CO2 pneumoperitoneum on an anaesthetized patient lying in a reverse Trendelenberg position are often interrelated and their individual contribution is difficult to disentangle. As a generalisation, pneumoperitoneum orchestrates a neurohormonal stress response which increases systemic vascular resistance, mean arterial blood pressure and heart rate [13–15]. These factors increase the afterload and myocardial oxygen consumption which are poorly tolerated by patients with cardiac dysfunction [16]. Elevated intra-abdominal pressure and reverse Trendelenburg position reduce venous return and preload and decrease cardiac output [17, 18]. This combination of decreased preload and increased afterload increase cardiac work load and could precipitate cardiac ischemia or infarction. Patients with ischemic heart disease are prone to develop atrial fibrillation, a condition which could be precipitated by CO2 pneumoperitoneum [19]. Most of the studies addressing cardiovascular effects of CO2 pneumoperitoneum have been performed in healthy subjects, who seem to tolerate pneumoperitoneum without untoward problem [14, 15, 20]. Similar issues have not been studied under the setting of randomized trial in patients with cardiac co-morbidities [21]. May be hence, there is considerable reticence among surgeons in performing laparoscopy in patients with compromised heart. However, laparoscopic cholecystectomy has been successfully performed in small series of 10 [22, 23] -14 patients [24] with ASA III/IV cardiac dysfunction. To avoid the complications of pneumoperitoneum, gasless laparoscopic cholecystectomy (abdominal wall lifting) has been used as an alternative to laparoscopy in high risk patients [25]. Although abdominal wall lifting is associated with less circulatory changes and improved postoperative cognitive function, there is increased risk of surgical error [26].
Meticulous history taking and subjective assessment of patients undergoing laparoscopic cholecystectomy is an important aspect of managing such patients. Scoring systems like ASA physical status, Goldman cardiac Index, Canadian Cardiac Scoring system, NYHA functional classification, Detskey Modified Multifactorial Index have been devised to assess the risk of intra- and postoperative cardiac complications. Of these, the NYHA is the most commonly used grading system of cardiac dysfunction [11, 27]. It is interesting to note that although the left ventricular ejection (LVEF) on resting transthoracic echocardiography is very commonly used to assess cardiac function, LVEF is not a part of any of these scoring systems. Hence there is little reason to discourage laparoscopic cholecystectomy on the basis of single LVEF value. Moreover, assessment of LVEF is operator dependant [28]. Despite these shortcomings, some textbooks continue to lay importance on LVEF, where a 40% cutoff value is considered safe for surgery [29, 30]. As a matter of policy it has been our practice to refer such patients for rigorous preoperative assessment by cardiologist and anesthesiologist. Premedication with Nitrazepam helps reduce anxiety and stress and the heart rate [31]. Preoperative hydration or volume loading is thought to preserve cardiac output during laparoscopic surgery [32]. But, in this study intravenous volume overloading was not practiced; instead fasting more than 4 hours was avoided and patients were operated early in the morning. Due to cardiac dysfunction, intravenous fluid was administered judiciously while oral fluid was commenced as early as 4–5 hours after surgery. To diminish the stress on the heart during induction and maintenance of anaesthesia, Sevoflurane and Isoflurane were used. These agents cause very little cardiac and circulatory changes. Tachycardia should be prevented and in 6 (21%) patients, intravenous Metoprolol, a cardio-specific betablocker, was used to control intraoperative tachycardia. Adequate depth of anaesthesia and pain control also helps prevent tachycardia.
The pressure effects of pneumoperitoneum could be partially offset by using a low insufflation pressure. Some study define ‘normal’ insufflation pressure as 12–15 mmHg and a ‘low’ pressure as 5–7 mmHg [21] while a recent review arbitrarily considered anything less than 12 mmHg as low pressure [33]. Studies in healthy individuals have shown less pronounced decrease in cardiac index using low pressure peritoneal insufflation, while the pulmonary parameters have remained more or less similar in both the groups [13, 34, 35]. By and large, the low pressure groups have also reported less post-operative pain and diminished analgesic requirement, but such was always not the case [34]. In patients with cardiac dysfunction, four non-randomised studies have shown less haemodynamic alterations under low insufflation pressure [5, 36–38]. In the present study, a peritoneal pressure of 8 mmHg was used and this figure was arbitrarily chosen. On occasions this low intra-peritoneal pressure prevented adequate exposure of the Calot’s triangle. In such situations either the peritoneal pressure was temporarily increased to 12 mmHg till the triangle was safely dissected and cystic duct/artery were clipped and then the pressure was decreased to 8 mmHg or a 5th port (5 mm) was introduced in the left midclavicular line at the level of umbilicus and a fan retractor was inserted to depress the omentum and bowel towards the spine. Rapid insufflation with CO2 stretches the peritoneum and could precipitate cardiac arrhythmia [39]. Therefore, a low rate of insufflation of 5 liters/min was routinely used in this study.
Conclusion
The present study showed that laparoscopic cholecystectomy may be safely performed in patients with significant cardiac dysfunction. Such patients need proper evaluation by cardiologists and anesthesiologists and a single trans-thoracic echocardiographic estimation of left ventricular ejection fraction should not be given undue importance. If considered safe to undergo general anaesthesia, such patients should not be denied the benefits of laparoscopic cholecystectomy. Optimization of cardiac status, administration of balanced anaesthesia and low-pressure pneumoperitoneum are essential steps to ensure patient safety. The chances of life threatening complications are rare, and in the eventuality, can be easily managed in a hospital with adequate cardiological support.
Acknowledgments
We gratefully acknowledge the help of our Cardiology and Anaesthesiology colleagues in pursuing this study.
Contributor Information
Sagar Sadhu, FAX: +91-033-24264204, Email: drsagar@rediffmail.com.
Manas Kr. Roy, Email: manas.roy@tatatea.co.in
References
- 1.Litynski GS. Profiles in laparoscopy: Mouret, Dudois, and Perissat: the laparoscopic breakthrough in Europe (1987–1988) JSLS. 1999;3(2):163–167. [PMC free article] [PubMed] [Google Scholar]
- 2.Barkun JS, Barkun AN, Sampalis JS, et al. Randomised controlled trial of laparoscopic versus mini cholecystectomy. The McGill Gallstone Treatment group. Lancet. 1992;340(8828):1116–1119. doi: 10.1016/0140-6736(92)93148-G. [DOI] [PubMed] [Google Scholar]
- 3.Nagle AP, Soper NJ, Hines JR. Cholecystectomy (Open and laparoscopic) In: Zinner MJ, Ashley SW, editors. Maingot’s abdominal operations. 11. New York: McGraw-Hill Medical; 2007. pp. 847–863. [Google Scholar]
- 4.Rosenthal RJ, Szomstein S, Kennedy CI, Soto FC, Zundel N. Laparoscopic surgery for morbid obesity: 1, 001 consecutive bariatric operations performed at the Bariatric Institute, Cleveland Clinic Florida. Obes Surg. 2006;16(2):119–124. doi: 10.1381/096089206775565230. [DOI] [PubMed] [Google Scholar]
- 5.Doste K, Lacoste L, Lehuede KJ, MS TD, Fusciardi J. Haemodynamic and ventricular changes during laparoscopic cholecystectomy in elderly ASA III patients. Can J Anaesthesia. 1996;43(8):783–788. doi: 10.1007/BF03013029. [DOI] [PubMed] [Google Scholar]
- 6.Barone JE, Bears S, Chen S, et al. Outcome study of cholecystectomy during pregnancy. Am J Surg. 1999;177:232–236. doi: 10.1016/S0002-9610(99)00007-0. [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin JG, Scheeres DE, Dean RJ, Bonnel BW. The adverse hemodynamic effects of laparoscopic cholecystectomy. Surg Endosc. 1995;9:121–124. doi: 10.1007/BF00191950. [DOI] [PubMed] [Google Scholar]
- 8.Glaser F, Sanwald GA, Buhr HJ, et al. General stress response to conventional and laparoscopic cholecystectomy. Ann Surg. 1995;221:372–380. doi: 10.1097/00000658-199504000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joris J, Cigarini I, Legrand M, et al. Metabolic and respiratory changes after cholecystectomy performed via laparotomy or laparoscopy. Br J Anaesth. 1992;69:341–345. doi: 10.1093/bja/69.4.341. [DOI] [PubMed] [Google Scholar]
- 10.Kloosterman T, von Blomberg BME, Borgstein P, et al. Unimpaired immune functions after laparoscopic cholecystectomy. Surgery. 1994;115:424–428. [PubMed] [Google Scholar]
- 11.Hunt SA, et al. ACC/AHA Guidelines for the Evaluation and Management of Chronic Heart Failure in the Adult: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2001;38:2101–2113. doi: 10.1016/S0735-1097(01)01683-7. [DOI] [PubMed] [Google Scholar]
- 12.Sadhu S, Jahangir TA, Sarkar S, Dubey Sanjoy Kr, Roy Manas Kr. Open port placement through the umbilical cicatrix. Indian J Surgery. 2009;71(5):273–275. doi: 10.1007/s12262-009-0069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dexter SP, Vucevic M, Gibson J, McMahan MJ. Haemodynamic consequences of high and low-pressure capnoperitoneum during laparoscopic technique. Surg Endosc. 1999;13:376–381. doi: 10.1007/s004649900993. [DOI] [PubMed] [Google Scholar]
- 14.Odeberg S, Ljunggvist O, Sevenberg T, et al. Haemodynamic effects of pneumoperitoneum and the influence of posture during anaesthesia for laparoscopic surgery. Acta Anaesthesiol Scand. 1994;38(3):276–283. doi: 10.1111/j.1399-6576.1994.tb03889.x. [DOI] [PubMed] [Google Scholar]
- 15.Joris JL, Noriot DP, Legrand MJ, Jacquet NJ, Lamy ML. Hemodynamic changes during laparoscopic cholecystectomy. Anesth Analg. 1993;76:1067–1071. doi: 10.1213/00000539-199305000-00027. [DOI] [PubMed] [Google Scholar]
- 16.Westerband A, Van de Water JM, Amzallag M, et al. Cardiovascular changes during laparoscopic cholecystectomy. Surg Gynecol Obstet. 1992;175(6):535–538. [PubMed] [Google Scholar]
- 17.Dorsay DA, Greene FL, Baysinger CL. Hemodynamic changes during laparoscopic cholecystectomy monitored with transesophageal echocardiography. Surg Endosc. 1995;9:128–134. doi: 10.1007/BF00191952. [DOI] [PubMed] [Google Scholar]
- 18.Willams MD, Murr PC. Laparoscopic insufflations of abdomen depresses cardiopulmonary function. Surg Endosc. 1993;7(1):12–16. doi: 10.1007/BF00591229. [DOI] [PubMed] [Google Scholar]
- 19.Bickel A, Marinovski M, Shturman A, Roguin N, Waksman I, Eitan A. Filtered signal-averaged P-wave duration during Pneumoperitoneum in patients undergoing laparoscopic cholecystectomy: a reflection of pathophysiological cardiac changes. Surg Endosc. 2008;22:221–227. doi: 10.1007/s00464-007-9676-z. [DOI] [PubMed] [Google Scholar]
- 20.Larsen JF, Svendsen FM, Pedersen V. Randomized clinical trial of the effect of pneumoperitoneum on cardiac function and haemodynamics during laparoscopic cholecystectomy. BJS. 2004;91(7):848–854. doi: 10.1002/bjs.4573. [DOI] [PubMed] [Google Scholar]
- 21.Neudecker J, Sauerland S, Neugebauer E, et al. The European Association for Endoscopic Surgery clinical practice guideline on the pneumoperitoneum for laparoscopic surgery. Surg Endosc. 2002;16(7):1121–1143. doi: 10.1007/s00464-001-9166-7. [DOI] [PubMed] [Google Scholar]
- 22.Zollinger A, Krayer S, Singer Th, et al. Haemodynamic effects of pneumoperitoneum in elderly patients with an increased cardiac risk. Eur J Anaesthesiol. 1997;14(3):266–275. doi: 10.1097/00003643-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Koivusalo A-M, Pere P, Valjus M, Scheinin T. Laparoscopic cholecystectomy with carbon dioxide pneumoperitoneum is safe even for high-risk patients. Surg Endosc. 2008;22:61–67. doi: 10.1007/s00464-007-9300-2. [DOI] [PubMed] [Google Scholar]
- 24.Gebhardt H, Bautz A, Ross M, Loose D, Wulf H, Schaube H. Pathophysiological and clinical aspects of CO2 pneumoperitoneum (CO2-PP) Surg Endosc. 1997;11:864–867. doi: 10.1007/s004649900473. [DOI] [PubMed] [Google Scholar]
- 25.Galizia G, Prizio G, Lieto E, et al. Hemodynamic and pulmonary changes during open, carbon dioxide pneumoperitoneum and abdominal wall lifting cholecystectomy. A prospective randomized study. Surg Endosc. 2001;15(5):477–483. doi: 10.1007/s004640000343. [DOI] [PubMed] [Google Scholar]
- 26.Alijani A, Hanna GB, Cuschieri A. Abdominal wall lift versus positive-pressure capnoperitoneum for laparoscopic cholecystectomy: randomized controlled trial. Ann Surg. 2004;239(3):388–394. doi: 10.1097/01.sla.0000114226.31773.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Remme WJ, Swedberg K. Guideline for the diagnosis and treatment of chronic heart failure. Eur Heart J. 2001;22(17):1527–1460. doi: 10.1053/euhj.2001.2783. [DOI] [PubMed] [Google Scholar]
- 28.Nishinura RA, Gibbons RJ, Glockner JF, Taijk AJ. Noninvasive cardiac imaging: echocardiography, nuclear cardiology, and MRI/CT imaging. In: Kasper DL, editor. Harrison’s principles internal medicine. 16. New York: McGraw-Hill Medical; 2005. pp. 1320–1327. [Google Scholar]
- 29.Foex P. Cardiological problems. In: Moris PJ, Wood WC, editors. Oxford textbook of surgery. 2. New York: Oxford University press; 2000. pp. 361–374. [Google Scholar]
- 30.Peters JH, DeMeester TR. Esophagus and diaphragmatic hernia. In: Schwartz SI, editor. Principles of surgery. 7. New York: McGraw-Hill medical; 1999. pp. 1081–1180. [Google Scholar]
- 31.Kangas L, Kanto J, Monsikka M. Nitrazepam premedication for minor surgery. Br J Anaesth. 1977;49:1153. doi: 10.1093/bja/49.11.1153. [DOI] [PubMed] [Google Scholar]
- 32.Korell M, Schmaus F, Strowitzki T, Schneeweiss SG, Hepp H. Pain intensity following laparoscopy. Surg Laparosc Endosc. 1996;6:375–379. doi: 10.1097/00019509-199610000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Gurusamy KS, Samraj K, Davidson BR (2009) Low pressure versus standard pressure pneumoperitoneum in laparoscopic cholecystectomy. Cochrane database of Systemic Reviews, Issue 2 Art No.: CD 006930 [DOI] [PubMed]
- 34.Pier A, Benedic M, Mann B, Buck V. Das postlaparoskopische schmerzsyndrom: Ergebnisseeiner prospektiven, randomisierten Studie. Chirurg. 1994;65:200–2008. [PubMed] [Google Scholar]
- 35.Wallace DH, Serpell MG, Baxter JN, O’Dwyre PJ. Randomized trial of different insufflation pressures for laparoscopic cholecystectomy. Br J Surg. 1997;84(4):455–458. doi: 10.1002/bjs.1800840408. [DOI] [PubMed] [Google Scholar]
- 36.Frank SM, Fleisher LA, Breslow MJ, Higgins MS, Olson KF, Kelly S, Beattie C. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. J Am Med Assoc. 1997;277:1127–1134. doi: 10.1001/jama.1997.03540380041029. [DOI] [PubMed] [Google Scholar]
- 37.Hendolin HI, Paakonen ME, Alhava EM, Tarvainen R, Kemppinen T, Lahtinen P. Laparoscopic or open cholecystectomy: a prospective randomized trial to compare postoperative pain, pulmonary function, and stress response. Eur J Surg. 2000;166:394–399. doi: 10.1080/110241500750008961. [DOI] [PubMed] [Google Scholar]
- 38.Sarac AM, Aktan AO, Baykan N, Yegen C, Yalin R. The effect and timing of local anesthesia in laparoscopic cholecystectomy. Surg Laparosc Endosc. 1996;6:362–366. doi: 10.1097/00019509-199610000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Reed DN, Jr, Duff JL. Persistence occurrence of bradycardia during laparoscopic cholecystectomies in low-risk patients. Dig Surg. 2000;17:513–517. doi: 10.1159/000051950. [DOI] [PubMed] [Google Scholar]


