Abstract
The recent establishment of high-throughput methods for culturing Drosophila provided a unique ability to screen compound libraries against complex disease phenotypes in the context of whole animals. However, as compound studies in Drosophila have been limited so far, the degree of conservation of compound activity between Drosophila and vertebrates or the effectiveness of feeding as a compound delivery system is not well known. Our comprehensive in vivo analysis of 27 small molecules targeting seven signaling pathways in Drosophila revealed a high degree of conservation of compound activity between Drosophila and vertebrates. We also investigated the mechanism of action of AY9944, one of the Hh pathway antagonists that we identified in our compound feeding experiments. Our epistasis analysis of AY9944 provided novel insights into AY9944’s mechanism of action and revealed a novel role for cholesterol transport in Hh signal transduction.
Electronic supplementary material
The online version of this article (doi:10.1007/s12154-010-0051-5) contains supplementary material, which is available to authorized users.
Keywords: Chemical genetics, Compound feeding, Drosophila, Drug discovery, Signal transduction
Introduction
In recent years, there has been growing interest in utilizing the genetic power of invertebrate model systems such as Drosophila in drug discovery as they provide high-throughput screening-compatible platforms that allow compound testing in a physiologically relevant environment [39]. Drosophila is a particularly attractive screening tool in this regard as many complex disease states associated with physiological defects in humans have been successfully modeled in flies, and these models mimic the pathology and specific symptoms of the diseases that are not easy to recapitulate in cell-based or in vitro assays [3, 5, 23, 25, 29]. Moreover, the reduced redundancy of the Drosophila genome could allow the identification of novel druggable targets that may be missed due to partially redundant activities of homologous proteins usually present in mammalian cells. Lastly, the availability of a large number of genetic tools allows rapid and detailed studies of compound activity in various mutant backgrounds and can provide novel insights into the compounds’ mechanism of action.
Only a small number of compound studies in Drosophila have been reported so far, mostly focusing on analyses of individual compounds [34, 42, 43]. In these studies, compounds are introduced into adult flies or developing larvae by feeding and assayed for their ability to induce or modify phenotypes. Some examples include the gamma-secretase inhibitor DAPT, whose activity as a Notch pathway inhibitor is conserved in Drosophila [34]; the tyrosine kinase inhibitor ZD6474, which is effective against the oncogenic forms of the receptor tyrosine kinase RET in both flies and mammals [42]; and the EGFR inhibitor gefitinib, which is recently shown to be effective in a Drosophila glioma model [43]. In addition to these studies of individual compounds, several novel hits have been identified in a recent compound screen in a Drosophila model of Fragile X syndrome [9]. Whether the activity of these hits will be conserved in mammalian systems still remains to be established; however, given the extensive conservation of biological processes between Drosophila and vertebrates, it is likely that there will be a high degree of conservation of compound activity as well.
One concern with the systemic introduction of compounds by feeding is that some compounds with desired biological activity could be missed due to bioavailability problems or poor compound stability in the food [39]. Since a limited number of compounds have been studied in Drosophila so far, and compounds that are not effective in vivo are usually not reported, whether the rate of false negatives poses a significant problem in compound screens in Drosophila is unclear. In vivo studies of more compounds will be useful to get a better understanding of the level of conservation of compound activity between Drosophila and mammalian systems, as well as the efficiency of feeding as a compound delivery strategy.
In order to evaluate how well compound activity is conserved in Drosophila and how effective feeding is as a compound delivery method, we tested 27 small molecules with known targets and/or previously established mechanisms of action in mammalian systems for in vivo activity in Drosophila. We focused our analysis on small molecules targeting Hh, Wg, EGFR/MAPK, insulin/PI3K, and JNK pathways as well as apoptosis and cell cycle since the developmental requirements for these pathways have been very well characterized in Drosophila. We tested compound effects using pathway-specific developmental phenotypes that were generated by the ectopic expression of several components of these pathways in a temporally controlled manner. We then confirmed the activity of several of these compounds directly on target tissues using pathway-specific target gene expression as read-outs. Our extensive gene–compound interaction study between these 27 compounds and the various components of the pathways that they target shows that the activity of 20 compounds as pathway modulators is conserved in Drosophila and demonstrates a high level of conservation of compound activity between Drosophila and mammals.
We then focused our analysis on one of the compounds with conserved activity in our system, the Hh inhibitor AY9944, as the mechanism of action of this compound is not very well understood. While the inhibitory effect of AY9944 on Hh signaling has been attributed to both its ability to inhibit cholesterol biosynthesis and trafficking of exogenously derived cholesterol, these pleiotropic effects on cholesterol metabolism hindered a clear understanding of its mechanism of action on Hh signaling [10, 22, 28]. The absence of cholesterol biosynthesis in Drosophila [37] provided us with a unique opportunity to separate the effects of AY9944 on cholesterol biosynthesis and trafficking and to study the mechanism of its inhibition of Hh signaling in the absence of cholesterol synthesis. Our genetic characterization of the mechanism of action of this compound revealed that AY9944 inhibits Hh-induced Ptc internalization, an immediate consequence of Hh binding to Ptc, as well as the expression of the Hh target gene engrailed (en). Epistasis analysis genetically placed AY9944 upstream of both pka and ptc, two negative regulators of Hh signaling. Furthermore, AY9944 treatment leads to the depletion of cholesterol from the plasma membrane and its intracellular accumulation in Drosophila tissues. Interestingly, the cholesterol moiety on the Hh protein is necessary for the inhibitory effect of AY9944 on Hh signaling; in its absence, Hh signaling is not sensitive to AY9944. We also show that two other structurally unrelated inhibitors of cholesterol transport [10, 28, 32] also inhibit Hh signaling in Drosophila and behave identically to AY9944 in our epistasis analysis. Our working model is that changes in cholesterol composition of cellular membranes caused by the inhibition of exogenously derived cholesterol trafficking by AY9944 interfere with the ability of the Hh protein to inhibit Ptc, leading to the inhibition of Hh signaling.
Results
Generation and selection of developmental phenotypes as read-outs of pathway activity
In order to generate developmental phenotypes for testing compound effects in vivo, we genetically manipulated the signaling pathway activity during Drosophila development using a targeted ectopic expression system (Gal4-UAS) [7] (Fig. S1). We focused our analysis on seven signaling pathways/cellular processes (Hh, Wg, EGFR/MAPK, insulin/PI3K, JNK, apoptosis, and cell cycle) based on the availability of transgenic lines to generate phenotypic read-outs and the presence of a large number of commercially available compounds targeting these pathways.
In order to generate the phenotypic read-outs to test the 27 compounds that we selected (Fig. S2), we ectopically expressed 19 UAS lines containing wild-type, dominant negative (DN), or constitutively active (CA) versions of several components of the seven pathways that we focused on (Fig. 1) throughout Drosophila development using two different Gal4 lines, primarily targeting the developing wing and the eye in the larva. In order to obtain phenotypes of different severity from each UAS/Gal4 combination, we took advantage of the temperature dependency of Gal4 activity [14] (Fig. S1) and set up each cross at three different temperatures to induce ectopic expression at three different levels for each pathway component (Fig. S3).
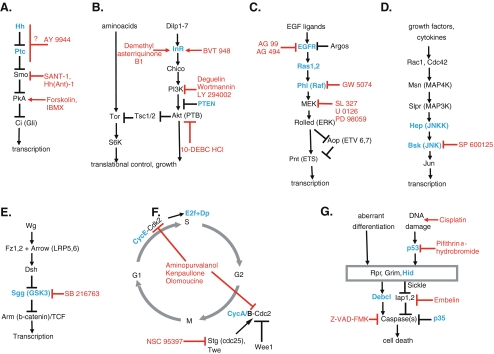
Fig. 1.
Signaling pathway components used to test gene–compound interactions. Key components of Hh (a), insulin/PI3K (b), Ras/MAPK (c), JNK (d), Wnt (e), cell cycle (f), and apoptosis (g) are depicted. Arrows indicate activation and T bars represent inhibition. For simplicity, only the core components of each pathway are shown. Pathway components used for gene–compound interaction experiments are in blue and compounds are in red
In order to avoid phenotypes that arise too early in larval development to be rescued by feeding compounds as characterized by irreversible changes such as cell death and excessive proliferation, we also generated phenotypes by inducing ectopic expression of pathway components during the different developmental stages and for different periods of time using a temperature-sensitive regulator of Gal4 activity, Gal80ts [33] (Fig. S3; also see “Experimental procedures”). We scored the progeny from these crosses for phenotypes and evaluated them for suitability as read-outs to test compound function (Fig. S4).
As we intended to test a relatively large number of compounds, the ability to quickly and easily identify phenotype modification by compounds was very important. For this reason, we only selected fully or highly penetrant phenotypes as read-outs in order to ensure that changes in relative severity of phenotypes were qualitatively obvious. Phenotypes that show multiple different phenotypic classes were avoided as scoring such complex phenotypes for changes in severity would be inconvenient for large-scale experiments. All crosses were repeated multiple times to ensure that the selected phenotypes did not show any variability, and a database of “baseline phenotypes” with a detailed description of the features of each selected phenotypic read-out was established.
In vivo analysis of compound activity using selected pathway-specific phenotypes
We evaluated the compound activity in Drosophila by testing each compound for its ability to modify phenotypes induced by the ectopic expression of several components of the pathway that it targets. The compounds were also tested for toxicity, by feeding to wild-type larvae, and specificity, by testing against a phenotype that is similar to the experimental phenotype being used to test their function, but generated by a genetic manipulation of a different pathway (Fig. 2). For example, the phenotypes that were selected as specificity controls for EGFR/Ras pathway antagonists were (1) a wing phenotype induced by the ectopic expression of the Hh pathway component Ptc (control for the EGFR.DN wing phenotype), (2) an eye phenotype induced by the ectopic expression of another Hh pathway component, Ci, (control for Ras.CA and Raf.CA eye phenotypes), and (3) larval lethality induced by Ci misexpression (control for larval lethal phenotypes induced by Ras.CA and Raf.CA).
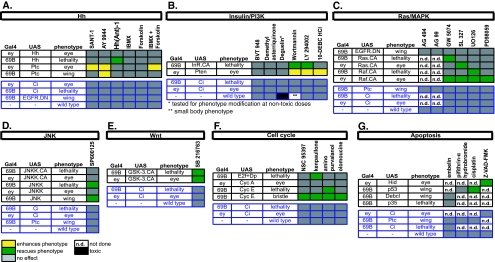
Fig. 2.
Summary of observed gene–compound interactions. Phenotypic read-outs tested for modification by compound feeding for Hh (a), insulin/PI3K (b), Ras/MAPK (c), JNK (d), and Wnt (e) pathways, as well as cell cycle (f) and apoptosis (g), are shown. The enhancement of a phenotype by a compound is shown in yellow and the suppression in green (n > 50 for each experiment). Phenotypes that are not modified by compound feeding are in gray and interactions that are not tested are in white. The phenotypes used in control experiments to test for specificity are in blue
Compound feeding was achieved by transferring the embryos of appropriate genotypes onto compound-treated food (see “Experimental procedures”). A total of 50–100 adult progeny of identical genotypes raised on compound-treated, DMSO-treated, or untreated food were scored blindly for phenotypes and assigned one of the following categories in comparison to the baseline phenotype previously established for that particular genotype: (1) no change, if the phenotype obtained is similar to the previously established baseline for that genotype; (2) enhanced, if there is a more severe phenotypic class that was never observed in the baseline phenotype for that genotype; and (3) suppressed if there is a less severe or wild-type phenotypic class that was never observed in the baseline phenotype for that genotype. A compound was identified as having activity if (1) the progeny raised on compound-treated progeny was classified into the “enhanced” or “suppressed” category and (2) the progeny of the same genotype raised on both DMSO-treated and untreated food was assigned into “no change” category. Overall, 20 of the 27 compounds that we tested successfully modified at least one of the pathway-specific phenotypes used, confirming their function as in vivo modulators of pathway activity in Drosophila. We have not observed any toxicity or phenotypic effects upon feeding compounds to wild-type larvae with two exceptions: The PI3K inhibitor deguelin was highly toxic and another PI3K inhibitor, wortmannin, induced a small body phenotype that is consistent with a reduction in the level of insulin/PI3K signaling [6]. The results of our analysis are summarized in Fig. 2 and examples of phenotypes modified by compound feeding can be found in Fig. S5.
All the compound effects that we observed were specific; we have not observed any non-specific enhancement or suppression of our phenotypes in specificity tests. Furthermore, in all cases, phenotype modification was in the direction expected based on the compound’s described activity on the targeted pathway. For instance, inhibiting endogenous Hh signaling in the developing wing imaginal disk by misexpression of the negative regulator Ptc leads to small wings with missing pattern elements in the central region of the wing, a phenotype that is consistent with the role of Hh signaling in wing development [41]. When larvae misexpressing Ptc were fed a Hh pathway antagonist, such as AY9944, this phenotype became much stronger, with wings exhibiting a more severe loss of central pattern elements accompanied by a further reduction in size (Fig. S5). A wing phenotype of this severity was never observed in untreated or DMSO-fed larvae of the same genotype, indicating that this enhancement is due to a further inhibition of endogenous Hh signaling by AY9944. Furthermore, this enhancement is specific to a phenotype generated by Ptc, as AY9944 had no effect on a wing phenotype generated by the overexpression of EGFR.DN (not shown).
Our feeding tests allowed us to detect not only the enhancement of pathway-specific phenotypes but also their suppression as well. For example, the ectopic activation of the MAPK pathway by the misexpression of activated Raf (Raf.CA) in the eye imaginal disk leads to bulgy eyes due to overproliferation of the developing eye disk, a previously documented outcome of ectopic Ras/MAPK activity in this tissue [26]. This phenotype was completely suppressed by feeding Raf or MEK inhibitors to the developing larvae, indicating that these compounds were able to block the ectopic MAPK activity in the developing eye disk.
In addition to confirming compound activity in vivo, our gene–compound interaction analysis also led to several interesting findings: First, we noticed that compounds with similar activity in cell-based or in vitro assays can behave differently in vivo. For instance, three different MEK inhibitors that we tested all rescued an eye phenotype induced by the misexpression of Raf.CA, yet only SL327 could suppress a similar but stronger eye phenotype that was obtained by misexpressing Ras.CA. Ras.CA-induced lethality, on the other hand, could not be rescued by any of the three, while Raf.CA-induced lethality was rescued by UO126 only (Fig. 2c). Clearly, SL327 and UO126 show stronger in vivo efficacy than PD98059 in our system. Such different profiles of compound activity may reflect differences in compound bioavailability, stability, and/or affinity of compound–target interactions. Interestingly, PD98059 inhibits MEK activity with an IC50 that is 10–30-fold higher than the other two compounds in cell-based assays [13, 40] (Fig. S2). The fact that we can pick up subtle differences in compound activity using phenotypes of different severity or those induced by different pathway components highlights the value of our experimental system in dissecting out compound function in vivo.
Testing compound effects on dissected tissues using molecular read-outs
Our gene–compound interaction study identified a large number of compounds with conserved in vivo activity in Drosophila, clearly demonstrating the effectiveness of feeding as a compound delivery tool. Our analysis also showed that pathway-specific developmental phenotypes provide excellent assays to test compound function in vivo; however, we also wanted to validate our observations based on phenotype modification by directly monitoring compound effects on tissues using molecular read-outs. To this end, we developed an alternative compound delivery method that allows the incubation of dissected larval tissues in compounds with no adverse effects on tissue morphology and integrity (see “Experimental procedures”). Furthermore, incubation conditions or DMSO alone did not lead to any changes in signaling pathway activity or cell death (Fig. 3; Fig. S6, not shown).
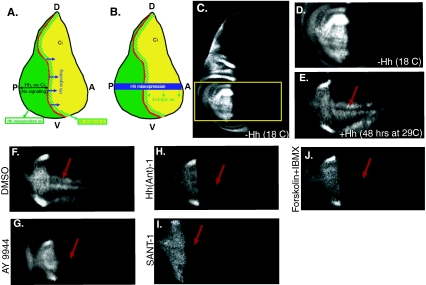
Fig. 3.
Hh pathway antagonists inhibit the expression of the Hh target gene engrailed in the wing imaginal disk. a Schematic representation of the expression patterns of Hh pathway components and domain of Hh signaling activity in the wing imaginal disk. Hh is expressed in the posterior compartment [12] (P, green) and travels into the anterior compartment (A, yellow) to activate signaling [47] (blue arrows). There is no Hh signaling in the posterior compartment as the transcriptional effector Ci is only expressed in the anterior compartment [35]. Hh signaling activates the expression of en in a narrow domain (yellow/green stripes) just anterior to the A/P boundary [47] (red line). en expression in the posterior compartment is independent of Hh signaling. b Graphic representation of the Hh misexpression assay. Misexpression of Hh throughout the wing imaginal disk (blue bar) leads to the ectopic activation of en expression throughout the anterior compartment (green arrows). c–len expression in wing disks from larvae of genotype 69B-gal4, gal80ts>UAShh. c, d No ectopic en is detectable in disks from larvae raised at 18 °C (gal80ts active). d–len expression in the wing pouch area of the wing imaginal disk (outlined by a yellow rectangle in c). e Ectopic en is induced in the anterior compartment as a result of Hh misexpression after a temperature shift to 29 °C for 48 h. f–len expression in disks misexpressing Hh incubated with DMSO (f), AY9944 (g), Hh(Ant)-1 (h), SANT-1 (i), as well as IBMX and forskolin in combination (j) for 24 h. AY9944, Hh(Ant)-1, and SANT-1 all lead to a strong reduction of ectopic en induced by Hh misexpression (g–i) while incubation with DMSO alone has no effect on ectopic en expression (f). IBMX and forskolin can only inhibit en expression together
We used this method to assess the effects of Hh pathway antagonists (Figs. 1a and 2a) on Hh signaling in wing imaginal disks using the endogenous Hh target gene en as a read-out [1, 30, 41, 46]. The misexpression of Hh for 48 h in wing imaginal disks was sufficient to induce the ectopic expression of en in the anterior compartment of the wing disk (Fig. 3a–e). We then incubated these disks with Hh pathway antagonists and tested their ability to suppress Hh-induced ectopic en expression in the anterior compartment. A total of 18–20 disks were blindly scored for each treatment condition and loss of anterior en expression in more than five disks was considered as successful inhibition. Consistent with results from feeding experiments, AY9944, SANT-1, and Hh(Ant)-1 all suppressed en expression in the anterior compartment (minimum penetrance of 25%, n = 18–20 disks, three independent experiments for each compound). Representative images from these experiments are shown in Fig. 3f–i. Furthermore, while forskolin and IBMX had no effect on en expression alone (not shown), they had a strong synergistic effect (Fig. 3j). These compounds lead to an increase in cellular cAMP levels by targeting different enzymes involved in cAMP production, which culminates in the activation of PKA, a negative regulator of the Hh pathway [36, 38]. The synergistic effect that we observed suggests that both compounds are active in our system yet neither one alone is effective in inhibiting Hh signaling. These results confirm the activity of these compounds as inhibitors of the Drosophila Hh signaling pathway and demonstrate the efficiency of pathway-specific developmental phenotypes as read-outs for testing compound activity in the context of a whole organism.
Genetic analysis of AY9944 mechanism of action
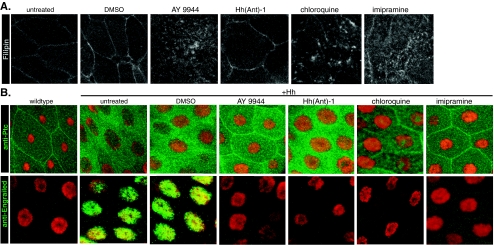
The inhibitory effect of AY9944 on Hh signaling has been attributed to both its ability to inhibit cholesterol biosynthesis and trafficking of exogenously derived cholesterol [28]. Since there is no cholesterol biosynthesis in Drosophila and 7-dehydrocholesterol Δ7 reductase (DHCR7), the enzyme targeted by AY9944, is not present [37], we reasoned that the inhibition of the Hh pathway in Drosophila by AY9944 must be mediated by its effects on cholesterol trafficking. AY9944 treatment of mammalian cells leads to the intracellular accumulation of free cholesterol, which can be detected by the fluorescent dye filipin [45]. In order to test whether AY9944 has an effect on cholesterol trafficking in Drosophila, we monitored filipin staining in AY9944-treated wing imaginal disks as well as another Hh-responsive larval tissue, larval salivary glands, as the large size of salivary gland cells allowed us to more easily visualize changes in cholesterol distribution within cellular membranes. Consistent with published data from mammalian cells, we also observed the intracellular accumulation of cholesterol, evident in intracellular vesicular structures, as well as a reduction in plasma membrane cholesterol in both wing imaginal disk and salivary gland cells (Fig. 4a, data not shown), clearly demonstrating that the ability of AY9944 to inhibit cholesterol trafficking is conserved in Drosophila. Furthermore, two structurally unrelated compounds, chloroquine and imipramine, that share the ability to inhibit cholesterol transport with AY9944 [10, 28, 32] showed a similar effect on cholesterol distribution (Fig. 4a) as well as on Hh signaling in wing imaginal disks (Fig. 6a).
Fig. 4.
Cholesterol transport inhibitors inhibit Hh-induced Ptc internalization and en expression. a Cholesterol distribution in compound-treated salivary glands monitored by filipin staining. b Wild-type salivary gland cells show membrane-localized Ptc (green, left-most panel, top) and no en expression (green, left-most panel, bottom). Right panels, Ptc localization (top) and en expression (bottom) in compound-treated salivary glands misexpressing Hh. Nuclei are shown in red
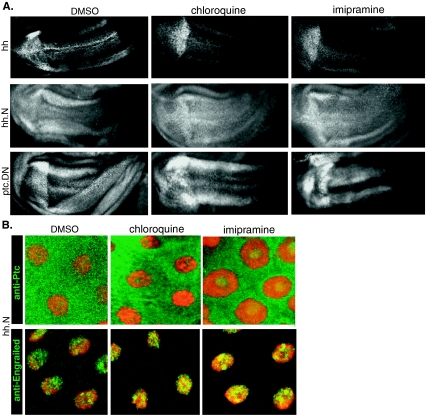
Fig. 6.
Cholesterol transport inhibitors chloroquine and imipramine behave identically to AY9944. aen expression in wing imaginal disks misexpressing Hh (top), hh.N (middle), and Ptc.DN (bottom) treated with DMSO, chloroquine, or imipramine. b Ptc localization (green top panels) and en expression (green, bottom panels) in salivary glands misexpressing Hh.N treated with DMSO, chloroquine, or imipramine. Nuclei are shown in red
In order to better understand where in the Hh signaling pathway AY9944 acts, we next turned to a more upstream read-out of Hh signaling: Ptc internalization. Ptc is predominantly localized to the plasma membrane in the absence of Hh, and it becomes internalized and targeted for degradation upon Hh binding [12, 47]. As expected, the ectopic expression of Hh in salivary glands led to the internalization of endogenous Ptc protein and activation of en expression (Fig. 4b, left-most panels). AY9944, chloroquine, and imipramine all blocked Hh-induced Ptc internalization as well as en expression (Fig. 4b), indicating that these compounds work upstream or at the level of Ptc. On the other hand, the Smo inhibitor Hh(Ant)-1 [2] whose cellular target is downstream of Ptc in the pathway only inhibited en expression, but it had no effect on Ptc internalization (Fig. 4b). Given the direct correlation between Hh binding and Ptc internalization, it is likely that AY9944 interferes with the ability of Hh protein to signal, possibly by blocking its interaction with Ptc.
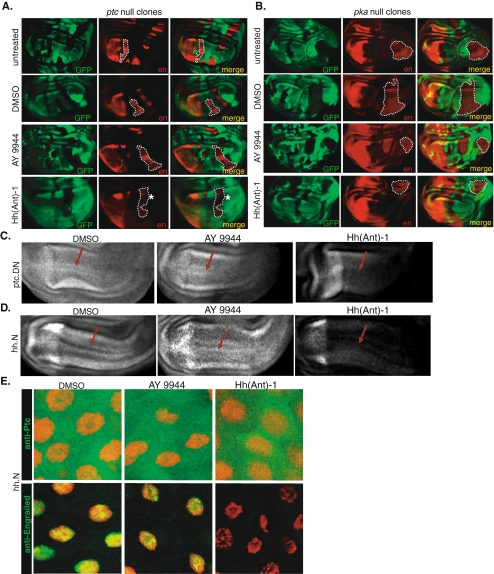
If AY9944 targets Hh signaling at the level or upstream of Ptc, it should not be able to suppress pathway activation as induced by the removal of ptc. To test this, we generated patches of ptc-/- cells (null clones) in wing imaginal disks using the FLP/FRT-mediated mitotic recombination [44] and treated these disks with AY9944 or the Smo antagonist Hh(Ant)-1 as a control (Fig. 5a). While Hh(Ant)-1 led to a reduction of ectopic en expression induced in ptc-/- clones, AY9944 did not (Fig. 5a). On the other hand, neither AY9944 nor Hh(Ant)-1 was able to inhibit en expression as induced by the removal of a further downstream negative regulator of Hh signaling, pka (Fig. 5b; also see Fig. 1a) [31, 41]. As expected, these findings genetically place Hh(Ant)-1 downstream of hh and ptc and upstream of pka, consistent with its cellular target. On the other hand, the behavior of AY9944 in our epistasis analysis indicates that it is upstream of pka and acts either upstream of or directly on ptc.
Fig. 5.
Epistasis analysis between AY9944 and Hh pathway components. a, b Wing imaginal disks carrying ptc-/- (a) or pka-/- (b) clones treated with AY9944 or Hh(Ant)-1 as control and labeled with anti-En antibody (red). Mutant cells are marked by the absence of GFP (green). c, d Wing imaginal disks misexpressing ptc.DN (c) or Hh.N (d) are treated with AY9944 or Hh(Ant)-1 and labeled with anti-En antibody. Red arrows indicate where en expression is ectopically activated. e Ptc localization (green, top panels) and en expression (green, bottom panels) in compound-treated salivary glands misexpressing Hh.N. Nuclei are shown in red
To test the possibility that AY9944 directly affects Ptc function, we next overexpressed a dominant negative version of the Ptc protein that is not able to repress Smo in the absence of Hh, leading to the ligand-independent activation of the pathway [24]. Consistent with the fact that Ptc.DN-induced Hh signaling still requires Smo function, Hh(Ant)-1 inhibited en expression induced by Ptc.DN misexpression but AY9944 did not (Fig. 5c). The inability of AY9944 to inhibit Hh signaling induced in a Hh-independent manner by Ptc.DN suggests that AY9944 must target the pathway at the level of the Hh ligand, possibly by interfering with the ability of the Hh ligand to find or bind to Ptc.
In order to further explore how AY9944 might be inhibiting Hh activity, we next asked whether the cholesterol modification on Hh is critical for AY9944 antagonism, using a truncated version of Hh protein that cannot be cholesterol-modified (Hh.N) [8, 17, 35]. While Hh.N is defective in the formation of higher-order Hh lipoprotein particles and interactions with the extracellular matrix, it is still able to activate Hh signaling [8, 17]. Like the wild-type, fully modified Hh protein, the misexpression of Hh.N also led to the ectopic activation of en expression in both the salivary glands and imaginal disks, as well as the internalization of Ptc (Fig. 5d, e). Interestingly, AY9944 did not inhibit en expression as induced by Hh.N in wing imaginal disks (Fig. 5d) nor did it block Ptc internalization or en expression in salivary glands (Fig. 5e). The inhibitory effect of Hh(Ant)-1, on the other hand, was not dependent on the presence of cholesterol (Fig. 5d, e). These observations lead us to conclude that the cholesterol moiety of Hh is critical for the inhibitory action of AY9944 and in its absence Hh pathway activity is not sensitive to AY9944.
Overall, our findings are consistent with the following model: the inhibition of cholesterol transport by AY9944 causes the intracellular accumulation of cholesterol and a concomitant reduction in plasma membrane cholesterol levels. These changes in cholesterol composition of cellular membranes interfere with the ability of Hh protein to bind to Ptc, leading to the inhibition of Hh signaling. If this is the case, we would expect chloroquine and imipramine, the other two inhibitors of cholesterol transport, to behave identically to AY9944 in all of our assays. In fact, both chloroquine and imipramine suppressed signaling induced by Hh, but not by Hh.N or Ptc.DN, in wing imaginal disks (Fig. 6a) and inhibited Ptc internalization and en expression as induced by Hh expression in salivary glands (Fig. 4b), but not by Hh.N (Fig. 6b). Taken together, these findings provide a strong support for the model that AY9944 inhibits Hh signaling by interfering with the ability of the Hh protein to signal and this inhibition is mediated by a defect in the trafficking of exogenously derived cholesterol.
Discussion
Here, we describe an extensive gene–compound interaction study between 27 small molecules targeting seven signaling pathways/cellular processes and genes encoding the various components of the signaling pathways that they target. We tested these compounds for in vivo activity in Drosophila using pathway-specific developmental phenotypes as generated by the ectopic expression of various pathway components in a temporally controlled manner as read-outs of signaling pathway activity. We were able to confirm the function of roughly 75% (20/27) of the compounds that we tested in vivo, a high success rate given that the degree of conservation of compound activity in mammalian and Drosophila cell lines is 60–65% (M.H., unpublished data). We also carried out a detailed genetic analysis of one of these compounds, Hh antagonist AY9944, and provided novel insights into its mechanism of action as well as a potential role for cholesterol transport in Hh signaling.
Developmental pathways provide sensitive read-outs for testing compound effects
Since most signaling pathways are repeatedly used during development, the ectopic expression of pathway components throughout development often leads to pleiotropic effects. In order to avoid such phenotypes, we chose to generate read-outs by controlling both the developmental timing and duration of our genetic manipulations using gal80ts. We then used the available information regarding the requirements for signaling pathways during development to select phenotypes that would be most sensitive to modification by compound feeding. The use of these well-characterized and highly sensitive phenotypes was an important factor in detecting compound effects in vivo. Even though ectopic expression throughout development also leads to robust phenotypes, these were not as effective in detecting compound effects: when tested at the same concentrations, only five out of 27 compounds were able to modify these phenotypes (not shown).
Interestingly, majority of the compounds that we tested did not have any significant effects on wild-type animals at the same concentrations that modified our phenotypic read-outs, suggesting that the compounds do not reach target tissues at levels high enough to induce a phenotype in a wild-type background. Poor compound bioavailability and stability must be in part responsible for this; however, another important factor is likely to be the resilience of developing tissues to environmental and genetic fluctuations due to compensatory mechanisms. Even though compounds may not be able to interfere with the activity of their target pathways at levels high enough to induce a phenotype in a wild-type background, their effects are easily revealed when the activity of the pathway in question is already compromised by a genetic manipulation.
It is important to note that not every compound identified in mammalian systems will have activity in Drosophila and vice versa due to the lack of conservation of compound targets/activity. Furthermore, some compounds with desired biological activity will be missed due to problems with bioavailability (i.e., false negatives). We only had seven compounds with no activity in our assays. One of these compounds, the PI3K inhibitor deguelin, was highly toxic. This toxicity is likely due to the strong pro-apoptotic effects of deguelin as it also inhibits ornithine decarboxylase and NADH/ubiquinone oxireductase activities [16]. It is possible that the non-toxic doses of this compound were not sufficient to inhibit the PI3K pathways at significant levels to modify our phenotypes.
Our goal was to generate multiple phenotypic read-outs, preferably using different pathway components, to test each compound. However, not all transgenic lines that we tested produced phenotypes that were robust and sensitive enough to be used as read-outs, a factor which may also have affected our success rate. For instance, we only had one phenotype generated by the ectopic expression of EGFR.DN to test the activity of the EGFR inhibitors AG99 and AG494 [18, 19]. We reasoned that the inhibition of endogenous EGFR by these compounds may lead to an enhancement of the EGFR.DN wing phenotype; however, neither AG99 nor AG494 was effective in modifying this phenotype. It is possible that the ectopic expression of wild-type or activated EGFR (EGFR.CA) would be better suited to test the activity of these compounds; however, the phenotypes generated by EGFR were too weak and those generated by EGFR.CA were either too strong or too variable to be used as read-outs (not shown). A similar argument can be made for the remaining four compounds with no effect in our assay. While we cannot distinguish whether these compounds were not effective in our system because of problems with compound delivery, lack of conservation of activity, or limitations of the phenotypic read-outs, the fact that we only had six such compounds in our analysis is encouraging. Furthermore, the degree of conservation that we observed in vivo is comparable to studies of compound activity in Drosophila and mammalian cell-based assays. For instance, in a recent study, 64% of small molecules that were identified as inhibitors of cytokinesis in Drosophila cells were also active in human HeLa cells [15]. Furthermore, more than 60% of compounds that were identified in mammalian cells as modulators of pathway activity were also active in Drosophila cell lines and vice versa (M.H., unpublished data). This high success rate in detecting compound effects also demonstrates the efficiency of our compound feeding approach and the sensitivity of our phenotypic read-outs.
Drosophila as a high-throughput drug screening platform
The high degree of conservation of signaling pathways between Drosophila and vertebrates, combined with the significant correlation of compound activity between flies and mammalian systems both in cell culture [15] and in vivo (Fig. 2), makes Drosophila a valuable platform for screening compound libraries against Drosophila models of human disease. The feasibility of such an approach has been recently demonstrated in a compound screen using a Drosophila model of Fragile X syndrome [9]. While the throughput of such in vivo screens may not be as high as that of cell-based screens, the ability to rapidly generate complex, multigenic animal models provides a unique ability to screen compound libraries against disease phenotypes in the context of a whole animal, making Drosophila a valuable drug discovery platform. Hits with conserved activity in mammalian cells can then be characterized using pathway-specific phenotypic read-outs as the ones described here in order to explore the signaling pathways that they may target, as well as their pathway specificity. Furthermore, the extensive genetic tools available in flies allows the testing of compounds in various mutant backgrounds to further explore their mechanisms of actions and provide hypotheses to be tested by direct biochemical methods. The phenotypic read-outs described here and compounds identified as having conserved activity will be invaluable tools in such studies.
Genetic dissection of AY9944 mechanism of action using gene–compound epistasis
Our initial compound feeding tests identified AY9944 as an inhibitor of Hh signaling in Drosophila and, in further experiments using both developmental phenotypes and endogenous molecular read-outs of Hh signaling, we clearly demonstrated that the function of AY9944 as a Hh pathway inhibitor is conserved in Drosophila (Figs. 2a and 3f–h). AY9944 is an inhibitor of cholesterol biosynthesis and cellular trafficking, and it has been shown to antagonize Hh signaling in a number of vertebrate model systems [21, 32, 45]. While the consequences of inhibition of cholesterol biosynthesis by AY9944 on Hh signaling have been well studied, the pleiotropic effects of this compound on cholesterol metabolism hindered the complete understanding of its mechanism of action [10, 22]. For instance, how the inhibition of cholesterol trafficking contributes to the antagonism of Hh signaling is not clear. The absence of cholesterol biosynthesis in Drosophila provided us with a unique opportunity to separate these two functions of AY9944 and study the effects of blocking cholesterol transport on Hh signaling.
Our genetic dissection of AY9944 function showed that AY9944 inhibited Hh-induced Ptc internalization, a direct and immediate consequence of Hh binding to Ptc, as well as the expression of the Hh target gene en. Furthermore, our epistasis analysis between AY9944 and various Hh signaling pathway components revealed that AY9944 acts upstream of ptc, at the level of hh, most likely by interfering with the ability of the Hh ligand to signal. Our working model is that changes in cholesterol composition of the plasma membrane in AY9944-treated tissues may interfere with the ability of native, cholesterol-modified Hh to closely associate with cellular membranes and find or interact with Ptc. In the absence of cholesterol modification, Hh behaves more like a freely diffusible ligand and is not sensitive to changes in membrane cholesterol composition.
There have been other models put forward to explain the mechanism of action of this compound. For instance, it has been shown that the inhibition of cholesterol biosynthesis by AY9944 leads to the accumulation of cholesterol precursors such as 7-dihydrocholesterol (7-DHC) and it has been suggested that 7-DHC or one of its derivatives like vitamin D3 can directly inhibit Smo activity [4]. This model suggests that it is the accumulation of these by-products, not the absence of cholesterol, that is responsible for the inhibition of Hh signaling. We also tested the ability of these compounds to block Hh signaling in our system, and neither 7-DHC nor vitamin D3 treatment had an effect on Hh signaling in Drosophila, nor did we see any synergistic effects between AY9944 and 7-DHC (not shown). It can be argued that this is the main mechanism by which AY9944 inhibits Hh signaling in mammalian cells and is not conserved in Drosophila. However, this is unlikely because the inhibitory effect of AY9944 can be rescued by cholesterol in mammalian cells [22], as well as in our system (not shown). This is inconsistent with the idea that 7-DHC accumulation is required to inhibit Hh signaling in AY9944-treated cells. While it is very likely that the inhibition of cholesterol biosynthesis also has a contribution to the antagonistic effects of AY9944 on Hh signaling in mammalian cells, observations from both our system and mammalian cells suggest that this mechanism involves a reduction of cellular cholesterol, not the accumulation of by-products of cholesterol biosynthesis pathway. Interestingly, observations from studies of human genetic disorders of cholesterol biosynthesis also support this proposal. For instance, patients affected by the Smith–Lemli–Opitz syndrome carry a mutation in the dhcr7 gene, have reduced cholesterol levels, accumulate 7-DHC, and exhibit developmental defects associated with perturbations in Hh signaling [20]. Fibroblasts isolated from these patients show reduced Hh signaling activity when deprived of exogenous cholesterol [11], suggesting that lowered cholesterol levels are at least partly responsible for impaired Hh signaling in these patients.
Our findings indicate that the change in cholesterol composition of cellular membranes due to the inhibition of exogenously derived cholesterol trafficking is responsible for the antagonistic effect of AY9944 on Hh signaling. The identical behavior of other cholesterol transport inhibitors to AY9944 in our assays and the observation that the inhibition of Hh signaling by AY9944 can be rescued by cholesterol in both mammalian cells and Drosophila suggest that this is a conserved mechanism. It remains to be seen how the epistatic behavior of AY9944 compares with that of other cholesterol transport inhibitors that do not inhibit cholesterol biosynthesis in mammalian cells. Given the link between impaired cholesterol transport and defective Hh signaling, it will also be interesting to see whether there are defects in Hh signaling in other genetic disorders of lipid/cholesterol trafficking, such as the Niemann–Pick C disease, a fatal progressive neurodegenerative disorder associated with neuronal death and dysfunction [27]. If this is the case, the pharmacological manipulation of Hh signaling may be a valuable therapeutic tool for the treatment of genetic disorders of cholesterol biosynthesis and trafficking.
Experimental procedures
Drosophila strains and crosses Flies were raised on standard cornmeal medium at 25 °C unless indicated otherwise. All mutant and transgenic lines used are described in Flybase (www.flybase.net).
Generation of developmental phenotypes by ectopic expression Ectopic expression throughout development was achieved using the Gal4-UAS system [7]. UAS lines were crossed to 69B-gal4 and ey-gal4 and progeny raised at 18, 25, and 29 °C were scored for phenotypes. Crosses with Gal80ts were set up at 18 °C and the progeny transferred to 25 or 29 °C during the following developmental stages: embryogenesis, first, second, early–mid third, and mid–late third larval instars. Progeny was either raised at higher temperatures until eclosion or shifted back to 18 °C after 24 h for “ectopic expression pulse” experiments.
Compound feeding Compound-treated food was prepared by adding 200 μl of compound solution in 10% DMSO on top of 3 ml of solidified instant food (Sigma) in standard Drosophila vials. The optimal volume of liquid necessary to uniformly cover the surface of the food was determined empirically to be 200 μl by adding different amounts of bromophenol blue solution on top of the food and monitoring the food surface for uniform absorption. The use of smaller volumes resulted in nonhomogenous absorption of the dye in patches on the food surface. Maximum tolerable DMSO concentration for feeding with this method was empirically determined to be 10%. Concentrations of compound solution added on top of the food range from 1 to 10 mM (Fig. S2), corresponding to the predicted compound concentrations at the range of 3–30 μM within the food assuming uniform distribution. The uniform distribution of compound on food surface was ensured by visual inspection (see methods in “Electronic supplementary material” for more information). Hh(Ant)-1 belongs to a family of benzimidazole-derived structures reported to antagonize Smo activity [2]. All other compounds used in this study were purchased from Tocris Inc. (for more information on compound structures and properties, see www.tocris.com). All compound stock solutions are in 100% DMSO, except for AY9944, which is water soluble. Feeding was achieved by transferring 100 μl of embryos (suspended in water) of indicated genotypes on compound-treated food. Embryos are obtained from 48-h egg lays at 18 °C and, after being transferred onto compound-treated food, the vials were shifted to higher temperatures at indicated times to induce ectopic expression.
Ex vivo compound incubation assay Dissected late third instar wing imaginal disks or salivary glands still attached to larval cuticles were incubated with compounds in 0.5% DMSO in serum-free Drosophila Schneider’s medium (SFM) at room temperature in 24-well plates (0.5% DMSO in SFM and SFM alone were used as controls). The tissues were incubated for 24 h to monitor en expression and for 8 h to monitor Ptc internalization. All Hh antagonists were used at 250 μM except for AY9944, which was used at 125 μM due to tissue toxicity at 250 μM.
Misexpression and clonal analysis In order to misexpress Hh pathway components in wing imaginal disks and salivary glands, the larvae of the following genotypes were generated at 18 °C: (1) w; tub-gal80ts/+; UAShh/69B-gal4, (2) w; tub-gal80ts/+; UAShh.N/69B-gal4, and (3) w; tub-gal80ts/UASptc.1130X (DN); +/69B-gal4. Ectopic expression was induced by a temperature switch to 29 °C for 2 days for Hh, 1 day for HhN, and 4 days for Ptc.DN, after which the larvae were dissected and compound incubation was carried out as described. The ptc and pka null clones were generated in the following genotypes: w hs-FLP1/(+ or Y); FRT42D ubi-GFP/FRT42D ptc16 and w hs-FLP1/(+ or Y); ubi-GFP FRT 40A/dcoB3FRT40A. The clones were induced by heat-shocking second instar larvae at 37 °C for 2 h to induce FLP recombinase expression. The ptc or pka mutant cells were identified by the absence of GFP expression.
Immunohistochemistry and imaging The dissected tissues were fixed in 4% formaldehyde for 10 min, blocked in 1% normal goat serum and 0.1% Triton-X in phosphate-buffered saline (PBS) for 1 h and incubated overnight at 4 °C with mouse anti-Engrailed (DSHB, 1:100) or mouse anti-Ptc (DSHB, 1:500) antibodies. Secondary antibodies (Alexa 488, Alexa 594; Molecular Probes) were used at 1:3,000. For filipin staining (Sigma, catalog number F4767), tissues were fixed and washed as indicated above, incubated for 1 h with filipin, rinsed three times with PBS, and mounted as indicated above. A working solution of filipin was made by dissolving a few crystals of the dye in 5 μl of 100% DMSO and immediately adding 500 μl of 1X PBS. Tissues were mounted in 80% glycerol and 0.5% N-propyl galate in PBS. Tissues incubated in compounds were first rinsed in PBS three times, followed by a 10-min wash in PBS, before they were fixed. Scoring of tissues and adult flies for phenotypes was done blindly using Zeiss AxioImager Z1 and Leica MZFL III microscopes, respectively. Fluorescence images were captured at identical settings using Zeiss AxioCam MRm and images of wing, eye, and notum phenotypes were captured with Nikon Digital Camera DXM1200.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 1,361 kb)
References
- 1.Basler K, Struhl G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature. 1994;368:208–214. doi: 10.1038/368208a0. [DOI] [PubMed] [Google Scholar]
- 2.Baxter ADA, Boyd Edward Andrew, Guicherit Oivin M, Price Stephen, Rubin Lee D (2003) Mediators of hedgehog signaling pathways, compositions and uses related thereto. Curtis, Inc., Cambridge, MA
- 3.Bier E, Bodmer R. Drosophila, an emerging model for cardiac disease. Gene. 2004;342:1–11. doi: 10.1016/j.gene.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Bijlsma MF, Spek CA, Zivkovic D, Water S, Rezaee F, Peppelenbosch MP. Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion. PLoS Biol. 2006;4:e232. doi: 10.1371/journal.pbio.0040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilen J, Bonini NM. Drosophila as a model for human neurodegenerative disease. Annu Rev Genet. 2005;39:153–171. doi: 10.1146/annurev.genet.39.110304.095804. [DOI] [PubMed] [Google Scholar]
- 6.Bohni R, Riesgo-Escovar J, Oldham S, Brogiolo W, Stocker H, Andruss BF, Beckingham K, Hafen E. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell. 1999;97:865–875. doi: 10.1016/S0092-8674(00)80799-0. [DOI] [PubMed] [Google Scholar]
- 7.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 8.Callejo A, Torroja C, Quijada L, Guerrero I. Hedgehog lipid modifications are required for Hedgehog stabilization in the extracellular matrix. Development. 2006;133:471–483. doi: 10.1242/dev.02217. [DOI] [PubMed] [Google Scholar]
- 9.Chang S, Bray SM, Li Z, Zarnescu DC, He C, Jin P, Warren ST. Identification of small molecules rescuing fragile X syndrome phenotypes in Drosophila. Nat Chem Biol. 2008;4:256–263. doi: 10.1038/nchembio.78. [DOI] [PubMed] [Google Scholar]
- 10.Cooper MK, Porter JA, Young KE, Beachy PA. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science. 1998;280:1603–1607. doi: 10.1126/science.280.5369.1603. [DOI] [PubMed] [Google Scholar]
- 11.Cooper MK, Wassif CA, Krakowiak PA, Taipale J, Gong R, Kelley RI, Porter FD, Beachy PA. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat Genet. 2003;33:508–513. doi: 10.1038/ng1134. [DOI] [PubMed] [Google Scholar]
- 12.Denef N, Neubuser D, Perez L, Cohen SM. Hedgehog induces opposite changes in turnover and subcellular localization of patched and smoothened. Cell. 2000;102:521–531. doi: 10.1016/S0092-8674(00)00056-8. [DOI] [PubMed] [Google Scholar]
- 13.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duffy JB. GAL4 system in Drosophila: a fly geneticist’s Swiss army knife. Genesis. 2002;34:1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- 15.Eggert US, Kiger AA, Richter C, Perlman ZE, Perrimon N, Mitchison TJ, Field CM. Parallel chemical genetic and genome-wide RNAi screens identify cytokinesis inhibitors and targets. PLoS Biol. 2004;2:e379. doi: 10.1371/journal.pbio.0020379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang N, Casida JE. Anticancer action of cube insecticide: correlation for rotenoid constituents between inhibition of NADH:ubiquinone oxidoreductase and induced ornithine decarboxylase activities. Proc Natl Acad Sci USA. 1998;95:3380–3384. doi: 10.1073/pnas.95.7.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallet A, Ruel L, Staccini-Lavenant L, Therond PP. Cholesterol modification is necessary for controlled planar long-range activity of Hedgehog in Drosophila epithelia. Development. 2006;133:407–418. doi: 10.1242/dev.02212. [DOI] [PubMed] [Google Scholar]
- 18.Gazit A, Osherov N, Gilon C, Levitzki A. Tyrphostins. 6. Dimeric benzylidenemalononitrile tyrophostins: potent inhibitors of EGF receptor tyrosine kinase in vitro. J Med Chem. 1996;39:4905–4911. doi: 10.1021/jm960225d. [DOI] [PubMed] [Google Scholar]
- 19.Gazit A, Osherov N, Posner I, Yaish P, Poradosu E, Gilon C, Levitzki A. Tyrphostins. 2. Heterocyclic and alpha-substituted benzylidenemalononitrile tyrphostins as potent inhibitors of EGF receptor and ErbB2/neu tyrosine kinases. J Med Chem. 1991;34:1896–1907. doi: 10.1021/jm00110a022. [DOI] [PubMed] [Google Scholar]
- 20.Guizzetti M, Costa LG. Sonic hedgehog in Smith–Lemli–Opitz syndrome and tumor development. J Pediatr Hematol Oncol. 2008;30:641–642. doi: 10.1097/MPH.0b013e318180bbb9. [DOI] [PubMed] [Google Scholar]
- 21.Incardona JP, Eaton S. Cholesterol in signal transduction. Curr Opin Cell Biol. 2000;12:193–203. doi: 10.1016/S0955-0674(99)00076-9. [DOI] [PubMed] [Google Scholar]
- 22.Incardona JP, Gaffield W, Kapur RP, Roelink H. The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development. 1998;125:3553–3562. doi: 10.1242/dev.125.18.3553. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs HT, Fernandez-Ayala DJ, Manjiry S, Kemppainen E, Toivonen JM, O’Dell KM. Mitochondrial disease in flies. Biochim Biophys Acta. 2004;1659:190–196. doi: 10.1016/j.bbabio.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Johnson RL, Milenkovic L, Scott MP. In vivo functions of the patched protein: requirement of the C terminus for target gene inactivation but not Hedgehog sequestration. Mol Cell. 2000;6:467–478. doi: 10.1016/S1097-2765(00)00045-9. [DOI] [PubMed] [Google Scholar]
- 25.Kango-Singh M, Halder G. Drosophila as an emerging model to study metastasis. Genome Biol. 2004;5:216. doi: 10.1186/gb-2004-5-4-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karim FD, Rubin GM. Ectopic expression of activated Ras1 induces hyperplastic growth and increased cell death in Drosophila imaginal tissues. Development. 1998;125:1–9. doi: 10.1242/dev.125.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Karten B, Peake KB, Vance JE. Mechanisms and consequences of impaired lipid trafficking in Niemann–Pick type C1-deficient mammalian cells. Biochim Biophys Acta. 2009;1791:659–670. doi: 10.1016/j.bbalip.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 28.Lange Y, Steck TL. Cholesterol homeostasis. Modulation by amphiphiles. J Biol Chem. 1994;269:29371–29374. [PubMed] [Google Scholar]
- 29.Lasko P. Diabetic flies? Using Drosophila melanogaster to understand the causes of monogenic and genetically complex diseases. Clin Genet. 2002;62:358–367. doi: 10.1034/j.1399-0004.2002.620502.x. [DOI] [PubMed] [Google Scholar]
- 30.Lawrence PA, Morata G. Compartments in the wing of Drosophila: a study of the engrailed gene. Dev Biol. 1976;50:321–337. doi: 10.1016/0012-1606(76)90155-X. [DOI] [PubMed] [Google Scholar]
- 31.Li W, Ohlmeyer JT, Lane ME, Kalderon D. Function of protein kinase A in hedgehog signal transduction and Drosophila imaginal disc development. Cell. 1995;80:553–562. doi: 10.1016/0092-8674(95)90509-X. [DOI] [PubMed] [Google Scholar]
- 32.Liscum L, Munn NJ. Intracellular cholesterol transport. Biochim Biophys Acta. 1999;1438:19–37. doi: 10.1016/s1388-1981(99)00043-8. [DOI] [PubMed] [Google Scholar]
- 33.McGuire SE, Mao Z, Davis RL (2004) Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE 2004, pl6 [DOI] [PubMed]
- 34.Micchelli CA, Esler WP, Kimberly WT, Jack C, Berezovska O, Kornilova A, Hyman BT, Perrimon N, Wolfe MS. Gamma-secretase/presenilin inhibitors for Alzheimer’s disease phenocopy Notch mutations in Drosophila. FASEB J. 2003;17:79–81. doi: 10.1096/fj.02-0394fje. [DOI] [PubMed] [Google Scholar]
- 35.Porter JA, Ekker SC, Park WJ, Kessler DP, Young KE, Chen CH, Ma Y, Woods AS, Cotter RJ, Koonin EV, et al. Hedgehog patterning activity: role of a lipophilic modification mediated by the carboxy-terminal autoprocessing domain. Cell. 1996;86:21–34. doi: 10.1016/S0092-8674(00)80074-4. [DOI] [PubMed] [Google Scholar]
- 36.Sakamoto N, Terai M, Takenaka T, Maeno H. Inhibition of cyclic AMP phosphodiesterase by 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylic acid 3-[2-(N-benzyl-N-methylamino)] ethyl ester 5-methyl ester hydrochloride (YC-93), a potent vasodilator. Biochem Pharmacol. 1978;27:1269–1274. doi: 10.1016/0006-2952(78)90462-8. [DOI] [PubMed] [Google Scholar]
- 37.Santos AC, Lehmann R. Isoprenoids control germ cell migration downstream of HMGCoA reductase. Dev Cell. 2004;6:283–293. doi: 10.1016/S1534-5807(04)00023-1. [DOI] [PubMed] [Google Scholar]
- 38.Seamon KB, Daly JW, Metzger H, Souza NJ, Reden J. Structure–activity relationships for activation of adenylate cyclase by the diterpene forskolin and its derivatives. J Med Chem. 1983;26:436–439. doi: 10.1021/jm00357a021. [DOI] [PubMed] [Google Scholar]
- 39.Segalat L. Invertebrate animal models of diseases as screening tools in drug discovery. ACS Chem Biol. 2007;2:231–236. doi: 10.1021/cb700009m. [DOI] [PubMed] [Google Scholar]
- 40.Selcher JC, Atkins CM, Trzaskos JM, Paylor R, Sweatt JD. A necessity for MAP kinase activation in mammalian spatial learning. Learn Mem. 1999;6:478–490. doi: 10.1101/lm.6.5.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strigini M, Cohen SM. A Hedgehog activity gradient contributes to AP axial patterning of the Drosophila wing. Development. 1997;124:4697–4705. doi: 10.1242/dev.124.22.4697. [DOI] [PubMed] [Google Scholar]
- 42.Vidal M, Wells S, Ryan A, Cagan R. ZD6474 suppresses oncogenic RET isoforms in a Drosophila model for type 2 multiple endocrine neoplasia syndromes and papillary thyroid carcinoma. Cancer Res. 2005;65:3538–3541. doi: 10.1158/0008-5472.CAN-04-4561. [DOI] [PubMed] [Google Scholar]
- 43.Witte HT, Jeibmann A, Klambt C, Paulus W. Modeling glioma growth and invasion in Drosophila melanogaster. Neoplasia. 2009;11:882–888. doi: 10.1593/neo.09576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- 45.Yoshikawa H. Effects of drugs on cholesterol esterification in normal and Niemann–Pick type C fibroblasts: AY-9944, other cationic amphiphilic drugs and DMSO. Brain Dev. 1991;13:115–120. doi: 10.1016/s0387-7604(12)80118-5. [DOI] [PubMed] [Google Scholar]
- 46.Zecca M, Basler K, Struhl G. Sequential organizing activities of engrailed, hedgehog and decapentaplegic in the Drosophila wing. Development. 1995;121:2265–2278. doi: 10.1242/dev.121.8.2265. [DOI] [PubMed] [Google Scholar]
- 47.Zhu AJ, Zheng L, Suyama K, Scott MP. Altered localization of Drosophila Smoothened protein activates Hedgehog signal transduction. Genes Dev. 2003;17:1240–1252. doi: 10.1101/gad.1080803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
(PDF 1,361 kb)