Abstract
It has been previously reported that serum levels of 70-kDa heat shock protein (Hsp70) are elevated in peripheral artery disease. The aim of the present study was to examine whether increased serum Hsp70 levels are related to the extent of arterial calcification and standard laboratory parameters of patients with peripheral artery disease, as well as to markers of inflammation (C-reactive protein), atherosclerosis (homocysteine), and calcification (fetuin-a). One hundred eighty chronic atherosclerotic patients with significant carotid stenosis and/or lower extremity vascular disease were enrolled in this cross-sectional study. Systemic atherosclerosis and calcification was assessed by ultrasound (carotid intima–media thickness (IMT), presence of calcification at the abdominal aorta, carotid and femoral bifurcations, and aortic and mitral cardiac valves). Standard serum markers of inflammation, diabetes, renal function, ankle-brachial indexes, and traditional risk factors for atherosclerosis were noted. Serum Hsp70 levels were measured with enzyme-linked immunosorbent assay. Standard laboratory parameters (clinical chemistry), C-reactive protein (CRP), and homocysteine levels were determined by an autoanalyzer using the manufacturer’s kits. Fetuin-a levels were measured by radial immunodiffusion. Patients’ median age was 64 (57–71) years, 69% were men, and 34.5% had diabetes. Serum heat shock protein 70 levels were significantly higher in patients with more severe arterial calcification (p < 0.02) and showed significant positive correlations with serum bilirubin (r = 0.23, p = 0.002) and homocysteine levels (r = 0.18, p = 0.02). Serum Hsp70 did not correlate with body mass index, IMT, CRP, or fetuin-a levels in this cohort. Logistic regression analysis confirmed the association between sHsp70 and calcification score (OR, 2.189; CI, 1.156–4.144, p = 0.016) and this correlation remained significant (OR, 2.264; CI, 1.021–5.020, p = 0.044) after the adjustment for age, sex, eGFR, smoking, CRP, and homocysteine levels. Our data show that serum Hsp70 levels correlate with the severity of atherosclerosis in patients with carotid artery disease and chronic lower limb ischemia. These data support a putative role for plasma Hsp70 in the development of arterial calcification. Nevertheless, further studies are required to investigate the usefulness of circulating Hsp70 level as a marker of atherosclerotic calcification.
Keywords: Heat shock protein 70, Vascular, Biomarker, Calcification, Peripheral, Atherosclerosis
Introduction
Ischemic cardiovascular diseases (CVD) of atherosclerotic origin are still leading cause of death in the USA (www.americanheart.org) and in Europe (www.heartstats.org). The presence of extended calcification in peripheral artery disease (PAD) of the lower extremities and carotid artery disease (CAD) is associated with a three- to fourfold higher risk for mortality and cardiovascular events (Norgren et al. 2007; Rennenberg et al. 2009). While the adverse cardiac and cerebrovascular complications are higher in persons with more severe PAD, there is still a significant risk in persons with mild and even asymptomatic disease (Eberhardt and Coffman 2004). Stress factors alone—such as smoking, hyperhomocysteinemia (Clarke et al. 1991; Norgren et al. 2007), or high C-reactive protein levels—do not always explain the extent of calcified vasculature and later cardiovascular risk in peripheral artery disease and CAD (Cao et al. 2007). Novel biomarkers of arterial calcification are therefore urgently needed to avoid later major CVD complications. Heat shock proteins (Hsp) are phylogenetically highly conserved molecules in their molecular structure, biochemical properties, and partly even in their immunological structure. Hsps are traditionally classified by their molecular weight, the 70-kDa family (Hsp70) including constitutively expressed Hsp-8 and inducible Hsp70-1, also called HspA1B (Hsp70 in the following refers to the inducible form) members. Hsp70 is traditionally considered as intracellular cytoprotective chaperone, and its level can increase several-fold in response to stress (Prohaszka and Fust 2004). It has been recognised that Hsp70 are present in the peripheral circulation of normal individuals (Pockley et al. 1998; Dhingra et al. 2006) providing the first evidence that Hsp70 may be released into the extracellular environment not only in response to stress but also under physiologic conditions. Soluble Hsp70 showed no apparent endogenous circadian rhythm in a rested state (Fortes and Whitham 2009). Elevated serum Hsp70 level was measured in pathologic pregnancies (Molvarec et al. 2009) such as pre-eclampsia and hemolytic anemia elevated liver enzymes low platelet count syndrome. Increased serum levels of Hsp70 levels were reported in patients with peripheral and renal vascular disease (Wright et al. 2000) and after acute myocardial infarction (Dybdahl et al. 2005; Satoh et al. 2006), whereas circulating Hsp70 levels were positively related to markers of tissue damage including creatine kinase MB and cardiac troponin T. In chronic heart failure (CHF) patients increased serum Hsp70 levels were found compared to healthy controls (Genth-Zotz et al. 2004). It was shown that Hsp70 levels positively correlate to NYHA (New York Heart Association) classes. Gombos et al. (2008) completed these findings in CHF by showing significant associations between the Hsp70 and markers of disease severity such as lower ejection fraction and higher NT-proBNP levels. Elevated Hsp70 and heme oxygenase-1 (HO-1) levels have been reported in response to LPS-induced endothelial injury (Bernardini et al. 2010) and in brain stem death triggered by hypoxia (Chang et al. 2009).
The source of the serum Hsp70 has not been fully clarified; however, studies suggest that Hsp70 may be released from viable endothelial cells within exosomes (Zhan et al. 2009). This mechanism can be induced by certain cytokines in breast adenocarcinoma (Bausero et al. 2005). Hsp70 expression was investigated in vitro in monocytes of patients suffering from obstructive sleep apnea syndrome (Lavie et al. 2009) and of peripheral artery disease (Luu et al. 2007; Madden et al. 2009) where lower inducible monocyte Hsp70 levels were found compared to healthy controls. Hsp70 is expressed in small arteries (Paier et al. 2009), explicated in dendritic cells of the arterial intima (Bobryshev and Lord 2002) and in smooth muscle cells (Zhu et al. 1995). Inducible Hsp70 can be released from the hepatosplanchnic tissue during exercise (Febbraio et al. 2002) or from the myocardium itself (St Rammos et al. 2002).
In the present study, we measured serum levels of Hsp70 in subjects with PAD of the lower extremities and with carotid artery disease and determined the possible correlation with the dimension of disease, and the presence of cardiovascular risk factors (homocysteine, CRP, smoking, and diabetes). We also aimed to investigate the association between serum Hsp70 and fetuin-a, a novel marker of arterial calcification (Fiore et al. 2007).
Research design and methods
Study population
In this cross-sectional study, 180 consecutive patients were recruited at the outpatient clinic of the Department of Vascular Surgery of Semmelweis University Budapest between January and June 2009. Consecutive patients with history or present symptoms of atherosclerotic chronic lower limb ischemia or chronic carotid artery stenosis were considered for inclusion. Patients who had acute onset of lower limb ischemia, clinical or laboratory signs of acute infection, myocardial infarction, stroke, trauma, and surgical procedure in the last 6 months were excluded in this study. Patients with coexisting malignant tumor, hepatic disease, end stage renal disease (dialysis) or immune suppression were also excluded. The full clinical record of the patients was registered at inclusion with the detailed physical status and routine clinical laboratory tests. Blood samples for the measurement of serum Hsp70 and fetuin-a were also collected at inclusion before the patients underwent surgery or percutaneous transluminal angioplasty (PTA). The study was carried out in accordance of the Helsinki Declaration at the Department of Vascular Surgery, Semmelweis University based on a study protocol approved by the Semmelweis University Regional and Institutional Committee of Science and Research Ethics. All patients provided written informed consent.
Clinical data and calcification score
All patients had a medical history interview and physical examination. A study questionnaire was used for recording the relevant demographic and clinical data (age, weight, height, smoking habit, medications, and concomitant disease). The traditional Fontaine classification was used to assess the clinical severity of the chronic lower extremity atherosclerotic disease (groups I, II/a, II/b, III, and IV). Group II was separated to “a” and “b” subgroups at walking distance of 200 m. Ankle–brachial index (ABI) measurement with Doppler ultrasound probe was performed by a medical doctor experienced in taking ABI. The patients lied in supine position after resting at normal room temperature; measurements were taken at each ankle over the posterior tibial and dorsal pedal arteries. ABI was calculated as the lowest pressure of the ankles divided by the higher of the left and right arm pressures (Espinola-Klein et al. 2008; Fowkes et al. 2008). Carotid IMT and the noncardiac part of the general calcification score was determined by a single experienced radiologist who was blinded to patients’ clinical information. IMT was measured at three points on a plaque free area of the dorsal wall of both common carotid arteries using linear (7.5–11 MHz) and convex (3.5–5 MHz) transducer of a Toshiba Aplio SSA-770 ultrasound system. The mean value and the maximum IMT was used for calculations (Higgins et al. 2005). At the same examination, carotid stenosis was also determined (Grant et al. 2003), stenosis with 70% or more were determined as significant. After having measured both carotid arteries, the more stenotic artery was determined as the maximal carotid stenosis. Body mass index (BMI) was calculated as weight (kg)/height2 (m). To assess the overall extent of systemic atherosclerosis a calcification score (CS) was calculated after examining the vascular system at seven sites: both carotid bifurcations, the infrarenal aorta, both common femoral arteries, and aortic and mitral valves by B-mode ultrasound (see technical details above at carotid IMT measurements). If calcification was noted, the spot was rated as one. Sites with no calcification received zero, so the calcification range was 0–7 (Guerin et al. 2000; Cozzolino et al. 2006). Transthoracic echocardiograms were performed by one experienced cardiologist blinded for other study information. According to the guidelines of the American Society of Echocardiography, complete two-dimensional examinations were performed including Doppler images in all standard views using phased array transducers (2.5–4.5 MHz) of a Toshiba Xario and Philips IE 33 ultrasound system. Mitral valve calcification was defined as an echodense (reaching epicardial density) structure of the anterior and posterior mitral leaflet and the mitral annulus on the parasternal short and long axis and apical four chamber view. Aortic valve calcification was determined if echodense structure (reaching epicardial density) was noticed at the aortic root on the parasternal short and long axis and apical five chamber view.
Blood samples and determination of serum soluble Hsp70
Blood samples were taken after 6 h of fasting between 8:00 a.m. and 10:00 a.m. by antecubital veinpuncture into native, ethylenediaminetetraacetic acid or sodium citrate anticoagulated tubes. The samples were centrifuged, and aliquots of serum and plasma were later stored at −70°C until further analysis.
Soluble Hsp70 level was measured by using R&D System (USA, Cat no. DYC1663E) enzyme-linked immunosorbent assay (ELISA) kit. For Hsp70 family nomenclature, the suggestions of Tavaria et al. (1996) were used. Ninety-six-well microtitre plates were coated with mouse anti-human Hsp70 capture antibodies (100 μl, 2 μg/ml) in carbonate buffer (pH 9.5) overnight at 4°C. Plates were washed with phosphate-buffered saline (PBS) containing 0.1% Tween 20 three times and non-specific binding sites blocked by incubation with 200 μl of PBS containing 0.5% gelatine and Tween 20 for 1 h at room temperature (RT). After washing, 100 μl of the reference preparation (recombinant human Hsp70, 0–10 ng/ml) or undiluted serum samples were added, and the plates were incubated for 2 h at RT. Plates were subsequently washed, and Hsp70 binding was determined using biotinylated rabbit anti-human antibodies (100 μl, 0.5 μg/ml) in PBS gelatine. After 1.5 h at room temperature, plates were washed and incubated with streptavidin–horseradish peroxidase (1:200) in PBS gelatine for 20 min at RT. Plates were washed, and 100 μl of o-phenylene-diamine (Sigma, St. Louis, MO, USA) in citrate buffer was added. The optical density was measured at λ = 490 nm (reference at λ = 620 nm). The detection range of the assay was 0.05–10 ng/ml, the intra/inter-assay variability <10/<16%, respectively.
Determination of other laboratory parameters
Serum levels of fetuin-a were determined by radial immunodiffusion. Five microlitres of patient’s serum diluted to 1:4 was applied in 11.5 ml of Litex agarose gel (Sigma). Serum samples (1:4 dilution) with known concentrations of fetuin-a served as standards. The incubation was done at room temperature for 48 h. We used two types of antibodies against fetuin-a as the protein is synthesized as a single chain and is rapidly converted to a dipeptide form following the cleavage of a connecting peptide. The commercially available product (anti-fetuin-a, IgG fraction, Incstar, Cat. no. 81931, 13.7 mg/ml, in a final concentration of 84 μl/11.5 ml gel) recognizes the dipeptide form. The other type of antibody binding to the newly synthesized single chain form of fetuin-a, was raised by immunizing a rabbit with recombinant human protein (final concentration of 568 μl/11.5 ml gel) (Kalabay et al. 2002).
Fasting serum samples were also used to examine standard clinical laboratory measurements, CRP, and homocysteine levels in the core laboratory of Semmelweis University (diagnostic instruments: D-Cell 5D—Diagon Ltd., Cobas Integra 400—Roche, STA-Compact—Diagnostica Stago). We used the Cockcroft-Gault formula for the calculation of glomerular filtration rate. Estimated glomerular filtration rate = ([140 − age] × weight in kg) × constant/(serum creatinine (μmol/L)), where constant was 1.23 for men and 1.04 for women.
Statistical analysis
As most of the variables had non-Gaussian distributions, data are presented in the text and in the tables as median (25th–75th percentile) or as number (percent). Non-parametric tests were used for group comparisons; continuous variables between two groups were compared with the Mann–Whitney U test. Spearman rank correlation coefficients were calculated for estimation of interrelations between sHsp70 and other variables. A power calculation was used to estimate the sample size in the correlation analysis between sHsp70 levels and CS (P = 0.62). Multiple logistic regression analysis was applied to estimate interrelationship between variables as categorical predictors and severity of peripheral artery disease. Analyses were carried out using STATISTICA 7.0 (StatSoft Inc., Tulsa, OK, USA), Prism for Windows v4.02 (GraphPad Software, San Diego, CA, USA) and SPSS for Windows 13.0.1 (SPSS Inc., Chicago, IL, USA) statistical software products. All statistical analyses were performed two-tailed and p < 0.05 was considered as significant.
Results
Patient characteristics
The mean age was 64 years in our 180 patient study population, 56 (31.1%) were female, 98 (54.4%) were current smokers and 55 (30.6%) were past smokers, only 27 (15%) patients smoked never before. The baseline clinical and laboratory characteristics of the study population are reported in Table 1. Thirty-seven (20.6%) patients suffered from significant carotid stenosis only, 91 (50.6%) participants had only lower extremity arterial disease, whereas 52 (28.9%) patients suffered from both diseases. The severity of symptoms of our patients required surgery or PTA in 30 (81%) carotid patients, in 80 (88%) PAD patients, and in 50 (96%) patients suffering from the both diseases. According to the Fontaine classification (I–IV) of chronic atherosclerotic lower extremity arterial disease, 129 patients (71.7%) belonged to Fontaine II/b-IV groups. The median values of ankle–brachial index was 0.50 (0.26–0.76), mean IMT was 0.83 mm (0.70–0.97), and worst IMT was 1.00 mm (0.80–1.30), respectively. Lipid profile results showed moderately higher than normal levels of triglyceride, cholesterol, and low-density lipoprotein (LDL). The median calcification score (CS) was 5 (4–6), this corresponds with the severe systemic arterial calcification of our cohort.
Table 1.
Clinical patient characteristics (n = 180)
| Variables | |
|---|---|
| Demographics and risk factors | |
| Age (years) | 64.1 (57.04–70.66) |
| Gender (male) | 124 (68.89%) |
| BMI | 26.1 (23.88–28.99) |
| Smoking (years) | 35 (25–41) |
| Consumed cigarettes/day | 20 (20–30) |
| Hypertension | 140 (77.78%) |
| Diabetes | 62 (34.44%) |
| Ischemic heart disease | 65 (36.11%) |
| Carotid stenosis significant (%) | 95 (52.78%) |
| Carotid stenosis maximum | 0.6 (0–0.9) |
| Fontaine stages I, II/a, II/b, III, IV (n = 143) | 14, 21, 83, 12, 13 |
| ABI | 0.50 (0.26–0.76) |
| IMT mean (mm) | 0.83 (0.70–0.97) |
| IMT maximum (mm) | 1.00 (0.80–1.30) |
| Calcification score | 5 (4–6) |
| Drug therapy | |
| Aspirin | 118 (65.56%) |
| Clopidogrel | 49 (27.22%) |
| Oral anticoagulants | 12 (6.67%) |
| ACE-inhibitors | 102 (56.67%) |
| Angiotensin receptor blockers | 102 (56.67%) |
| β-blockers | 83 (46.11%) |
| Oral hypoglycemics | 43 (23.89%) |
| Insulin | 18 (10.00%) |
| Statins | 105 (58.33%) |
| Laboratory findings | |
| Serum sodium (mmol/l) | 141 (138–143) |
| Serum potassium (mmol/l) | 4.5 (4.3–4.8) |
| Serum calcium (mmol/l) | 2.47 (2.38–2.53) |
| Serum phosphate (mmol/l) | 1.11 (0.97–1.21) |
| Serum creatinine (μmol/l) | 89 (80–103) |
| Serum carbamide (mmol/l) | 6.6 (5.20–7.85) |
| eGFR (ml/min) | 75.8 (57.17–94.36) |
| Serum bilirubin (μmol/l) | 9.65 (7.25–13.05) |
| AST (U/l) | 21 (17–26) |
| ALT (U/l) | 21 (14–28) |
| Gamma-GT (U/l) | 33 (22–55) |
| Serum albumin (g/l) | 46.5 (43.20–49.00) |
| CRP (mg/l) | 2.7 (0.9–6.5) |
| Homocysteine (μmol/l) | 16.1 (13.20–19.30) |
| Triglyceride (mmol/l) | 1.7 (1.20–2.40) |
| Total cholesterol (mmol/l) | 5.2 (4.30–6.40) |
| LDL (mmol/l) | 3.1 (2.61–4.07) |
| HDL (mmol/l) | 1.4 (1.20–1.58) |
| Fetuin-a (μg/ml) | 732 (648–804) |
| Soluble Hsp70 (ng/ml) | 0.63 (0.52–0.81) |
Values are median (interquartile range) or number (%)
BMI body mass index, ABI ankle–brachial index, IMT intima–media thickness, AST aspartate aminotransferase, ALT alanine aminotransferase, Gamma-GT gamma-glutamyl transpeptidase, CRP C-reactive protein, eGFR estimated glomerular filtration rate, LDL low-density lipoprotein-cholesterol, HDL high-density lipoprotein-cholesterol
Association of sHsp70 levels with calcification severity and clinical variables
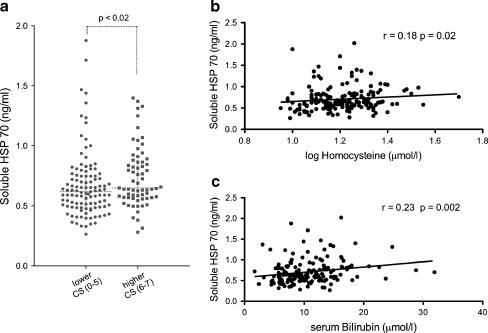
We investigated the association between serum Hsp70 levels and calcification score, clinical characteristics, and laboratory parameters of patients with peripheral artery disease. Analysis of clinical characteristics and other laboratory parameters revealed a significant correlation between serum heat shock protein 70 levels and age and serum bilirubin (Table 2). Patients with higher calcification scores had higher soluble heat shock protein levels (Mann–Whitney probe, p < 0.02). The significant relationships between soluble Hsp70 levels and homocysteine, serum bilirubin levels, and calcification scores are visualized on Fig. 1a–c. There were no significant correlations between sHsp70 concentrations and other markers of liver injury [aspartate aminotransferase (AST), alanine aminotransferase (ALT), and γGT]. No significant association was found between serum Hsp70 levels and clinical characteristics such as gender, BMI, hypertension, diabetes, presence of ischemic heart disease, mean IMT, and ankle–brachial index. Most importantly, no correlation with CRP, triglyceride, cholesterol, serum creatinine, serum carbamide levels, and eGFR was observed (Table 2). No correlation was found between sHsp70 levels and any of the medications used at the time the blood samples were taken (data not shown). There were no significant differences in Hsp70 (p = 0.588) and fetuin-a levels between the three patient groups (p = 0.431). No significant differences have been found between the three patient groups in age, gender, BMI, diabetes, smoking, or calcification scores (p = n.s., respectively). The examination of Hsp70 levels (p = 0.993) or calcification scores (p = 0.909) in diabetic and non-diabetic patients resulted no differences. In a univariate analysis, patients with sHsp70 level above the 75th percentile (0.7296 ng/ml) had an almost 2.2-fold risk to belong to the seriously calcified group (CS 6–7). To evaluate the association of sHsp70 level and the extent of arterial calcification, logistic regression analysis were performed. We examined the possible effect of three models (model 1: age, gender, eGFR; model 2: model 1 + smoking (years); model 3: model 2 + CRP; and model 4: model 3 + homocysteine) on the odds ratio. After correction for those major confounding factors the significant correlation between circulating Hsp70 and arterial calcification still remained significant (Table 3). Adjustment for type II diabetes mellitus with age, gender, and eGFR has also not influenced the significant risk (OR = 2.136 (1.077–4.237) p = 0.03) for extended calcification in patients with higher soluble Hsp70 levels.
Table 2.
Correlation coefficients with p values between serum Hsp70 levels and clinical and laboratory variables (total n = 180)
| Variable | r | p value |
|---|---|---|
| Age | 0.150549 | 0.045485 |
| Gender (male) | −0.026178 | 0.729442 |
| BMI | 0.083219 | 0.270798 |
| Smoking (years) | 0.090170 | 0.269263 |
| Consumed cigarettes/day | 0.031008 | 0.704520 |
| Hypertension | 0.097433 | 0.196998 |
| Diabetes | −0.007125 | 0.925012 |
| Ischemic heart disease | 0.099890 | 0.185885 |
| Carotid stenosis significant (%) | 0.055731 | 0.461263 |
| Carotid stenosis maximum | 0.061359 | 0.417191 |
| Fontaine stages I, II/a, II/b, III, IV (n = 143) | −0.157684 | 0.062791 |
| ABI | 0.062841 | 0.406005 |
| IMT mean (mm) | 0.076109 | 0.325362 |
| IMT maximum (mm) | −0.031641 | 0.682991 |
| Calcification score | 0.168998 | 0.024535 |
| Serum creatinine (μmol/l) | 0.111446 | 0.139731 |
| Serum carbamide (mmol/l) Urea | 0.030041 | 0.691422 |
| eGFR (ml/min) | −0.094085 | 0.212905 |
| Serum bilirubin (μmol/l) | 0.233000 | 0.002036 |
| AST (U/l) GOT | 0.126953 | 0.094095 |
| ALT (U/l) GPT | 0.101128 | 0.184258 |
| Gamma-GT (U/l) | 0.150425 | 0.046923 |
| Serum albumin (g/l) | −0.045852 | 0.551502 |
| CRP (mg/l) | −0.085874 | 0.262677 |
| Homocysteine (μmol/l) | 0.179437 | 0.017829 |
| Triglyceride (mmol/l) | −0.026359 | 0.729904 |
| Total cholesterol (mmol/l) | −0.028605 | 0.707898 |
| LDL (mmol/l) | −0.027531 | 0.728840 |
| HDL (mmol/l) | 0.020420 | 0.795849 |
| Fetuin-a (μg/l) | 0.003335 | 0.964965 |
BMI body mass index, ABI ankle–brachial index, IMT intima–media thickness, AST aspartate aminotransferase, ALT alanine aminotransferase, Gamma-GT gamma-glutamyl transpeptidase, CRP C-reactive protein, eGFR estimated glomerular filtration rate, LDL low-density lipoprotein-cholesterol, HDL high-density lipoprotein-cholesterol
Fig. 1.
Association of serum Hsp70 levels with calcification score (a) and homocysteine levels (b) and serum bilirubin levels (c) in patients with peripheral artery disease and carotid stenosis
Table 3.
Association between sHsp70 and arterial calcification score
| Odds of more severe arterial calcification | p value | |
|---|---|---|
| (Odds ratio and 95% confidence intervals) | ||
| Unadjusted | 2.189 (1.156–4.144) | 0.016 |
| Model 1 | 2.110 (1.066–4.175) | 0.032 |
| Model 2 | 2.233 (1.054–4.730) | 0.036 |
| Model 3 | 2.403 (1.115–5.181) | 0.025 |
| Model 4 | 2.264 (1.021–5.020) | 0.044 |
Odds ratio and 95% confidence intervals (CI) were obtained by logistic regression model
Model 1 demographics: age, gender, eGFR, Model 2 Model 1 + smoking (years), Model 3 Model 2 + CRP, Model 4 Model 3 + homocysteine
Discussion
The novel finding of the present study is that we reported a significant increase in serum heat shock protein 70 levels in patients with more severe systemic arterial calcification scores in a cohort with severe chronic lower extremity atherosclerosis and severe carotid stenosis. We also observed significant correlation between serum Hsp70 and homocysteine levels. The detailed characterization of the patient population allowed us to identify significant correlations between sHsp70 levels and age and serum bilirubin. There was, however, no relationship between soluble Hsp70 and the acute phase reactants C-reactive protein and fetuin-a.
A soluble heat-shock-mediated component to cardiovascular disease has been suggested by a number of studies. Previous works have shown an inverse correlation between circulating Hsp70 levels and atherosclerotic disease progression in cardiac (Zhu et al. 2003; Zhang et al. 2010) and in extracardiac vascular calcifications, such as peripheral artery disease of the lower extremities and carotid artery disease (Wright et al. 2000; Martin-Ventura et al. 2007). In their elegant study, Martin-Ventura et al. (2007) showed significantly decreased Hsp70 levels in plasma of patients with CAD in relation to matched healthy subjects. In contrast, Wright et al. had shown that Hsp70 serum levels were increased in patients with PAD (Wright et al. 2000). This discrepancy could have been due to the degree and localization of atherosclerosis (peripheral versus carotid) other samples (serum versus plasma) and different type of ELISA used. However, potential mechanisms which could explain this inverse relation have not yet been explored.
Our observations corroborate the results of Wright et al. (2000) and Zhang et al. (2010) inasmuch as the higher concentration of Hsp70 was related to disease severity in peripheral artery and in acute coronary syndrome patients. In our study group, Hsp70 levels significantly correlated to the extent of arterial calcification in patients with PAD, carotid artery disease, or in those suffering from both localizations of atherosclerosis. The increasing serum concentration of Hsp70 with the severity of arterial calcification seemed to be independent of the condition of the kidneys and the inflammation, proven by a logistic regression model. Patient groups divided according to lower and higher calcification score classes (1–5 vs. 6–7) showed marked significant difference in sHsp70 levels and were compared using a logistic regression adjusted for the following variables: age, gender, estimated glomerular filtration rate, smoking, diabetes type II, C-reactive protein, and homocysteine levels. The risk to belong to the more severe calcified group was more than two times higher for those having high (>75% percentile, 0.7296 ng/ml) serum Hsp70 levels as compared to those with low levels. This association was independent from the above mentioned variables. Furthermore, lack of significant correlation was observed between sHsp70 and serum creatinine and serum carbamide, indicating that the elevation of sHsp70 parallel with calcification severity is not due to the impaired renal clearance of Hsp70.
In a prospective study, Pockley et al. (2003) demonstrated that increased concentrations of circulating Hsp70 correlated with decreased changes in intima/media thickness in hypertensive patients. It was also implicated by the same authors that serum Hsp70 levels reflect tissue expression, and elevated levels might therefore reflect the presence of an antiatherogenic state in the vasculature. We could not strengthen these findings with IMT because of our cross-sectional study design and more severe atherosclerotic cohort. In their well-designed study, Dulin et al. (2010) have not detected any correlations between the concentrations of circulating Hsp70 and classical vascular risk factors such as homocysteine and C-reactive protein. In our present study, a positive correlation between serum Hsp70 and homocysteine levels was found. Hyperhomocysteinemia is a well-known and independent risk factor for vascular disease (Clarke et al. 1991) and triggers atherosclerotic lesion development in apolipoprotein E-deficient mice involving Hsp70 stress pathway (Zhou et al. 2004). Another interesting animal study showed that heat shock protein 70 enhanced vascular bone morphogenetic protein-4 (BMP) signaling by binding matrix Gla protein, enhanced BMP-induced calcium deposition. In addition, Hsp70 mediated the procalcific effect of interleukin-6 on calcifying vascular cells (Yao et al. 2009).
The source of the serum Hsp70 has not been fully elucidated. Febbraio et al. (2002) found inducible Hsp70 release from splanchnic tissues into the blood stream during exercise. This elevation in plasma Hsp70 levels after acute exercise stress has disappeared after longer intensive exercise and heat acclimation suggesting a thermoregulatory role on the cellular level (Magalhaes et al. 2010). This finding is not in contradiction with our study since we aimed to investigate the possible chronic effects of hsp70 in vascular calcification. Our patients were investigated and their blood samples collected in a rested state. No patients had surgical or traumatic stress or suffered from any acute disease in the last 6 months before their blood samples were taken. We also observed positive correlation between soluble Hsp70 and serum bilirubin levels. This result may reflect the possible relation between hepatosplanchnic tissue damage and circulating Hsp70, suggesting that one source of heat shock protein 70 is maybe in the liver. This hypothesis is in concordance with our previous results (Gombos et al. 2008; Madach et al. 2008); however, other markers of hepatic damage were not correlated with sHsp70 in our present study. The positive correlation between elevated soluble Hsp70 and serum bilirubin levels indicates that both factors increase due to chronic atherosclerotic inflammatory and oxidative stress. HO-1, also known as Hsp32, an enzyme in heme degradation, indirectly elevates blood bilirubin levels in response to oxidative stress (Vitek and Ostrow 2009; Grochot-Przeczek et al. 2010). Bilirubin is a potent antioxidant by scavenging RONS directly, and suppressing the activity of NADPH oxidase indirectly (McCarty 2007; Jansen et al. 2010). Sources of circulating Hsp70 can be endothelial cells induced by oxidative low-density lipoprotein and homocysteine (Zhan et al. 2009). Hyperhomocysteinemia triggers apoptosis in vascular endothelial cells acting as an endoplasmic reticulum stress inducer (Kyriakakis et al. 2010). Moreover, this extracellular serum Hsp70 may represent a danger signal of cellular death or lysis-activating innate immunity through a strong classical complement pathway activation (Prohaszka et al. 2002). These elevated levels of circulating Hsp70 may also originate from necrotic cells, and stimulate macrophages to secrete cytokines, and induce expression of antigen-presenting and co-stimulatory molecules on the dendritic cells, in response to cell death. The role of sHsp70 in atherosclerosis is controversial. It is not clear, whether higher serum Hsp70 levels are a causative or an atheroprotective factor due to severe systemic calcification. Evidence suggests that soluble Hsp70 is likely to be involved in cytoprotection (Bielecka-Dabrowa et al. 2009) due to the chronic stress of atherosclerosis (Pockley et al. 2009). The significance of this inverse relation between Hsp70 and atherosclerosis remains to be clarified.
Study limitations
There are two major limitations of our study. The first one is the different patient numbers in the three cohorts. The different pathology of carotid and lower extremity atherosclerosis is well-known and widely investigated. The relatively low number of our carotid artery patient group compared to the other two groups and the severity of the atherosclerotic disease in our whole cohort might have influenced our analysis regarding this issue. The other limitation of our study is the relatively small number of the whole patient cohort. However, this is the first, small number investigation related to vascular calcification and serum Hsp70 levels. Further investigations are needed and a larger number of atherosclerotic cohorts with differing extents of the disease should be investigated in the future to confirm the novel findings of our study.
Conclusions
This is the first human study investigating serum heat shock protein 70 in association with the extent of arterial calcification in atherosclerosis. Our findings indicate that higher numbers of calcified plaques are closely correlated with higher Hsp70 levels. Further prospective studies are required to establish the possible biomarker role of Hsp70 in arterial calcification, in order to clarify whether high circulating levels of Hsp70 are a consequence of chronic atherosclerotic disease or a predisposing factor for later cardiovascular events.
Acknowledgments
We thank Judit Skopál for her help in handling serum specimens, Renáta Dudás for data management and Dr. Gábor Széplaki for assistance in statistical consultation.
Financial disclosures The authors report no conflicts of interest.
Abbreviations
- Hsp
Heat shock protein
- BMI
Body mass index
- AST
Aspartate aminotransferase
- ALT
Alanine aminotransferase
- γGT
Gamma-glutamyl transpeptidase
- CRP
C-reactive protein
- LPS
Lypopolysaccharide
Footnotes
Miklós Krepuska and Zoltán Szeberin contributed equally to this work.
References
- Bausero MA, Gastpar R, Multhoff G, Asea A. Alternative mechanism by which IFN-gamma enhances tumor recognition: active release of heat shock protein 72. J Immunol. 2005;175(5):2900–2912. doi: 10.4049/jimmunol.175.5.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardini C, Zannoni A, Bacci ML, Forni M. Protective effect of carbon monoxide pre-conditioning on LPS-induced endothelial cell stress. Cell Stress Chaperones. 2010;15(2):219–224. doi: 10.1007/s12192-009-0136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielecka-Dabrowa A, Barylski M, Mikhailidis DP, Rysz J, Banach M. HSP 70 and atherosclerosis—protector or activator? Expert Opin Ther Targets. 2009;13(3):307–317. doi: 10.1517/14728220902725149. [DOI] [PubMed] [Google Scholar]
- Bobryshev YV, Lord RS. Expression of heat shock protein-70 by dendritic cells in the arterial intima and its potential significance in atherogenesis. J Vasc Surg. 2002;35(2):368–375. doi: 10.1067/mva.2002.121067. [DOI] [PubMed] [Google Scholar]
- Cao JJ, Arnold AM, Manolio TA, Polak JF, Psaty BM, Hirsch CH, Kuller LH, Cushman M. Association of carotid artery intima-media thickness, plaques, and C-reactive protein with future cardiovascular disease and all-cause mortality: the Cardiovascular Health Study. Circulation. 2007;116(1):32–38. doi: 10.1161/CIRCULATIONAHA.106.645606. [DOI] [PubMed] [Google Scholar]
- Chang AY, Chan JY, Cheng HL, Tsai CY, Chan SH. Hypoxia-inducible factor 1/heme oxygenase 1 cascade as upstream signals in the prolife role of heat shock protein 70 at rostral ventrolateral medulla during experimental brain stem death. Shock. 2009;32(6):651–658. doi: 10.1097/SHK.0b013e3181a71027. [DOI] [PubMed] [Google Scholar]
- Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, Fowler B, Graham I. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991;324(17):1149–1155. doi: 10.1056/NEJM199104253241701. [DOI] [PubMed] [Google Scholar]
- Cozzolino M, Galassi A, Biondi ML, Turri O, Papagni S, Mongelli N, Civita L, Gallieni M, Brancaccio D. Serum fetuin-A levels link inflammation and cardiovascular calcification in hemodialysis patients. Am J Nephrol. 2006;26(5):423–429. doi: 10.1159/000095782. [DOI] [PubMed] [Google Scholar]
- Dhingra R, Larson MG, Benjamin EJ, Lipinska I, Gona P, Corey D, Keaney JF, Jr, Vasan RS. Cross-sectional correlates of serum heat shock protein 70 in the community. Am J Hypertens. 2006;19(2):227–231. doi: 10.1016/j.amjhyper.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Dulin E, Garcia-Barreno P, Guisasola MC. Extracellular heat shock protein 70 (HSPA1A) and classical vascular risk factors in a general population. Cell Stress Chaperones. 2010;15:929–937. doi: 10.1007/s12192-010-0201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybdahl B, Slordahl SA, Waage A, Kierulf P, Espevik T, Sundan A. Myocardial ischaemia and the inflammatory response: release of heat shock protein 70 after myocardial infarction. Heart. 2005;91(3):299–304. doi: 10.1136/hrt.2003.028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt RT, Coffman JD. Cardiovascular morbidity and mortality in peripheral arterial disease. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4(3):209–217. doi: 10.2174/1568006043336230. [DOI] [PubMed] [Google Scholar]
- Espinola-Klein C, Rupprecht HJ, Bickel C, Lackner K, Savvidis S, Messow CM, Munzel T, Blankenberg S. Different calculations of ankle-brachial index and their impact on cardiovascular risk prediction. Circulation. 2008;118(9):961–967. doi: 10.1161/CIRCULATIONAHA.107.763227. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Ott P, Nielsen HB, Steensberg A, Keller C, Krustrup P, Secher NH, Pedersen BK. Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J Physiol. 2002;544(Pt 3):957–962. doi: 10.1113/jphysiol.2002.025148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore CE, Celotta G, Politi GG, Pino L, Castelli Z, Mangiafico RA, Signorelli SS, Pennisi P. Association of high alpha2-Heremans-Schmid glycoprotein/fetuin concentration in serum and intima-media thickness in patients with atherosclerotic vascular disease and low bone mass. Atherosclerosis. 2007;195(1):110–115. doi: 10.1016/j.atherosclerosis.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Fortes MB, Whitham M. No endogenous circadian rhythm in resting plasma Hsp72 concentration in humans. Cell Stress Chaperones. 2009;14(3):273–280. doi: 10.1007/s12192-008-0082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton-Tyrrell K, Lee AJ, Price JF, d’Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodriguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300(2):197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genth-Zotz S, Bolger AP, Kalra PR, Haehling S, Doehner W, Coats AJ, Volk HD, Anker SD. Heat shock protein 70 in patients with chronic heart failure: relation to disease severity and survival. Int J Cardiol. 2004;96(3):397–401. doi: 10.1016/j.ijcard.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Gombos T, Forhecz Z, Pozsonyi Z, Janoskuti L, Prohaszka Z. Interaction of serum 70-kDa heat shock protein levels and HspA1B (+1267) gene polymorphism with disease severity in patients with chronic heart failure. Cell Stress Chaperones. 2008;13(2):199–206. doi: 10.1007/s12192-007-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, Carroll BA, Eliasziw M, Gocke J, Hertzberg BS, Katanick S, Needleman L, Pellerito J, Polak JF, Rholl KS, Wooster DL, Zierler RE. Carotid artery stenosis: gray-scale and Doppler US diagnosis–Society of Radiologists in Ultrasound Consensus Conference. Radiology. 2003;229(2):340–346. doi: 10.1148/radiol.2292030516. [DOI] [PubMed] [Google Scholar]
- Grochot-Przeczek A, Dulak J, Jozkowicz A. Heme oxygenase-1 in neovascularisation: a diabetic perspective. Thromb Haemost. 2010;104(3):424–431. doi: 10.1160/TH09-12-0825. [DOI] [PubMed] [Google Scholar]
- Guerin AP, London GM, Marchais SJ, Metivier F. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant. 2000;15(7):1014–1021. doi: 10.1093/ndt/15.7.1014. [DOI] [PubMed] [Google Scholar]
- Higgins CL, Marvel SA, Morrisett JD. Quantification of calcification in atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2005;25(8):1567–1576. doi: 10.1161/01.ATV.0000172017.79441.73. [DOI] [PubMed] [Google Scholar]
- Jansen T, Hortmann M, Oelze M, Opitz B, Steven S, Schell R, Knorr M, Karbach S, Schuhmacher S, Wenzel P, Munzel T, Daiber A. Conversion of biliverdin to bilirubin by biliverdin reductase contributes to endothelial cell protection by heme oxygenase-1-evidence for direct and indirect antioxidant actions of bilirubin. J Mol Cell Cardiol. 2010;49(2):186–195. doi: 10.1016/j.yjmcc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Kalabay L, Jakab L, Prohaszka Z, Fust G, Benko Z, Telegdy L, Lorincz Z, Zavodszky P, Arnaud P, Fekete B. Human fetuin/alpha2HS-glycoprotein level as a novel indicator of liver cell function and short-term mortality in patients with liver cirrhosis and liver cancer. Eur J Gastroenterol Hepatol. 2002;14(4):389–394. doi: 10.1097/00042737-200204000-00009. [DOI] [PubMed] [Google Scholar]
- Kyriakakis E, Philippova M, Joshi MB, Pfaff D, Bochkov V, Afonyushkin T, Erne P, Resink TJ. T-cadherin attenuates the PERK branch of the unfolded protein response and protects vascular endothelial cells from endoplasmic reticulum stress-induced apoptosis. Cell Signal. 2010;22:1308–1316. doi: 10.1016/j.cellsig.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Lavie L, Dyugovskaya L, Golan-Shany O, Lavie P. Heat-shock protein 70: expression in monocytes of patients with sleep apnoea and association with oxidative stress and tumour necrosis factor-alpha. J Sleep Res. 2010;19:139–147. doi: 10.1111/j.1365-2869.2009.00789.x. [DOI] [PubMed] [Google Scholar]
- Luu NT, Madden J, Calder PC, Grimble RF, Shearman CP, Chan T, Tull SP, Dastur N, Rainger GE, Nash GB. Comparison of the pro-inflammatory potential of monocytes from healthy adults and those with peripheral arterial disease using an in vitro culture model. Atherosclerosis. 2007;193(2):259–268. doi: 10.1016/j.atherosclerosis.2006.08.050. [DOI] [PubMed] [Google Scholar]
- Madach K, Molvarec A, Rigo J, Jr, Nagy B, Penzes I, Karadi I, Prohaszka Z. Elevated serum 70 kDa heat shock protein level reflects tissue damage and disease severity in the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Eur J Obstet Gynecol Reprod Biol. 2008;139(2):133–138. doi: 10.1016/j.ejogrb.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Madden J, Coward JC, Shearman CP, Grimble RF, Calder PC. Hsp70 expression in monocytes from patients with peripheral arterial disease and healthy controls: monocyte Hsp70 in PAD. Cell Biol Toxicol. 2010;26:215–223. doi: 10.1007/s10565-009-9134-x. [DOI] [PubMed] [Google Scholar]
- Magalhaes FD, Amorim FT, Passos RL, Fonseca MA, Oliveira KP, Lima MR, Guimaraes JB, Ferreira-Junior JB, Martini AR, Lima NR, Soares DD, Oliveira EM, Rodrigues LO. Heat and exercise acclimation increases intracellular levels of Hsp72 and inhibits exercise-induced increase in intracellular and plasma Hsp72 in humans. Cell Stress Chaperones. 2010;15:885–895. doi: 10.1007/s12192-010-0197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Ventura JL, Leclercq A, Blanco-Colio LM, Egido J, Rossignol P, Meilhac O, Michel JB. Low plasma levels of HSP70 in patients with carotid atherosclerosis are associated with increased levels of proteolytic markers of neutrophil activation. Atherosclerosis. 2007;194(2):334–341. doi: 10.1016/j.atherosclerosis.2006.10.030. [DOI] [PubMed] [Google Scholar]
- McCarty MF. “Iatrogenic Gilbert syndrome”—a strategy for reducing vascular and cancer risk by increasing plasma unconjugated bilirubin. Med Hypotheses. 2007;69(5):974–994. doi: 10.1016/j.mehy.2006.12.069. [DOI] [PubMed] [Google Scholar]
- Molvarec A, Tamasi L, Losonczy G, Madach K, Prohaszka Z, Rigo J., Jr Circulating heat shock protein 70 (HSPA1A) in normal and pathological pregnancies. Cell Stress Chaperones. 2009;15:237–247. doi: 10.1007/s12192-009-0146-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, T. I. W. Group Inter-society consensus for the management of peripheral arterial disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- Paier A, Agewall S, Kublickiene K. Expression of heat shock proteins and nitrotyrosine in small arteries from patients with coronary heart disease. Heart Vessels. 2009;24(4):260–266. doi: 10.1007/s00380-008-1117-y. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Shepherd J, Corton JM. Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Invest. 1998;27(6):367–377. doi: 10.3109/08820139809022710. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Georgiades A, Thulin T, Faire U, Frostegard J. Serum heat shock protein 70 levels predict the development of atherosclerosis in subjects with established hypertension. Hypertension. 2003;42(3):235–238. doi: 10.1161/01.HYP.0000086522.13672.23. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Calderwood SK, Multhoff G. The atheroprotective properties of Hsp70: a role for Hsp70-endothelial interactions? Cell Stress Chaperones. 2009;14(6):545–553. doi: 10.1007/s12192-009-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohaszka Z, Fust G. Immunological aspects of heat-shock proteins-the optimum stress of life. Mol Immunol. 2004;41(1):29–44. doi: 10.1016/j.molimm.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Prohaszka Z, Singh M, Nagy K, Kiss E, Lakos G, Duba J, Fust G. Heat shock protein 70 is a potent activator of the human complement system. Cell Stress Chaperones. 2002;7(1):17–22. doi: 10.1379/1466-1268(2002)007<0017:HSPIAP>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennenberg RJ, Kessels AG, Schurgers LJ, Engelshoven JM, Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag. 2009;5(1):185–197. doi: 10.2147/VHRM.S4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh M, Shimoda Y, Akatsu T, Ishikawa Y, Minami Y, Nakamura M. Elevated circulating levels of heat shock protein 70 are related to systemic inflammatory reaction through monocyte Toll signal in patients with heart failure after acute myocardial infarction. Eur J Heart Fail. 2006;8(8):810–815. doi: 10.1016/j.ejheart.2006.03.004. [DOI] [PubMed] [Google Scholar]
- St Rammos K, Koullias GJ, Hassan MO, Argyrakis NP, Voucharas CG, Scarupa SJ, Cowte TG. Low preoperative HSP70 atrial myocardial levels correlate significantly with high incidence of postoperative atrial fibrillation after cardiac surgery. Cardiovasc Surg. 2002;10(3):228–232. doi: 10.1016/S0967-2109(01)00138-7. [DOI] [PubMed] [Google Scholar]
- Tavaria M, Gabriele T, Kola I, Anderson RL. A hitchhiker’s guide to the human Hsp70 family. Cell Stress Chaperones. 1996;1(1):23–28. doi: 10.1379/1466-1268(1996)001<0023:AHSGTT>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitek L, Ostrow JD. Bilirubin chemistry and metabolism; harmful and protective aspects. Curr Pharm Des. 2009;15(25):2869–2883. doi: 10.2174/138161209789058237. [DOI] [PubMed] [Google Scholar]
- Wright BH, Corton JM, El-Nahas AM, Wood RF, Pockley AG. Elevated levels of circulating heat shock protein 70 (Hsp70) in peripheral and renal vascular disease. Heart Vessels. 2000;15(1):18–22. doi: 10.1007/s003800070043. [DOI] [PubMed] [Google Scholar]
- Yao Y, Watson AD, Ji S, Bostrom KI. Heat shock protein 70 enhances vascular bone morphogenetic protein-4 signaling by binding matrix Gla protein. Circ Res. 2009;105(6):575–584. doi: 10.1161/CIRCRESAHA.109.202333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan R, Leng X, Liu X, Wang X, Gong J, Yan L, Wang L, Wang Y, Qian LJ. Heat shock protein 70 is secreted from endothelial cells by a non-classical pathway involving exosomes. Biochem Biophys Res Commun. 2009;387(2):229–233. doi: 10.1016/j.bbrc.2009.06.095. [DOI] [PubMed] [Google Scholar]
- Zhang X, Xu Z, Zhou L, Chen Y, He M, Cheng L, Hu FB, Tanguay RM, Wu T. Plasma levels of Hsp70 and anti-Hsp70 antibody predict risk of acute coronary syndrome. Cell Stress Chaperones. 2010;15:675–686. doi: 10.1007/s12192-010-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Werstuck GH, Lhotak S, Koning AB, Sood SK, Hossain GS, Moller J, Ritskes-Hoitinga M, Falk E, Dayal S, Lentz SR, Austin RC. Association of multiple cellular stress pathways with accelerated atherosclerosis in hyperhomocysteinemic apolipoprotein E-deficient mice. Circulation. 2004;110(2):207–213. doi: 10.1161/01.CIR.0000134487.51510.97. [DOI] [PubMed] [Google Scholar]
- Zhu WM, Roma P, Pirillo A, Pellegatta F, Catapano AL. Oxidized LDL induce hsp70 expression in human smooth muscle cells. FEBS Lett. 1995;372(1):1–5. doi: 10.1016/0014-5793(95)00834-V. [DOI] [PubMed] [Google Scholar]
- Zhu J, Quyyumi AA, Wu H, Csako G, Rott D, Zalles-Ganley A, Ogunmakinwa J, Halcox J, Epstein SE. Increased serum levels of heat shock protein 70 are associated with low risk of coronary artery disease. Arterioscler Thromb Vasc Biol. 2003;23(6):1055–1059. doi: 10.1161/01.ATV.0000074899.60898.FD. [DOI] [PubMed] [Google Scholar]