Abstract
Chronic repeated exposure to hyperthermia in humans results in heat acclimation (HA), an adaptive process that is attained in humans by repeated exposure to hyperthermia and is characterized by improved heat elimination and increased exercise capacity, and acquired thermal tolerance (ATT), a cellular response characterized by increased baseline heat shock protein (HSP) expression and blunting of the acute increase in HSP expression stimulated by re-exposure to thermal stress. Epidemiologic studies in military personnel operating in hot environments and elite athletes suggest that repeated exposure to hyperthermia may also exert long-term health effects. Animal models demonstrate that coincident exposure to mild hyperthermia or prior exposure to severe hyperthermia can profoundly affect the course of experimental infection and injury, but these models do not represent HA. In this study, we demonstrate that CD-1 mice continuously exposed to mild hyperthermia (ambient temperature ~37°C causing ~2°C increase in core temperature) for 5 days and then exposed to a thermal stress (42°C ambient temperature for 40 min) exhibited some of the salient features of human HA, including (1) slower warming during thermal stress and more rapid cooling during recovery and (2) increased activity during thermal stress, as well as some of the features of ATT, including (1) increased baseline expression of HSP72 and HSP90 in lung, heart, spleen, liver, and brain; and (2) blunted incremental increase in HSP72 expression following acute thermal stress. This study suggests that continuous 5-day exposure of CD-1 mice to mild hyperthermia induces a state that resembles the physiologic and cellular responses of human HA. This model may be useful for analyzing the molecular mechanisms of HA and its consequences on host responsiveness to subsequent stresses.
Keywords: Heat acclimation, Mouse model, Heat shock protein, Acquired thermal tolerance
Introduction
Heat acclimation (HA) is an adaptive physiological process that is attained in humans by repeated exposure to hyperthermia such as occurs during strenuous activity at high ambient temperatures (Sawka et al. 1996; McClung et al. 2008). This process confers benefits during subsequent heat stress by reducing the incidence and severity of serious heat illnesses through reduced cardiovascular, thermal, and metabolic strain. Well-controlled programs designed to induce HA have been actively employed by athletes and the military since the early 1940s to improve endurance and performance under strenuous conditions and elevated temperatures (Sawka et al. 1985, 1996, 2001; Nielsen et al. 1993; McClung et al. 2008). Despite the persistent use of HA protocols and its known effects on the physiologic response to stress, significant gaps remain in our understanding of the molecular mechanisms and biological consequences of this adaptive process. Our laboratory (McClung et al. 2008) and others (Dietz and Somero 1992; Flanagan et al. 1995; Maloyan et al. 1999; Buckley et al. 2001; Tomanek and Somero 2002; Horowitz et al. 2004; Lund et al. 2006; Marshall et al. 2007; Yamada et al. 2007) have recently demonstrated that human HA and experimental animal models of HA are accompanied by elements of acquired thermal tolerance (ATT), characterized by modifications in constitutive and heat-inducible expression levels of heat shock proteins (HSPs). However, a broader understanding of the effects of HA on gene expression and cell function and the ultimate effects on human health is lacking.
Previous studies, including from our own laboratory, demonstrated that acute exposure of mice to moderate, febrile-range hyperthermia (FRH; ~39.5°C core temperature) concurrent with infection or treatment with non-infectious inflammatory agonists had previously unsuspected effects on immunologic function and cell survival (Jiang et al. 1999a, b, 2000; Ostberg et al. 2000, 2001; Evans et al. 2001; Hasday et al. 2003; Ellis et al. 2005; Rice et al. 2005; Chen et al. 2006; Singh et al. 2008), including increased trafficking of neutrophils (Hasday et al. 2003; Ellis et al. 2005; Rice et al. 2005; Singh et al. 2008) and lymphocytes (Jiang et al. 1999a, b, 2000; Ostberg et al. 2000; Evans et al. 2001; Chen et al. 2006), and altered expression of proinflammatory cytokines (Hasday et al. 2003; Ellis et al. 2005; Rice et al. 2005; Chen et al. 2006; Singh et al. 2008) and adhesion molecules (Evans et al. 2001; Hasday et al. 2001; Chen et al. 2006). Other studies have shown that a single pre-exposure to hyperthermia in the heat shock range (~42°C) followed by several hours of normothermic recovery confers protection against subsequent tissue injury, an effect attributed to activation of the heat shock response and increased HSP generation (Villar et al. 1993; Javadpour et al. 1998; Slutsky 2002). Furthermore, the heat shock response not only protects against subsequent thermal stress but also induces cross tolerance to other unique insults such as ischemia, hypoxia (Christians et al. 2002), endotoxemia (Chu et al. 1997), and burns (Meyer et al. 2000). Although neither of these acute heating models reflects the long-term, recurrent exposure to moderate hyperthermia (e.g., troops deployed to hot environments, athletes in training, and individuals who work in high-temperature environments), these studies indicate the complex, potentially profound effects of hyperthermia on host responses to stress, including immune responses to infections and injury, and underscores the importance of the heat shock response pathway in mediating many of these effects. In fact, studies in rats (Maloyan et al. 1999) and humans (Yamada et al. 2007; McClung et al. 2008) demonstrated that HA is accompanied by reprogramming of HSP gene expression, and that induction of HSPs is likely concurrent with HA and is an integral component of the HA process.
Compared with the acute effects of short-term heat exposure, little is known about the delayed health effects of chronic exposure to hyperthermia. The available data are limited to observations made in patients with acute heat-related illness (Sonna et al. 2004) and epidemiologic studies of athletes during prolonged training programs and troops returning from high-temperature military theaters (Peters and Bateman 1983; Linde 1987; Fitzgerald 1991; Peters et al. 1993; Gleeson et al. 1995; Riddle et al. 2007; Roop et al. 2007). Several survey studies suggest that prolonged intense exercise increases the risk of upper respiratory tract infection (Peters and Bateman 1983; Linde 1987; Fitzgerald 1991; Peters et al. 1993). A survey study of troops participating in Operation Bright Star during the summer in Egypt showed respiratory illnesses reported in 47% of 1,454 deployed troops or 73 episodes per 100 person-months (Riddle et al. 2007). In a survey of troops participating in Operation Enduring Freedom in Iraq from April to July 2003, Roop et al. (2007) reported new or increased symptoms of shortness of breath during deployment in 13% of 155 survey respondents. Elite swimmers undergoing 7 months (Gleeson et al. 1995) of intensive training exhibited a 57% reduction in circulating NK cells. Collectively, these studies suggest that repeated exposure to exertional hyperthermia, which leads to HA in humans, also exerts important effects that may modify the capacity of individuals to cope with subsequent stresses. While the individuals in these studies were exposed to different environmental factors and variable degrees of physical exercise, they likely have in common chronic, recurrent exposure to hyperthermia. That these uncontrolled studies are only correlative in nature demonstrates the limitations of human studies, but the results underscore the importance of a more complete understanding of the biological effects of such exposures.
A fundamental obstacle to progress in this field is the lack of a facile animal model that mimics the physiologic and molecular features of humans exposed to chronic recurrent hyperthermia. The absence of such a model not only limits our understanding of the cellular and molecular changes that occur during such exposures but it also restricts our efforts to study the effects of chronic hyperthermia on the response to defined environmental challenges and pathologic states. The best-studied model of human HA to date, the Sabra mouse model (Shein et al. 2008) requires a 30-day exposure to hyperthermia to achieve an HA-like state. In this study, we have modified our short-term hyperthermic CD-1 mouse model to develop a continuous 5-day heating protocol that maintained core temperature ~2°C above basal levels and elicited physiological responses and HSP gene expression profiles similar to that of humans exposed to a standard 10-day HA protocol (McClung et al. 2008).
Materials and methods
Five-day heat acclimation protocol and acute thermal stress exposures Male CD-1 mice weighing 30–35 g were purchased from Charles River and housed in the Animal Care Facility in the Veterans Administration Medical Center, Baltimore under AALAC-approved conditions and under the supervision of a full-time veterinarian. Mice were adapted to standard plastic cages with standard bedding and a plastic igloo for at least 7 days before the study and were used within 8 weeks of arrival. To avoid the influence of diurnal cycling, all experiments were started at approximately the same time each day (between 8:00 and 10:00 AM). Lights were cycled on and off at 7:00 AM and 7:00 PM daily. Mice in which physiologic response to HA and thermal stress protocols was measured were implanted with intraperitoneal telemetric thermistors (Data Sciences International, DSI; St. Paul, MN, USA; ETA-F10) 7 days prior to HA exposure. A sterilized ETA-F10 transmitter was placed into the peritoneal cavity and subcutaneous electrodes secured under isoflurane anesthesia as described in the manufacturer’s protocol. The mice received 0.1 mg/kg buprenorphine analgesia s.c. q12h for two postoperative days, housed one mouse per cage, and were provided with food and water ad libitum immediately after surgery and allowed to recover for 7 days at ~25°C ambient temperature. Additional mice were exposed to the same protocols in parallel and used for analysis of HSP expression.HA was achieved by transferring the mice in their standard cages into modified Air Shields™ infant incubators set to 37°C for 5 days. The bedding and igloo were maintained and food and water were supplied ad libitum. Core temperature, heart rate, and a semi-quantitative assessment of activity were continuously monitored using the DSI Automated Data Acquisition System. Activity was measured using the DSI proprietary algorithm that predominantly assesses locomotor activity by measuring change in transmitter signal strength. Unacclimated controls were housed under identical conditions except at standard room temperature (~25°C).The response to thermal stress was analyzed by exposing HA and unacclimated mice to 42°C ambient temperature for 40 min. An Air Shields™ infant incubator was modified to reach ambient temperatures of 42°C using three radiant heating panels (Boaphile model 1611) controlled with an electronic temperature controller (Ranco, London, UK). To ensure similar pre-thermal stress core temperatures in the unacclimated and HA mice prior to acute thermal stress, the HA mice were maintained at 25°C ambient temperature until core temperature returned to the level in unacclimated mice (~36.5°C). Mice implanted with transmitters were transferred to a standard mouse cage in the 42°C incubator for 40 min with continuous monitoring of core temperature, heart rate, and activity. The igloo and most of the bedding was removed to allow even heat distribution within the cage and water but not food was available ad libitum during the thermal stress exposure. Mice were then transferred back to their original cages with bedding, igloo, water, and food and allowed to recover at room temperature (~25°C) with continued monitoring of physiologic parameters (shown in Fig. 3) and euthanized 4 h later. Following euthanasia by exsanguination via cardiac puncture and cervical dislocation under deep isoflurane anesthesia, lung, heart, spleen, liver, and brain were harvested and snap frozen in liquid nitrogen for Western blot analysis and serum was collected for analysis of circulating HSP72. Other groups of HA and unacclimated mice were euthanized without exposure to thermal stress and organs harvested and processed using the same protocol. Body weight was measured manually using an electronic balance before surgery, prior to and following HA, and prior to and following thermal stress exposure. In some experiments, the food and water bottles were weighed prior to and immediately following the 5-day HA exposure and at comparable times in the unacclimated controls. All procedures were approved by the Baltimore VA and the University of Maryland, Baltimore, Institutional Animal Care and Use Committee.
Fig. 3.
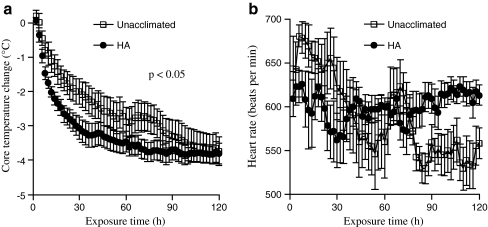
Effect of 5-day HA protocol on rate of cooling and heart rate normalization during post-thermal stress recovery. Following 40-min exposure to acute thermal stress, unacclimated and 5-day HA mice were transferred to standard room temperature (~25°C) and core temperature (a) and heart rate (b) were continuously monitored during recovery. The change in core temperature compared with immediate post-thermal stress levels were calculated and means of each 2-min interval for change in core temperature or for heart rate were calculated. Means ± SE of eight mice per group are shown. The difference between groups was significant for change in core temperature with a p value <0.05 by repeated measures ANOVA
Heat shock protein analysis Frozen organs were homogenized (Precellys™24; Bertin Technologies, Montigny-le-Bretonneux, France) using three 20-s cycles at 6,000 rpm in RIPA solution containing 10 μg/ml 4-(2-aminoethyl benzenesulfenylofluoride hydrochloride), 20 μg/ml aprotinin (20 μg/μl), phosphatase inhibitors 1 and 2 (Sigma; St. Louis, MO), and protease inhibitors (Roche Scientific, Indianapolis, IN, USA). The samples were clarified by centrifugation. Protein levels were measured using a commercial Bradford reagent (Thermo Fisher Pierce, Rockford, IL, USA); 10–20 μg of protein per lane was separated by 10% SDS–PAGE and electrostatically transferred to PVDF membrane. The membranes were blocked for 1 h at room temperature in blocking buffer (TBS-T) (10 mM Tris–HCl, pH 7.5, 136 mM NaCl, 2.0 mM KCl, 0.1% Tween 20) containing 5% non-fat dry milk. Following blocking, the membranes were washed with TBS-T and incubated with antibodies against HSP72 (Stressgen, Ann Arbor, MI, USA; cat. no. SPA812; 1:10,000 dilution), HSP90 (Santa Cruz Biotechnology, Santa Cruz, CA, USA; cat. no. SC 7947; 1:10,000 dilution), or the control housekeeping gene β-tubulin (Millipore, Billerica, MA, USA; cat. no. MAB3408; 1:20,000 dilution) in blocking buffer overnight. After incubation with primary antibody, the membranes were washed with TBS-T, incubated for 1 h with horseradish peroxidase-conjugated secondary antibody, developed with a chemiluminescence detection system (Western Lightning Plus—ECL; Perkin-Elmer, Waltham, MA, USA), and quantified by direct imaging (Fuji LS4000 gel documentation system and ImageGauge software). Density of each HSP band was normalized to β-tubulin density after stripping and probing the same gel. HSP72 in serum was measured by ELISA in the University of Maryland Cytokine Core Laboratory using paired antibodies from R&D Systems and having a lower detection limit of 150 pg/ml.
Data analysis Data are presented as mean ± standard error. For physiologic measurements during the 5-day HA exposure in Fig. 1, we calculated the means for each 2-h interval during the 5-day exposure period. For core temperature and heart rate during thermal stress in Fig. 2, we calculated the means for each 1-min interval during the 40-min exposure. For the physiologic changes during the post-thermal stress recovery period in Fig. 3, we calculated the means for each 2-min interval. Physiological data was analyzed by repeated measures two-way analysis of variance using the JMP statistical package (SAS, Cary, NC, USA). Differences in Western blot protein quantification between two groups were analyzed by unpaired Student t test. Differences among more than two groups were analyzed by applying the post hoc Tukey honestly significant difference (HSD) test to a one-way analysis of variance (ANOVA). For activity during the 40-min thermal stress protocol, area under the activity–time curve was calculated using the trapezoidal rule (Atkinson 1989) for each third of the 40-min stress period, and differences between groups were analyzed by ANOVA/Tukey HSD.
Fig. 1.
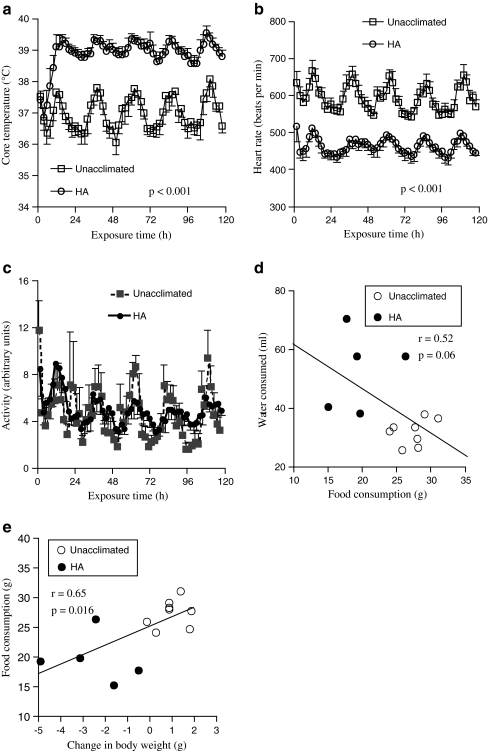
Core temperature, heart rate, and activity level during passive hyperthermia (5-day HA protocol). Mice were implanted with intraperitoneal sensors, recovered for 7 days, and core temperature (a), heart rate (b), and activity level (c) were continuously monitored during 5-day continuous exposure to either 25°C (unacclimated controls) or 37°C ambient temperature (HA) and the means for each 2-h interval were calculated. Two experiments, each with four mice per group, were pooled. Data are presented as means ± SE. The core temperature and heart rate were different between the heated and unacclimated control mice with p values <0.001 by repeated measures ANOVA. d, e In five mice exposed to the 5-day HA protocol and eight control mice, food and water consumption and body weight were determined by measuring the weights of each before and following the 5-day HA and control exposure. Correlations between food consumed and (d) water consumed or (e) change in body weight are shown
Fig. 2.
Effect of 5-day HA protocol on physiologic response to acute thermal stress. Five-day-heated mice (HA) were returned to standard room temperature (~25°C) until core temperature returned to normal baseline (36.5°C). The five HA protocol-exposed mice and unacclimated mice were individually exposed to acute thermal stress (42°C for 40 min) while continuously monitoring core temperature (a), heart rate (b), and activity (c). The mean values for change in core temperature and heart rate were determined for each 1-min exposure interval. The mean activity level for each tertile was calculated. Means ± SE for eight 5-day-heated and unacclimated mice are shown. The difference in core temperatures between groups was significant with a p value <0.001 by repeated measures ANOVA. *p < 0.05 vs. activity in unacclimated mice during the same time period
Results
Physiological responses during 5-day exposure to moderate passive hyperthermia In each of two experiments, groups of four mice were implanted with intraperitoneal thermistors, allowed to recover 7–10 days, then maintained at either standard room temperature (~25°C) or 37°C ambient temperature for 5 days while telemetrically monitoring core temperature, heart rate, and a global estimate of locomotor activity. As we have previously reported, an increase in ambient temperature to ~37°C reduces the temperature gradient for heat elimination and leads to an increase in core temperature above the ambient temperature (Hasday et al. 2003; Rice et al. 2005). Exposure to 37°C ambient temperature did not alter the activity level (Fig. 1c) of the mice or the circadian pattern of the measured physiologic parameters, but did cause ~2°C increase in core temperature (Fig. 1a) over the first 10 h of exposure and ~25% decrease in heart rate within the first hour of exposure (Fig. 1b). These patterns were sustained during the 5-day exposure. Mice lost 5.6 ± 1.2% body weight during the 5-day exposure compared with a gain of 3.8 ± 1.2% during the same time period in the unacclimated mice despite ad libitum access to food and water and comparable activity levels in the two groups. Food intake was reduced by ~30% (19.6 ± 1.8 vs. 27.3 ± 0.8 g) and water intake increased ~65% (52.7 ± 6 vs. 31.8 ± 1.6 ml) during the 5-day HA exposure compared with unacclimated mice over the same time period. Food intake was negatively correlated with both water intake (Fig. 1d) and positively correlated with change in body weight (Fig. 1e).
Effect of 5-day heating protocol on physiologic responses to thermal stress and recovery We have previously demonstrated that humans undergoing a 10-day HA protocol comprising a 100-min treadmill walk at 49°C ambient temperature exhibited the following physiologic changes when compared to their pre-HA state: (1) slower rise in core temperature, (2) lower heart rate, and (3) increased exercise capacity at 49°C (McClung et al. 2008). At the end of 5 days of continuous passive warming, mice were allowed to recover at standard room temperature (~25°C) until their core temperature returned to basal levels, which required approximately 45 min. Unacclimated and 5-day-heated mice were then exposed to 42°C ambient temperature for 40 min to analyze the effect of 5-day exposure to hyperthermia on physiologic response to acute thermal stress (Fig. 2). Both 5-day-heated mice and unacclimated mice increased core temperature (Fig. 2a) and initially increased activity (Fig. 2c) during the exposure to thermal stress. However, in comparison to unacclimated mice, 5-day-heated mice had a significantly slower increase in core temperature (Fig. 2a) despite exhibiting more sustained activity (Fig. 2c). Prior to thermal shock, HR was approximately 20% slower in the 5-day-heated mice (Fig. 2b), but upon transfer to the thermal stress cage, HR increased ~20% in both groups of mice. HR slowed to pre-thermal stress levels in the 5-day-heated mice within 7 min and remained at this level for the duration of the thermal stress. Control mice exhibited a gradual slowing of HR, reaching a nadir of 550 by 22 min of thermal stress. When switched back to standard room temperature (~25°C) following the exposure to thermal stress, core temperature decreased to baseline levels more quickly in the 5-day-heated mice than in the unacclimated mice (Fig. 3a). While the heart rate of the HA-exposed mice appeared to be less variable during the recovery period compared with the unacclimated mice, the difference did not reach statistical significance (Fig. 3b).
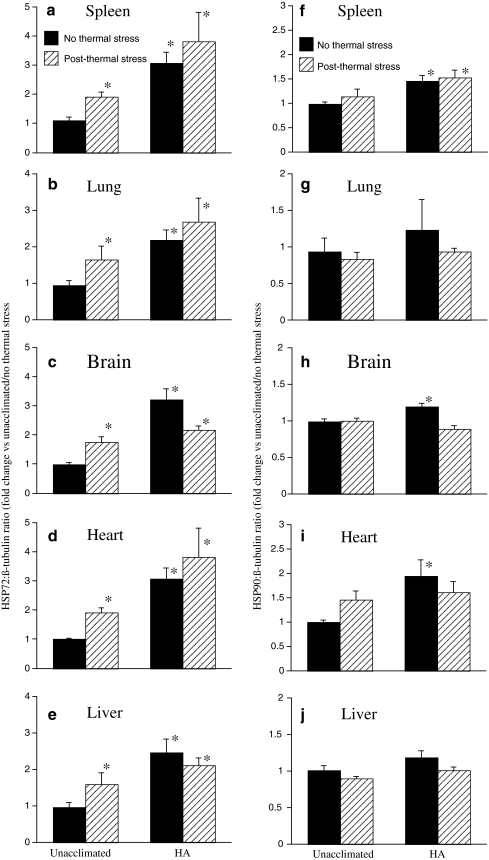
Effect of 5-day heating protocol on baseline and post-thermal stress HSP expression In our previous study of human HA, we showed that baseline HSP72 and HSP90 expression in peripheral blood mononuclear cells (PBMC) was higher following HA, but further induction following ex vivo heat shock was blunted in cells from the HA group (McClung et al. 2008). To determine whether our continuous 5-day heating protocol induced similar changes in HSP gene expression in mice, we measured HSP72 and HSP90 protein levels in lung, liver, spleen, brain, and heart of mice exposed to the 5-day heating protocol and unacclimated control mice in the presence or absence of an additional 40 min 42°C thermal stress and 4-h normothermic recovery. Protein levels were quantified by Western blotting and the band densities of HSP bands normalized to β-tubulin and expressed as fold-change compared with levels in unacclimated mice without acute thermal stress exposure (Fig. 4). In the unacclimated mice, exposure to acute thermal stress induced 1.5- to 2-fold increases in HSP72 levels in all five tissues (Fig 4a–e). In the mice exposed to the 5-day heating protocol, pre-thermal stress HSP72 levels in the same five organs were 2- to 3-fold higher than pre-thermal stress levels in the unacclimated mice, but exposure to acute thermal stress did not induce a further increase in HSP72 expression in the 5-day-heated mice. Pre-thermal stress levels of HSP90 were increased in spleen, brain, and heart of 5-day-heated mice compared with unacclimated controls, but exposure to acute thermal stress did not induce significant increases in HSP90 expression in either unacclimated or 5-day-heated mice (Fig. 4f–j). Soluble HSP72 was undetectable in serum from unacclimated to 5-day-heated mice, indicating the 5-day heating protocol increases baseline HSP72 levels in multiple tissues without stimulating detectable release of HSP72 into the circulation.
Fig. 4.
Effect of 5-day HA protocol on baseline and acute thermal stress-induced expression of HSP72 and HSP90 protein in multiple organs. Unacclimated mice and 5-day HA mice were either euthanized immediately following the 5-day HA protocol or were exposed to acute thermal stress (42°C ambient temperature for 40 min) and allowed to recover at standard room temperature (~25°C) for 4 h prior to euthanasia. Spleen (a, f), lung (b, g), brain (c, h), heart (d, i), and liver (e, j) were collected, snap frozen in liquid nitrogen, homogenized, and analyzed by Western blot for levels of HSP72 (a–e) and HSP90 (f–j). The intensity of the chemiluminescent bands was analyzed by direct imaging, standardized to β-tubulin band intensities, and expressed as fold change vs. unacclimated mice without exposure to acute thermal stress. Means ± SE of eight mice in each of the four groups. *p < 0.05 vs. unacclimated mice without exposure to acute thermal stress
Discussion
We sought to develop a mouse model that exhibited the key physiological and cellular heat shock responses of human undergoing a previously characterized human HA protocol (McClung et al. 2008). The human protocol consisted of repeated daily exercise at high ambient temperature for 10 days and resulted in increased exercise tolerance, slower rise in core temperature and lower maximal temperature, lower maximal heart rate, and increased sweating rates during subsequent exposure to exercise and hyperthermia (Sawka et al. 1996; Sawka and Young 2005; McClung et al. 2008).
While HA in humans was achieved by repeated intermittent increases in core temperature achieved by encouraging physical exercise at high ambient temperature (49°C), mice were subjected to continuous passive hyperthermia. Both models achieved an approximate 2°C maximal increase in core temperature. Aside from a 5.6% decrease in body weight, there were no detectable adverse effects observed in mice during the 5-day HA exposure. Considering HA mice exhibited activity levels and circadian rhythms that were similar to control mice, increased their water intake, and failed to demonstrate any physical signs of distress, the observed weight loss was not likely caused by dehydration. Rather, it is likely caused by adaptation to the approximate 20% increase in metabolism that is expected to accompany their 2°C increase in core temperature (Schumacker et al. 1987; Manthous et al. 1995) and a reduction in food intake, which has previously been shown to occur in humans and rodents during exposure to high ambient temperature (Esher and Wolfe 1979; Herman 1993). The potential contribution of reduced calorie intake to the physiologic and cell biologic changes observed in the 5-day-HA-exposed mice is beyond the scope of the present study.
Humans undergoing a 10-day HA protocol exhibit improved capacity to defend against hyperthermia and increased exercise capacity on the tenth day of exercise/heat exposure compared with the first day (Sawka et al. 1996; Sawka and Young 2005; McClung et al. 2008). In our previous study, subjects demonstrated a higher exercise time until exhaustion (95.5 ± 11 min) following HA than before HA (79.3 ± 21.1 min) (McClung et al. 2008). The subjects demonstrated a lower mid-exercise core temperature following HA (37.77 ± 0.40°C) than prior to HA (38.32 ± 0.52°C), demonstrating a slower rise in core temperature during thermal stress in heat-acclimated individuals. As previously reported (Sawka et al. 1996; Sawka and Young 2005; McClung et al. 2008), the slower increase in core temperature following 10 days of intermittent exercise/heat exposure is predominantly due to improved heat elimination as reflected by increased sweating rate (1.30 ± 0.20 vs. 1.09 ± 0.21 l/h). To analyze the effect of continuous 5-day moderate passive hyperthermia (5-day HA protocol) on the capacity of mice to tolerate thermal stress, we exposed 5-day-heated mice and unacclimated mice to an acute thermal stress at 42°C ambient temperature for 40 min. To ensure similar core temperatures at the beginning of thermal stress, the 5-day-heated mice were allowed to return to the same core temperature (36.5°C) as in the unacclimated state, which occurred over 45 min. Both unacclimated and 5-day-heated mice increased their activity level when transferred to the 42°C cage, an expected response to handling and a new environment. However, the 5-day-heated mice were able to sustain the higher activity level longer than the unacclimated mice, suggesting that, like heat-acclimated humans, the 5-day-heated mice exhibit enhanced exercise capacity during heat exposure. However, unlike humans undergoing HA, mice were not forced to maintain high activity levels. It is possible that the more sustained activity in the 5-day-heated mice was caused by factors other than increased exercise capacity.
As occurs with heat-acclimated humans, mice exposed to the 5-day heating protocol exhibited a slower rise in core temperature during acute thermal stress than unacclimated mice. After the first 15 min of thermal stress exposure, core temperature had increased 4.09 ± 0.42°C in the unacclimated mice compared with only 3.52 ± 0.23°C in the 5-day-heated mice. That core warming was delayed despite higher activity levels in the 5-day-heated mice suggests a greater capacity for heat elimination in the 5-day-heated animals. The accelerated cooling during recovery in the 5-day-heated mice provides further support that these mice have enhanced cooling capacity. However, while improved heat elimination in heat-acclimated humans is known to be a result of enhanced sweating rates, the mechanisms responsible for increased heat elimination capacity in the 5-day-heated mice have not yet been delineated. Collectively, these data suggest that mice continuously exposed to moderate hyperthermia for 5 days exhibit improved heat elimination and increased exercise capacity during thermal stress, which are the hallmark physiologic characteristics of human HA.
In this study, mice exhibited a rapid and sustained ~25% decrease in heart rate upon transfer to 37°C ambient temperature. Transfer of 5-day-heated mice to 42°C environment did not reduce heart rate further. Unacclimated mice reduced their heart rate by ~25% during the 40-min thermal stress. We have previously shown that humans participating in a 10-day HA protocol exhibited a 12% decrease in mid-exercise heart rate after day 5 of the HA protocol compared with their pre-acclimated state. Since the human subjects in this study were exercising while the mice in the present study were exposed to passive hyperthermia, it is not clear that the negative chronotropic effect of thermal stress was mediated by the same mechanisms in the two species. Spielman and Lyman (1971) previously reported a similar rapid onset of bradycardia in rats in response to thermal stress, which has been shown to be mediated by both direct effects on intrinsic pacemaker activity (Spielman and Lyman 1971) and by altered autonomic tone (Horowitz and Meiri 1993).
We (McClung et al. 2008) and others (Yamada et al. 2007) have demonstrated that humans undergoing HA also exhibit the cellular changes characteristic of acquired thermal tolerance, specifically increased baseline expression of HSPs. Our previous study of HSP gene expression in PBMCs from human subjects participating in a 10-day HA protocol showed that levels of HSP72 and HSP90 increased by 17.6 ± 6.1% and 21.1 ± 6.5%, respectively, on the tenth day of HA conditioning compared with the first. However, the further induction of HSP72 in response to an ex vivo 43°C heat shock was blunted in the day-10 cells (McClung et al. 2008). Yamada et al. (2007) reported similar effects of human HA on HSP72 expression in PBMCs. In the present study, mice that were continuously exposed to moderate hyperthermia for 5 days exhibited increased baseline levels of HSP72 protein in all organs and increased HSP90 levels in brain, spleen, and heart. To determine whether the 5-day HA protocol suppressed further induction of HSP expression following heat shock, we subjected unacclimated and 5-day HA mice to 42°C ambient temperature for 40 min, which increased core temperature to 40.83 ± 0.10°C. Mice were allowed to recover at 25°C ambient temperature for 4 h to allow time for generation of HSPs. As expected, we found that exposure to 42°C for 40 min stimulated a 1.5- to 2-fold induction of HSP72 protein levels in all organs tested in the unacclimated mice, but in 5-day-heated mice, HSP72 levels, which were higher at baseline than the heat-induced levels in unacclimated mice, were not further increased by acute thermal stress. These results suggest that the heat shock response is maximally activated in the 5-day-heated mice and is similar to the results of heat shock expression analysis in human heat acclimation (McClung et al. 2008; Magalhaes et al. 2010). We failed to detect induction of HSP90 levels following acute thermal stress in either the unacclimated mice or the 5-day-heated mice. This is also consistent with our previous report of HSP expression in human PBMCs (McClung et al. 2008). Prior to HA, human PBMCs exhibited 4-fold higher induction of HSP72 than HSP90 following ex vivo heat shock, whereas ex vivo heat shock failed to induce HSP90 expression in PBMCs isolated after HA. These results are consistent with prior studies in ectothermic and endothermic animals that demonstrate an overlap between HA stimulated by continuous exposure to hyperthermia and expression of genes that comprise the heat shock response (Dietz and Somero 1992; Flanagan et al. 1995; Maloyan et al. 1999; Buckley et al. 2001; Tomanek and Somero 2002; Horowitz et al. 2004; Lund et al. 2006). Furthermore, this analysis demonstrates that our 5-day continuous hyperthermia exposure mouse model reproduces with high fidelity the critical heat shock protein gene expression pattern associated with human heat shock.
In summary, we have developed a convenient mouse model of human HA, which requires only 5-day continuous exposure to moderate hyperthermia, and which recapitulates some of the physiological responses and the heat shock gene expression patterns exhibited by heat-acclimated humans. We propose that this mouse model may be useful for analyzing the molecular mechanisms that produce the heat-acclimated phenotype and to analyze how recurrent exposure to hyperthermia, such as occurs during heat acclimation, may alter the capacity of the host to cope with future stresses.
Acknowledgments
Grants Supported by NIH grants GM069431 (ISS) and GM066855, HL69057 and HL085256 (JDH), and by VA Merit Review grants to JDH and ISS.
Conflicts of interest No conflicts of interest, financial or otherwise, are declared by the authors.
References
- Atkinson KA. An introduction to numerical analysis. New York: Wiley; 1989. [Google Scholar]
- Buckley BA, Owen ME, Hofmann GE. Adjusting the thermostat: the threshold induction temperature for the heat-shock response in intertidal mussels (genus Mytilus) changes as a function of thermal history. J Exp Biol. 2001;204:3571–3579. doi: 10.1242/jeb.204.20.3571. [DOI] [PubMed] [Google Scholar]
- Chen Q, Fisher DT, Clancy KA, Gauguet JM, Wang WC, Unger E, Rose-John S, Andrian UH, Baumann H, Evans SS. Fever-range thermal stress promotes lymphocyte trafficking across high endothelial venules via an interleukin 6 trans-signaling mechanism. Nat Immunol. 2006;7:1299–1308. doi: 10.1038/ni1406. [DOI] [PubMed] [Google Scholar]
- Christians ES, Yan LJ, Benjamin IJ. Heat shock factor 1 and heat shock proteins: critical partners in protection against acute cell injury. Crit Care Med. 2002;30:S43–S50. doi: 10.1097/00003246-200201001-00006. [DOI] [PubMed] [Google Scholar]
- Chu EK, Ribeiro SP, Slutsky AS. Heat stress increases survival rates in lipopolysaccharide-stimulated rats. Crit Care Med. 1997;25:1727–1732. doi: 10.1097/00003246-199710000-00025. [DOI] [PubMed] [Google Scholar]
- Dietz TJ, Somero GN. The threshold induction temperature of the 90-kDa heat shock protein is subject to acclimatization in eurythermal goby fishes (genus Gillichthys) Proc Natl Acad Sci U S A. 1992;89:3389–3393. doi: 10.1073/pnas.89.8.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis G, Carlson D, Hester L, Bagby G, Singh IS, Hasday J. G-CSF, but not corticosterone mediates circulating neutrophilia induced by febrile-range hyperthermia. J Appl Physiol. 2005;98:1799–1804. doi: 10.1152/japplphysiol.01376.2004. [DOI] [PubMed] [Google Scholar]
- Esher RJ, Wolfe JL. The effects of temperature and housing on water balance in a burrowing mouse, Peromyscus polionotus. J Comp Physiol B. 1979;133:241–245. doi: 10.1007/BF00691472. [DOI] [Google Scholar]
- Evans SS, Wang WC, Bain MD, Burd R, Ostberg JR, Repasky EA. Fever-range hyperthermia dynamically regulates lymphocyte delivery to high endothelial venules. Blood. 2001;97:2727–2733. doi: 10.1182/blood.V97.9.2727. [DOI] [PubMed] [Google Scholar]
- Fitzgerald L. Overtraining increases the susceptibility to infection. Int J Sports Med. 1991;12 Suppl 1:S5–S8. doi: 10.1055/s-2007-1024742. [DOI] [PubMed] [Google Scholar]
- Flanagan SW, Ryan AJ, Gisolfi CV, Moseley PL. Tissue-specific HSP70 response in animals undergoing heat stress. Am J Physiol. 1995;268:R28–R32. doi: 10.1152/ajpregu.1995.268.1.R28. [DOI] [PubMed] [Google Scholar]
- Gleeson M, McDonald WA, Cripps AW, Pyne DB, Clancy RL, Fricker PA. The effect on immunity of long-term intensive training in elite swimmers. Clin Exp Immunol. 1995;102:210–216. doi: 10.1111/j.1365-2249.1995.tb06658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasday JD, Bannerman D, Sakarya S, Cross AS, Singh IS, Howard D, Drysdale B-E, Goldblum SE. Exposure to febrile temperature modifies endothelial cell response to tumor necrosis factor-α. J Appl Physiol. 2001;90:90–98. doi: 10.1152/jappl.2001.90.1.90. [DOI] [PubMed] [Google Scholar]
- Hasday J, Garrison A, Singh I, Standiford T, Ellis G, Rao S, He J-R, Rice P, Frank M, Goldblum S, Viscardi R. Febrile-range hyperthermia augments pulmonary neutrophil recruitment and amplifies pulmonary oxygen toxicity. Am J Pathol. 2003;162:2005–2017. doi: 10.1016/S0002-9440(10)64333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman CP. Effects of heat on appetite. In: Marriott BM, editor. Nutritional needs in hot environment. Washington: National Academy Press; 1993. pp. 187–213. [Google Scholar]
- Horowitz M, Meiri U. Central and peripheral contributions to control of heart rate during heat acclimation. Pflugers Arch. 1993;422:386–392. doi: 10.1007/BF00374295. [DOI] [PubMed] [Google Scholar]
- Horowitz M, Eli-Berchoer L, Wapinski I, Friedman N, Kodesh E. Stress-related genomic responses during the course of heat acclimation and its association with ischemic-reperfusion cross-tolerance. J Appl Physiol. 2004;97:1496–1507. doi: 10.1152/japplphysiol.00306.2004. [DOI] [PubMed] [Google Scholar]
- Javadpour M, Kelly CJ, Chen G, Stokes K, Leahy A, Bouchier-Hayes DJ. Thermotolerance induces heat shock protein 72 expression and protects against ischaemia–reperfusion-induced lung injury. Br J Surg. 1998;85:943–946. doi: 10.1046/j.1365-2168.1998.00722.x. [DOI] [PubMed] [Google Scholar]
- Jiang Q, DeTolla L, Kalvakolanu I, Fitzgerald B, Hasday JD. Fever upregulates expression of pyrogenic cytokines in endotoxin-challenged mice. Am J Physiol. 1999;276:R1653–R1660. doi: 10.1152/ajpregu.1999.276.6.R1653. [DOI] [PubMed] [Google Scholar]
- Jiang Q, DeTolla L, Kalvakolanu I, Roojien N, Singh IS, Fitzgerald B, Cross AS, Hasday JD. Febrile range temperature modifies early systemic TNFα expression in mice challenged with bacterial endotoxin. Infect Immun. 1999;67:1539–1546. doi: 10.1128/iai.67.4.1539-1546.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Cross AS, Singh IS, Chem TT, Viscardi RM, Hasday JD. Febrile core temperature is essential for optimal host defense in bacterial peritonitis. Infect Immun. 2000;68:1265–1270. doi: 10.1128/IAI.68.3.1265-1270.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde F. Running and upper respiratory tract infections. Scand J Sport Sci. 1987;9:21–23. [Google Scholar]
- Lund SG, Ruberte MR, Hofmann GE. Turning up the heat: the effects of thermal acclimation on the kinetics of hsp70 gene expression in the eurythermal goby, Gillichthys mirabilis. Comp Biochem Physiol A Mol Integr Physiol. 2006;143:435–446. doi: 10.1016/j.cbpa.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Magalhaes F, Amorim FT, Passos RL, Fonseca MA, Oliveira KP, Lima MR, Guimaraes JB, Ferreira-Junior JB, Martini AR, Lima NR, Soares DD, Oliveira EM, Rodrigues LO. Heat and exercise acclimation increases intracellular levels of Hsp72 and inhibits exercise-induced increase in intracellular and plasma Hsp72 in humans. Cell Stress Chaperones. 2010;15:885–895. doi: 10.1007/s12192-010-0197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloyan A, Palmon A, Horowitz M. Heat acclimation increases the basal HSP72 level and alters its production dynamics during heat stress. Am J Physiol. 1999;276:R1506–R1515. doi: 10.1152/ajpregu.1999.276.5.R1506. [DOI] [PubMed] [Google Scholar]
- Manthous C, Hall J, Olson D, Singh M, Chatila W, Pohlman A, Kushner R, Schmidt G, Wood L. Effect of cooling on oxygen consumption in febrile critically ill patients. Am J Respir Crit Care Med. 1995;151:10–14. doi: 10.1164/ajrccm.151.1.7812538. [DOI] [PubMed] [Google Scholar]
- Marshall H, Campbell S, Roberts C, Nimmo M. Human physiological and heat shock protein 72 adaptations during the initial phase of humid-heat acclimation. J Therm Biol. 2007;32:341–348. doi: 10.1016/j.jtherbio.2007.04.003. [DOI] [Google Scholar]
- McClung JP, Hasday JD, He JR, Montain SJ, Cheuvront SN, Sawka MN, Singh IS. Exercise-heat acclimation in humans alters baseline levels and ex vivo heat inducibility of HSP72 and HSP90 in peripheral blood mononuclear cells. Am J Physiol Regul Integr Comp Physiol. 2008;294:R185–R191. doi: 10.1152/ajpregu.00532.2007. [DOI] [PubMed] [Google Scholar]
- Meyer TN, Silva AL, Vieira EC, Alves AC. Heat shock response reduces mortality after severe experimental burns. Burns. 2000;26:233–238. doi: 10.1016/S0305-4179(99)00139-4. [DOI] [PubMed] [Google Scholar]
- Nielsen B, Hales JR, Strange S, Christensen NJ, Warberg J, Saltin B. Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. J Physiol. 1993;460:467–485. doi: 10.1113/jphysiol.1993.sp019482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostberg JR, Taylor SL, Baumann H, Repasky EA. Regulatory effects of fever-range whole-body hyperthermia on the LPS-induced acute inflammatory response. J Leukoc Biol. 2000;68:815–820. [PubMed] [Google Scholar]
- Ostberg JR, Gellin C, Patel R, Repasky EA. Regulatory potential of fever-range whole body hyperthermia on langerhans cells and lymphocytes in an antigen-dependent cellular immune response. J Immunol. 2001;167:2666–2670. doi: 10.4049/jimmunol.167.5.2666. [DOI] [PubMed] [Google Scholar]
- Peters EM, Bateman ED. Ultramarathon running and upper respiratory tract infections. An epidemiological survey. S Afr Med J. 1983;64:582–584. [PubMed] [Google Scholar]
- Peters EM, Goetzsche JM, Grobbelaar B, Noakes TD. Vitamin C supplementation reduces the incidence of postrace symptoms of upper-respiratory-tract infection in ultramarathon runners. Am J Clin Nutr. 1993;57:170–174. doi: 10.1093/ajcn/57.2.170. [DOI] [PubMed] [Google Scholar]
- Rice P, Martin E, He J-R, Frank M, DeTolla L, Hester L, O’Neill T, Manka C, Singh I, Hasday J. Febrile-range hyperthermia augments neutrophil accumulation and enhances lung injury in experimental gram-negative bacterial pneumonia. J Immunol. 2005;174:3676–3685. doi: 10.4049/jimmunol.174.6.3676. [DOI] [PubMed] [Google Scholar]
- Riddle MS, Halvorson HA, Shiau D, Althoff J, Monteville MR, Shaheen H, Horvath EP, Armstrong AW. Acute gastrointestinal infection, respiratory illness, and noncombat injury among US military personnel during Operation Bright Star 2005, in Northern Egypt. J Travel Med. 2007;14:392–401. doi: 10.1111/j.1708-8305.2007.00159.x. [DOI] [PubMed] [Google Scholar]
- Roop SA, Niven AS, Calvin BE, Bader J, Zacher LL. The prevalence and impact of respiratory symptoms in asthmatics and nonasthmatics during deployment. Mil Med. 2007;172:1264–1269. doi: 10.7205/milmed.172.12.1264. [DOI] [PubMed] [Google Scholar]
- Sawka MN, Young AJ. Physiological systems and their responses to conditions of heat and cold. In: Tipton CM, Sawka MN, Tate CA, Trejung RL, editors. ACSM’s advanced exercise physiology textbook. Baltimore: Lippincott, Williams & Wilkins; 2005. pp. 535–563. [Google Scholar]
- Sawka MN, Young AJ, Cadarette BS, Levine L, Pandolf KB. Influence of heat stress and acclimation on maximal aerobic power. Eur J Appl Physiol Occup Physiol. 1985;53:294–298. doi: 10.1007/BF00422841. [DOI] [PubMed] [Google Scholar]
- Sawka MN, Wenger CB, Pandolf KB. Thermoregulatory responses to acute exercise-heat stress and heat acclimation. In: Fregly MJ, Blatteis CM, editors. Handbook of physiology section 4, environmental physiology. New York: Oxford University Press; 1996. pp. 157–185. [Google Scholar]
- Sawka MN, Latzka WA, Montain SJ, Cadarette BS, Kolka MA, Kraining KK, Gonzalez RR. Physiologic tolerance to uncompensable heat: intermittent exercise, field vs laboratory. Med Sci Sports Exerc. 2001;33:422–430. doi: 10.1097/00005768-200103000-00014. [DOI] [PubMed] [Google Scholar]
- Schumacker P, Rowland J, Saltz S, Nelson D, Wood L. Effects of hyperthermia and hypothermia on oxygen extraction by tissues during hypovolemia. J Appl Physiol. 1987;63:1246–1252. doi: 10.1152/jappl.1987.63.3.1246. [DOI] [PubMed] [Google Scholar]
- Shein NA, Grigoriadis N, Horowitz M, Umschwief G, Alexandrovich AG, Simeonidou C, Grigoriadis S, Touloumi O, Shohami E. Microglial involvement in neuroprotection following experimental traumatic brain injury in heat-acclimated mice. Brain Res. 2008;1244:132–141. doi: 10.1016/j.brainres.2008.09.032. [DOI] [PubMed] [Google Scholar]
- Singh IS, Gupta A, Nagarsekar A, Cooper Z, Manka C, Hester L, Benjamin IJ, He J-E, Hasday JD. Heat shock co-activates interleukin-8 transcription. Am J Respir Cell Mol Biol. 2008;39:235–242. doi: 10.1165/rcmb.2007-0294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutsky AS. Hot new therapy for sepsis and the acute respiratory distress syndrome. J Clin Invest. 2002;110:737–739. doi: 10.1172/JCI16551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonna LA, Wenger CB, Flinn S, Sheldon HK, Sawka MN, Lilly CM. Exertional heat injury and gene expression changes: a DNA microarray analysis study. J Appl Physiol. 2004;96:1943–1953. doi: 10.1152/japplphysiol.00886.2003. [DOI] [PubMed] [Google Scholar]
- Spielman RS, Lyman CP. Thermal bradycardia in the mildly stressed rat. Am J Physiol. 1971;221:948–951. doi: 10.1152/ajplegacy.1971.221.3.948. [DOI] [PubMed] [Google Scholar]
- Tomanek L, Somero GN. Interspecific- and acclimation-induced variation in levels of heat-shock proteins 70 (hsp70) and 90 (hsp90) and heat-shock transcription factor-1 (HSF1) in congeneric marine snails (genus Tegula): implications for regulation of hsp gene expression. J Exp Biol. 2002;205:677–685. doi: 10.1242/jeb.205.5.677. [DOI] [PubMed] [Google Scholar]
- Villar J, Edelson JD, Post M, Mullen JB, Slutsky AS. Induction of heat stress proteins is associated with decreased mortality in an animal model of acute lung injury. Am Rev Respir Dis. 1993;147:177–181. doi: 10.1164/ajrccm/147.1.177. [DOI] [PubMed] [Google Scholar]
- Yamada PM, Amorim FT, Mosley PL, Robergs RR, Schneider SM. Effect of heat acclimation on heat shock protein 72 and interleukin-10 in humans. J Appl Physiol. 2007;103:1196–1204. doi: 10.1152/japplphysiol.00242.2007. [DOI] [PubMed] [Google Scholar]