Abstract
Hyperbaric oxygen (HBO) is thought to confer protection to cells via a cellular response to free radicals. This process may involve increased expression of heat shock proteins, in particular the highly inducible heat shock protein 72 (Hsp72). Healthy male volunteers (n = 16) were subjected to HBO for 1 h at 2.8 ATA. Inducible Hsp72 expression was measured by flow cytometry pre-, post- and 4 h-post HBO. Peripheral blood mononuclear cells (PBMC) were isolated from whole blood via density centrifugation pre-, post- and 4 h post-HBO. PBMC were then subjected to an in vitro heat shock at 40°C or hypoxia at 37°C (5% O2) with a control at 37°C. Cells were then analysed for Hsp72 expression by flow cytometry. Monocytes showed no significant changes in Hsp72 expression following HBO. No detectable Hsp72 was seen in lymphocytes or neutrophils. Following in vitro hypoxic exposure, a significant increase in Hsp72 expression was observed in monocytes isolated immediately post- (p = 0.006) and 4 h post-HBO (p = 0.010) in comparison to control values. HBO does not induce Hsp72 expression in PBMC. The reported benefits of HBO in terms of pre-conditioning are not due to inducement of Hsp72 expression in circulating blood cells, but may involve an enhancement of the stress response.
Keywords: Hyperbaric oxygen, Heat shock, Hypoxia, Monocytes
Introduction
Hyperbaric oxygen (HBO) is thought to confer cellular protection via a stress response linked to free radical generation in vivo (Thom 2009). This response may include inducing increased heat shock protein expression (Dennog et al. 1999; Thom 2009). We have previously shown HBO prior to cardiac surgery confers protection against neuro-sequelae (Alex et al. 2005) and improves outcome measures (Yogaratnam et al. 2007) following surgery. It was proposed that protection against ischemia-reperfusion injury involves phenotype changes of circulating blood cells and the endothelium (Yogaratnam et al. 2007). Most studies have focused on HBO in disease and despite studies demonstrating potential beneficial clinical effects (Alex et al. 2005; Roeckl-Wiedmann et al. 2005; Yogaratnam et al. 2007), the mechanism of HBO treatment at a cellular and biochemical level remains to be elucidated. However, HBO preconditioning results in increased oxygen dissolving in the plasma and improves the elasticity of red blood cells, enabling them to reach ischemic tissues (Mathieu et al. 1984). This can result in enhanced tissue oxygenation and increased metabolism and has led to studies involving the potential use of HBO in cerebral ischemic disease and their sequelae (Clifton 1995; Veltkamp and Tooke 1997), myocardial ischemia and infarct size reduction (Shandling et al. 1997; Thomas et al. 1990), and improvement of myocardial function (Dekleva et al. 2004). Hyperbaric oxygen has been demonstrated to increase reactive oxygen species (ROS) generation (Benedetti et al. 2004; Conconi et al. 2003; Gregorevic et al. 2001) and these may play a role in inducing expression of cellularly protective heat shock proteins (Hsp; Dennog et al. 1999; Rothfuss et al. 2001). The intracellular concentration of Hsp has been demonstrated to be closely correlated to antioxidant enzyme activity under conditions of oxidative stress, suggesting that Hsp are induced by an increase in ROS (Currie et al. 1988; Mocanu et al. 1993) to attenuate ROS-dependent cellular and tissue damage.
Hsp are ubiquitous and perform an essential cellular function, the chaperoning of nascent proteins, preventing them from degradation (Bukau and Horwich 1998). Inducible Hsp72 is a member of the highly conserved Hsp70 family of proteins and is well characterised (Madden et al. 2008). Hsp72 has a role in re-folding damaged proteins, inhibiting protein aggregation and translocation of proteins (Kregel 2002). Hsp72 expression is increased markedly in response to heat shock (Vince et al. 2010) and has shown to be protective against subsequent stresses such as heat (Locke and Noble 1995; Vince et al. 2010), hypoxia (Patel et al. 1995) and oxidative stress (Kukreja et al. 1994). Monocytes have been demonstrated to be the most sensitive blood cell for Hsp72 expression analysis (Bachelet et al. 1998; Sandstrom et al. 2009; Vince et al. 2010).
The aim of this study was to investigate whether the mechanism of HBO preconditioning involves inducing Hsp72 expression within circulating peripheral blood mononuclear cells (PBMC) and whether any protection against a subsequent in vitro applied stress was conferred.
Methods
Ethical approval
Healthy male volunteers (n = 16, 18–36 years) participated in this study. The University’s Department of Sport, Health and Exercise Science ethical committee granted approval for the study and written informed consent was gained. All subjects were treated in accordance with the Declaration of Helsinki.
Hyperbaric oxygenation
Volunteers, in groups of five or six breathed 100% oxygen for 1 h at 2.8 ATA. This consisted of 20 min oxygen followed by 5 min air, by two cycles. Subjects were fasting on the day of the study and refrained from caffeine intake, HBO exposure began at 12 pm. The study was spread over three consecutive weeks.
Blood sampling
Blood was drawn by venipuncture into potassium EDTA tubes (Greiner, Stonehouse, UK). Blood was taken immediately prior- and post-HBO and a further sample was taken 4 h-post HBO.
Inducible Hsp72 assay
Inducible Hsp72 was measured by flow cytometry following blood sampling. Whole blood (100 μL) was incubated for 10 min with red cell lysing buffer (2 mL, Erythrolsye, AbD Serotec, UK). Cells were then fixed and permeabilised using commercial buffers (AbD Serotec, UK) and incubated for 30 min with 5 μL of either isotype matched negative control/FITC (AbD Serotec, Oxford, UK) or anti-Hsp72/FITC (Assay Designs, Ann Arbor, MI, USA) to the same final concentration, as described previously (Vince et al. 2010).
PBMC isolation
PBMC were isolated by density gradient centrifugation (lymphocyte separation medium, PAA, UK) according to manufacturers’ instructions. Cells underwent controlled freezing to −80°C and were used within 24 h of isolation.
In vitro stress assay
PBMC were defrosted on ice, washed with PBS (20 mL) and resuspended in RPMI1640 media (PAA, Yeovil, UK) supplemented with 10% (v/v) foetal calf serum (Biosera, UK). The assay was performed as previously detailed (Vince et al. 2010). Briefly, washed PBMC were divided into 500-μL aliquots and transferred to 4 mL sterile tubes (BD Biosciences, UK) one was placed within a hypoxic incubator (5% O2, 5% CO2, nitrogen balance, BOC speciality gases, UK) at 37°C for 90 min. A further aliquot was placed in a normoxic (5% CO2, air balance) incubator at 37°C for 90 min and a final aliquot placed in a heating block at 40°C (± 0.1°C) for 90 min.
Flow cytometry analysis
Samples were analysed on BDFACSCalibur (Becton Dickinson, Oxford, UK) running CELLQuest software and 20,000 events were counted. Monocytes were gated according to their scatter properties and Hsp72 expression determined by mean fluorescence intensity of samples incubated with the anti-Hsp72 antibody relative to that of the negative control, thus accounting for any differences in autofluorescence between and within subjects. Therefore, a value of 1 indicates no increase over that of the negative control.
Cell viability
A subset of samples (n = 6) were analysed for viability post in vitro assay conditions, by flow cytometry, using an Annexin V apoptosis detection kit (BD Biosciences, UK) in accordance with manufacturers’ instructions.
Statistical analyses
All statistical analyses were completed using PASW statistics 18 (SPSS Inc., Chicago, IL, USA). The effects of condition (live, control, heat and hypoxia) and time (pre-HBO, immediately post-HBO, and 4 h post-HBO) on Hsp72 expression were analysed using linear mixed models. Condition and time were treated as fixed repeated measures effects and subject as a random effect. Pair-wise comparisons were obtained in the event of a significant F ratio. Associations between Hsp72 in the control and hypoxia conditions were investigated using the Pearson correlation coefficient. The family-wise type 1 error rate was controlled using Sidak-adjusted p values. Two-tailed statistical significance was accepted as p < 0.05.
Results
Hsp72 live assay
There were no significant changes observed in monocyte Hsp72 expression following HBO exposure. Pre-exposure the mean fluorescence intensity of monocytes labelled with anti-Hsp72 was 1.17 ± 0.24, immediately post-exposure the value was 1.20 ± 0.24 and 4 h post-HBO this had reduced non-significantly to 1.09 ± 0.20.
In vitro stimulation assay
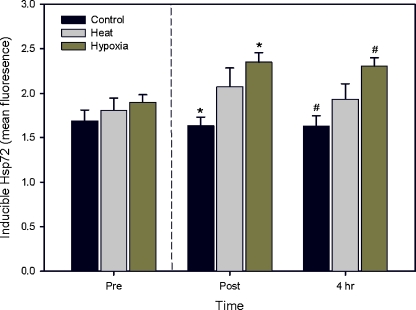
There were no significant changes in mean Hsp72 across time in the control (37°C) or heat (40°C) conditions, although an increase in Hsp72 expression after heat shock was observed in comparison to the control which was greater post-HBO (Fig. 1). In the hypoxia condition, Hsp72 increased by 24% from pre-HBO exposure to immediately post-exposure (p = 0.006) and was significantly elevated 4 h post-HBO (p = 0.010) when compared to the control response. The amount of Hsp72 in response to hypoxia post-HBO was not significantly different between samples isolated immediately post-HBO or 4 h post-HBO.
Fig. 1.
Mean (SEM) Hsp72 expression within monocytes before, immediately after and 4 h after hyperbaric exposure (represented by the vertical dashed line) in the control, heat and hypoxia conditions (in vitro). Asterisks and number signs indicate significantly different Hsp72 levels (p < 0.05)
The Hsp72 in the control condition was positively correlated with that in the heat condition pre-HBO exposure (r = 0.65, p = 0.033). A similar relationship was also observed between the control and hypoxia conditions post-HBO exposure (r = 0.64, p = 0.021).
Across all time points, the hypoxic insult was seen to generate a significantly (p < 0.001) increased expression (mean 18 ± 6%) of Hsp72 when compared to the heat shock insult. Cell viability was assessed across in vitro conditions and time on six subjects and no significant differences were observed (overall mean viability 70.4 ± 11.9%). A lower viability was noted 4 h post-HBO across all conditions (60.8 ± 9.4%), however this was non-significant (p = 0.20).
Discussion
No increased expression of Hsp72 was observed in PBMC following HBO in a healthy male population. Furthermore, no protection against a subsequent hypoxic stress was observed in isolated PBMC after a single session of HBO, as seen by the increases in Hsp72 expression after the applied stress. Hsp72 is rapidly induced and can remain elevated for a number of hours post-stressor (Locke and Noble 1995). Exposure of isolated PBMC to hypoxia resulted in a significantly increased expression of Hsp72 in samples taken post-HBO when compared to PBMC isolated prior to HBO. Therefore, the applied stress post-HBO appeared to result in an enhanced Hsp72 response to hypoxia, which may be beneficial. We have recently shown that a single HBO exposure was sufficient to increase NFκBp50 expression within nuclei of human peripheral blood mononuclear cells (Madden et al. 2010a), suggesting that physiological changes within monocytes may be induced by HBO.
A recent study demonstrated significant increases in tissue oxygen saturation during HBO although saturation did not reach 100% and returned to baseline values within 2 min of cessation of treatment (Larsson et al. 2010). Any increase in oxygen within the circulation comes from the increased alveolar partial pressure of oxygen during HBO exposure (Weaver et al. 2009) and no changes in rates of oxygen delivery and consumption have been reported after HBO (Weaver et al. 2009). Therefore, one could reasonably argue that HBO does not induce a significant stress response within the blood cell population. We have demonstrated previously that any heat stress response is inversely proportional to the amount of Hsp72 already present within the cell at the time of the stress (Vince et al. 2010). A correlation previously reported between the heat shock response after 90 min incubation at 37°C and 40°C with no intervention (Vince et al. 2010) was again observed here prior to HBO. Post-HBO this relationship was not significantly correlated, suggesting an alteration of the stress response may occur after HBO. However, the hypoxic exposure was seen to significantly elevate Hsp72 levels after a single session of HBO, which may suggest that a hypoxic insult is the stronger stressor. This hypothesis is supported by previous findings where hypoxia in vivo was shown to result in a time-dependent increase in baseline Hsp72 over seven consecutive days (Taylor et al. 2010).
HBO preconditioning strategies are used primarily to oxygenate soft tissues (Larsson et al. 2010) and usually involve comparison between randomised groups. Any changes in heat shock expression that have been previously determined have taken place after surgery and the consensus is that higher amounts of Hsp72 are protective (Madden et al. 2008). Data presented here shows that post-HBO there is no change in monocyte expressed Hsp72, however a subsequent hypoxic stress may result in an enhanced stress response, resulting in higher amounts of Hsp72 being produced. If the subsequent hypoxic stress involved was, for example caused by surgical intervention then enhanced Hsp72 expression may prove beneficial.
The cellular response to HBO is not fully characterised. It is presumed an oxidative stress resulting in free radical generation should result in Hsp expression (Yogaratnam et al. 2007), however the present study demonstrates no inducement of Hsp72 expression within PBMC following a single HBO exposure in healthy males. One previous human study (Dennog et al. 1999) found that the relative concentration of Hsp72 was significantly induced in the lymphocytes of human subjects after a single treatment with HBO. However, this increase was noted after 24 h and it is unclear how the Hsp72 was accurately determined in the four people tested. The wide range of antioxidants also tested in the study showed no significant changes in concentration or antioxidant capacity 24 h after a single HBO session. We have also previously observed no changes in serum thiobarbituric reactive substances, a measure of lipid peroxidation, following HBO in a previous study (Vince et al. 2009). In the present study, Hsp72 was determined twice in a four-hour period (a total of 32 measurements) following HBO exposure and showed no change. This data coupled with the previous antioxidant study does suggest that a single 1 h HBO session does not cause a significant homeostatic shift within the circulation, although molecular adaptations may occur (Rothfuss et al. 2001). A study on human lymphocytes showed that Hsp72 expression was increased following an in vitro oxygen stress (Shinkai et al. 2004). The Jurkat human T-cell line (Oh et al. 2005) and a mouse neuroblastoma cell line (Shyu et al. 2004) have also been shown to produce Hsp72 in response to HBO exposure.
The endothelium is sensitive to oxidative stress and perhaps HBO-mediated cellular protection may be conferred here as the primary barrier between the blood and surrounding tissues. Our previous studies support this hypothesis, showing HBO-mediated endothelial protection prior to cardiac surgery in patients (Alex et al. 2005; Yogaratnam et al. 2007) and an improvement in reactive hyperaemia following HBO in healthy subjects (Madden et al. 2010b).
In conclusion, HBO in healthy male subjects does not cause an increase in inducible Hsp72 within circulating PBMC although the stress response may be enhanced following a subsequent stress. It seems therefore that the protective effects of HBO are therefore more likely seen within tissues and this is where further work is required.
Acknowledgements
The authors wish to thank the staff of the Hull Hyperbaric Unit for their expertise and assistance, in particular Carol Compton-Mudd, and also Lee Taylor and Lars McNaughton for their input. The work described was internally funded.
Conflict of interest None declared
References
- Alex J, Laden G, Cale ARJ, Bennett S, Flowers K, Madden L, Gardiner E, McCollum PT, Griffin SC. Pretreatment with hyperbaric oxygen and its effect on neuropsychometric dysfunction and systemic inflammatory response after cardiopulmonary bypass: a prospective randomized double-blind trial. J Thorac Cardiovasc Surg. 2005;130:1623–1630. doi: 10.1016/j.jtcvs.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Bachelet M, Mariethoz E, Banzet N, Souil E, Pinot F, Polla CZ, Durand P, Bouchaert I, Polla BS. Flow cytometry is a rapid and reliable method for evaluating heat shock protein 70 expression in human monocytes. Cell Stress Chaperones. 1998;3:168–176. doi: 10.1379/1466-1268(1998)003<0168:FCIARA>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti S, Lamorgese A, Piersantelli M, Pagliarani S, Benvenuti F, Canestrari F. Oxidative stress and antioxidant status in patients undergoing prolonged exposure to hyperbaric oxygen. Clin Biochem. 2004;37:312–317. doi: 10.1016/j.clinbiochem.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/S0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- Clifton GL. Hypothermia and hyperbaric oxygen as treatment modalities for severe head injury. New Horiz. 1995;3:474–478. [PubMed] [Google Scholar]
- Conconi MT, Baiguera S, Guidolin D, Furlan C, Menti AM, Vigolo S, Belloni AS, Parnigotto PP, Nussdorfer GG. Effects of hyperbaric oxygen on proliferative and apoptotic activities and reactive oxygen species generation in mouse fibroblast 3 T3/J2 cell line. J Investigativ Med. 2003;51:227–232. doi: 10.2310/6650.2003.39201. [DOI] [PubMed] [Google Scholar]
- Currie RW, Karmazyn M, Kloc M, Mailer K. Heat shock response is associated with enhanced post-ischemic ventricular recovery. Circ Res. 1988;63:543–549. doi: 10.1161/01.res.63.3.543. [DOI] [PubMed] [Google Scholar]
- Dekleva M, Neskovic A, Vlahovic A, Putnikovic B, Beleslin B, Ostojic M. Adjunctive effect of hyperbaric oxygen treatment after thrombolysis on left ventricular function in patients with acute myocardial infarction. Am Heart J. 2004;148:E14. doi: 10.1016/j.ahj.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Dennog C, Radermacher P, Barnett YA, Speit G. Antioxidant status in humans after exposure to hyperbaric oxygen. Mutat Res Fundam Mol Mech Mutagen. 1999;428:83–89. doi: 10.1016/S1383-5742(99)00034-4. [DOI] [PubMed] [Google Scholar]
- Gregorevic P, Lynch GS, Williams DA. Hyperbaric oxygen modulates antioxidant enzyme activity in rat skeletal muscles. Eur J Appl Physiol. 2001;86:24–27. doi: 10.1007/s004210100503. [DOI] [PubMed] [Google Scholar]
- Kregel KC. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol. 2002;92:2177–2186. doi: 10.1152/japplphysiol.01267.2001. [DOI] [PubMed] [Google Scholar]
- Kukreja RC, Kontos MC, Loesser KE, Batra SK, Qian YZ, Gbur CJ, Naseem SA, Jesse RL, Hess ML. Oxidant stress increases heat-shock protein-70 messenger-RNA in isolated-perfused rat-heart. Am J Physiol Heart Circ Physio. 1994;267:H2213–H2219. doi: 10.1152/ajpheart.1994.267.6.H2213. [DOI] [PubMed] [Google Scholar]
- Larsson A, Uusijärvi J, Eksborg S, Lindholm P. Tissue oxygenation measured with near-infrared spectroscopy during normobaric and hyperbaric oxygen breathing in healthy subjects. Eur J Appl Physiol. 2010 doi: 10.1007/s00421-010-1403-0. [DOI] [PubMed] [Google Scholar]
- Locke M, Noble EG. Stress proteins—the exercise response. Can J Appl Physiol. 1995;20:155–167. doi: 10.1139/h95-011. [DOI] [PubMed] [Google Scholar]
- Madden LA, Sandstrom ME, Lovell RJ, McNaughton L. Inducible heat shock protein 70 and its role in preconditioning and exercise. Amino Acids. 2008;34:511–516. doi: 10.1007/s00726-007-0004-7. [DOI] [PubMed] [Google Scholar]
- Madden LA, Vince RV, Laden G. The effect of acute hyperoxia in vivo on NF kappa B expression in human PBMC. Cell Biochem Func. 2010 doi: 10.1002/cbf.1712. [DOI] [PubMed] [Google Scholar]
- Madden LA, Chrismas B, Mellor D, Vince RV, Midgley AW, McNaughton L, Atkin SL, Laden G. Endothelial function and stress response after simulated dives to 18msw breathing air or oxygen. Aviat Space Environ Med. 2010;81:41–45. doi: 10.3357/ASEM.2610.2010. [DOI] [PubMed] [Google Scholar]
- Mathieu DCJ, Vinckier F, Saulnier A, Durocher ET, Wattel F. Red blood cell deformability and hyperbaric oxygen. Med Subaquatique Hyperbar. 1984;3:100–104. [Google Scholar]
- Mocanu MM, Steare SE, Evans MCW, Nugent JH, Yellon DM. Heat-stress attenuates free-radical release in the isolated-perfused rat-heart. Free Radical Biol Med. 1993;15:459–463. doi: 10.1016/0891-5849(93)90046-W. [DOI] [PubMed] [Google Scholar]
- Oh E, Oh S, Im H, Lee J, Kim J, Moon J, Hong E, Kim Y, Yang M, Lim Y, Park S, Lee E, Sul D. Effects of hyperbaric pressure on cellular morphology, proliferation and protein expression of Jurkat cell. Mol Cellul Toxicol. 2005;1:116–123. [Google Scholar]
- Patel B, Khaliq A, JarvisEvans J, Boulton M, Arrol S, Mackness M, McLeod D. Hypoxia induces HSP 70 gene expression in human hepatoma (HEP G2) cells. Biochem Mol Biol Int. 1995;36:907–912. [PubMed] [Google Scholar]
- Roeckl-Wiedmann I, Bennett M, Kranke P. Systematic review of hyperbaric oxygen in the management of chronic wounds. Brit J Surg. 2005;92:24–32. doi: 10.1002/bjs.4863. [DOI] [PubMed] [Google Scholar]
- Rothfuss A, Radermacher P, Speit G. Involvement of heme oxygenase-1 (HO-1) in the adaptive protection of human lymphocytes after hyperbaric oxygen (HBO) treatment. Carcinogen. 2001;22:1979–1985. doi: 10.1093/carcin/22.12.1979. [DOI] [PubMed] [Google Scholar]
- Sandstrom ME, Madden LA, Taylor L, Siegler JC, Lovell RJ, Midgley A, McNaughton L. Variation in basal heat shock protein 70 is correlated to core temperature in human subjects. Amino Acids. 2009;37:279–284. doi: 10.1007/s00726-008-0144-4. [DOI] [PubMed] [Google Scholar]
- Shandling AH, Ellestad MH, Hart GB, Crump R, Marlow D, VanNatta B, Messenger JC, Strauss M, Stavitsky Y. Hyperbaric oxygen and thrombolysis in myocardial infarction: the “HOT MI” Pilot Study. Am Heart J. 1997;134:544–550. doi: 10.1016/S0002-8703(97)70093-0. [DOI] [PubMed] [Google Scholar]
- Shinkai M, Shinomiya N, Kanoh S, Motoyoshi K, Kobayashi H. Oxygen stress effects on proliferation rates and heat shock proteins in lymphocytes. Aviat Space Environ Med. 2004;75:109–113. [PubMed] [Google Scholar]
- Shyu WC, Lin SZ, Saeki K, Kubosaki A, Matsumoto Y, Onodera T, Chiang MF, Thajeb P, Li H. Hyperbaric oxygen enhances the expression of prion protein and heat shock protein 70 in a mouse neuroblastoma cell line. Cellul Mol Neurobiol. 2004;24:257–268. doi: 10.1023/B:CEMN.0000018620.41913.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L, Midgley AW, Chrismas B, Madden LA, Vince RV, McNaughton LR. The effect of acute hypoxia on heat shock protein 72 expression and oxidative stress in vivo. Eur J Appl Physiol. 2010;109:849–855. doi: 10.1007/s00421-010-1430-x. [DOI] [PubMed] [Google Scholar]
- Thom SR. Oxidative stress is fundamental to hyperbaric oxygen therapy. J Appl Physiol. 2009;106:988–995. doi: 10.1152/japplphysiol.91004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MP, Brown LA, Sponseller DR, Williamson SE, Diaz JA, Guyton DP. Myocardial infarct size reduction by the synergistic effect of hyperbaric oxygen and recombinant tissue plasminogen activator. Am Heart J. 1990;120:791–800. doi: 10.1016/0002-8703(90)90194-3. [DOI] [PubMed] [Google Scholar]
- Veltkamp R, Tooke JF. Hyperbaric oxygen—a neuroprotective adjunct for hyperacute ischemic stroke? J Neurol Sci. 1997;150:1–2. doi: 10.1016/S0022-510X(97)05478-6. [DOI] [PubMed] [Google Scholar]
- Vince RV, McNaughton LR, Taylor L, Midgley AW, Laden G, Madden LA. Release of VCAM-1 associated endothelial microparticles following simulated SCUBA dives. Eur J Appl Physiol. 2009;105:507–513. doi: 10.1007/s00421-008-0927-z. [DOI] [PubMed] [Google Scholar]
- Vince RV, Oliver K, Midgley A, McNaughton L, Madden LA. In vitro heat shock of human monocytes results in a proportional increase of inducible Hsp70 expression according to the basal content. Amino Acids. 2010;38:1423–1428. doi: 10.1007/s00726-009-0354-4. [DOI] [PubMed] [Google Scholar]
- Weaver LK, Howe S, Snow GL, Deru K. Arterial and pulmonary arterial hemodynamics and oxygen delivery/extraction in normal humans exposed to hyperbaric air and oxygen. J Appl Physiol. 2009;107:336–345. doi: 10.1152/japplphysiol.91012.2008. [DOI] [PubMed] [Google Scholar]
- Yogaratnam JZ, Laden G, Madden LA, Guvendik L, Cowen M, Greenman J, Seymour A-M, Cale A, Griffin S. Hyperbaric oxygen preconditioning safely improves myocardial function, promotes pulmonary vascular flow, and protects the endothelium from ischemic reperfusion injury. Cardiovasc Revascul Med. 2007;8:148–151. doi: 10.1016/j.carrev.2007.03.167. [DOI] [Google Scholar]