Abstract
Grp94 is the main endoplasmic reticulum-resident heat shock protein (HSP) that besides chaperoning native proteins, displays important modulatory effects on both the innate and adaptive immune response. Since the knowledge of a direct influence of Grp94 on the humoral response is lacking, in this work we tested the effect of Grp94 on Ig secretion from peripheral blood mononuclear cells (PBMCs) of five normal volunteers. The concentration of Ig secreted in the medium after incubation of 15 days was found increased in a dose-dependent manner in the presence of Grp94, used at the final concentrations of 10 and 100 ng/ml. However, by measuring the Ig secretion at different incubation times, it was apparent that maximal percent stimulation by Grp94 occurred at 7 days, decreasing thereafter. In addition, the pattern of Ig secretion in time significantly differed in the presence of Grp94 with respect to that of control PBMCs. Grp94 also stimulated in a dose-dependent manner the PBMC proliferation, an effect that preceded the Ig secretion and was accompanied by morphological changes of cells similar to those induced by the pokeweed mitogen. Effects of Grp94 on PBMCs were mediated by an intense activation of the MEK-ERK1/2 pathway and by an increased expression of HSP90. Results indicate that Grp94 can activate the humoral response by a cytokine-like, cell-mediated mechanism that leads to an accelerated process of B cell maturation and differentiation.
Keywords: Antibody production, Cell proliferation, Extracellular signal-regulated MAP kinases, Heat shock proteins, Lymphocyte activation
Introduction
Glucose-regulated protein94 (Grp94), the most important endoplasmic reticulum-resident heat shock protein (HSP), besides its main function to chaperon native proteins for attaining the correct folding, displays the unique specialized function to modulate the immune system by influencing both native and adaptive immune responses (Li et al. 2002). Stimulation of the innate immunity is due to the activation of antigen-presenting cells (APC), macrophages and natural killer cells through upstream involvement of specific receptors of the innate immunity. These effects are displayed by Grp94 when expressed on cell surface or liberated extracellularly (Reed et al. 2003; Baker-LePain et al. 2004), a condition sensed as “immunological danger” due to the un-physiological location of Grp94 that renders Grp94 a potent immunogen (Baker-LePain et al. 2002). The adaptive immune response elicited by Grp94 is more specifically attributed to its capacity to bind antigenic peptides in the cell and to present them to the MHC class I processing pathway on APCs, thereby activating cytotoxic T lymphocytes (Suto and Srivastava 1995). This effect has extensively been elucidated in its molecular aspects in several in vitro and in vivo studies (Srivastava et al. 1986; Binder et al. 2000; Tamura et al. 1997), and also effectively exploited for the development of anti-tumor (Tamura et al. 1997; Heike et al. 1999) and anti-viral therapies (Navaratnam et al. 2001). However, Grp94 may be able to elicit an adaptive immune response independently of its function of chaperoning peptides, by simply stimulating the innate immune system (More et al. 1999; Breloer et al. 1999; Singh-Jasuja et al. 2000). In this view, the receptor-mediated activation of APCs (Singh-Jasuja et al. 2000) and macrophages (Reed et al. 2003) might lead to the downstream production of cytokines that are per se sufficient to generate the immunological environment, favoring the stimulation of cellular immune response (Nicchitta 2003).
Although most studies have investigated in detail the effects of Grp94 on cellular immunity and the related molecular mechanisms, much fewer addressed the question of whether and to what extent Grp94 might directly affect humoral immune response. Recently, Liu B. and Li Z. (Liu and Li 2008) investigated the function of Grp94 as immunoglobulin (Ig) chaperone in a model of B cell-selective Grp94-deficient mice. Although the main result of the work suggested a more restricted role of Grp94 in B cell functioning, questioning the involvement of Grp94 in the Ig assembly and secretion, the role that Grp94 might play in modulating antibody (Ab) secretion and function in physiological conditions, in humans, has not conclusively been addressed as yet.
We have observed that Grp94 can induce the growth and promote the differentiation of human umbilical vein endothelial cells (HUVECs) with a cytokine-like mechanism (Tramentozzi et al. 2008). Since HUVECs are cells of the innate immunity (Carmeliet 2000), we speculated that Grp94 might activate the same mechanism in other, more specialized cells of immunity, thereby eliciting a different panel of effects. We tested this possibility on human peripheral blood mononuclear cells (PBMCs) incubated with native Grp94, by measuring the cell growth and Ab secretion at different incubation times. Results indicate that Grp94 affects the humoral immune response by stimulating the process of B cell maturation and differentiation with the consequent increase in the rate of Ig secretion.
Materials and methods
Reagents and antibodies
Ficoll–Paque was from Amersham Pharmacia (Amersham Pharmacia, Uppsala, Sweden), Roswell Park Memorial Institute medium 1640 (RPMI) from Euroclone (Euroclone Life Sciences Division, Milan, Italy), fetal bovine serum (FBS) from Life Technologies (Life Technologies, Gaithersburg, MD, USA), gelatin, l-glutamine, pokeweed mitogen (PWM), phytohemagglutinin (PHA), puromycin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and bovine serum albumin (BSA) from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA). The following primary Abs were used: anti-CD19 monoclonal (Coulter Electronics, FL, USA), mouse anti-β-actin monoclonal (Cell Signaling and Neurosciences, St. Louis, MI, USA), rabbit anti-human HSP90α/β polyclonal, and mouse anti-human HSP70 monoclonal (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). All other reagents were of analytical grade from Sigma.
Grp94 solutions
Grp94 was purified from rat hepatocyte microsomal fraction through sequential chromatography on DEAE-Sepharose and Heparin-Sepharose columns, followed by gel-filtration on a FPLC-Superdex 200, as described (Brunati et al. 2000). The purified fractions of Grp94 were submitted to ultra-filtration on Amicon Centriplus YM-3 (3,000 MWCO) and to the QCL-1000 chromogenic LAL end-point assay (Cambrex BioScience Inc., Walkersville, MD, USA) that excluded any contamination with endotoxin.
Purification of peripheral blood mononuclear cells
PBMCs were purified from the freshly drawn peripheral blood of healthy donors, using Ficoll–Paque density gradient, according to the manufacturer’s instructions. Subjects were regular blood donors at the Transfusion Center of the Hospital (Padua, Italy). They all gave informed consent for blood drawing and treatment of clinical and laboratory data. Blood was diluted with an equal volume of RPMI medium, layered over the Ficoll-Paque solution and centrifuged at 600×g for 40 min. The mononuclear cell layer was collected, washed with the medium and centrifuged at 600×g for 15 min. After discarding the supernatant, the pellet was washed twice with the medium and centrifuged at 300×g for 10 min. PBMCs were counted with the Türk dye method.
In vitro antibody production
PBMCs were plated at the concentration of 2 × 106 cells/ml in a 24- or 48-well flat-bottomed plates with the medium supplemented with 10% FBS, 1% l-glutamine, and incubated at 37°C in a humidified 95% air and 5% CO2 atmosphere, in the absence (control) and presence of native Grp94 (10 and 100 ng/ml, final concentrations). Additional controls included cells treated with puromicine (100 μg/ml), for excluding non-de novo synthesis of Ig, and PWM (final concentration of 20 μg/ml) used as positive control, i.e., as the mitogen of reference for inducing a polyclonal response in standardized test of functional activity of B cells (Hauser et al. 1984). At time intervals of 3, 7, and 15 days, cells were harvested and centrifuged at 1,500×g for 15 min, supernatants collected and stored at −20°C for further analysis. Aliquots of PBMCs, both before and after each incubation time, were used for the cytofluorimetric determination of CD19+ lymphocytes.
Radio immuno assay
Ig were measured by radio immuno assay following the method previously reported (Indraccolo et al. 1993), with some modifications. Specifically, wells (200 μl) of a 96-well flat-bottomed Optiplate (Packard) were covered with 50 μl of goat anti-human Ig Abs (Sera-Lab, West Sussex, UK; 5 μg/ml in NaHCO3/Na2CO3 buffer, pH 9.6) and after drying, a solution (200 μl) of phosphate buffered saline (PBS) with 3% BSA and 0.02% NaN3 was added to saturate non-specific binding sites. The supernatants collected from PBMC cultures were diluted (serial dilutions) in PBS/BSA/NaN3 and added to wells after washing with PBS. A 50-μl aliquot of sheep anti-human Ig Fab2-I125 (Amersham Pharmacia, Uppsala, Sweden; 1 μCi/ml dissolved in PBS/BSA/NaN3) was then added to each well and left in incubation for 2 h. After repeated washings, the plate was dried and radioactivity measured in a TOP Count Perkin Elmer scintillator after the addition of 30 μl of Microscint 20 (Hewlett-Packard). The concentration of Ig in the supernatant was calculated on the calibration curve obtained by properly diluting a solution of standard human serum with a known concentration of Ig, and expressed as ng/ml × 10−5 B cells, as determined by cytofluorimetric analysis with anti-CD19 monoclonal Abs.
Peripheral blood mononuclear cell proliferation assay
The MTT test (Mosmann 1983) was used for measuring the PBMC proliferation. PBMCs were seeded in a 96-well plate (200 μl/well) at the concentration of 5 × 105/ml with the addition of RPMI, 10% FBS and 1% l-glutamine. Quadruplicate wells were used for both control and treatment. Both untreated cells and cells treated with the standard mitogen PHA (final concentration, 10 μg/ml) served as negative and positive controls, respectively. After incubation at 37°C for 3 and 7 days, 20 μl of MTT (5 mg/ml in PBS) were added to each well and left in incubation for 4 h. The plate was then centrifuged at 1,800 rpm for 5 min, the supernatant discarded, and 100 μl of 2-propanol HCl added for solubilization of formazan crystals. Absorbance was read at 570 and 630 nm and the difference between the two wavelengths taken as the value expressing the entity of proliferation.
Analyses of PBMC lysates
After removal of the supernatant, cells were washed twice with 1 ml PBS and lysed with the Laemmli lysis buffer (50 mM Tris–HCl, pH 8.9, 5 mM EDTA, 380 mM glycine, 4% SDS, 10% glycerol) followed by boiling for 3 min. Cell lysates were analyzed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 10% acrylamide gel) and probed with anti-human HSP90α/β, HSP70, and IgG (whole molecule) Abs in Western blot analysis. Immunodetection was obtained using either phosphatase alkaline-conjugate, affinity-purified IgG (from Sigma) or biotin conjugate, affinity-purified IgG (Vector Laboratories, Burlingame, CA) coupled with affinity-purified egg white avidin conjugated to alkaline phosphatase (ABC system). Secondary Abs alone served as controls for excluding any false positive reaction.
In separate experiments, PBMCs were seeded at the density of 2 × 106 cells/ml in a 48-well plate in absence of FBS. After incubation for 3 and 7 days, the supernatant was collected, cells lysed in the Laemmli lysis buffer, as above, with the addition of 7 mM β-mercaptoethanol and lysates analyzed in SDS-PAGE followed by blotting on a nitrocellulose membrane for measuring activity of P-ERK1/2 with specific polyclonal Abs (Cell Signaling and Neuroscience). Immunodetection was attained by ECL system. Abs against β-actin were used as control for protein loading after stripping of the same membrane.
Statistical analysis
All data are presented as mean ± SD unless otherwise stated. Statistical analysis (linear regression analysis) was performed using GraphPad Prism 3 (GraphPad Software, Inc. San Diego, CA, USA). Variations in both Ig secretion and cell proliferation following treatments were calculated as percentage over values of control (in the absence of treatment). Cell aggregates visualized at the optical microscopy were measured using ImageJ 1.42q (NIH, USA) and the area expressed as percentage on that of the entire field analyzed (100%). Values of aggregate areas are the mean (±SD) of measurements made on seven different fields in the well.
Results
Ig secretion by Grp94
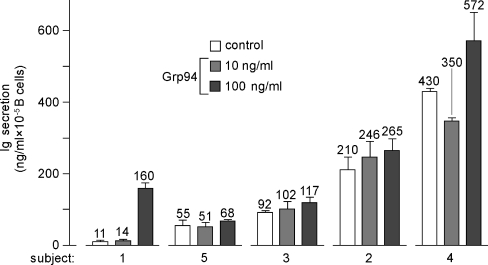
The characteristics of subjects analyzed in the study are summarized in Table 1. The basal Ig secretion after 15 days ranged from 11 to 430 ng/ml, as calculated on the individual number of B cells (Fig. 1). The Ig stimulation obtained with PWM was intense in any subject, thus proving that B cells of any subject were functionally responsive to the activator. Ig concentration following PWM ranged from 138 ng/ml (subject 5) to 6,394 ng/ml (subject 3), showing an inter-individual variability lesser than that commonly reported in the literature in the functional test of activation of B cells with this mitogen (Hauser et al. 1984). Grp94 at 10 ng/ml yielded only slight or no stimulations at all (subjects 4 and 5) of the Ig secretion whereas at 100 ng/ml stimulations ranged from 24% to 33% (mean ± SD, 27.5% ± 3.35; subjects 2–5), reaching the peak of 1,454% in subject 1 (Fig. 1). The entity of stimulation did not appear to depend on either the plasma concentration of B cells or the basal number of PBMCs.
Table 1.
Characteristics of subjects analyzed in the study
| Subject | Sex | Age (year) | Basal value of | |
|---|---|---|---|---|
| CD19+ (%) | PBMCs (nr. × 106/ml) | |||
| 1 | M | 52 | 7.7 | 3.4 |
| 2 | M | 25 | 11.7 | 1.0 |
| 3 | F | 34 | 3.8 | 2.0 |
| 4 | M | 62 | 3.2 | 2.0 |
| 5 | F | 56 | 7.7 | 1.6 |
The percentage of CD19+ is calculated on the PBMC value. The number of PBMCs is per milliliter of whole blood
Fig. 1.
Basal and Grp94-dependent Ig secretion from PBMCs of normal subjects. Igs were measured in the supernatant of PBMCs after 15 days incubation, as specified in the “Materials and methods”. Values of the Ig concentration in both absence (control) and presence of Grp94 (10 and 100 ng/ml) are indicated above histograms in each subject and represent the mean (±SD) of measurements made in duplicate
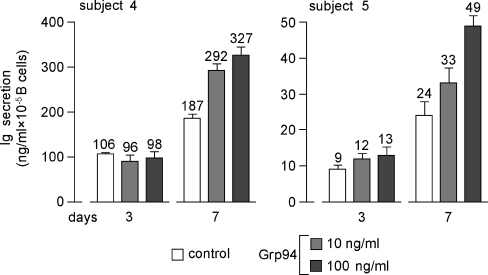
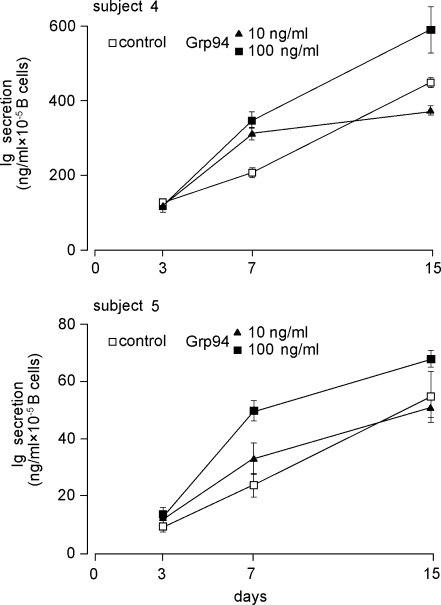
Considering the inconstant stimulatory effect of Grp94 at 10 ng/ml, it was of interest to see whether effects of Grp94 varied in time, a reason for which Ig secretion was also measured at 3 and 7 days. As shown in Fig. 2, where subjects 4 and 5 are chosen as representative of others, maximal percent stimulation by Grp94 occurred at 7 days, thereafter decreasing. In particular, the stimulations of 33% and 24% observed at 15 days with 100 ng/ml Grp94 in subjects 4 and 5, respectively, (Fig. 1) reached 75% and 104% at 7 days (Fig. 2). By plotting values of the Ig concentration against the incubation time, it was apparent that the pattern of Ig secretion, in both absence and presence of Grp94, was strikingly similar in any subject (Fig. 3, for subjects 4 and 5, and data not shown for others), and that the linear trend of basal Ig secretion became exponential in the presence of Grp94. Time-course experiments of Ig secretion allowed us to explain the unexpected reduction in Ig secretion noted with 10 ng/ml Grp94 in subjects 4 and 5 at 15 days (Fig. 1). Indeed, at 7 days, 10 ng/ml Grp94 still stimulated the Ig secretion (by 56% and 38%, respectively, in subjects 4 and 5), but thereafter the stimulatory effect ceased so that the curve reached the plateau, remaining below that of the control (Fig. 3). These results are consistent with an early and intense acceleration of B cell maturation by Grp94, an effect responsible for the highest percent stimulation of Ig secretion observed at 7 days.
Fig. 2.
Time-dependent Ig secretion from PBMCs in absence and presence of Grp94. Igs were measured in the supernatant of PBMCs, incubated without (control) and with Grp94 (10 and 100 ng/ml), for 3 and 7 days, as specified in the “Materials and methods”. Values of the Ig concentration above each histogram are the mean (±SD) of measurements made in duplicate
Fig. 3.
Pattern of Ig secretion in time, in absence and presence of Grp94. Patterns of Ig secretion in absence (control) and presence of 10 and 100 ng/ml Grp94, in the same subjects whose values of Ig concentrations are specified in both Figs. 1 and 2. The time-course curve shows the different behavior of the basal compared with Grp94-dependent secretion of Igs. Each point corresponds to the mean (±SD) of Ig concentration expressed as the height of histograms in both Figs. 1 and 2
Grp94 induces the proliferation of PBMCs
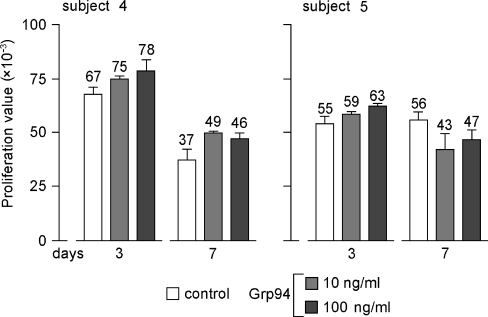
To verify whether, and to what extent, the increase in Ig secretion was dependent on an active PBMC proliferation, cell growth was measured at 3 and 7 days. Longer incubation times were not considered, since it was observed that cell viability decreased significantly. Indeed, at 15 days the mean (±SD) survival of control PBMCs was 28% (±2.9) in the five subjects. A Grp94-dependent cell growth stimulation was observed at 3 days (increases of 16% and 15%, with 100 ng/ml Grp94, for subjects 4 and 5, respectively) whereas at 7 days stimulation persisted only in subject 4 (32% and 24% with 10 and 100 ng/ml Grp94, respectively) (Fig. 4). Similar percent stimulation of cell proliferation at 3 days, followed by reduction at 7 days were also observed in other subjects (data not shown), indicating that cell proliferation and Ig secretion had distinct kinetics, the latter process peaking when the former already started to decline.
Fig. 4.
Time-dependent PBMC proliferation in absence and presence of Grp94. The measurement of cell proliferation in absence (control) and presence of Grp94 (10 and 100 ng/ml) was made by means of MTT test, as detailed in the Materials and methods. Proliferation value (on the ordinate) is the difference between the wavelength at 570 and 630 nm. Values above histograms in each subject are the mean (±SD) of measurements made in quadruplicate. The subjects 4 and 5 are shown as representative of others
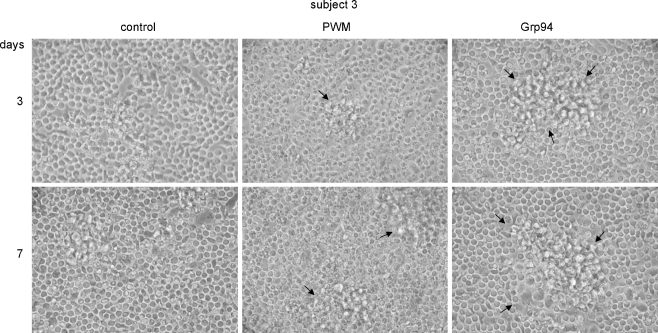
The microscopic analysis of PBMC morphology supported results of cell proliferation induced by Grp94 treatment, showing pictures characterized by the appearance of big agglomerates of larger cells suggesting the activation of paracrine signaling mechanisms (Fig. 5). In particular, whereas in control PBMCs the mean (±SD) value of the agglomerate area was 1.6% (±0.19) and 2.75% (±0.04) at 3 and 7 days, respectively, it reached 22.3% (±0.08) and 24.0% (±0.32) with Grp94 10 ng/ml at 3 and 7 days, respectively. Interestingly also, agglomerates of PBMCs in the presence of Grp94 were much larger, although less numerous, than those induced by PWM (Fig. 5) so that the overall area occupied by formers was higher than that measured with the latter at any incubation time.
Fig. 5.
Morphological changes of PBMCs in the presence of Grp94. PBMCs were cultured as specified in the “Materials and methods” and at the indicated incubation times morphology was analyzed at the optical microscopy. Representative pictures of many others made on the same well for subjects 3 (as representative of others) are shown in each panel, in the absence (control) and presence of Grp94 (10 ng/ml) and PWM. Big aggregates of large and adherent cells (arrows) characterize PBMCs in the presence of Grp94. More numerous aggregates of similar size were also observed with 100 ng/ml Grp94 (data not shown)
Effects of Grp94 on PBMCs are mediated by activation of the ERK1/2 pathway
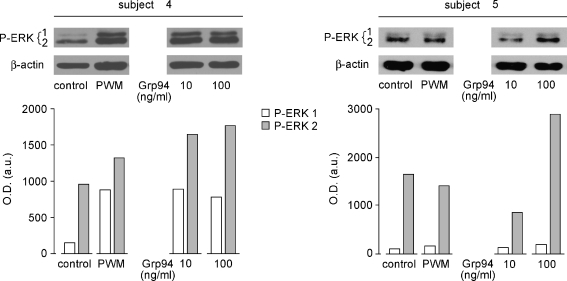
Since Grp94 promotes the proliferation and differentiation of cells of the innate immunity by stimulating the ERK1/2 phosphorylation—an effect that leads to the downstream activation of cytokine-mediated immune responses (Reed et al. 2003; Tramentozzi et al. 2008)—it was reasonable to hypothesize that the Grp94-mediated proliferation of PBMCs could also be sustained by the activation of the MEK-ERK1/2 pathway. The expression of P-ERK1/2 was measured in cell lysates after incubation at 1.5, 3, and 7 days in absence of FBS. In both control and Grp94-treated PBMCs, a faint signal was visible at 1.5 days (data not shown) that was significantly increased in a dose-dependent manner by Grp94 at 3 days, more evident for the P-ERK2 isoform (Fig. 6, subjects 4 and 5, representative of others). Any signal almost completely disappeared at 7 days (data not shown). It is worth noting that the stimulation induced by Grp94 (100 ng/ml) (especially for the ERK2 isoform) was even higher than that caused by PWM.
Fig. 6.
Grp94-dependent stimulation of P-ERK1/2 in PBMCs. PBMCs were cultured in absence of FBS for 1.5 and 3 days and, after removal of the supernatant, cells were lysed, as specified in the “Materials and methods”, and an equal volume of lysates loaded in the gel (10% polyacrylamide) for SDS-PAGE and Western blotting with anti-P-ERK1/2 polyclonal Abs. The same membranes were then stripped and probed with anti-β actin Abs for normalization of the protein content. Immunoblotting at 3 days only is presented since no signal was detected at 1.5 and 7 days. Graphs represent the intensity of P-ERK1- and P-ERK2-positive bands measured by densitometric analysis (ImageJ 1.36b software, NIH, USA) and expressed as arbitrary units of optical density (in the ordinate). A representative immunoblotting of two separate experiments is presented for each subject
Basal and Grp94-induced expression of HSPs in PBMCs
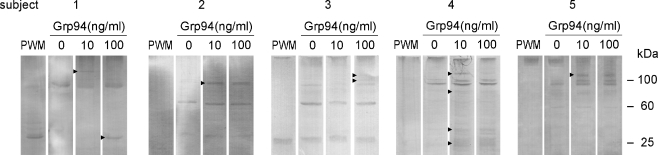
Since the activation of human lymphocytes by mitogenic stimuli is known to induce an increased expression of the two major HSPs, HSP90, and HSP70 (Ferris et al. 1988), that can also be secreted extracellularly and act as paracrine agents in sustaining cell proliferation and differentiation (Aligue et al. 1994; Liang and MacRae 1997), we sought whether the same occurred in PBMCs challenged with Grp94. The expression of HSP90 and HSP70 was thus measured in cell lysates after incubation of PBMCs at 3, 7, and 15 days. Whereas the pattern of the HSP70 expression did not change significantly following the Grp94 treatment in any subject and at any incubation time (data not shown), HSP90 was significantly modified by Grp94, being scarcely expressed at 3 days, becoming more evident at 7 days (data not shown), and reaching its maximum at 15 days (Fig. 7). It was noted that besides being present at its expected mass at about 100 kDa, HSP90 also focused at both lower (70 and 30–40 kDa) and higher masses (above 100 kDa), in both control and Grp94-treated PBMC lysates. This is likely explained by the fact that HSP90 is the chaperone for numerous and diverse cellular client proteins (McLaughlin et al. 2002) with which forms big complexes. Under denaturing conditions of electrophoresis these complexes undergo variable disaggregation, originating HSP90-positive fragments that focus in bands at different molecular weight. However, despite the expected variability of the HSP90 bands in control cell lysates, it was apparent that in any subject Grp94 induced the expression of different HSP90 bands (although to a variable extent from subject to subject), consistent with the activation of processes regulating cell growth and differentiation.
Fig. 7.
Basal and Grp94-dependent expression of HSP90 in PBMCs of all subjects. PBMCs were cultured in the presence of FBS, with and without 10 and 100 ng/ml Grp94, as specified in the “Materials and methods”, and after 15 days incubation, supernatant was removed and cells lysed in the lysis buffer. The same quantity of proteins was loaded in each lane for SDS-PAGE followed by Western blotting with specific primary Abs (see the “Materials and methods”). Arrowheads on the left of lanes mark the bands that differ from the control due to the treatment. On right are molecular masses in kDa
Basal and Grp94-induced Ig secretion is inversely correlated with intra-cellular IgG
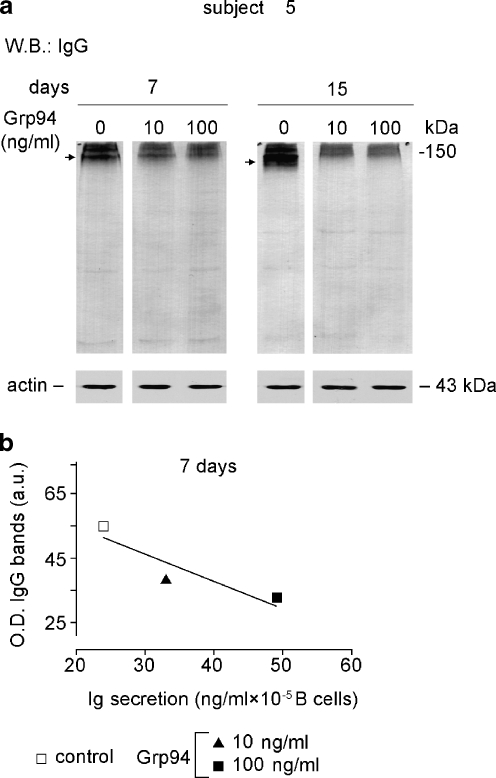
In order to shed further light on the mechanisms by which Grp94 stimulated the Ig secretion, whether due to an enhanced intra-cellular Ig production or an increased rate of Ig secretion, we measured the expression of IgG in PBMC lysates of subject 5 as representative of others. The measurement of the cumulative optical density of IgG-positive bands revealed that the IgG content in control PBMCs increased in time (higher at 15 than at 7 days) whereas in Grp94-treated PBMCs the opposite was observed (Fig. 8a), so that the pattern of intra-cellular IgG content mirrored the increase in the rate of Ig secretion by Grp94. In addition, the intense staining at 150 kDa demonstrated that IgG were mostly present in their assembled form (Fig. 8a, arrow), being thus ready for secretion (Melnick et al. 1994).
Fig. 8.
Time- and Grp94-dependent changes in the IgG content of PBMCs. The IgG expression in PBMC lysates was measured at the indicated time intervals in subjects 5, as specified in the “Materials and methods”. a Western blotting with anti-human IgG (H&L) polyclonal Abs following SDS-PAGE (10% acrylamide gel) in which samples were loaded in non-reducing conditions. Arrows on the left mark bands that show differences in lanes on right. Blotting of β-actin made on the same samples is reported below as control for protein loading. b Cumulative measurement of the intensity of IgG bands in each lane was performed with densitometric analysis and, at each incubation time, values of cumulative optical density (O.D.) plotted against the corresponding concentrations of secreted Igs. The linear regression analysis was applied for establishing the goodness of fit among points and deviation of the curve from zero (GraphPad Prism 3). The correlation coefficient (r2) was 0.82
By plotting the optical density of IgG bands for each condition (both control and Grp94-treated PBMCs) against the concentration of Ig secreted at any incubation time, it was apparent that the Ig secretion correlated negatively with the IgG content, this relationship reaching significance at 7 days (Fig. 8b).
Discussion
A number of studies have demonstrated the property of Grp94 to activate receptors of the innate immunity (Baker-LePain et al. 2002; Vabulas et al. 2002) and to induce secretion of cytokines from APCs (Reed et al. 2003) and T lymphocytes (Li et al. 2002; Srivastava et al. 1986), thus affecting both innate and adaptive immune responses (Li et al. 2002). Only scarce and indirect evidences instead exist about the role played by Grp94 in modulating B cells response and Ab secretion. The aim of this work was thus to investigate this issue with a particular attention to the effect of Grp94 on Ig secretion from human PBMCs, as this might be of physiopathological relevance for any therapeutic application of Grp94 in vivo.
Our results show that Grp94 is able to stimulate the Ig secretion in a concentration and time-dependent manner. Although basal Ig secretion varied from subject to subject, due to expected inter-individual differences in the functioning and reactivity of the immune system, it is worth noting that in four subjects out of five, overlapping percent stimulations were observed with Grp94 at 100 ng/ml (Fig. 1). In the correlation analysis, the entity of stimulations did not show any significant relationship with the basal value of Ig in all five subjects. However, the finding that the most intense stimulation of Ig secretion (1,454%) occurred in the subject with the lowest basal Ig concentration (11 ng/ml), suggests that the entity of stimulation might depend on basal humoral response, especially when this is exceptionally low.
By investigating the pattern of Ig secretion and cell proliferation in time, both in absence and presence of Grp94, it was possible to shed light on the mechanisms underlying effects of Grp94 on PBMCs. Grp94 caused a dose-dependent stimulation of cell growth that peaked at 3 days whereas maximal percent stimulation of Ig secretion occurred at 7 days. Thus, the processes of cell growth and Ig secretion from B cells appeared to follow distinct kinetics, the activation of mechanisms regulating the cell growth preceding those of secretion. Moreover, in the presence of Grp94, Ig secretion followed an exponential pattern, consistent with being the rate of secretion higher at shorter times, although the Ig concentration was maximal at 15 days due to time-dependent Ig accumulation in the media. This pattern differed from the linear trend of Ig secretion in control PBMCs (Fig. 3), suggesting the involvement of specific (receptor-mediated?) mechanisms. The additional result showing saturation of the stimulatory effect at 15 days with 10 ng/ml Grp94, with an apparent inhibitory effect on the Ig secretion (Fig. 3) might be explained by considering that distinct targets on the membrane of immune cells are affected by Grp94 at different concentrations. Thus, whereas at 10 ng/ml, occupancy and activation of receptors with a higher affinity for Grp94 might occur, at 100 ng/ml additional, less specific sites are likely involved (possibly also on different cells among those forming the PBMC pool), that concur in sustaining and enhancing the stimulatory effect of Grp94. This possibility is supported by the evidence of a higher percent stimulation of cell growth at 100 ng/ml than at 10 ng/ml of Grp94, demonstrating the recruitment of a larger number of cells accompanying the most intense stimulation of Ig secretion. Since the number of CD19+ B cells dropped in time by a similar value in both control and Grp94-treated PBMCs (Table 2), as expected due to the B cell differentiation into Ig-secreting plasma cells, it is reasonable to suppose that the effect of Grp94 on Ig secretion does not affect B cells directly, rather, it is a T cell-mediated effect that induces a more rapid maturation and differentiation of B cells.
Table 2.
Values (%) of CD19+ cells after incubation of PBMCs at 3, 7, and 15 days
| Incubation (days) | CD19+ (%) | ||
|---|---|---|---|
| Control | Grp94 (ng/ml) | ||
| 10 | 100 | ||
| 3 | 3.0 | 3.1 | 3.1 |
| 7 | 2.7 | 2.8 | 2.7 |
| 15 | 1.7 | 2.1 | 1.8 |
Data are referred to subject 4 as representative of others. Similar % decreases in CD19+ cells after incubation were observed in other subjects. The percent of CD19+ cells was calculated by means of cytofluorimetric determination made on an aliquot of PBMCs (4 × 106 cells in 2-ml wells) at the indicated times
Our results support the specificity of mechanisms involved in mediating the effects of Grp94 on PBMCs: besides the very low Grp94 concentrations at which effects are measured, the finding that Grp94 activated in a dose-dependent manner the MEK-ERK1/2 pathway in PBMCs (Fig. 6) proves that the cell signaling targeted by Grp94 is specific and adds further evidence to previous observations showing that Grp94 is able to regulate the proliferation and differentiation of cells of the innate and adaptive immunity by a fine, cytokine-like mechanism (Reed et al. 2003; Tramentozzi et al. 2008).
It is known that Grp94 and other HSPs not only stimulate the secretion of cytokines from T cells (Suto and Srivastava 1995), but also the reciprocal is true, i.e., cytokines are capable of inducing the expression of HSPs, in particular HSP90 and HSP70, in lymphocytes (Ferris et al. 1988; Stephanou et al. 1997). If effects of Grp94 on PBMCs were mediated by a cytokine-like mechanism, then, changes might be expected to occur in the expression of HSPs specifically involved in the process of proliferation and differentiation, such as HSP90 (Aligue et al. 1994; Liang and MacRae 1997; Shinozaki et al. 2006). We observed that at variance with HSP70, the expression of which did not change at different incubation times and following the Grp94 treatment, HSP90 expression was stimulated in time and even more by Grp94, being visible in various bands referred to HSP90 associated with different client proteins (Fig. 6). Increases in the expression of HSP90 have been demonstrated to occur in the presence of Grp94 in HUVECs, in which HSP90 plays a paracrine role in the Grp94-dependent cell growth stimulation and angiogenic differentiation (Tramentozzi et al. 2008). Considering that the stimulation of HSP90 expression by Grp94 appeared at 7 days and lasted up to 15 days, it is possible that also in PBMCs HSP90 is involved in mediating the effects of Grp94, by promoting the growth of T cells that in turn affect maturation and differentiation of B cells.
Although we did not explore the finest molecular mechanisms by which Grp94 treatment leads to Ig secretion, the measurement of intra-cellular content of IgG allowed us to exclude the involvement of mechanisms regulating the Ig production in the cell. Indeed, if this were the case, a positive relationship between the cellular content of IgG and the extracellular Ig concentration would be expected to occur in time. This actually occurred in control PBMCs in which the IgG content positively correlated with the Ig secretion (Fig. 8). Instead, the result of a close inverse relationship between cellular IgG content and extracellular Ig concentration in the presence of Grp94 (Fig. 8) rather supports the possibility that Grp94 stimulates the humoral response by increasing the rate of Ig secretion from the cell, consistent with a higher rate of differentiation of already existing immature B cells.
In conclusion, our work confirms and extends to cells of immunity, the cytokine-like property of Grp94 that is able to induce an increased Ab response in human PBMCs. This effect might be usefully exploited for therapeutic applications of Grp94 in pathologies in which the stimulation of the humoral response represents the main therapeutic target.
Acknowledgments
We would like to thank Dr. Alberto Marotti of the Servizio Immunotrasfusionale (Azienda Ospedaliera of Padua) for his helpful collaboration in collecting laboratory and clinical data of subjects participating in the study. This work was supported by MURST (Ministero dell’Università e della Ricerca Scientifica e Tecnologica) grants “ex-60%”.
Abbreviations
- Ab
Antibody
- APC
Antigen-presenting cell
- Grp94
Glucose-regulated protein94
- HSP
Heat shock protein
- HUVEC
Human umbilical vein endothelial cell
- MMP
Matrix-metallo protease
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PBMC
Peripheral blood mononuclear cell
- PWM
Pokeweed mitogen
- RPMI
Roswell Park Memorial Institute medium 1640
References
- Aligue R, Akhavan-Niak H, Russell P. A role for Hsp90 in cell cycle control: Weel tyrosine kinase activity requires interaction with Hsp90. EMBO J. 1994;13:6099–6106. doi: 10.1002/j.1460-2075.1994.tb06956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-LePain JC, Sarzotti M, Fields TA, Li CY, Nicchitta CV. GRP94 (gp96) and GRP94 N-terminal geldanamycin binding domain elicit tissue nonrestricted tumor suppression. J Exp Med. 2002;196:1447–1459. doi: 10.1084/jem.20020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-LePain JC, Sarzotti M, Nicchitta CV. Glucose-regulated protein 94/glycoprotein 96 elicits bystander activation of CD4+ T cell Th1 cytokine production in vivo. J Immunol. 2004;172:4195–4203. doi: 10.4049/jimmunol.172.7.4195. [DOI] [PubMed] [Google Scholar]
- Binder RJ, Anderson KM, Basu S, Srivastava PK. Cutting edge: heat shock protein gp96 induces maturation and migration of CD11c+ cells in vivo. J Immunol. 2000;165:6029–6035. doi: 10.4049/jimmunol.165.11.6029. [DOI] [PubMed] [Google Scholar]
- Breloer M, Fleischer B, Bonin A. In vivo and in vitro activation of T cells after administration of ag-negative heat shock proteins. J Immunol. 1999;162:3141–3147. [PubMed] [Google Scholar]
- Brunati AM, Contri A, Muenchbach M, James P, Marin O, Pinna LA. GRP94 (endoplasmin) co-purifies with and is phosphorylated by golgi apparatus casein kinase. FEBS Lett. 2000;471:151–155. doi: 10.1016/S0014-5793(00)01378-8. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Ferris DK, Harel-Bellan A, Morimoto RI, Welch WJ, Farrar WL. Mitogen and lymphokine stimulation of heat shock proteins in T lymphocytes. Proc Natl Acad Sci USA. 1988;85:3850–3854. doi: 10.1073/pnas.85.11.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser C, Wilhelm JA, Matter L, Schopfer K. Spontaneous and pokeweed mitogen-induced in vitro IgG production specific for S. aureus cell wall determinants in man. Clin Exp Immunol. 1984;57:227–233. [PMC free article] [PubMed] [Google Scholar]
- Heike M, Weinmann A, Bethke K, Galle PR. Stress protein/peptide complexes derived from autologous tumor tissue as tumor vaccines. Biochem Pharmacol. 1999;58:1381–1387. doi: 10.1016/S0006-2952(99)00178-1. [DOI] [PubMed] [Google Scholar]
- Indraccolo S, Zamarchi R, Veronese ML, Mazza MR, Mion M, Veronesi A, Panozzo M, Colombatti M, Barelli A, Rocchetto P. Standardization of in vitro synthesis and detection of HIV-1-specific antibodies. J Immunol Methods. 1993;157:105–115. doi: 10.1016/0022-1759(93)90076-J. [DOI] [PubMed] [Google Scholar]
- Li Z, Menoret A, Srivastava P. Roles of heat-shock proteins in antigen presentation and cross-presentation. Curr Opin Immunol. 2002;14:45–51. doi: 10.1016/S0952-7915(01)00297-7. [DOI] [PubMed] [Google Scholar]
- Liang P, MacRae TH. Molecular chaperones and the cytoskeleton. J Cell Sci. 1997;110(Pt 13):1431–1440. doi: 10.1242/jcs.110.13.1431. [DOI] [PubMed] [Google Scholar]
- Liu B, Li Z. Endoplasmic reticulum HSP90b1 (gp96, grp94) optimizes B-cell function via chaperoning integrin and TLR but not immunoglobulin. Blood. 2008;112:1223–1230. doi: 10.1182/blood-2008-03-143107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin SH, Smith HW, Jackson SE. Stimulation of the weak ATPase activity of human hsp90 by a client protein. J Mol Biol. 2002;315:787–798. doi: 10.1006/jmbi.2001.5245. [DOI] [PubMed] [Google Scholar]
- Melnick J, Dul JL, Argon Y. Sequential interaction of the chaperones BiP and GRP94 with immunoglobulin chains in the endoplasmic reticulum. Nature. 1994;370:373–375. doi: 10.1038/370373a0. [DOI] [PubMed] [Google Scholar]
- More S, Breloer M, Fleischer B, Bonin A. Activation of cytotoxic T cells in vitro by recombinant gp96 fusion proteins irrespective of the ‘fused’ antigenic peptide sequence. Immunol Lett. 1999;69:275–282. doi: 10.1016/S0165-2478(99)00100-5. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Navaratnam M, Deshpande MS, Hariharan MJ, Zatechka DS, Jr, Srikumaran S. Heat shock protein-peptide complexes elicit cytotoxic T-lymphocyte and antibody responses specific for bovine herpesvirus 1. Vaccine. 2001;19:1425–1434. doi: 10.1016/S0264-410X(00)00381-9. [DOI] [PubMed] [Google Scholar]
- Nicchitta CV. Re-evaluating the role of heat-shock protein-peptide interactions in tumour immunity. Nat Rev Immunol. 2003;3:427–432. doi: 10.1038/nri1089. [DOI] [PubMed] [Google Scholar]
- Reed RC, Berwin B, Baker JP, Nicchitta CV. GRP94/gp96 elicits ERK activation in murine macrophages. A role for endotoxin contamination in NF-kappa B activation and nitric oxide production. J Biol Chem. 2003;278:31853–31860. doi: 10.1074/jbc.M305480200. [DOI] [PubMed] [Google Scholar]
- Shinozaki F, Minami M, Chiba T, Suzuki M, Yoshimatsu K, Ichikawa Y, Terasawa K, Emori Y, Matsumoto K, Kurosaki T, Nakai A, Tanaka K, Minami Y. Depletion of hsp90beta induces multiple defects in B cell receptor signaling. J Biol Chem. 2006;281:16361–16369. doi: 10.1074/jbc.M600891200. [DOI] [PubMed] [Google Scholar]
- Singh-Jasuja H, Scherer HU, Hilf N, Arnold-Schild D, Rammensee HG, Toes RE, Schild H. The heat shock protein gp96 induces maturation of dendritic cells and down-regulation of its receptor. Eur J Immunol. 2000;30:2211–2215. doi: 10.1002/1521-4141(2000)30:8<2211::AID-IMMU2211>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Srivastava PK, DeLeo AB, Old LJ. Tumor rejection antigens of chemically induced sarcomas of inbred mice. Proc Natl Acad Sci USA. 1986;83:3407–3411. doi: 10.1073/pnas.83.10.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephanou A, Amin V, Isenberg DA, Akira S, Kishimoto T, Latchman DS. Interleukin 6 activates heat-shock protein 90 beta gene expression. Biochem J. 1997;321(Pt 1):103–106. doi: 10.1042/bj3210103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117–120. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- Tramentozzi E, Montopoli M, Orso G, Pagetta A, Caparrotta L, Frasson M, Brunati AM, Finotti P. Stable complexes formed by Grp94 with human IgG promoting angiogenic differentiation of HUVECs by a cytokine-like mechanism. Mol Immunol. 2008;45:3639–3648. doi: 10.1016/j.molimm.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Braedel S, Hilf N, Singh-Jasuja H, Herter S, Ahmad-Nejad P, Kirschning CJ, Costa C, Rammensee HG, Wagner H, Schild H. The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the toll-like receptor 2/4 pathway. J Biol Chem. 2002;277:20847–20853. doi: 10.1074/jbc.M200425200. [DOI] [PubMed] [Google Scholar]