Abstract
It has been reported that pretreatment of rats with lipopolysaccharide (LPS) increases myocardial functional recovery in ischemia/reperfusion (I/R) hearts. However, the mechanisms by which LPS induces cardioprotection against I/R injury have not been fully elucidated. In this study, we pretreated rats with LPS (1.0 mg/kg) 24 h before they were subjected to I/R injury, and then examined the roles of heat shock protein-70 (HSP70) and nucleus factor-κB (NF-κB) in LPS-induced cardioprotection. We observed that pretreatment with low-dose LPS resulted in significantly increased levels of HSP70 in the myocardium, which could dramatically inhibit NF-κB translocation and reduce degradation of inhibitory κB. Inhibition of NF-κB, in turn, attenuated release of inflammatory cytokines (tumor necrosis factor-α, interleukin (IL)-1β, and IL-6) and reduced apoptosis of myocardium and infarct area following I/R injury. Moreover, HSP70 could ameliorate oxidative stress following I/R injury. To further investigate whether increase of HSP70 might be responsible for protection of the myocardium against I/R injury, we co-administered the HSP70 inhibitor, quercetin, with LPS before I/R injury. We found that LPS-induced cardioprotection was attenuated by co-administration with quercetin. Herein, we concluded that increased levels of HSP70 through LPS pretreatment led to inhibition of NF-κB activity in the myocardium after I/R injury. Our results indicated that LPS-induced cardioprotection was mediated partly through inhibition of NF-κB via increase of HSP70, and LPS pretreatment could provide a means of reducing myocardial I/R injury.
Keywords: Lipopolysaccharide, Heat shock protein 70, NF-κB, Ischemia/reperfusion injury
Introduction
Reperfusion after a period of ischemia has deleterious effects on the myocardium, ranging from contractile impairment to actual necrosis. A substantial amount of evidence supports the idea that ischemia/reperfusion (I/R)-induced injury to the heart is due to the release of reactive oxygen species (ROS; Pchejetski et al. 2007; Oshima et al. 2005). As an intracellular target of ROS, nucleus factor-κB (NF-κB) is sequestered in the cytoplasm in an inactive state due to its association with a class of inhibitory proteins termed inhibitory κB (IκB). Ischemia/reperfusion injury causes a rapid degradation of IκBα. Then, NF-κB translocates into the nucleus and activates κB containing genes such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6; Cepinskas et al. 2002; Li et al. 1999). These locally overexpressed myocardial cytokines may play a critical role in the progression of myocardial dysfunction, including myocardial remodeling, cardiac hypertrophy, and heart failure (Deten et al. 2002). Herein, NF-κB plays a pivotal role in I/R injury, and inhibition of NF-κB can protect myocardium from I/R injury.
Lipopolysaccharide (LPS), the antigenic component of the gram-negative bacterial cell wall, is known as the exogenous ligand of Toll-like receptor-4 (Chow et al. 1999). Combination of LPS and its receptor leads to the activation of MyD88-dependent signal transduction pathway and nuclear translocation of NF-κB. The dysregulation of NF-κB may lead to the excessive production of pro-inflammatory mediators, resulting in myocardium damage, heart failure, and even death (Nemoto et al. 2002). Excessive stimulation of cardiac cells by LPS results in necrosis and apoptosis of myocardium in gram-negative septic shock. Interestingly, it has been reported that pretreatment of rats with low-dose LPS increases myocardial functional recovery in ischemia/reperfusion hearts (Brown et al. 1989; Song et al. 1996; Ha et al. 2008). Our previous study also has shown that LPS could protect mesenchymal stem cells (MSCs) against oxidative stress-induced apoptosis, and LPS pretreatment enhances the efficacy of MSCs transplantation in a rat model of acute myocardial infarction (Wang et al. 2009a, b; Yao et al. 2009). However, the mechanisms by which LPS induce cardioprotection against I/R injury have not been fully elucidated.
Heat shock proteins (HSPs) are highly conserved cellular stress proteins which are present in every organism from bacteria to mammalian animals. Many studies have shown the importance of HSPs for the survival of cells under stress conditions (Bao and Liu 2008; Shinohara et al. 2007). HSP70, as molecular chaperon, could respond to a wide variety of stress, such as heat shock, ischemia, and inflammation (Zhang et al. 2009). Overexpression of HSP70 could inhibit the translocation of NF-κB, attenuate the release of inflammatory factors, and reduce the apoptosis of myocardium (Dokladny et al. 2010). In the present study, we examined the role of HSP70 and NF-κB in LPS-induced cardioprotection. We observed that pretreatment with low-dose LPS resulted in significantly increased levels of HSP70 in the myocardium, which could dramatically inhibit NF-κB translocation and reduce release of inflammatory cytokines in the following I/R injury. LPS-induced cardioprotection was attenuated by co-administration with a pharmacological inhibitor of HSP70. Our results indicated that LPS-induced cardioprotection was mediated partly through inhibition of NF-κB via increase of HSP70.
Materials and methods
Animal preparation
Adult male Wistar rats (wild type, 210–250 g) were provided by Slac Company (Shanghai, China). The procedure was performed in accordance with the “Guide for the Care and Use of Laboratory Animals” (NIH Publication No. 85-23, National Academy Press, Washington, DC, revised 1996). The study protocol was approved by the Animal Care and Use Committee of Jiangsu University.
Experimental protocols
Rats in the LPS pretreatment group (LPS + I/R group, n = 12) were pretreated with LPS (1.0 mg/kg) by intraperitoneal injection. To examine the role of HSP70 in the LPS-induced cardioprotection, another group of rats (Q-LPS + I/R group, n = 12) was intraperitoneally injected with quercetin (100 mg/kg), inhibitor of HSP70, 2 h before LPS administration. The third group of rats (I/R group, n = 12) was given the same volume of normal saline. Twenty-four hours later, three groups of rats were anesthetized with ketamine (100 mg/kg) by intraperitoneal injection and mechanically ventilated. Under a sterile condition, the heart was exposed through a left thoracotomy, and the left anterior descending coronary artery was ligated with silk suture (7.0) 2 mm to its origin with a slipknot. After completion of the 30 min of occlusion, the coronary artery was reperfused by releasing the knot for 3 h. Control group (n = 12) was intraperitoneally injected with the same volume of normal saline and given a sham surgical operation. After that, rats were sacrificed, and blood and hearts were collected for the following experiments.
TUNEL assay
To detect apoptotic cardiomyocytes in the heart, the LV myocardium (n = 5, each) was fixed in 4% paraformaldehyde, cut transversely, and embedded in paraffin. Apoptotic cardiomyocytes were evaluated by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using an in situ Cell Death Detection Kit (Roche, Germany) according to the manufacturer’s instructions. Apoptotic cells were identified by brown color in their nuclei. Tissue sections were examined microscopically, and the percentage of apoptotic cells per total cells was determined in eight randomly chosen fields.
Determination of myocardial infarct size
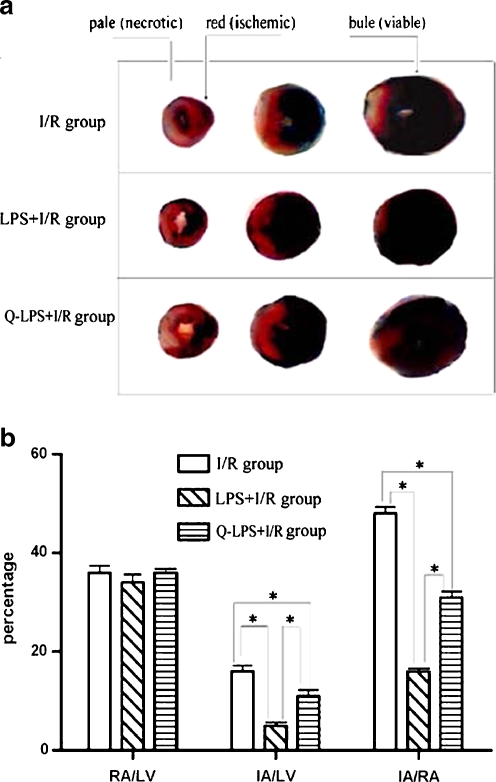
To assess the ischemic area at risk, 1% Evans blue was infused into the aorta and coronary arteries in retrograde fashion. Hearts were excised and sliced into five 1-mm cross sections. The heart sections were incubated with 1% triphenyltetrazolium chloride (TTC) solution (Sigma-Aldrich) at 37°C for 15 min. Ischemic myocardium, which was still viable, was stained red with TTC, whereas the necrotic myocardium was not stained and appeared pale white. The infarct area (white) and the area at risk (red and white) from each section were measured using NIH Image and Spot software. Ratios of risk area vs. left ventricle (RA/LV) and infarct area vs. risk area (IA/RA) were calculated and expressed as a percentage.
Serum concentrations of TNF-α, IL-1β, and IL-6
Blood was collected before and after ischemia/reperfusion injury, and serum was prepared. Concentration of TNF-α, IL-1β, and IL-6 in serum was determined by ELISA kits (R&D Systems) according to the instruction of the manufacturer. Experiments were repeated three times for verification of results.
Electrophoretic mobility shift assay
Heart nuclear extracts for electrophoretic mobility shift assay were prepared using nuclear-cytosol extraction kit (Applygen Technologies Inc.). Double-stranded NF-κB consensus oligonucleotide probe (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) was end-labeled with biotin. Electrophoretic mobility shift assay kit (Pierce) was used to perform the reaction. Binding reactions (20 μl in total) consisted of 35 fmol of biotin-labeled DNA, 10 μg of nuclear protein, 2.5% glycerol, 5 mM MgCl2, and 50 ng/μl poly(dI–dC) and incubated at room temperature for 20 min. DNA protein complexes were subjected to non-denaturing 4% polyacrylamide gel in low ionic strength buffer (45 mM Tris-borate, 1 mM EDTA) at 75 mV/8 mA for approximately 3 h. Gels were vacuum-dried and exposed to X-ray film at −70°C. The quantification of the radioactive intensity of autoradiographic signals was performed by using an enhanced chemiluminescence (Amersham, USA).
Western blot analysis of IκBα, caspase-3, and HSPs
Cytoplasmic protein was prepared from heart tissue, and immunoblot was performed as described previously (Hu et al. 2008). Protein was analyzed using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Bio-Rad). Membranes were then incubated with primary antibodies including IκBα (1:1,000, Cell Signaling); cleaved caspase-3 (1:1,000, Cell Signaling); HSP27, HSP60, HSP70, and HSP90 (1:1,000, Cell Signaling); and β-actin (1:5,000, Sigma) at 4°C overnight, respectively. The membranes were then incubated with peroxidase-labeled secondary antibody (1:1,000, Santa Cruz, USA) at 37°C for 2 h. Signals were detected by enhanced chemiluminescence (Amersham, USA). Densitometric analysis for the blots was performed with NIH image software. Experiments were repeated six times for verification of results.
Oxidative stress assay
After I/R, the hearts were collected to detect homogenate lipid peroxides by measuring malondialdehyde (MDA) concentration with MDA ELISA kit (Uscn Life Science Inc.). Antioxidant enzymes were measured by Superoxide Dismutase (SOD) Assay Kit (sigma) according to the illustration of the manufacturer.
Statistical analysis
Data are expressed as mean ± SEM and analyzed with SPSS11.0 statistical software. Comparisons of data between groups were made using one-way analysis of variance with Bonferroni’s post-test. Statistical significance was set at <0.05 level.
Result
LPS pretreatment attenuates myocardial apoptosis following ischemia/reperfusion injury
In order to study myocardial apoptosis following I/R injury and anti-apoptotic effect of LPS pretreatment, TUNEL assay was performed. TUNEL-positive nuclei staining (brown color in their nuclei) was detectable in both control and experimental groups (Fig. 1a). As shown in Fig. 1b, cardiac myocyte apoptosis was greatly increased in I/R group compared with that in control group (28.6 ± 2.2% vs. 2.3 ± 0.4%, P < 0.01). LPS pretreatment significantly decreased the number of TUNEL-positive cells in LPS + I/R group compared with that in I/R group (20.0 ± 2.6% vs. 28.6 ± 2.2%, P < 0.01). To investigate the role of HSP70 in the LPS pretreatment, quercetin, the inhibitor of HSP70, was used in the experiment. We could see that quercetin partly canceled the anti-apoptotic effect of LPS in Q-LPS + I/R group (25.5 ± 1.5%).
Fig. 1.
LPS pretreatment attenuates myocardial apoptosis following I/R injury. a Representative photomicrographs of TUNEL-positive nuclei staining (brown) in ischemic cardiac muscle cells (×400). b Cardiac myocyte apoptosis was increased after ischemic/reperfusion injury. LPS pretreatment significantly decreased the number of TUNEL-positive cells in the heart following I/R injury. Quercetin partly canceled the anti-apoptotic effect of LPS (n = 5 in each group and eight randomly chosen fields from each tissue section were calculated). c Caspase-3 activity by Western blot analysis with specific anti-cleaved caspase-3 antibody (n = 6 in each group). Corresponding β-actin blots are shown as a control for sample loading. d The protein levels for each sample were determined as a ratio to their corresponding β-actin levels. LPS pretreatment significantly decreased the level of cleaved caspase-3 in the heart following I/R injury. However, quercetin attenuated the anti-apoptotic effect of LPS pretreatment. Data are mean ± SEM. *P < 0.01 compared with indicated groups
To further explore the anti-apoptotic effect of LPS pretreatment, caspase-3 cleavage and activity were measured by Western blot (Fig. 1c). The protein levels for each sample were determined as a ratio to their corresponding β-actin levels (Fig. 1d). Compared with control group, I/R injury induced great activation of pro-caspase-3 in I/R group (0.23 ± 0.02 vs. 2.10 ± 0.08, P < 0.01). LPS pretreatment dramatically inhibited the pro-caspase-3 cleavage in LPS + I/R group compared with that in I/R group (0.81 ± 0.04 vs. 2.10 ± 0.08, P < 0.01). However, inhibition of pro-caspase-3 cleavage was attenuated by quercetin administration in Q-LPS + I/R group (1.24 ± 0.03).
LPS pretreatment reduces myocardial infarct size following ischemia/reperfusion injury
As shown in Fig. 2, there was no significant difference in the risk area among the three experimental groups (RA/LV), averaging 36.3 ± 1.0% (I/R group), 34.5 ± 1.8% (LPS + I/R group), and 36.7 ± 0.6% (Q-LPS + I/R group; P > 0.05). I/R injury resulted in a significant myocardial injury which was indexed as infarct area/risk area (IA/RA, 48.5 ± 1.8%). However, infarct area in LPS + I/R group was significantly reduced compared with that in I/R group (15.8 ± 0.7 vs. 48.5 ± 1.8%, P < 0.01). To investigate whether increase of HSP70 may be responsible for myocardial protection from I/R injury, we administered the HSP70 inhibitor, quercetin, before I/R. Heart sections from Q-LPS + I/R group showed a larger necrotic infarct area than that in LPS + I/R group (IA/RA 31.7 ± 0.9 vs. 15.8 ± 0.7%, P < 0.01).
Fig. 2.
LPS pretreatment reduces myocardial infarct size following ischemia/reperfusion injury. a The hearts in three experimental groups were harvested and infarct size was determined by triphenyltetrazolium chloride (TTC) staining (n = 8 in each group). Viable myocardium stains blue with TTC. Ischemic myocardium, which is still viable, stains red with TTC. Necrotic or dead myocardium does not stain and appears pale white. b Bar graph of infarct size and risk area in the three groups. The infarct area (white) and the area at risk (red and white) from each section were measured using an imagine analyzer. Ratio of infarct area vs. risk area (IA/RA) and risk area vs. left ventricle area (RA/LV) were calculated and are presented in the graph. I/R group hearts developed large infarcts that represented 48.5 ± 1.8% of risk zone size. As compared with I/R group, LPS + I/R group and Q-LPS + I/R group had significantly smaller infarcts, representing 15.8 ± 0.7% and 31.7 ± 0.9% of risk zone, respectively. The risk area was similar among the three groups, averaging 36.3 ± 1.0% (I/R group), 34.5 ± 1.8% (LPS + I/R group), and 36.7 ± 0.6% (Q-LPS + I/R group). Data are mean ± SEM. *P < 0.01 compared with indicated groups
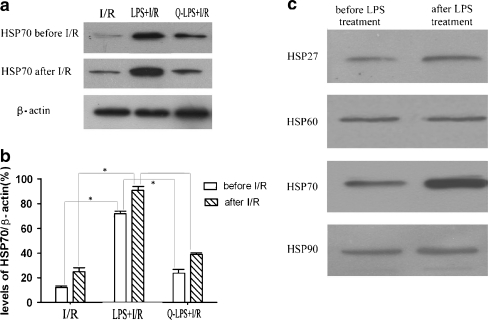
LPS pretreatment increases HSP70 levels in the myocardium before and after ischemia/reperfusion injury
Because HSPs are reported to protect heart against ischemia/reperfusion injury (Williamson et al. 2008), induction of HSP70 may be responsible for LPS-induced myocardial protection. To confirm our supposition, we investigated the levels of HSP70 in three experimental groups before and after I/R injury (Fig. 3a). As shown in Fig. 3b, before I/R injury, immunoblotting analysis of HSP70 showed that LPS pretreatment resulted in a higher content of HSP70 in LPS + I/R group than that in I/R group (72.3 ± 2.6% vs. 12.3 ± 1.9%, P < 0.01). However, level of HSP70 was inhibited significantly by its inhibitor, quercetin, in Q-LPS + I/R group (24.6 ± 2.9%). After I/R injury, levels of HSP70 were increased in every experimental groups. Interestingly, elevated HSP70 in LPS + I/R group was still more than that in I/R group (91.4 ± 2.8% vs. 25.3 ± 3.1%, P < 0.01). The protein levels for each sample were determined as a percentage to their corresponding β-actin levels.
Fig. 3.
Levels of HSP70 in three experimental groups before and after ischemia/reperfusion injury. a Representative immunoblots for HSP70 protein in hearts before and after I/R injury. β-actin was also examined as an internal control (n = 6 in each group). b Summary of densitometric analyses of the immunoblots of HSP70 protein in hearts. The protein levels for each sample were determined as a percentage to their corresponding β-actin levels. Before I/R injury, LPS pretreatment could induce the expression of HSP70 in myocardium, and quercetin administration decreased the level of HSP70 in Q-LPS + I/R group. After I/R injury, levels of HSP70 were increased in every experimental groups. However, level of HSP70 in LPS + I/R group was still more than that in I/R group. c To investigate the possible effects of other HSP molecules, we performed Western blot analysis of other HSPs, including HSP27, HSP60, and HSP90. In contrast to HSP70, HSP27, HSP60, and HSP90 showed a minimal increase after LPS pretreatment in heart. Data are means ± SEM. *P < 0.01 compared with indicated groups
To investigate the possible effects of other HSP molecules, we performed Western blot analysis of other HSPs, including HSP27, HSP60, and HSP90. In contrast to HSP70, HSP27, HSP60, and HSP90, showed a minimal increase after LPS pretreatment in heart (Fig. 3c).
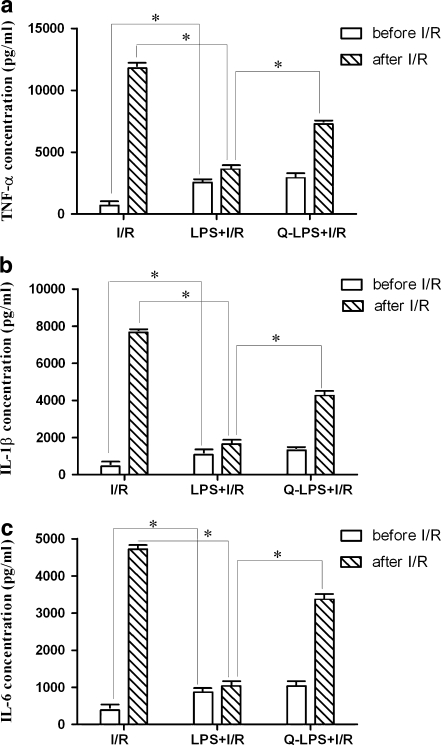
LPS pretreatment reduces expression of inflammatory factors following ischemia/reperfusion injury
I/R injury could induce release of inflammatory factors, such as TNF-α, IL-1β, and IL-6, which play an important role in the fibrosis of myocardium and heart failure (Deten et al. 2002). Reduction of inflammatory factors could protect hearts from I/R injury. Serum concentrations of TNF-α, IL-1β, and IL-6 were measured before and after I/R injury in three experimental groups. Before I/R injury, concentrations of inflammatory factors (TNF-α, IL-1β, IL-6) from LPS + I/R group had moderately increased compared with that in I/R group (TNF-α 2,556 ± 30 vs. 698 ± 12 pg/ml, IL-1β 1,077 ± 15 vs. 458 ± 8 pg/ml, IL-6 872 ± 13 vs. 384 ± 6 pg/ml, P < 0.01, respectively). However, after ischemia for 30 min and reperfusion for 3 h, serum from LPS + I/R group rats had decreased concentrations of inflammatory factors compared with serum from I/R group rats (TNF-α 3,643 ± 46 vs. 11,785 ± 146 pg/ml, IL-1β 1,643 ± 124 vs. 7,663 pg/ml, IL-6 1,038 ± 13 vs. 4,720 ± 53 pg/ml, P < 0.01, respectively). LPS pretreatment could induce the release of inflammatory factors moderately before I/R but reduce the release of inflammatory factors significantly in the following I/R injury. As an inhibitor of HSP70, quercetin incurred higher level of inflammatory factors in Q-LPS + I/R group than that in LPS + I/R group (TNF-α 7,267 ± 46 vs. 3,643 ± 46 pg/ml, IL-1β 4,274 ± 67 vs. 1,643 ± 124 pg/ml, IL-6 3,380 ± 35 vs. 1,038 ± 13 pg/ml, P < 0.01, respectively). These results indicated that HSP70 induced by LPS pretreatment was responsible for the reduction of inflammatory factors following I/R injury (Fig. 4).
Fig. 4.
LPS pretreatment reduces inflammatory factors following I/R injury. Serum concentrations of TNF-α, IL-1β, and IL-6 were measured before and after I/R injury in three experimental groups (n = 12 in each group). Before ischemia/reperfusion, concentrations of inflammatory factors (TNF-α, IL-1β, IL-6) from rats pretreated with LPS had moderately increased compared with untreated rats. However, after ischemia for 30 min and reperfusion for 3 h, serum from LPS + I/R group rats had decreased inflammatory factors concentrations dramatically compared with serum from I/R group rats. LPS pretreatment could induce the release of inflammatory factors moderately before I/R but reduce the release of inflammatory factors greatly in the following ischemia/reperfusion injury. As an inhibitor of HSP70, quercetin could incur higher level of inflammatory factors in Q-LPS + I/R group compared with that in LPS + I/R group. These results indicated that HSP70 induced by LPS pretreatment was responsible for the reduction of inflammatory factors following I/R injury. Data are means ± SEM. *P < 0.01 compared with indicated groups
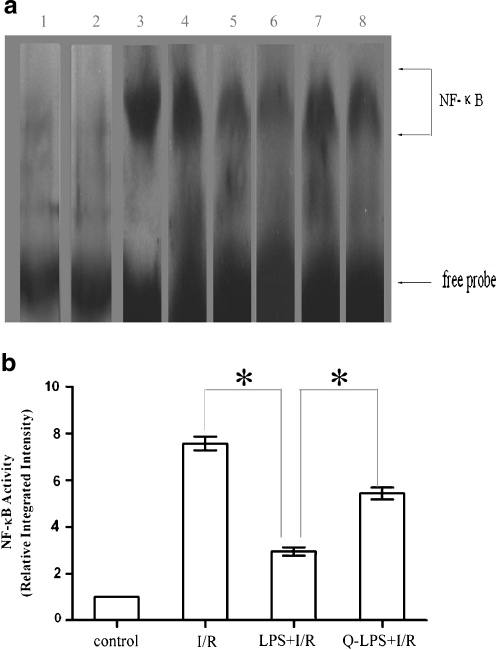
LPS pretreatment attenuates activation of NF-κB and degradation of IκBα following ischemia/reperfusion injury
NF-κB activation in the nuclear extracts of myocardium was determined by electrophoretic mobility shift assay (EMSA). As shown in Fig. 5a, NF-κB binding activity was present at very low levels in control group but significantly increased after the hearts were subjected to I/R injury. When the rats were given LPS pretreatment before I/R injury, NF-κB binding activity was attenuated. It has been reported that overexpressed HSP70 could inhibit the translocation of NF-κB (Senf et al. 2008). To confirm the role of HSP70 in LPS-induced cardioprotection, quercetin was administrated before LPS pretreatment in Q-LPS + I/R group. Quercetin could increase NF-κB binding activity in Q-LPS + I/R group compared with that in LPS + I/R group. The relative integrated intensity of NF-κB binding activity for each group was determined as a ratio to the control group (Fig. 5b).
Fig. 5.
LPS pretreatment attenuates activation of NF-κB following I/R injury. Following 30 min of ischemia/3 h of reperfusion, nuclear proteins were extracted from left ventricular tissue and analyzed for NF-κB DNA binding activity by EMSA (n = 6 in each group). a Representative EMSA results are shown and the NF-κB and the free probe bands are labeled. Lanes 1 and 2, control group; lanes 3 and 4, I/R group; lanes 5 and 6, LPS + I/R group; lanes 7 and 8, Q-LPS + I/R group. b Relative integrated intensity of NF-κB activity was shown as graph bar. Data are means ± SEM. *P < 0.01 compared with indicated groups
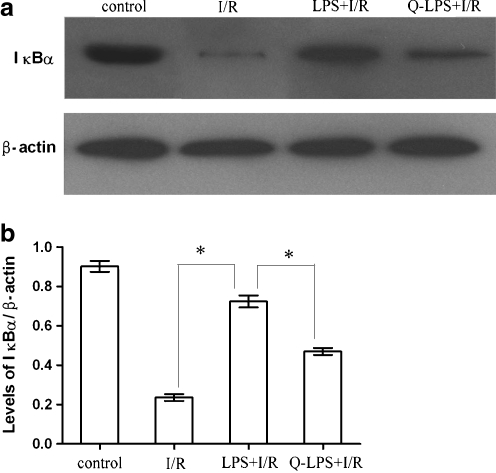
Because ischemia rapidly induces NF-κB binding activity in the myocardium, the effects of I/R injury on the dynamics of IκBα protein in the cytoplasm were investigated. Hearts were subjected to I/R injury, and the cytoplasmic extracts were assayed by Western blot analysis with an antibody specific to IκBα. As shown in Fig. 6, the kinetics of NF-κB binding activity in the nuclear extracts paralleled the kinetics of IκBα decrement in the cytoplasm. The data suggested that I/R injury induced the loss of IκBα protein from the cytoplasm and activated translocation of NF-κB to the nucleus. LPS pretreatment ameliorated degradation of IκBα and reduced activity of NF-κB following I/R injury.
Fig. 6.
LPS pretreatment prevents cytoplasmic IκBα degradation. The cytoplasmic proteins from every group were subjected to immunoblotting analysis of IκBα and β-actin protein levels with specific antibodies. The tissue samples from control group were taken from the same regions as the ischemic hearts (n = 6 in each group). a Representative immunoblotting gel showing IκBα and β-actin levels is presented. b The protein levels for each sample were determined as a ratio to their corresponding β-actin levels. Ischemia/reperfusion injury induced the loss of IκBα protein from the cytoplasm in I/R group, and LPS pretreatment attenuated the degradation of IκBα in LPS + I/R group. Administration of quercetin before I/R injury attenuated the effect of LPS pretreatment. The results were expressed as means ± SEM. *P < 0.01 compared with indicated groups
LPS pretreatment ameliorates oxidative stress following ischemia/reperfusion injury
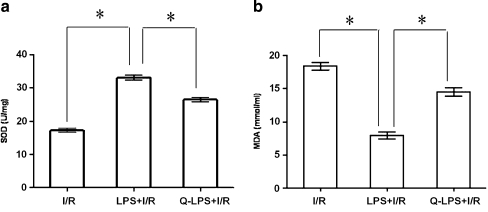
I/R injury has previously been suggested to be caused by increased oxidative stress. To determine if LPS pretreatment might be accompanied by the induction of antioxidant pathways, which detoxify ROS, we measured the activities of antioxidant enzymes and lipid peroxidation in heart homogenates. The enzymatic activity of SOD was significantly increased in LPS + I/R group, compared with that in I/R group (33.1 ± 0.7 vs. 17.2 ± 0.5 U/mg, P < 0.01). MDA is a product of lipid peroxidation induced by a diversity of oxidative injury. It has been used as a biomarker of cardiac oxidative damage (Col et al. 2010). The result presented in Fig. 7 showed the effect of LPS pretreatment on MDA concentrations in hearts. I/R injury dramatically elevated the MDA concentrations in I/R group (18.4 ± 0.6 mmol/ml). However, the concentration of MDA in the LPS + I/R group was significantly lower than that in I/R group (8.0 ± 0.5 vs. 18.4 ± 0.6 mmol/ml, P < 0.01). The protective effect of LPS pretreatment was attenuated by quercetin administration (14.5 ± 0.7 mmol/ml).
Fig. 7.
LPS pretreatment ameliorates oxidative stress following ischemia/reperfusion injury. After I/R, the blood was collected to detect serum lipid peroxides by measuring malondialdehyde (MDA) concentration with MDA ELISA kit. Antioxidant enzymes were measured by Superoxide Dismutase (SOD) Assay Kit according to the illustration of the manufacturer (n = 12 in each group). a Concentration of SOD in three experimental groups. The enzymatic activity of SOD was significantly increased in LPS + I/R group compared with that in I/R group. However, quercetin decreased the activity of SOD in Q-LPS + I/R group. b Concentration of MDA in three experimental groups. I/R injury dramatically elevated the MDA concentrations in I/R group. However, the concentration of MDA in the LPS + I/R group was significantly lower than that in I/R group. The protective effect of LPS pretreatment was attenuated by quercetin administration. The results were expressed as means ± SEM. *P < 0.01 compared with indicated groups
Discussion
Despite recent advances in cardiovascular research, ischemic heart disease remains the major cause of death in the world (Lopez et al. 2006). Reperfusion therapy, which allows the rapid return of blood flow to the ischemic zone of the myocardium, however, may lead to further complications such as diminished cardiac contractile function and irreversible tissue necrosis (Turer and Hill 2010). Therefore, development of cardioprotective strategies to improve myocardial function, decrease the onset of necrosis, and limit the total extent of infarction during I/R injury is of great clinical importance. Ischemic preconditioning is a well-described cardioprotective strategy in which brief exposure to ischemia remarkably enhances the ability of heart to withstand a subsequent ischemia/reperfusion injury (Cepinskas et al. 2001; Churchill et al. 2010). Numerous studies also have shown that gram-negative bacterial LPS induced myocardial protection (Zacharowski et al. 2000; Meng et al. 1997). Specifically, pretreatment of rats with LPS for 24 h increases myocardial functional recovery in isolated ischemia/reperfusion hearts (Ha et al. 2008). However, the underlying mechanisms have not been fully elucidated. In this study, we demonstrated that (1) LPS pretreatment could reduce apoptosis of myocardium and infarct area following I/R injury; (2) I/R injury activated cardiac NF-κB and induced inflammatory cytokines expression, whereas pretreatment with LPS resulted in attenuation of both NF-κB activation and release of inflammatory cytokines; and (3) LPS pretreatment increase the levels of HSP70 in myocardium, which played a protective role in the subsequent I/R injury. Inhibitor of HSP70, quercetin, could attenuate the effect of LPS pretreatment.
In response to a wide variety of stresses, such as heat shock, hypoxia, inflammation, and hydrogen peroxide, there is an increase in the expression of HSPs. HSP70 is one of the most fundamental and prominent stress proteins induced in almost all cell types. It plays a role in cell survival by having a capacity to bind to misfolded or denatured proteins and thereby prevent their irreversible denaturation (Latchman 2001). As bacterial endotoxin, LPS could induce the expression of HSP70 in myocardium, which had been confirmed in our study. We also observed that pretreatment of rats with low-dose LPS significantly attenuated cardiac myocyte apoptosis, caspase-3 activity, and reduced infarct area in LPS pretreatment group. Accordingly, we speculated that high levels of HSP70 in LPS-pretreated rats may be responsible for the cardioprotection against I/R injury.
In the heart, I/R injury induces release of ROS. ROS are thought to initiate leukocyte recruitment with resultant inflammatory mediators and heart dysfunction (Rui et al. 2001). Because these inflammatory mediators, such as TNF-α, IL-1β, and IL-6, are known to have κB-binding motifs in their promoter regions, their transcriptions are thought to be under the control of NF-κB. Thereby, modulation of NF-κB activity might provide a means of reducing inflammatory factors following I/R injury. NF-κB is normally sequestered in the cytoplasm by the inhibitory protein IκB family, including IκBα, IκBβ, and IκBγ (Guttridge et al. 1999), among which, IκBα is the most widely investigated. Ischemia/reperfusion injury causes a rapid degradation of the IκBα. This allows NF-κB to translocate into the nucleus, where it binds to its target sequences and induces transcription of inflammation genes (Dogra et al. 2006; Chao et al. 2005). In our study, we found that LPS pretreatment significantly inhibited I/R-induced IκBα degradation and reduced NF-κB translocation ability. Recently, several studies suggested that one of the protective mechanisms of HSP70 is its inhibitory effect on NF-κB-mediated inflammatory reaction (Dokladny et al. 2010; Sun et al. 2005). Herein, we concluded that increased levels of HSP70 through LPS pretreatment led to downregulation of NF-κB in the myocardium after ischemia/reperfusion injury.
Ischemia/reperfusion injury has previously been demonstrated to be caused by increased oxidative stress. Oral intake pretreatment with ebselen enhances HSP72 expression and reduces ROS induced by I/R injury (Baljinnyam et al. 2006). To determine if increased levels of HSP70 might contribute to the induction of antioxidant pathways, which detoxify ROS, we measured the activities of antioxidant enzymes and lipid peroxidation in heart homogenates. The enzymatic activity of SOD was significantly increased after LPS pretreatment. MDA is a product of lipid peroxidation induced by a diversity of oxidative injury. The result presented in our study showed that MDA concentration was significantly decreased after LPS pretreatment. Herein, we concluded that increased levels of HSP70 through LPS pretreatment attenuated the oxidative stress induced by I/R injury.
The aforementioned findings suggest that the effect of LPS pretreatment might be mediated via increase of HSP70 and inhibition of NF-κB. However, there still are uncertainties as to the possible effects of other HSP molecules. We therefore performed Western blot analysis of other HSPs, including HSP27, HSP60, and HSP90. In contrast to HSP70, HSP27, HSP60, and HSP90 showed a minimal increase after LPS pretreatment in heart. To further investigate whether increase of HSP70 might be responsible for protection of myocardium from I/R injury, we administered the HSP70 inhibitor, quercetin, before I/R (Wang et al. 2009a, b). As shown in Fig. 3, quercetin administration reduced the level of HSP70. Inhibition of HSP70 resulted in more apoptotic myocardium and infarct area in Q-LPS + I/R group than that in LPS + I/R group (Fig. 1). Next, we checked the expression levels of inflammatory factors, such as TNF-α, IL-1β, and IL-6 after quercetin treatment. As shown in Fig. 4, LPS pretreatment obviously decreased the release of inflammatory factors following I/R injury, and this reduction was partially inhibited by co-administration of quercetin. This effect of inhibition by quercetin was also confirmed by NF-κB binding activity and immunostaining of IκBα (Fig. 6). These observations strongly suggested that the beneficial effect of LPS pretreatment was mediated partly by the increase of HSP70, and HSP70 induction was correlated with the inhibition of NF-κB. It has been reported that quercetin inhibits NF-κB directly in some kinds of cells and tissue (Panicker et al. 2010; Granado-Serrano et al. 2010). However, whether quercetin inhibits NF-κB activation in the following I/R injury is still unknown and needs a further investigation.
It has been reported that pretreatment of rats with LPS increase myocardial recovery in I/R hearts. However, the underlying mechanisms have not been fully elucidated. Recently, Li et al. reported that LPS-induced cardioprotection in I/R injury was mediated through a PI3K/Akt-dependent mechanism (Ha et al. 2008). In their study, they observed that LPS pretreatment could increase the levels of HSP27, and there was a link between PI3K/Akt and HSP27. However, we did not observe a dramatic increase in the expression of HSP27 in myocardium after LPS pretreatment. The difference between the two studies may be due to different experimental protocols, such as different animals and different dose of LPS used in experiment. As for the link between PI3K/Akt and HSP70 in LPS pretreatment, it needs further investigation. In summary, the present study showed that increased levels of HSP70 through LPS pretreatment led to downregulation of NF-κB activity in the myocardium after ischemia/reperfusion injury. This might be one of the mechanisms by which LPS pretreatment led to better myocardial protection. Thus, LPS pretreatment could provide a means of reducing myocardial ischemia/reperfusion injury.
Acknowledgments
This project was supported by grants from the Technology Bureau Foundation of Zhenjiang City, Jiangsu Province (No. SH2008043).
Contributor Information
Yong-wei Yao, Email: ywyao78@sina.com.cn.
Guo-hui Zhang, Email: laoyao_1978@sina.com.cn.
References
- Baljinnyam E, Hasebe N, Morihira M, Sumitomo K, Matsusaka T, Fujino T, Fukuzawa J, Ushikubi F, Kikuchi K. Oral pretreatment with ebselen enhances HSP72 expression and reduces myocardial infarct size. Hypertens Res. 2006;29(11):905–913. doi: 10.1291/hypres.29.905. [DOI] [PubMed] [Google Scholar]
- Bao XQ, Liu GT. Bicyclol: a novel antihepatitis drug with hepatic heat shock protein 27/70-inducing activity and cytoprotective effects in mice. Cell Stress Chaperones. 2008;13(3):347–355. doi: 10.1007/s12192-008-0034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Grosso MA, Terada LS, Whitman GJR. Endotoxin pretreatment increases endogenous myocardial catalase activity and decrease ischemia–reperfusion injury of isolated rat heart. Proc Natl Acad Sci USA. 1989;86:2516–2520. doi: 10.1073/pnas.86.7.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepinskas G, Rui T, Kvietys PR. Neutrophil–endothelial cell interactions during the development of tolerance to ischaemia/reperfusion in isolated cells. Acta Physiol Scand. 2001;173(1):23–33. doi: 10.1046/j.1365-201X.2001.00881.x. [DOI] [PubMed] [Google Scholar]
- Cepinskas G, Rui T, Kvietys PR. Interaction between reactive oxygen metabolites and nitric oxide in oxidant tolerance. Free Radic Biol Med. 2002;33(4):433–440. doi: 10.1016/S0891-5849(02)00962-0. [DOI] [PubMed] [Google Scholar]
- Chao W, Shen Y, Zhu X, Zhao H, Novikov M, Schmidt U, Rosenzweig A. Lipopolysaccharide improves cardiomyocytes survival and function after serum deprivation. J Biol Chem. 2005;280(23):21997–22005. doi: 10.1074/jbc.M413676200. [DOI] [PubMed] [Google Scholar]
- Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274(16):10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- Churchill EN, Ferreira JC, Brum PC, Li S, Mochly-Rosen D. Ischaemic preconditioning improves proteasomal activity and increases the degradation of deltaPKC during reperfusion. Cardiovasc Res. 2010;85(2):385–394. doi: 10.1093/cvr/cvp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Col C, Dinler K, Hasdemir O, Buyukasik O, Bugdayci G. Oxidative stress and lipid peroxidation products: effect of pinealectomy or exogenous melatonin injections on biomarkers of tissue damage during acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2010;9(1):78–82. [PubMed] [Google Scholar]
- Deten A, Volz HC, Briest W, Zimmer HG. Cardiac cytokine expression is upregulated in the acute phase after myocardial infarction. Experimental studies in rats. Cardiovasc Res. 2002;55(2):329–340. doi: 10.1016/S0008-6363(02)00413-3. [DOI] [PubMed] [Google Scholar]
- Dogra C, Changotra H, Mohan S, Kumar A. Tumor necrosis factor-like weak inducer of apoptosis inhibits skeletal myogenesis through sustained activation of nuclear factor-κB and degradation of myoD protein. J Biol Chem. 2006;281(15):10327–10336. doi: 10.1074/jbc.M511131200. [DOI] [PubMed] [Google Scholar]
- Dokladny K, Lobb R, Wharton W, Ma TY, Moseley PL. LPS-induced cytokine levels are repressed by elevated expression of HSP70 in rats: possible role of NF-κB. Cell Stress Chaperones. 2010;15(2):153–163. doi: 10.1007/s12192-009-0129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granado-Serrano AB, Martin MA, Bravo L, Goya L, Ramos S. Quercetin modulates NF-kappa B and AP-1/JNK pathways to induce cell death in human hepatoma cells. Nutr Cancer. 2010;62(3):390–401. doi: 10.1080/01635580903441196. [DOI] [PubMed] [Google Scholar]
- Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cel Biol. 1999;19(8):5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha T, Hua F, Liu X, Ma J, McMullen JR, Shioi T, Izumo S, Kelley J, Gao X, Browder W, Williams DL, Kao RL, Li C. Lipopolysaccharide-induced myocardial protection against ischaemia/reperfusion injury is mediated through a PI3K/Akt-dependent mechanism. Cardiovasc Res. 2008;78(3):546–553. doi: 10.1093/cvr/cvn037. [DOI] [PubMed] [Google Scholar]
- Hu Z, Zhang F, Yang Z, Yang N, Zhang D, Zhang J, Cao K. Combination of simvastatin administration and EPC transplantation enhances angiogenesis and protects against apoptosis for hindlimb ischemia. J Biomed Sci. 2008;15:509–517. doi: 10.1007/s11373-008-9243-1. [DOI] [PubMed] [Google Scholar]
- Latchman DS. Heat shock proteins and cardiac protection. Cardiovasc Res. 2001;51:637–646. doi: 10.1016/S0008-6363(01)00354-6. [DOI] [PubMed] [Google Scholar]
- Li C, Browder W, Kao RL. Early activation of transcription factor NF-κB during ischemia in perfused rat heart. Am J Physiol Heart Circ Physiol. 1999;276:543–552. doi: 10.1152/ajpheart.1999.276.2.H543. [DOI] [PubMed] [Google Scholar]
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factor, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- Meng XZ, Ao LH, Brown JM, Meldrum DR, Sheridan BC, Cain BS, Banerjee A, Harken AH. LPS induces late cardiac functional protection against ischemia independent of cardiac and circulating TNF-α. Am J Physiol Heart Circ Physiol. 1997;273:1894–1902. doi: 10.1152/ajpheart.1997.273.4.H1894. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Vallejo JG, Knuefermann P, Misra A, Defreitas G, Carabello BA, Mann DL. Escherichia coli LPS-induced LV dysfunction: role of toll-like receptor-4 in the adult heart. Am J Physiol Heart Circ Physiol. 2002;282:H2316–H2323. doi: 10.1152/ajpheart.00763.2001. [DOI] [PubMed] [Google Scholar]
- Oshima Y, Fujio Y, Nakanishi T, Itoh N, Yamamoto Y, Negoro S, Tanaka K, Kishimoto T, Kawase I, Azuma J. STAT3 mediates cardioprotection against ischemia/reperfusion injury trough metallothionein induction in the heart. Cardiovasc Res. 2005;65(2):428–435. doi: 10.1016/j.cardiores.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Panicker SR, Sreenivas P, Babu MS, Karunagaran D, Kartha CC. Quercetin attenuates monocyte chemoattractant protein-1 gene expression in glucose primed aortic endothelial cells through NF-kappa B and AP-1. Pharmacol Res. 2010;62(4):328–336. doi: 10.1016/j.phrs.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Pchejetski D, Kunduzova O, Dayon A, Calise D, Seguelas MH, Leducq N, Seif I, Parini A, Cuvillier O. Oxidative stress-dependent sphingosine kinase-1 inhibition mediates monoamine oxidase A-associated cardiac cell apoptosis. Circ Res. 2007;100:41–49. doi: 10.1161/01.RES.0000253900.66640.34. [DOI] [PubMed] [Google Scholar]
- Rui T, Cepinskas G, Feng Q, Ho YS, Kvietys PR. Cardiac myocytes exposed to anoxia-reoxygenation promote neutrophil transendothelial migration. Am J Physiol Heart Circ Physiol. 2001;281(1):H440–H447. doi: 10.1152/ajpheart.2001.281.1.H440. [DOI] [PubMed] [Google Scholar]
- Senf SM, Dodd SL, McClung JM, Judge AR. Hsp70 overexpression inhibits NF-kappaB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy. FASEB J. 2008;22(11):3836–3845. doi: 10.1096/fj.08-110163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T, Takahashi N, Kohno H, Yamanaka K, Ooie T, Wakisaka O, Murozono Y, Taniguchi Y, Hara M, Shimada T, Saikawa T, Yoshimatsu H. Mitochondria are targets for geranylgeranylacetone-induced cardioprotection against ischemia/reperfusion in the rat heart. Am J Physiol Heart Circ Physiol. 2007;293(3):1892–1899. doi: 10.1152/ajpheart.00493.2007. [DOI] [PubMed] [Google Scholar]
- Song W, Furman BL, Parratt JR. Delayed protection against ischaemia-induced ventricular arrhythmias and infarct size limitation by the prior administration of Escherichia coli endotoxin. Br J Pharmacol. 1996;118(8):2157–2163. doi: 10.1111/j.1476-5381.1996.tb15657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Chen D, Du B, Pan J. Heat shock response inhibits NF-κB activation and cytokine production in murine Kupffer cells. J Surg Res. 2005;129(1):114–121. doi: 10.1016/j.jss.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Turer AT, Hill JA. Pathogenesis of myocardial ischemia–reperfusion injury and rationale for therapy. Am J Cardiol. 2010;106(3):360–368. doi: 10.1016/j.amjcard.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RE, Kao JL, Hilliard CA, Pandita RK, Roti Roti JL, Hunt CR, Taylor JS. Inhibition of heat shock induction of HSP70 and enhancement of HSP27 phosphorylation by quercetin derivatives. J Med Chem. 2009;52(7):1912–1921. doi: 10.1021/jm801445c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZJ, Zhang FM, Wang LS, Yao YW, Zhao Q, Gao X. Lipopolysaccharides can protect mesenchymal stem cells from oxidative stress-induced apoptosis and enhance proliferation of MSCs via Toll-like receptor(TLR)-4 and PI3K/Akt. Cell Biol Int. 2009;33:665–674. doi: 10.1016/j.cellbi.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Williamson CL, Dabkowski ER, Dillmann WH, Hollander JM. Mitochondria protection from hypoxia/reoxygenation injury with mitochondria heat shock protein 70 overexpression. Am J Physiol Heart Circ Physiol. 2008;294(1):H249–H256. doi: 10.1152/ajpheart.00775.2007. [DOI] [PubMed] [Google Scholar]
- Yao YW, Zhang FM, Wang LS, Zhang GH, Wang ZJ, Chen JM, Gao X. Lipopolysaccharide preconditioning enhances the efficacy of mesenchymal stem cells transplantation in a rat model of acute myocardial infarction. J Biomed Sci. 2009;16:74. doi: 10.1186/1423-0127-16-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharowski K, Frank S, Otto M, Chatterjee PK, Cuzzocrea S, Hafner G, Pfeilschifter J, Thiemermann C. Lipoteichoic acid induces delayed protection in the rat heart: a comparison with endotoxin. Arterioscler Thromb Vasc Biol. 2000;20:1521–1528. doi: 10.1161/01.atv.20.6.1521. [DOI] [PubMed] [Google Scholar]
- Zhang K, Zhao T, Huang X, Liu ZH, Xiong L, Li MM, Wu LY, Zhao YQ, Zhu LL, Fan M. Preinduction of HSP70 promotes hypoxic tolerance and facilitates acclimatization to acute hypobaric hypoxia in mouse brain. Cell Stress Chaperones. 2009;14(4):407–415. doi: 10.1007/s12192-008-0094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]