Abstract
Expression of the tight junction protein junctional adhesion molecule-A (JAM-A) has been linked to proliferation and tumour progression. However, a direct role for JAM-A in regulating proliferative processes has not been shown. By using complementary in vivo and in vitro approaches, we demonstrate that JAM-A restricts intestinal epithelial cell (IEC) proliferation in a dimerization-dependent manner, by inhibiting Akt-dependent β-catenin activation. Furthermore, IECs from transgenic JAM-A−/−/β-catenin/T-cell factor reporter mice showed enhanced β-catenin-dependent transcription. Finally, inhibition of Akt reversed colonic crypt hyperproliferation in JAM-A-deficient mice. These data establish a new link between JAM-A and IEC homeostasis.
Keywords: junctional adhesion molecule, Akt, β-catenin, cell proliferation, tight junction
Introduction
Junctional adhesion molecule-A (JAM-A) is a tight junction-associated protein that is responsible for several functions in epithelial and endothelial cells. Recent studies from our lab and others show that JAM-A is required for the maintenance of intestinal epithelial cell (IEC) homeostasis and barrier function (Laukoetter et al, 2007; Vetrano et al, 2008). JAM-A contains two extracellular immunoglobulin loops, a transmembrane domain and a short cytoplasmic tail. The distal extracellular immunoglobulin loop mediates homodimerization between JAM-A proteins, which form intercellular contacts (Kostrewa et al, 2001; Prota et al, 2003; Severson et al, 2008). The cytoplasmic tail of JAM-A interacts with several intracellular scaffolding molecules including ZO-1, AF6, Par3 and PDZ–GEF2 (reviewed by Severson & Parkos, 2009). Indeed, JAM-A is believed to stimulate outside-in signalling through dimerization-dependent scaffold protein recruitment (Severson & Parkos, 2009; Severson et al, 2009). However, the signalling events through which JAM-A mediates many of its biological functions are unclear.
Akt/protein kinase B is a serine/threonine-specific kinase that is involved in several pathways regulating cell growth, differentiation and proliferation (Dummler & Hemmings, 2007). Aberrant activation of Akt is often observed in human cancers, and a direct role for Akt signalling in the regulation of cell proliferation has been shown in several transgenic mouse models (Luo et al, 2003; Dillon et al, 2007). In the intestine, β-catenin not only functions as a downstream effector of canonical Wnt signalling to promote crypt stem-cell proliferation (Li & Clevers, 2010), but also can be transactivated by Akt-mediated phosphorylation at Ser 552 (Fang et al, 2007).
In this study, we investigate the role of JAM-A in IEC proliferation and epithelial homeostasis. We observe that loss of JAM-A expression enhances IEC proliferation in vivo and in vitro, which requires JAM-A dimerization. Investigations into the signalling mechanisms involved reveal that increased proliferation requires phosphatidylinositol 3-kinase (PI3K) and phosphatase and tensin homologue (PTEN)-dependent, Akt-mediated β-catenin transcriptional activation, as inhibition of Akt is sufficient to rescue epithelial hyperproliferation. In light of these results, we propose that JAM-A contributes to intestinal epithelial homeostasis by regulating β-catenin signalling.
Results And Discussion
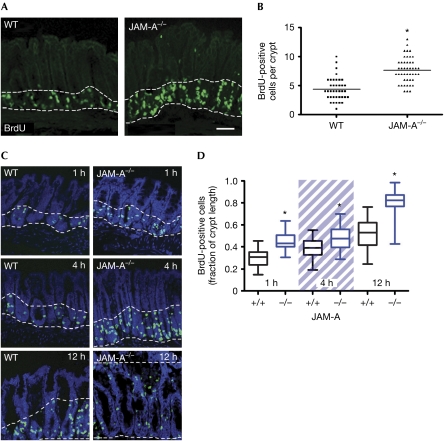
JAM-A-deficient mice (JAM-A−/−) show enhanced colonic crypt Ki67 staining (Laukoetter et al, 2007). Therefore, we investigated the role of JAM-A in cell proliferation by using 5-bromo-2-deoxyuridine (BrdU) incorporation in wild-type and JAM-A−/− mice. Mice were analysed 4 h after injection and a significant increase in proliferation was found in colonic crypts of JAM-A−/− mice (Fig 1A,B). Our observations indicate that loss of JAM-A results in either an increase in the rate of colonic stem-cell division, combined with enhanced progenitor cell migration, or in increased proliferation within transient amplifying cell populations. To distinguish between these possibilities, we performed BrdU pulse-chase experiments (Fig 1C,D). Increased cell migration is shown by the height of BrdU-positive cells along the crypt-to-surface axis, which is increased in JAM-A−/− mice at all time points. Increased BrdU incorporation was seen at 1 h, at which time cells are seen at higher positions along the crypt-to-surface axis. At this time, cell migration has a small role in determining cell position along the crypt-to-surface axis. Therefore, the increased cell proliferation in JAM-A−/− mice seen after 1 h BrdU incorporation is due to increased proliferation in the transient amplifying zone. Crypt length was unchanged (data not shown), probably because of a compensatory increase in IEC apoptosis at the luminal interface (supplementary Fig S1A online; Vetrano et al, 2008). Taken together, these results show that JAM-A is a negative regulator of IEC proliferation and migration in vivo.
Figure 1.
Junctional adhesion molecule-A-deficient mice show increased intestinal epithelial cell proliferation. (A) Confocal images of murine distal colon tissue illustrate the presence of BrdU (green) in proliferating IECs of JAM-A−/− and WT mice (4 h after i.p. injection of 50 mg/kg BrdU). Scale bar, 50 μM. (B) BrdU-positive cells per crypt: JAM-A−/−=7.6±2.2 BrdU cells per crypt, n=46; WT=4.4±2 BrdU cells per crypt, n=37; *P<0.05. (C) BrdU pulse-chase assays at the indicated times after i.p. injection. (D) Box and whisker plots showing the migration of BrdU-positive cells at 1 h, 4 h and 12 h post-injection, as a fraction of the total length of the crypt to surface axis in WT and JAM-A−/− mice. n=60 crypts per group, WT compared with JAM-A−/− at each time point, *P<0.001. BrdU, 5-bromo-2-deoxyuridine; IEC, intestinal epithelial cell; i.p., intraperitoneal; JAM-A, junctional adhesion molecule-A; WT, wild type.
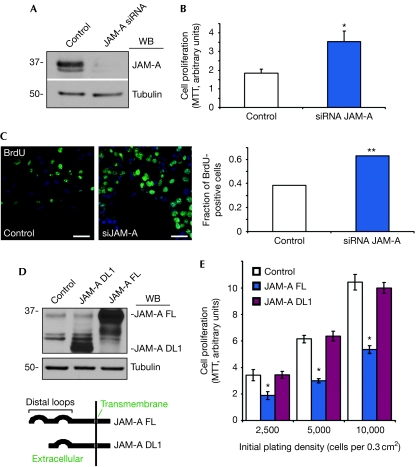
JAM-A−/− mice have decreased IEC barrier function and increased mucosal inflammation (Laukoetter et al, 2007; Vetrano et al, 2008). To exclude the possibility that increased proliferation in vivo is due to mitogenic paracrine signalling in response to immune activation, we conducted complementary experiments with model human IECs, SKCO15. As shown in Fig 2A, JAM-A small interfering RNA (siRNA) strongly downregulated protein expression 48 h after transfection. Consistent with in vivo data, JAM-A downregulation was sufficient to induce IEC proliferation, as indicated by increased tetrazolium salt metabolism (MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay; Fig 2B) and increased BrdU incorporation (Fig 2C). The above findings indicate that JAM-A directly regulates IEC proliferation. JAM-A intercellular signalling requires protein homodimerization (Severson & Parkos, 2009). To investigate whether this is required to suppress cell proliferation, we assessed HEK 293T cells stably expressing full-length or dimerization-defective mutant JAM-A lacking the distal immunoglobulin loop (Fig 2D,E). Only full-length JAM-A reduced cell growth. These findings were confirmed by rescue experiments in SKCO15 cells, in which expression of full-length, but not dimerization-defective JAM-A, attenuated the increase in cell proliferation after JAM-A depletion (supplementary Fig S1B–E online). These data show that JAM-A dimerization is required to suppress epithelial cell proliferation.
Figure 2.
Junctional adhesion molecule-A regulates intestinal epithelial cell proliferation. (A) Immunoblotting shows efficient depletion of JAM-A in SKCO15 cells (siRNA target 1). (B) Increased proliferation in cells lacking JAM-A, as measured by MTT assay (s.e.m., n=3, *P<0.001). (C) SKCO15 cells transfected with control or JAM-A siRNA were incubated with BrdU for 30 min. Scale bar, 20 μm. Graph: quantitative analysis of the fraction of cells with BrdU incorporation (38.2%, control: n=195; 63.1%, siRNA JAM-A: n=310; P<0.05; Fisher's exact test). (D) Immunoblot analysis of stable HEK 293T cell lines expressing full-length (FL) or dimerization-deficient (DL1) JAM-A. Control cells express an empty vector. (E) MTT activity in HEK 293T with JAM-A FL or DL1 overexpression (s.d.: n=3, *P<0.05, FL compared with control and DL1, analysis of variance). BrdU, 5-bromo-2-deoxyuridine; IEC, intestinal epithelial cell; JAM-A, junctional adhesion molecule-A; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; siRNA, small interfering RNA; WB, western blot.
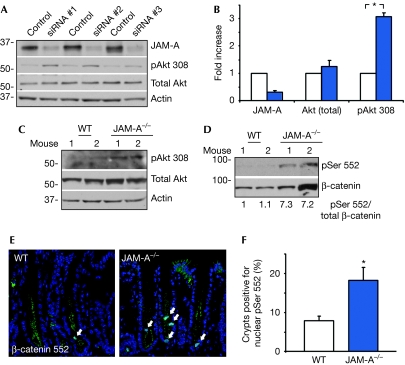
To identify signalling pathways that control IEC proliferation downstream of JAM-A, we analysed the activity of Akt, a known regulator of epithelial homeostasis (Itoh et al, 2002; Cao et al, 2006). Depletion of JAM-A in SKCO15 cells using different siRNA constructs resulted in a significant increase in Akt activity, as indicated by phosphorylation of Thr 308 (Fig 3A,B). Similarly, increased Akt activity was observed in mucosal lysates of JAM-A−/− mice, compared with controls (Fig 3C). Previous studies have shown that Akt can enhance β-catenin signalling in two ways: by phosphorylation of β-catenin at Ser 552, thereby promoting its nuclear translocation and association with T-cell factor (TCF)/lymphoid enhancing factor family transcription factors (Behrens et al, 1996; Daniels & Weis, 2005; Fang et al, 2007), and by inhibition of GSK-3β, a potent negative regulator of β-catenin protein stability (Cross et al, 1995). Analysis of mucosal lysates showed a consistent increase in β-catenin pSer 552 in JAM-A−/− mice (Fig 3D), and immunolocalization showed a significantly increased number of proliferating IECs with nuclear pSer 552 in these animals (Fig 3E,F). By contrast, we observed no difference in mucosal GSK-3β activity, and total β-catenin expression was variable between animals (Fig 3D, data not shown). Thus, although we cannot exclude a possible contribution of increased β-catenin protein levels to increased IEC proliferation in these mice, our findings indicate that altered activity, rather than the stability of β-catenin, accounts for the findings in this study. Taken together, these results show that JAM-A regulates epithelial cell proliferation by inhibiting Akt/β-catenin signalling.
Figure 3.
Enhanced Akt/β-catenin phosphorylation after junctional adhesion molecule-A depletion. (A) SKCO15 cells were transfected with three different JAM-A siRNAs. All oligos increased the activity of Akt (phosphorylation of Thr 308), compared with control. Densitometric analysis is shown in (B). pAkt 308: s.e.m., 3.1±0.3-fold, n=3, *P<0.001 compared with control. (C) Representative immunoblots of tissue lysates from WT and JAM-A−/− distal colon tissue, identifying an increase in pAkt Thr 308. (D) Immunoblot of increased β-catenin phosphorylation (Ser 552) in the same lysates. (E) Immunolocalization of pSer 552 β-catenin in distal colon sections of WT or JAM-A−/− mice. Arrows indicate nuclear pSer 552. (F) The number of crypts with nuclear β-catenin pSer 552 is increased in JAM-A−/− mice (s.e.m., n=60/group, *P<0.01). BrdU, 5-bromo-2-deoxyuridine; JAM-A, junctional adhesion molecule-A; siRNA, small interfering RNA; WT, wild type.
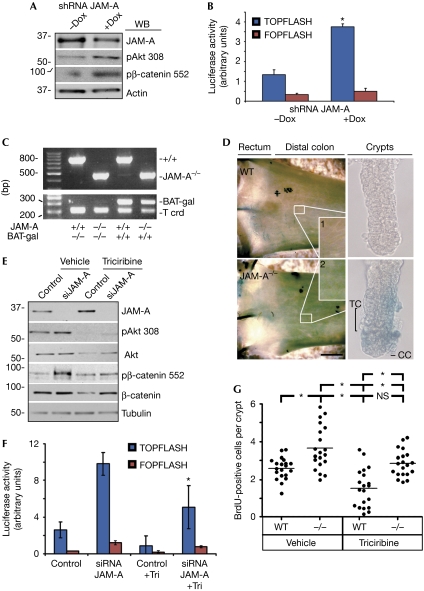
To corroborate these observations in vitro, we generated an SKCO15 cell line stably expressing a doxycyclin-inducible JAM-A short-hairpin RNA. In agreement with the data from JAM-A−/− mice, depletion of JAM-A by doxycyclin treatment increased the phosphorylation of Akt and β-catenin (Fig 4A). Loss of JAM-A resulted in nuclear translocation of β-catenin pSer 552, in agreement with previous studies (supplementary Fig S2A online). In addition, we used a luciferase-based β-catenin/TCF transcriptional activity assay (TOPFLASH/FOPFLASH) to determine β-catenin-mediated gene transcription. As shown in Fig 4B, loss of JAM-A on doxycyclin exposure was associated with a significant increase in β-catenin transcriptional activity, which was not the case in control cells treated with doxycyclin (supplementary Fig S2B online), and which was additionally confirmed by transient depletion using siRNA (data not shown). To further support our conclusions, we cross-bred JAM-A−/− mice with previously described β-catenin reporter mice that express the lacZ gene under the control of β-catenin/TCF responsive elements (BAT-gal; Maretto et al, 2003). The BAT-gal transgene, as well as JAM-A deletion, was confirmed by PCR, as shown in Fig 4C. In agreement with the in vitro data, strong β-galactosidase activity was observed in the distal colon of JAM-A−/− mice (Fig 4D). To further localize reporter activity within the intestine, we isolated and analysed colonic crypts. Similar to previous reports, BAT-gal colonic crypts showed minimal lacZ signal in the base of the crypt (Fig 4D, right panel; Maretto et al, 2003). However, a striking increase in lacZ staining was observed in JAM-A−/−/BAT-gal crypts in both transient amplifying cells and crypt cells (Fig 4D). We thus conclude that JAM-A regulates β-catenin/TCF-mediated gene transcription in vivo.
Figure 4.
Junctional adhesion molecule-A depletion enhances β-catenin/T-cell factor signalling. (A) Western blot analysis of SKCO15 cells stably expressing doxycycline-inducible JAM-A short-hairpin RNA. Doxycycline treatment (4 μg/ml, 48 h) suppresses JAM-A protein levels and increases Akt Thr 308/β-catenin Ser 552 phosphorylation. (B) Depletion of JAM-A in SKCO15 cells enhances β-catenin/TCF transcriptional activity, as shown by TOPFLASH reporter assay. Control (FOPFLASH) has mutated TCF binding sites (s.e.m., n=3, *P<0.001 compared with −Dox). (C) PCR analysis of JAM-A−/−/BAT-gal transgenic mice. (D) Representative image showing increased lacZ expression in JAM-A−/−/BAT-gal mice compared with WT/BAT-gal, indicative of increased β-catenin transcriptional activity. Right panel: isolated colonic crypts with highlighted progenitor transient amplifying cells (TC) and crypt cells (CC). (E) Immunoblot analysis of SKCO15 cells shows that Akt inhibitor Triciribine (20 μM) prevents β-catenin phosphorylation after loss of JAM-A. (F) Akt inhibition suppresses JAM-A depletion-induced β-catenin/TCF transcriptional activity. SKCO15 cells transfected with TCF reporter constructs and control or JAM-A-directed siRNA, with or without Triciribine treatment (20 μM, s.d., *P<0.01, ANOVA). (G) IEC proliferation due to JAM-A deficiency is attenuated by inhibition of Akt in vivo. WT and JAM-A−/− mice were intraperitoneally injected daily with Triciribine (1 mg/kg) before in vivo BrdU labelling (4 h; *P<0.01, ANOVA). ANOVA, analysis of variance; BrdU, 5-bromo-2-deoxyuridine; IEC, intestinal epithelial cell; JAM-A, junctional adhesion molecule-A; shRNA, short-hairpin RNA; siRNA, small interfering RNA; TCF, T-cell factor; Tri, Triciribine; WB, western blot; WT, wild type.
The above results indicate that Akt/β-catenin is the key regulator of IEC proliferation downstream of JAM-A. To test this hypothesis, Akt activity was inhibited by using Triciribine in cells treated with scramble or JAM-A siRNA, which efficiently reduced phosphorylation of Akt and β-catenin (Fig 4E). Under these conditions, β-catenin transcriptional activity was reduced after Triciribine treatment, as indicated by TOPFLASH assay (Fig 4F). Furthermore, we observed a significant reduction in SKCO15 proliferation after Triciribine treatment (data not shown). As Triciribine reduced total Akt expression, we confirmed our findings by using a peptide inhibitor of Akt, Akt-in and Akt siRNA. Importantly, both treatments yielded results similar to those observed with Triciribine, and Akt-in did not change Akt expression (supplementary Fig S3A,B online). Treatment of controls with Triciribine also suppressed Akt downstream effectors, which is consistent with the presence of functioning Akt-dependent pathways in control cells that are potentiated after JAM-A suppression. Taken together, these findings suggest that Akt is the main effector of JAM-A-mediated mitogenic signalling.
To corroborate our in vitro results, we analysed the effect of Akt inhibition on IEC proliferation in JAM-A−/− mice. Wild-type or JAM-A-knockout mice were treated with the Akt inhibitor Triciribine, injected with BrdU and analysed 4 h post-injection (Fig 4G). Consistent with our in vitro findings, Akt inhibitor treatment significantly decreased IEC proliferation in JAM-A−/− mice, which coincided with a decrease in Akt Thr 308 phosphorylation (supplementary Fig S3C online). Indeed, inhibition of Akt activity is sufficient to attenuate the increased cell proliferation seen in JAM-A−/− animals.
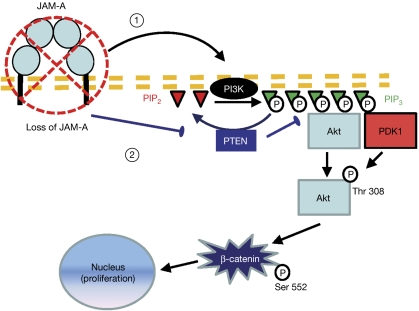
To explain the way in which JAM-A engages with Akt to regulate β-catenin transcriptional activity, we examined the levels of Akt activator phosphatidylinositol (3,4,5)-trisphosphate (PIP3) in our experimental system. Depletion of JAM-A in SKCO15 cells resulted in a notable increase in the total pool of PIP3 (supplementary Fig S4A online). PIP3 is generated by PI3K. To determine the role of PI3K in Akt regulation by JAM-A, we used siRNA to deplete PI3K subunit p110α in IEC. As shown in supplementary Fig S4B online, loss of PI3K inhibited activation of Akt and β-catenin transcriptional activity following JAM-A depletion. In support of our conclusions, expression of a dominant-negative kinase-dead Akt construct completely blocked β-catenin-dependent gene transcription, independently of JAM-A expression (supplementary Fig S4C online). An alternative cause of increased Akt activity might be inhibition of PTEN, which converts PIP3 to PIP2. As shown in supplementary Fig S5A online, depletion of JAM-A resulted in increased PTEN phosphorylation at the inhibitory residues Ser 380, Thr 382 and Thr 383. In addition, we observed consistently decreased expression of PTEN in mucosal lysates of JAM-A−/− mice (supplementary Fig S5B online). We also found that depletion of PTEN increased Akt phosphorylation and β-catenin transcriptional activity, which did not rescue the effect of Akt inhibition, confirming that PTEN functions upstream of Akt (supplementary Fig S5C,D online). Finally, additional studies of alternative pathways linking JAM-A to Akt showed that integrin-linked kinase and Rap1 were not involved in Akt activation after loss of JAM-A (data not shown). It is important to note that this observation does not exclude the fact that other signalling pathways that are not investigated here affect epithelial homeostasis following loss of JAM-A. Similarly, it is possible that β-catenin transcriptional activity is additionally regulated by other proteins such as protein kinase A (Taurin et al, 2006). Nevertheless, the results presented here indicate that JAM-A regulates Akt activity by controlling PIP3 levels in a PI3K- and PTEN-dependent manner. As schematically summarized in Fig 5, we thus conclude that homodimerization of JAM-A in IECs decreases cell proliferation by dampening Akt activity and β-catenin transcriptional activation.
Figure 5.
Schematic representation of the proposed signalling pathway. Loss of JAM-A increases the abundance of PIP3 through (1) the activation of PI3K and (2) inactivation of PTEN. Consequently, in the absence of JAM-A, Akt is recruited to the membrane and phosphorylated at Thr 308 probably by its activating kinase PDK1. Active Akt phosphorylates β-catenin at Ser 552, which promotes its nuclear translocation and enhances its activity as a transcription cofactor for TCF. Thus, we propose that JAM-A (dimerization) restricts epithelial-cell proliferation by negatively regulating Akt/β-catenin signalling. JAM-A, junctional adhesion molecule-A; PDK1, 3-phosphoinositide-dependent protein kinase 1; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; PI3K, phosphatidylinositol 3-kinase; PTEN, phosphatase and tensin homologue; TCF, T-cell factor.
Methods
Animal experiments. JAM-A−/− mice were a gift from T. Sato, Cornell University (New York, NY, USA) and C57BL/6 (wild-type) mice were obtained from The Jackson Laboratory. JAM-A−/− mice were genotyped as described previously (Cera et al, 2004). lacZ integration in BAT-gal reporter mice (The Jackson Laboratory) was confirmed by PCR, as reported previously (Maretto et al, 2003). Akt was inhibited by daily intraperitoneal injection of 1 mg/kg body weight Triciribine for 7 days, with dimethylsulphoxide as control. All procedures using animals were reviewed and approved by the Emory University Institutional Animal Care and Use Committee, according to the criteria of the National Institutes of Health.
Cell proliferation assays. BrdU (Sigma-Aldrich) was administered by intraperitoneal injection of 50 mg/kg body weight. BrdU was detected after ethanol and glycine fixation of intestinal frozen sections. Specimens were digested with 0.05% trypsin and antigen retrieved by denaturation with 4 M HCl. Slides were incubated with fluorophore-labelled BrdU antibody (Roche Pharmaceuticals). Proliferation in vitro was assessed by BrdU or MTT assay (Roche Pharmaceuticals). For BrdU scoring, longitudinal sections were selected such that both crypt base and luminal surface cells were visible. Apoptosis in vivo was determined by detecting cleaved Caspase-3 (R&D Systems).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank K. den Beste. This study was supported by grants from the National Institutes of Health (DK72564, DK61379 and DK79392 to C.A.P.; DK53202, DK55679 and DK59888 to A.N.; and DK64399; NIH DDRC tissue culture and morphology grant), the Crohn's and Colitis Foundation of America (fellowship award to P.N., S.K. and C.T.C.) and the American Gastroenterological Association (Research Scholar Award to P.N.).
Author contributions: P.N., C.T.C., K.K. and A.N. planned the experiments; P.N., C.T.C., S.K., K.K., C.R.R., A.E.F. and M.E.F. conducted the experiments; P.N., C.T.C., C.A.P. and A.N. analysed the data; C.T.C., J.K. and A.N. wrote the manuscript; C.A. supervised animal breeding; L.L. provided reagents; and C.A.P. and A.N. supervised the study.
Footnotes
The authors declare that they have no conflict of interest.
References
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W (1996) Functional interaction of β-catenin with the transcription factor LEF-1. Nature 382: 638–642 [DOI] [PubMed] [Google Scholar]
- Cao Y, Deng C, Townsend CM Jr, Ko TC (2006) TGF-β inhibits Akt-induced transformation in intestinal epithelial cells. Surgery 140: 322–329 [DOI] [PubMed] [Google Scholar]
- Cera MR et al. (2004) Increased DC trafficking to lymph nodes and contact hypersensitivity in junctional adhesion molecule-A-deficient mice. J Clin Invest 114: 729–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378: 785–789 [DOI] [PubMed] [Google Scholar]
- Daniels DL, Weis WI (2005) β-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat Struct Mol Biol 12: 364–371 [DOI] [PubMed] [Google Scholar]
- Dillon RL, White DE, Muller WJ (2007) The phosphatidyl inositol 3-kinase signaling network: implications for human breast cancer. Oncogene 26: 1338–1345 [DOI] [PubMed] [Google Scholar]
- Dummler B, Hemmings BA (2007) Physiological roles of PKB/Akt isoforms in development and disease. Biochem Soc Trans 35: 231–235 [DOI] [PubMed] [Google Scholar]
- Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z (2007) Phosphorylation of β-catenin by AKT promotes β-catenin transcriptional activity. J Biol Chem 282: 11221–11229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N, Semba S, Ito M, Takeda H, Kawata S, Yamakawa M (2002) Phosphorylation of Akt/PKB is required for suppression of cancer cell apoptosis and tumor progression in human colorectal carcinoma. Cancer 94: 3127–3134 [DOI] [PubMed] [Google Scholar]
- Kostrewa D et al. (2001) X-ray structure of junctional adhesion molecule: structural basis for homophilic adhesion via a novel dimerization motif. EMBO J 20: 4391–4398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukoetter MG et al. (2007) JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med 204: 3067–3076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Clevers H (2010) Coexistence of quiescent and active adult stem cells in mammals. Science 327: 542–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Manning BD, Cantley LC (2003) Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell 4: 257–262 [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S (2003) Mapping Wnt/β-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci USA 100: 3299–3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prota AE, Campbell JA, Schelling P, Forrest JC, Watson MJ, Peters TR, Aurrand-Lions M, Imhof BA, Dermody TS, Stehle T (2003) Crystal structure of human junctional adhesion molecule 1: implications for reovirus binding. Proc Natl Acad Sci USA 100: 5366–5371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson EA, Jiang L, Ivanov AI, Mandell KJ, Nusrat A, Parkos CA (2008) Cis-dimerization mediates function of junctional adhesion molecule A. Mol Biol Cell 19: 1862–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson EA, Lee WY, Capaldo CT, Nusrat A, Parkos CA (2009) Junctional adhesion molecule A interacts with Afadin and PDZ-GEF2 to activate Rap1A, regulate β1 integrin levels, and enhance cell migration. Mol Biol Cell 20: 1916–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson EA, Parkos CA (2009) Mechanisms of outside-in signaling at the tight junction by junctional adhesion molecule A. Ann NY Acad Sci 1165: 10–18 [DOI] [PubMed] [Google Scholar]
- Taurin S, Sandbo N, Qin Y, Browning D, Dulin NO (2006) Phosphorylation of β-catenin by cyclic AMP-dependent protein kinase. J Biol Chem 281: 9971–9976 [DOI] [PubMed] [Google Scholar]
- Vetrano S et al. (2008) Unique role of junctional adhesion molecule-a in maintaining mucosal homeostasis in inflammatory bowel disease. Gastroenterology 135: 173–184 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.