Abstract
Modification of proteins by ubiquitin (Ub) and Ub-like (Ubl) modifiers regulates a variety of cellular functions. The ability of Ub to form chains of eight structurally and functionally distinct types adds further complexity to the system. Ub-specific proteases (USPs) hydrolyse polyUb chains, and some have been suggested to be cross-reactive with Ubl modifiers, such as neural precursor cell expressed, developmentally downregulated 8 (NEDD8) and interferon-stimulated gene 15 (ISG15). Here, we report that USP21 cleaves Ub polymers, and with reduced activity also targets ISG15, but is inactive against NEDD8. A crystal structure of USP21 in complex with linear diUb aldehyde shows how USP21 interacts with polyUb through a previously unidentified second Ub- and ISG15-binding surface on the USP domain core. We also rationalize the inability of USP21 to target NEDD8 and identify differences that allow USPs to distinguish between structurally related modifications.
Keywords: ISG15, NEDD8, ubiquitin-specific protease, linkage specificity, cross-reactivity
Introduction
Modification of proteins with ubiquitin (Ub) is a principal mechanism of cellular regulation. Sophisticated enzymatic machineries attach the carboxy-terminus of Ub to lysine residues in substrates (Dye & Schulman, 2007). Most Ub in cells exists in a polymeric form, in which Ub molecules are linked through one of seven lysine residues (K6, K11, K27, K29, K33, K48 or K63) or through the amino-terminus (linear linkages). Differently linked Ub chains have distinct functions in cells (Komander, 2009). In addition to Ub, mammalian cells encode approximately 16 Ub-like (Ubl) modifiers, which are attached to proteins by similar mechanisms and have independent functions in cellular regulation (Hochstrasser, 2009). The closest relative of Ub is neural precursor cell expressed, developmentally downregulated 8 (NEDD8), which has key roles in the activation of Cullin E3 ligases and other cellular pathways (Rabut & Peter, 2008). Another Ubl modifier, interferon-stimulated gene 15 (ISG15), contains two Ubl domains and modifies proteins in response to viral infection (Dao & Zhang, 2005).
Dedicated enzymes remove Ub and Ubl modifications. Approximately 85 deubiquitinases (DUBs), comprising five structurally distinct families, are encoded in human cells (Komander et al, 2009; Reyes-Turcu et al, 2009). Ub-specific proteases (USPs) are the largest of these families (56 members) and most are large proteins with complicated domain architectures, sharing a catalytic USP domain (Ye et al, 2009). USPs are often involved in the regulation of cellular signalling pathways, and the family comprises tumour suppressors and oncogenes (Komander et al, 2009; Reyes-Turcu et al, 2009). Interestingly, several USPs have been reported to also target Ubl modifications. USP18 (also known as UBP43) is an ISG15-specific enzyme (Malakhov et al, 2002), and several other USPs can target both Ub and ISG15 (Catic et al, 2007). USP21 is the only USP with reported NEDD8 cross-reactivity (Gong et al, 2000). This DUB has been implicated in deubiquitination of histone H2A (Nakagawa et al, 2008) and receptor-interacting protein 1 (Xu et al, 2010). Proteomic analysis identified MARK (microtubule affinity-regulating) protein kinases and phosphatases as USP21 interactors (Sowa et al, 2009), suggesting roles for USP21 in cell signalling.
To further understand cross-reactivity in USP enzymes, we studied the ability of USP21 to target Ub, NEDD8 and ISG15. Biochemical analysis shows that USP21 can function on Ub and ISG15, but not on NEDD8. A crystal structure of the USP21 catalytic domain in complex with linear diUb aldehyde identified a second Ub-binding site on the USP core. Mutational analysis showed that this site contributes as an S2 site to polyUb chain binding and also to interaction with ISG15. The structure showed molecular determinants that prevent NEDD8 hydrolysis by USP21 and related USP domains.
Results and Discussion
USP21 acts against Ub and ISG15, not NEDD8
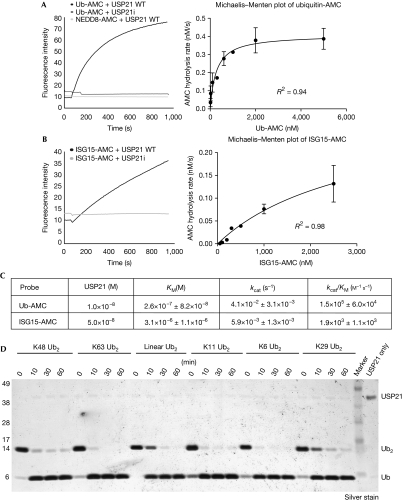
Human USP21 comprises 565 amino acids and a C-terminal catalytic USP domain (370 residues), as well as regions of high flexibility in the N-terminus. Full-length USP21 is unstable when expressed in bacteria, but the catalytic domain (residues 196–565, referred to as USP21) could be purified to homogeneity. USP21 hydrolysed the fluorogenic model substrate Ub-AMC (7-amino-4-methylcoumarin), but not NEDD8-AMC. Mutation of the catalytic Cys 221 to Ala (USP21i) rendered the protein inactive (Fig 1A). Furthermore, Ub-based suicide inhibitors covalently modified USP21, whereas NEDD8-based suicide inhibitors did not modify USP21 (supplementary Fig S1A online, and see below). By contrast, the Ub C-terminal hydrolase-L3 was active against both Ub- and NEDD8-AMC (supplementary Fig S1B online) and was similarly modified by suicide probes (supplementary Fig S1A online). The Cullin components of SCF (Skp1/Cul1/F-box) ligases are well-characterized substrates of NEDD8 modification in cells. USP21 was unable to function on NEDD8-modified Cul1 in vitro, in contrast to the COP9 signalosome (Enchev et al, 2010; supplementary Fig S1C online).
Figure 1.
Analysis of USP21 cross-reactivity against Ub, NEDD8 and ISG15. (A) USP21 and a catalytically inactive mutant USP21i were tested against Ub-AMC and NEDD8-AMC. Right panel: Michaelis–Menten kinetic analysis for Ub-AMC. Error bars represent s.d. from the mean. (B) USP21 activity against ISG15-AMC, as tested in (A). Right panel: Michaelis–Menten kinetic analysis for ISG15-AMC. (C) Kinetic parameters of USP21 cleavage of Ub-AMC and ISG15-AMC, derived from the plots in A and B. (D) Specificity analysis of USP21 against diUb of indicated linkages. Time course assays were resolved on 4–12% NuPAGE gels, followed by silver staining. ISG15, interferon-stimulated gene 15; NEDD8, neural precursor cell expressed, developmentally downregulated 8; PAGE, polyacrylamide gel electrophoresis; Ub, ubiquitin; USP, Ub-specific protease; WT, wild type.
Interestingly, USP21 was able to hydrolyse ISG15-AMC, albeit with lower activity (Fig 1B). Detailed kinetic analysis of USP21 against Ub-AMC produced parameters comparable to the related enzyme USP2 (Renatus et al, 2006; Fig 1C). Km and kcat values for ISG15-AMC cleavage by USP21 were 12-fold higher and 6-fold lower, respectively, compared with Ub-AMC cleavage, resulting in 70-fold specificity difference (kcat/Km) between ISG15 and Ub (Fig 1C). The reduction in kcat is intriguing, as the C-terminal tail of ISG15 is identical to Ub, and no other part of the modifier reaches the active site. This indicates that more allosteric activation mechanisms are active in USP enzymes that are induced by binding to a modifier.
Next, we analysed USP21 Ub chain linkage specificity. USP21 cleaved K6-, K11-, K29-, K48-, K63- and linear diUb (Fig 1D), as well as K11-, K48-, K63- and linear tetraUb, with similar activity (supplementary Fig S2 online). Hence, USP21 is a highly active deubiquitinating enzyme that might also deISGylate proteins.
Structure of USP21 in complex with linear diUb aldehyde
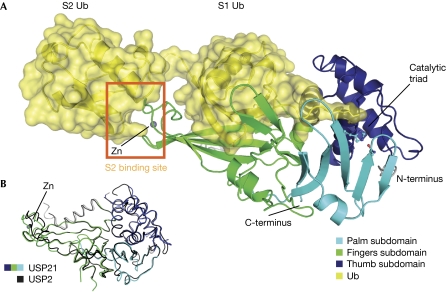
To understand the molecular basis for USP21 activity, a covalent complex of USP21 with non-cleavable linear diUb aldehyde was purified and crystallized. Data at 2.7 Å resolution were collected at the European Synchrotron Radiation Facility (ESRF) synchrotron (Grenoble, France), and the structure was determined by molecular replacement and subsequently refined (final statistics reported in supplementary Table S1 online). The asymmetric unit contained two USP21–diUb complexes. USP21 adopts the common three-subdomain architecture of USP enzymes, comprising thumb, finger and palm domains (Fig 2A), and is structurally similar to USP2 (root mean square deviation of 1.2 Å over 303 residues; Renatus et al, 2006; Fig 2B; supplementary Fig S3 online). The covalently attached proximal moiety of linear diUb binds in the S1 site of the enzyme, as in previous USP–Ub structures (Renatus et al, 2006). The distal Ub molecule extends from the moiety bound in the S1 site and is located at two positions within the two complexes in the asymmetric unit (supplementary Fig S4A online). In one arrangement, the distal molecule wraps around the fingers subdomain (Fig 2A; supplementary Fig S4A online), forming a second binding site with the back and tip of the fingers subdomain. In the second arrangement, the distal Ub cannot bind to the back of the fingers subdomain because of crystal packing, and projects away from the USP21 core. The electron density of this moiety is weak, indicating high mobility (supplementary Fig S4B online).
Figure 2.
Structure of USP21 in complex with linear diubiquitin aldehyde. (A) Structure of the human USP21 USP domain in complex with linear diUb aldehyde, shown in a cartoon representation. A yellow, semi-transparent surface covers the Ub molecules. Subdomains in USP21 are shown in dark blue (thumb), green (fingers) and light blue (palm). Residues of the catalytic triad and the zinc ion bound at the fingers subdomain are indicated. (B) Superposition of USP21 (coloured as in A) with USP2 (black, Protein Data Bank ID: 2hd5; Renatus et al, 2006). Ub, ubiquitin; USP, Ub-specific protease.
An S2 binding site in USP21 for linear Ub chains
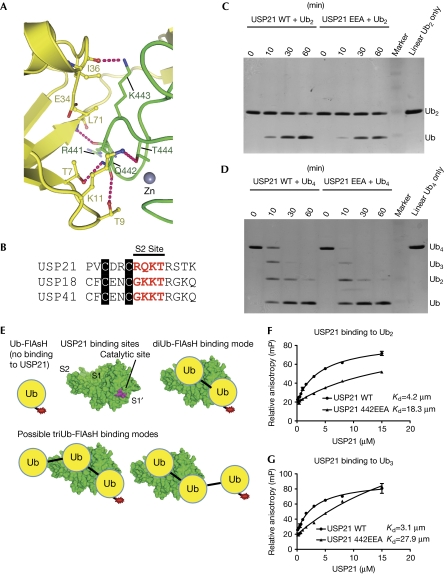
We further analysed the putative S2 binding site at the tip and the back of the fingers subdomain (Figs 2,3A), as an S2 binding site would have implications for activity. DUBs can cleave Ub polymers with exo activity (that is, hydrolyse single Ub moieties from the distal or proximal end of a chain) or with endo activity within a polymer. An S2 binding site in USP21 suggested endo activity against linear Ub chains (also see supplementary Fig S5A online).
Figure 3.
An S2 ubiquitin binding site in USP21 for linear diubiquitin and ISG15. (A) Structural detail of the interaction of the distal Ub in the USP21–diUb complex, corresponding to the boxed area in Fig 2. Hydrogen bonds are indicated by pink dotted lines. (B) Sequence alignment of the S2 site residues in USP21, USP18 and USP41. (C,D) DUB assays of USP21WT and USP21EEA against linear di- and tetraUb. (E) Possible interaction modes for di- and triUb (yellow circles) in USP21 (green). (F,G) USP21WT and USP21EEA binding to (F) linear diUb-FlAsH and (G) triUb-FlAsH measured by fluorescence anisotropy. Error bars represent s.d. from mean. DUB, deubiquitinase; Ub, ubiquitin; USP, Ub-specific protease; WT, wild type.
The USP21 S2 site is small (387 Å2) and consists of Arg 441, Gln 442, Lys 443 and Thr 444, which contact residues surrounding the Ile36 patch of Ub (Thr 7, Thr 9, Lys 11, Glu 34 and Leu 71; Fig 3A,B). USP21 residues forming the S2 site do not contribute to the S1 site. An USP21 triple mutant in which Gln 442, Lys 443 and Thr 444 were mutated to Glu, Glu and Ala, respectively, (USP21EEA) cleaved di- and tetraUb of any linkage similar to USP21WT (Fig 3C,D and data not shown). This indicated that the S2 binding site does not contribute to tetraUb hydrolysis. However, USP21EEA is still an active exo-DUB, that is able to processively cleave polymers from the distal end.
To understand the contribution of the S2 site to binding Ub polymers, we examined the binding of different fluorescent Ub chains to inactivated USP21i. Five residues of Ub at the C-terminus were replaced with a FlAsH-tag sequence preceded by Trp (WCCPGCC), which can be labelled by fluorescein derivatives. Fluorescently labelled monoUb does not bind to the S1 Ub-binding site of USP21i, presumably because the bulky fluorescent group does not fit the active-site groove (supplementary Fig S6 online). By contrast, a linear diUb with this sequence added to the proximal moiety can bind to the S1 and S1′ sites and a linear triUb can bind to the S2, S1 and S1′ sites of the enzyme (Fig 3E). Linear triUb could also only interact with the S1/S1′ sites, not benefitting from an S2 site (Fig 3E). Differences between di- and triUb binding therefore partly reflect a contribution of the S2 binding site.
Anisotropy measurements revealed a small but reproducible difference between di- and triUb binding to USP21i, in which triUb bound with 1.4-fold higher affinity (Fig 3F,G). By contrast, the USP21iEEA mutant bound to triUb with 1.5-fold lower affinity, compared with diUb (Fig 3F,G).
These different binding affinities suggest a small contribution of an S2 Ub binding site, as seen in the crystal structure (Fig 2; supplementary Fig S4 online). The S2 binding site is not essential for deubiquitination, as USP21 can cleave Ub chains with exo activity. However, more weak interactions with the S2 site might benefit endo cleavage of linear and perhaps also of K63-, K27- and K33-linked chains (see supplementary Fig S5A online). The S2 binding site might not, therefore, directly contribute to the catalytic efficiency, at least in vitro, but it probably participates in binding to longer Ub chains, for example on USP21 substrates. The development of further tools—particularly longer polyUb chains of defined linkages attached to model substrates—will help us to fully understand USP specificity and endo activity.
The S2 binding site is involved in deISGylation
ISG15 comprises two tandem Ubl domains and thereby structurally resembles non-cleavable linear diUb. It is possible that the Ubl moieties interact with both S1 and S2 sites of USP21.
Furthermore, the ISG15-specific enzyme USP18 and its homologue USP41 share a high degree of sequence conservation in the S2 site with USP21 (Fig 3B), whereas this sequence is not conserved in the remaining USPs (Ye et al, 2009). This supports the hypothesis that the S2 binding site might be involved in interactions with ISG15.
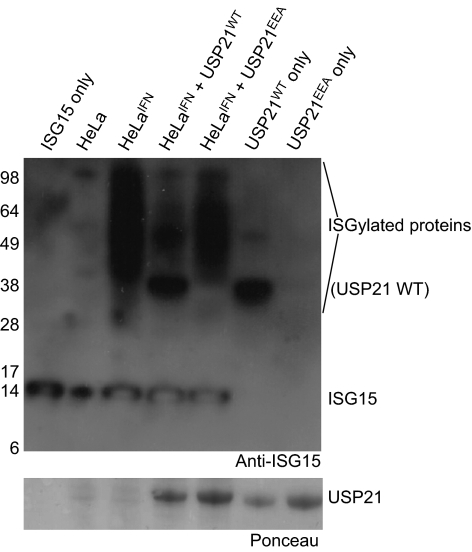
We examined the role of the USP21 S2 binding site in hydrolysing ISG15 from physiologically relevant targets. Interferon-β-treated HeLa cell lysates that are enriched in ISGylated proteins (Durfee et al, 2010; Fig 4) were incubated with USP21WT or with the S2-site mutant USP21EEA, and ISG15 modification was detected by western blotting. This analysis showed that USP21WT can function on endogenous ISG15-modified substrates; however, a mutant USP21 lacking the S2 Ub-binding site was impaired in its ability to hydrolyse ISG15 modifications (Fig 4).
Figure 4.
ISG15 deconjugation in HeLa cell lysates. HeLa cells were stimulated with IFN-β to induce ISG15 modification of endogenous proteins. Cell lysates were incubated with USP21WT or USP21EEA, resolved by SDS–polyacrylamide gel electrophoresis and western-blotted with an ISG15 antibody. USP21WT but not USP21EEA crossreacts with the polyclonal ISG15 antibody, possibly because of a QK sequence in USP21WT and ISG15, which is mutated in USP21EEA. The Ponceau-stained membrane below shows equal loading of the USP21 proteins. IFN, interferon; ISG15, interferon-stimulated gene 15; USP, Ub-specific protease; WT, wild type.
Overall, our data suggested that USP21 interacts similarly with ISG15 and linear diUb, and that the S2 site contacts the distal ISG15 Ubl-fold. Such a conformation of ISG15 would differ from the ISG15 crystal structure that displayed an interface between the two Ubl moieties (Narasimhan et al, 2005; supplementary Fig S5B online). It will be interesting to see how USP18 binds to ISG15, and whether it uses the S2 site in this interaction.
USP21 distinguishes Ub/Ubl C-termini
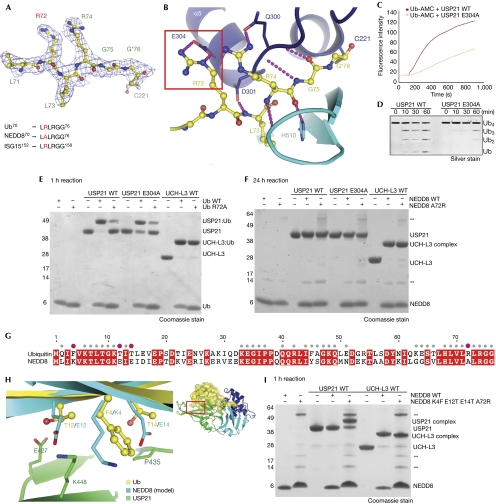
In the USP21–diUb structure, the Ub C-terminal LRLRGG sequence is well defined in the electron density maps (Fig 5A), and interacts extensively with the enzyme (Fig 5B), in a similar manner as observed for other USP family members. Most Ubl modifiers have divergent C-terminal sequences (for example, SUMO1: QEQTGG) that contribute to the specificity of their respective deconjugating enzymes. ISG15 contains the same C-terminus as Ub, whereas NEDD8 contains Ala 72 instead of Arg 72 (LALRGG, Fig 5A). This difference determines the specificity of NEDP1, the NEDD8-specific hydrolase (Shen et al, 2005).
Figure 5.
Structural basis for lack of USP21 activity against NEDD8. (A) C-terminus of Ub bound to the catalytic Cys residue of USP21. Residues are shown in stick representation with nitrogen atoms in blue and oxygen atoms in red. A 2∣Fo∣-∣Fc∣ electron density map is shown, contoured at 1 σ. An asterisk indicates the tetrahedral thiohemiacetal resulting from the aldehyde reaction. The C-terminal sequences of Ub, NEDD8 and ISG15 are shown below. (B) Interaction of the C-terminal region of Ub (yellow) with USP21 (blue). Purple dotted lines indicate hydrogen bonds. The Glu 304–Arg 72 interaction is highlighted. (C,D) Mutation of Glu 304 in USP21 leads to reduced deubiquitinase activity against (C) Ub-AMC and (D) linear tetraUb. (E,F) Suicide probe assays performed for indicated times at 23°C and visualized on SDS–polyacrylamide gel electrophoresis gels by Coomassie staining. Numbers to the left indicate molecular weight markers. (E) USP21WT and USP21E304A modification by Ub-C2Cl and UbR72A-C2Cl. UCH-L3 acts as a positive control. (F) USP21WT and USP21E304A modification by NEDD8-C2Cl and NEDD8A72R-C2Cl. (G) Sequence alignment of human NEDD8 and Ub. Residues marked by grey dots interact with the USP21 S1 site and purple dots indicate key differences. (H) Structural detail of the interaction between Ub β1/β2-strands and USP21, corresponding to the boxed area in the figure on the right. In the left image, NEDD8 (light blue) was superimposed onto Ub (yellow) at the S1 site. Differing interface residues in the β1/β2-loop of Ub/NEDD8 are shown in a stick representation. (I) USP21 reactivity towards suicide probes on the basis of NEDD8-C2Cl and a mutant NEDD8A72R, K4F, E12T, E14T-C2Cl. **Denotes contaminant bands originating from the mutant NEDD8 purification. ISG15, interferon-stimulated gene 15; NEDD8, neural precursor cell expressed, developmentally downregulated 8; Ub, ubiquitin; USP, Ub-specific protease; UCH, Ub C-terminal hydrolase; WT, wild type.
Ub Arg 72 interacts with an invariant Glu residue of the USP thumb domain (Glu 304 in USP21; Fig 5B). Mutation of Glu 304 to Ala (USP21E304A) significantly decreases USP21 activity against Ub-AMC and tetraUb (Fig 5C,D). We further analysed the ability of wild-type USP21 (USP21WT) and USP21E304A to be modified by a Ub-based suicide probe (UbC2Cl; Wilkinson et al, 2005) or by a Ub R72A mutant probe (UbR72AC2Cl; Fig 5E). USP21WT was quantitatively modified by UbC2Cl, however, modification of USP21E304A with UbC2Cl or of USP21WT with UbR72AC2Cl was incomplete, indicating reduced affinity (Fig 5E). Both suicide probes modified Ub C-terminal hydrolase-L3, which does not interact with Arg 72 (Misaghi et al, 2005; Fig 5E). Similar observations were made with ISG15-based suicide probes (supplementary Fig 7 online). Hence, interaction between Arg 72 (or Arg 153 in ISG15) and USP21 Glu 304, which is conserved in all active USP domains, is essential for processing Ub (and ISG15) modifications.
NEDD8 β1/β2 residues preclude USP binding
Surprisingly, we were unable to generate a mutant NEDD8 that was able to bind to USP21 by simply mutating Ala 72 to Arg, to mimic the Ub/ISG15 C-terminus (Fig 5F). This indicated that other differences between Ub and NEDD8 restrict USP21 activity towards NEDD8. Superposition of NEDD8 onto the S1 Ub indicated more differences in the β1- and β2-strands between the modifiers (Fig 5G,H). In this region, Ub Phe 4, Thr 12 and Thr 14 are replaced with Lys 4, Glu 12 and Glu 14 in NEDD8 (Fig 5G,H). USP21 and other USP domains (for example, USP2; Renatus et al, 2006) contact this Ub surface with charged residues from the first two β-strands of the fingers subdomain (Fig 5H). Modelling of wild-type NEDD8 into the S1 site results in steric clashes and charge repulsion (Fig 5H). However, a NEDD8 suicide probe in which four residues were changed to their Ub equivalents (NEDD8R72A, K4F, E12T, E14T; Fig 5I) was able to react with USP21. Hence, both the C-terminal Arg 72 and residues on the β1/β2-strand of Ub contribute to the ability of USP21 to discriminate between Ub and NEDD8. As far as we know, this is the first description of the β1/β2-loop of the Ubl-fold as a key determinant enabling enzymes to distinguish between modifiers such as NEDD8 and Ub. The USP domain is the only Ub-binding fold known to interact with this Ub surface. It will be interesting to see whether other proteins use similar mechanisms to distinguish between Ub and NEDD8.
Conclusions
Ub-specific proteases are key regulators of cellular signalling pathways. However, most USPs remain poorly characterized, and their cross-reactivity with other Ubl modifiers is not well-understood. We characterize USP21 as a DUB that hydrolyses all Ub chain types and might also target ISG15 modifications. An S2 site allows endo-DUB activity and interaction with both Ubl-folds on ISG15. Newly synthesized proteins are cotranslationally modified with ISG15 in response to viral infection (Durfee et al, 2010). It is tempting to speculate that the cellular substrates of USP21 are protected from ubiquitination and also, during viral infection, from ISG15 modification. It is now important to identify these substrates. USP21 comprises a unique N-terminal extension that might determine substrate specificity and/or localization of the enzyme. Our analysis of the structural and catalytic properties of USP21 might benefit future studies of this enzyme.
Methods
Crystallization of USP21–diUb. USP21 (residues 196–565) was expressed in Rosetta2 pLysS cells and affinity purified by using an N-terminal His6-SUMO-tag. After cleaving the SUMO tag with Sentrin-specific protease (SENP1), a final gel-filtration step in buffer A (25-mM Tris, 200-mM NaCl, 5-mM dithiothreitol, pH 7.4) resulted in pure protein. For crystallization of a diUb complex, purified USP21 was incubated with a slight excess of diUb aldehyde in buffer A, and again subjected to gel filtration. Complex crystals were grown at a concentration of 6.7 mg ml−1 from 15% (v/v) PEG 8000 and 0.2-M ammonium sulphate. Diffraction data at 2.7 Å resolution collected at the ESRF synchrotron were phased by molecular replacement using USP2 (Protein Data Bank ID: 2hd5; Renatus et al, 2006) and monoUb (Protein Data Bank ID: 1ubq; Vijay-Kumar et al, 1987) as search models. Refinement statistics can be found in supplementary Table S1 online.
Analysis of linear Ub-chain binding to inactivated USP21. MonoUb, and linear di- and triUb with C-terminal FlAsH-tag sequence was produced according to Akutsu et al (2011). Binding assays were performed in a 384-well format using a PheraStar FS fluorescence spectrometer. Linear chains were diluted to 80 nM in FlAsH buffer (50-mM Tris, 50-mM NaCl, 0.1% β-mercaptoethanol, pH 7.6). USP21WT or USP21i were serially diluted in FlAsH buffer to the indicated concentration range (Fig 3F,G). A volume of 10 μl of the fluorescent Ub chain was mixed with an equal volume of USP21i at different concentrations and incubated at 23°C for 1 h before measurement. A control was used for either linear di- or triUb molecules in which 10 μl of FlAsH buffer was added instead. This control was also used for the normalization of anisotropy readings.
Detailed experimental procedures can be found in the supplementary material online.
Coordinates and structure factors have been deposited with the Protein Data Bank, accession code 2y5b.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank Sylvie Urbe and Michael Clague (University of Liverpool, UK) for constructs and for sharing unpublished data, and Anja Bremm, Yogesh Kulathu, Satpal Virdee, Georg Blaser, Stephen McLaughlin and Martin Busch for reagents and discussions.
Footnotes
The authors declare that they have no conflict of interest.
References
- Akutsu M, Ye Y, Virdee S, Chin J, Komander D (2011) Molecular basis for ubiquitin and ISG15 cross-reactivity in viral OTU domains. Proc Natl Acad Sci USA 108: 2228–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catic A, Fiebiger E, Korbel GA, Blom D, Galardy PJ, Ploegh HL (2007) Screen for ISG15-crossreactive deubiquitinases. PLoS ONE 2: e679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao CT, Zhang DE (2005) ISG15: a ubiquitin-like enigma. Front Biosci 10: 2701–2722 [DOI] [PubMed] [Google Scholar]
- Durfee LA, Lyon N, Seo K, Huibregtse JM (2010) The ISG15 conjugation system broadly targets newly synthesized proteins: implications for the antiviral function of ISG15. Mol Cell 38: 722–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye BT, Schulman BA (2007) Structural mechanisms underlying posttranslational modification by ubiquitin-like proteins. Annu Rev Biophys Biomol Struct 36: 131–150 [DOI] [PubMed] [Google Scholar]
- Enchev RI, Schreiber A, Beuron F, Morris EP (2010) Structural insights into the COP9 signalosome and its common architecture with the 26S proteasome lid and eIF3. Structure 18: 518–527 [DOI] [PubMed] [Google Scholar]
- Gong L, Kamitani T, Millas S, Yeh ET (2000) Identification of a novel isopeptidase with dual specificity for ubiquitin- and NEDD8-conjugated proteins. J Biol Chem 275: 14212–14216 [DOI] [PubMed] [Google Scholar]
- Hochstrasser M (2009) Origin and function of ubiquitin-like proteins. Nature 458: 422–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komander D (2009) The emerging complexity of protein ubiquitination. Biochem Soc Trans 37: 937–953 [DOI] [PubMed] [Google Scholar]
- Komander D, Clague MJ, Urbé S (2009) Breaking the chains: structure and function of the deubiquitinases. Nat Rev Mol Cell Biol 10: 550–563 [DOI] [PubMed] [Google Scholar]
- Malakhov MP, Malakhova OA, Kim KI, Ritchie KJ, Zhang DE (2002) UBP43 (USP18) specifically removes ISG15 from conjugated proteins. J Biol Chem 277: 9976–9981 [DOI] [PubMed] [Google Scholar]
- Misaghi S, Galardy PJ, Meester WJN, Ovaa H, Ploegh HL, Gaudet R (2005) Structure of the ubiquitin hydrolase UCH-L3 complexed with a suicide substrate. J Biol Chem 280: 1512–1520 [DOI] [PubMed] [Google Scholar]
- Nakagawa T et al. (2008) Deubiquitylation of histone H2A activates transcriptional initiation via trans-histone cross-talk with H3K4 di- and trimethylation. Genes Dev 22: 37–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan J, Wang M, Fu Z, Klein JM, Haas AL, Kim J-JP (2005) Crystal structure of the interferon-induced ubiquitin-like protein ISG15. J Biol Chem 280: 27356–27365 [DOI] [PubMed] [Google Scholar]
- Rabut G, Peter M (2008) Function and regulation of protein neddylation. ‘Protein modifications: beyond the usual suspects’ review series. EMBO Rep 9: 969–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renatus M et al. (2006) Structural basis of ubiquitin recognition by the deubiquitinating protease USP2. Structure 14: 1293–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Turcu FE, Ventii KH, Wilkinson KD (2009) Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu Rev Biochem 78: 363–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L-n, Liu H, Dong C, Xirodimas D, Naismith JH, Hay RT (2005) Structural basis of NEDD8 ubiquitin discrimination by the deNEDDylating enzyme NEDP1. EMBO J 24: 1341–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowa ME, Bennett EJ, Gygi SP, Harper JW (2009) Defining the human deubiquitinating enzyme interaction landscape. Cell 138: 389–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay-Kumar S, Bugg CE, Cook WJ (1987) Structure of ubiquitin refined at 1.8 A resolution. J Mol Biol 194: 531–544 [DOI] [PubMed] [Google Scholar]
- Wilkinson KD, Gan-Erdene T, Kolli N (2005) Derivitization of the C-terminus of ubiquitin and ubiquitin-like proteins using intein chemistry: methods and uses. Meth Enzymol 399: 37–51 [DOI] [PubMed] [Google Scholar]
- Xu G et al. (2010) Ubiquitin-specific peptidase 21 inhibits tumor necrosis factor alpha-induced nuclear factor κB activation via binding to and deubiquitinating receptor-interacting protein 1. J Biol Chem 285: 969–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Scheel H, Hofmann K, Komander D (2009) Dissection of USP catalytic domains reveals five common insertion points. Mol Biosyst 5: 1797–1808 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.