Abstract
Cranial neural crest cells (NCCs) require neuropilin signaling to reach and invade the branchial arches. Here, we use an in vivo chick model to investigate whether the neuropilin-1 knockdown phenotype is specific to the second branchial arch (ba2), changes in NCC behaviors and phenotypic consequences, and whether neuropilins work together to facilitate entry into and invasion of ba2. We find that cranial NCCs with reduced neuropilin-1 expression displayed shorter protrusions and decreased cell body and nuclear length-to-width ratios characteristic of a loss in polarity and motility, after specific interaction with ba2. Directed NCC migration was rescued by transplantation of transfected cells into rhombomere 4 of younger hosts. Lastly, reduction of neuropilin-2 expression by shRNA either solely or with reduction of neuropilin-1 expression did not lead to a stronger head phenotype. Thus, NCCs, independent of rhombomere origin, require neuropilin-1, but not neuropilin-2 to maintain polarity and directed migration into ba2.
Keywords: Neural crest, cell migration, chick, neuropilins, confocal, time-lapse
Introduction
During vertebrate development, cranial neural crest cells (NCCs) delaminate from the neural tube, travel long distances to specific branchial arches and contribute to important head and neck structures (Trainor and Krumlauf, 2001; Santagati and Rijli, 2003). The cranial NCC migratory pattern emerges in a rostrocaudal manner as cells exit from all segments (rhombomeres (r)) of the hindbrain and are dynamically sculpted into three discrete migratory streams (Le Douarin and Kalcheim, 1999; Farlie et al., 1999). Within each migratory stream, cranial NCCs move in a directed manner by responding to microenvironmental and cell-to-cell contact cues (Teddy and Kulesa, 2004; Olesnicky-Killian et al., 2009; Carmona-Fontaine et al., 2008). While our understanding of how cranial NCCs exit the neural tube and select a migratory pathway has advanced (Graham et al., 2004; Kulesa et al., 2004; Taneyhill, 2008; Berndt et al., 2008), it is still unclear how signals regulate NCC entry into and invasion of the branchial arches.
Multiple studies have implicated neuropilins in the proper segregation of cranial NCCs into discrete streams, in zebrafish (Yu and Moens, 2005), chick (Osborne et al., 2005; Eickholt et al., 1999; McLennan and Kulesa, 2007) and mouse (Gammill et al., 2007; Schwarz et al., 2008). Both neuropilin-1 and neuropilin-2 are expressed by cranial NCCs and have been shown to be involved in the initial segmental migration of cranial NCCs from the neural tube (Eickholt et al., 1999; Chilton and Guthrie, 2003; Osborne et al., 2005; Gammill et al., 2007; McLennan and Kulesa, 2007; Schwarz et al., 2008). Recently, it was shown that neuropilin-1 plays a role in the invasion of cranial NCCs into second branchial arch (ba2), the target site of these NCCs (McLennan and Kulesa, 2007). When neuropilin-1 expression was knocked down in premigratory cranial NCCs in vivo, by transfection with a neuropilin-1 siRNA-EGFP construct (Np-1 siRNA) (Bron et al., 2004), the NCCs failed to fully invade the ba2 microenvironment. Instead, Np-1 siRNA transfected NCCs arrived at the entrance into ba2 and stopped, as other non-transfected cranial NCCs properly invaded the proximal and distal portions of the ba2 microenvironment (McLennan and Kulesa, 2007).
What is unclear is whether this observed neuropilin-1 knockdown phenotype is specific to the second branchial arch and whether loss of the neuropilin-1 function causes sustained loss of cranial NCC polarity and migratory ability. If cranial NCCs display changes in directed cell migration at the entrance to ba2, it is not known whether they recover to participate in cranial NC-derived tissues, including the cranial ganglia and bones of the face. Lastly, since neuropilin-1 and neuropilin-2 are expressed by r4 NCCs, it is unclear whether they cooperate to facilitate proper NCC entry into and invasion of ba2. That is, whether loss of either neuropilin-2 expression solely or with reduction of neuropilin-1 expression leads to a stronger head phenotype than observed with reduction of neuropilin-1 expression alone. Here, we combine loss of function, chick embryo microsurgery, and in vivo confocal time-lapse microscopy to address these questions. By analyzing cell behaviors of Np-1 siRNA transfected cranial NCCs either in electroporated embryos or embryos receiving electroporated cranial NCC tissue transplants, we determine the dynamics of loss of neuropilin-1 function and phenotypic consequences. By development of an Np-2 shRNA and electroporation of a combination of Np-1 siRNA and Np-2 shRNA constructs into premigratory cranial NCCs, we study the functions of multiple neuropilins in cranial NCC migration.
Results
The failure of Np-1 siRNA transfected NCCs to invade ba2 is specific to the local microenvironment and not the NCC rhombomere of origin
We have previously shown that Np-1 siRNA transfected r4 NCCs transplanted directly into or near the ba2 microenvironment failed to spread out from the transplant site (McLennan and Kulesa, 2007). Our interpretation of this data was that neuropilin-ligand interactions were important throughout the ba2 microenvironment, rather than solely near the arch entrance.
To determine the specificity of the Np-1 siRNA r4 NCC phenotype to the second branchial arch, we moved NCCs that do not typically populate ba2, into the second branchial arch microenvironment. Specifically, we transplanted tissue containing premigratory r7 NCCs transfected with Np-1 siRNA and DiI-labeled, directly onto the r4 NCC migratory pathway near the ba2 entrance in HH Stage 10–12 embryos (Fig. 1A). We selected r7 NCCs, since in Np-1 siRNA transfected chick embryos, we only observed an aberrant NC-free zone in the anterior portion of ba3, but normal r7 NCC invasion into ba4 (Fig. S1). In embryos receiving tissue electroporated with Np-1 siRNA, Np-1 siRNA transfected r7 NCCs remained at or near the transplant site (Fig. 1D-F; green-colored cells). Some transplanted NCCs moved back towards the neural tube (Fig. 1D,E; arrowhead) and DiI-labeled non-transfected NCCs entered into ba2 (Fig. 1D-F; red-colored cells). In contrast, both co-transplanted, non-transfected DiI-labeled r7 NCCs and control EGFP transfected transplanted r7 NCCs migrated statistically further from the center of the transplant site (80.6% compared to 46.1% of the distance DiI-labeled cranial NCCs migrated respectively) (Fig. 1B, C, F; arrows).
Figure 1. r7 NCCs transfected with Np-1 siRNA fail to be invasive when transplanted directly into the ba2 microenvironment entrance.

(A) A schematic representation of the r7 to ba2 transplantation technique and how the quantitative measurements were calculated. (B-C) A static confocal image of a typical host embryo, 24 hours after a subpopulation of DiI-labeled (red), EGFP-transfected (green) r7 NCCs were transplanted directly into the ba2 microenvironment entrance, n=9. Both the transfected (green) and untransfected (red) NCCs were able to migrate from the transplant site. (D-E) A static confocal image of a host embryo, 24 hours after a subpopulation of DiI-labeled (red), Np-1 siRNA-transfected (green) r7 NCCs were transplanted into the ba2 microenvironment entrance, n=11. Only untransfected NCCs (red) were able to migrate from the transplant site, into the ba2 microenvironment. (F) Quantitative analysis of the length the transfected (green) NCCs migrated from the center of the transplant site as a percentage of the length the untransfected (red) NCCs migrated from the center of the transplant site. The scale bars are 100um. The notations are r, rhombomere, ba, branchial arch, L, length from the center of the transplant, *, significantly different, p<0.05.
To test whether transplanted Np-1 siRNA transfected r7 NCCs failed to migrate independent of the neural tube microenvironment, we transplanted premigratory r7 NCCs and surrounding tissue, directly into r4 of unlabeled host embryos. The transplanted r7 NCCs were able to migrate from r4 towards the ba2 microenvironment appropriately when transfected with control EGFP or with Np-1 siRNA (Fig. S1).
Np-1 siRNA transfected cranial NCCs that fail to invade the second branchial arch display characteristics of loss of cell polarity and motility
We have shown that Np-1 siRNA transfected r4 NCCs stopped after encountering the ba2 microenvironment and have fewer filopodia than EGFP only transfected cranial NCCs (McLennan and Kulesa, 2007). To determine the extent to which neuropilin-ligand interactions affected cranial NCC behaviors, we measured changes in filopodial dynamics, cell and nuclear shape, and cell motility at the entrance to ba2 in detail (Fig. 2A,B). Time-lapse confocal analysis revealed that as Np-1 siRNA transfected cranial NCCs entered the ba2 microenvironment), they stopped and collapsed leading edge filopodia (compare Fig. 2C-F; asterisk). Instead of long filopodial extensions and translocation of the cell body to follow the cell’s leading edge, we observed rounded NCC shapes and multiple, shortened filopodial protrusions (Fig. 2C-F, arrows). Transfected cranial NCCs showed severely reduced motility (Fig. 2C-F) and did not re-route their trajectories and move to other locations.
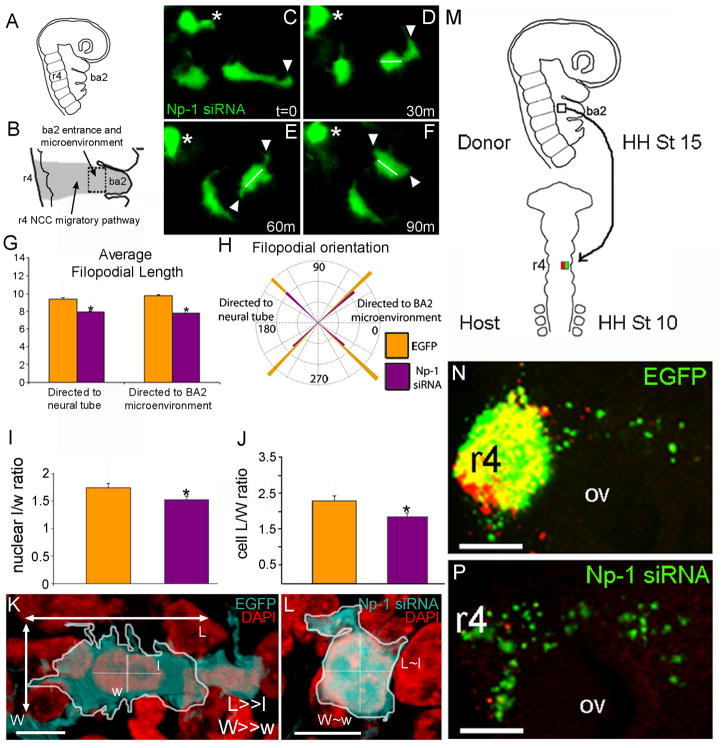
Figure 2. Np-1 siRNA transfected r4 NCCs display characteristics consistent with loss of polarity and motility, but recover directed migration after transplantation into rhombomere 4 of younger host embryos.
(A, B) Schematic representation of the ba2 microenvironment showing the location of the Np-1 siRNA phenotype. (C-F) Selected images from a time-lapse imaging session. The Np-1 siRNA transfected cranial NCC (green) stopped and collapsed leading edge filopodia (asterisk, t=0–90m; arrowheads, t=0–30m), remained in the same location and displayed short, dynamic filopodia in random directions (arrows, 30m–60m). Np-1 siRNA transfected cranial NCCs did not reroute trajectories, but cell body orientation actively changed (white line, 30–90m). (G) Quantitative analysis of the average length of filopodia, n=703 EGFP filopodia, n=385 Np-1 siRNA filopodia. (H) Quantitative analysis of filopodia orientation, n=703 EGFP filopodia, n=385 Np-1 siRNA filopodia. (I-J) Quantitative analysis of the nuclear and cell L/W aspect ratios. (K) Static confocal image of a cryostat section showing typical EGFP transfected cranial NCCs stained with DAPI to calculate the cell body and nuclear aspect ratios, n=41 cranial NCCs. (L) Static confocal image of typical Np-1 siRNA transfected cranial NCC in cryostat section stained with DAPI to calculate the same ratios, n=40 cranial NCCs. (M) A schematic representation of the ba2 to r4 transplantation technique. (N) A static confocal image of a host embryo, 24 hours after a subpopulation of DiI-labeled (red), EGFP-transfected (green) migratory r4 cranial NCCs from the ba2 microenvironment entrance were transplanted heterochronically and heterotopically into r4, n=8. Both the transfected (green) and untransfected (red) cranial NCCs were able to migrate from the transplant site towards the ba2 microenvironment. (P) A static confocal image of a host embryo, 24 hours after a subpopulation of DiI-labeled (red), Np-1 siRNA-transfected (green) migratory r4 cranial NCCs from the ba2 microenvironment entrance were transplanted heterochronically and heterotopically into r4, n=10. Both the transfected (green) and non-transfected (red) cranial NCCs were able to migrate from the transplant site towards the ba2 microenvironment. The notations are r, rhombomere, ba, branchial arch, ov, otic vesicle. The scale bars are 10um in K-L, and are 50um in M-P. The notations are W, width, L, length *, significantly different, p<0.05.
Static 3D confocal imaging showed that r4 NCCs transfected with Np-1 siRNA had shorter filopodia when compared to r4 NCCs transfected with EGFP only, at the ba2 microenvironment entrance (Fig. 2G). This morphology was particularly significant for filopodia directed towards the ba2 microenvironment (Fig. 2H). However, we found no significant difference in the orientation of filopodia when r4 NCCs transfected with control EGFP or Np-1 siRNA were compared (Fig. 2H).
To confirm a loss in individual NCC polarity of Np-1 siRNA transfected r4 NCCs, we analyzed the cell body and nuclear shape changes in 3D static confocal images of the entrance to the ba2 microenvironment (Fig. 2I-L). Specifically, we calculated the length (L) and width (W) of each cranial NCC nucleus and cell body and compared the L/W ratios for each case (Fig. 2I-L). An L/W ratio of 1 represented a rounded nucleus and/or rounded cell body. We found that the nuclei and cell bodies of Np-1 siRNA transfected r4 NCCs were significantly rounder than the nuclei and cell bodies of EGFP transfected r4 NCCs (Fig. 2I-L; 1.53±0.1 and 1.75±0.1 respectively for nuclei, and 1.85±0.1 and 2.30±0.1, respectively for cell bodies). These results showed that both the cell body and nuclear shape of Np-1 siRNA transfected NCCs were altered after contact with the ba2 microenvironment.
Np-1 siRNA transfected cranial NCCs regain their migratory ability and direction after transplantation into rhombomere 4 of younger host embryos
Cranial NCCs that stopped at the second branchial arch entrance showed severely reduced motility (Fig. 2C-F). To test whether cranial NCC migratory ability could be rescued, Np-1 siRNA or EGFP only transfected cranial NCCs (18 hours after transfection) were excised from the ba2 microenvironment entrance and transplanted heterochronically and heterotopically into r4 of younger, unlabeled HH Stage 8–9 host embryos (Fig. 2M-P). After 24 hours of re-incubation, transplanted Np-1 siRNA and EGFP only transfected cranial NCCs were distributed along the typical r4 migratory pathway (Fig. 2N,P), demonstrating that these cells were able to regain their migratory ability and undergo directed migration towards ba2.
Cranial NCCs transfected with Np-1 siRNA do not contribute to NCC-derived tissues, however these tissues form appropriately
To investigate the extent Np-1 siRNA transfected r4 NCCs contributed to normal NC-derived tissue, we examined cranial ganglia and bone formation (Fig. 3). Together with placodes, cranial NCCs contribute to the formation of the cranial ganglia (McCabe and Bronner-Fraser, 2008). Twenty-four hours after re-incubation, embryos were screened for high levels of r4 NCC transfection and then re-incubated to E4.5. Cranial regions were excised from these embryos, cryostat sectioned and the cranial ganglia were labeled with BEN (a marker for neuronal cell surface glycoprotein; Pourquie et al., 1990). When r4 NCCs were transfected with control EGFP, EGFP-positive r4 NCCs were observed in the forming cranial ganglia (Fig. 3A). Surprisingly, when r4 NCCs were transfected with Np-1 siRNA, very few, EGFP-positive cells were found in the cranial ganglia (Fig. 3B). Even though Np-1 siRNA transfected cranial NCCs appeared to fail to survive at these later developmental stages, the cranial ganglia were still able to form, as indicated by BEN staining (Fig. 3B).
Figure 3. R4 NCC-derived structures form appropriately even though Np-1 siRNA transfected r4 NCCs do not survive long term.
(A) A cryostat section through the cranial ganglia of an EGFP transfected embryo at E4.5, stained with BEN, n=6. (B) A cryostat section through the cranial ganglia of an Np-1 siRNA transfected embryo at E4.5, stained with BEN, n=8. (C, E) A cryostat section through ba2 of an EGFP transfected embryo at E3.5, n=5 and E4.5, n=8. (D, F) A cryostat section through ba2 of an Np-1 siRNA transfected embryo at E3.5, n=6 and E4.5, n=10. (G, H) Cartilage stains of the lower jaw of E9.5 embryos. (G) EGFP, n=9. (H) Np-1 siRNA, n=4. The scale bars are 100um for A-G, 50um for H-I. The notations are ba, branchial arch, CG, cranial ganglia, Mc, Meckel’s cartilage, Cb, ceratobranchial, Bh, basihyal, Bb, basibranchial, *, electroporated side of embryo.
To further investigate the timing of the death of Np-1 siRNA transfected cranial NCCs, we examined transfected cells in E3.5 and E4.5 embryos. At E3.5, there were fewer Np-1 siRNA transfected cranial NCCs (compare Fig. 3C,D). At E4.5, the Np-1 siRNA transfected cranial NCCs were very few in number along the r4 migratory route (Fig. 3F). In contrast, control EGFP transfected r4 NCCs were present throughout the ba2 microenvironment and along the migratory route in both E3.5 and E4.5 embryos (Fig. 3C,E).
To examine bone formation for possible defects, we studied the formation of the lower jaw and hyoid bones at E9.5. Components of Meckel’s cartilage, ceratobranchial, basihyal and basibranchial are derived from r3-r5 NCCs (Couly et al., 1996; Schneider and Helms, 2003). The hyoid bone formed normally when transfected with either control EGFP or Np-1 siRNA, (compare structures in Fig. 3G, H). Thus, Np-1 siRNA transfected r4 NCCs that stopped after encountering the ba2 microenvironment lost polarity and motility, survived through E3.5, and did not participate in NC-derived tissues.
Neuropilin-2 knockdown does not affect cranial NCC entry into the second branchial arch, but causes trunk NCCs to invade caudal somite-halves
To study the in vivo function of neuropilin-2 during the cranial NCC entry into and invasion of the second branchial arch, we labeled premigratory r4 NCCs with DiI, co-electroporated in neuropilin-2 shRNA (Np-2 shRNA) and examined the r4 NCC migratory stream 14–24 hours after re-incubation (Fig. 4). Our Np-2 shRNA was showed to effectively cause neuropilin-2 expression knockdown (Fig. 4A). In embryos transfected with Np-2 shRNA, we observed no abnormal phenotype for NCC entry into and invasion of the second branchial arch (Fig. 4B; compare the positions of the green- and red-colored cells in ba2). The dimensions of the r4 NCC migratory stream, composed of Np-2 shRNA transfected r4 NCCs, were statistically equivalent to the r4 NCC migratory stream composed of DiI-labeled r4 NCCs (Fig. 4C). Specifically, DiI-labeled cranial NCCs migrated 99.6% (±0.4%) of the distance from the neural tube to the distal tip of ba2 and populated 100% the width of ba2 (Fig. 4C). Np-2 shRNA transfected cranial NCCs migrated 97.1% (±3%) of the distance from the neural tube to the distal tip of ba2 and 90.9% (±5.7%) of the width of ba2 (Fig. 4C).
Figure 4. Knockdown of neuropilin-2 expression alone or in combination with neuropilin-1 does not show a stronger branchial arch phenotype.
(A) RT-PCR demonstrated that Np-2 shRNA transfected cells had reduced neuropilin-2 expression levels. (D,E) Static confocal images of the initial migration of trunk NCCs, 18 hours after electroporation. (D) Trunk NCCs transfected with EGFP only, n=7. The NCCs have started their initial migration towards and through the rostral half somite. (E) Trunk NCCs transfected with Np-2 shRNA, n=8. NCCs have entered both the rostral and caudal half somites. (B) Static confocal image of the r4 NCC stream in which cranial NCCs were transfected with Np-2 shRNA and labeled with DiI, n=16. (F) Static confocal image of the r4 NCC stream in which cranial NCCs were transfected with Np-1 siRNA and Np-2 shRNA and labeled with DiI, n=19. (C) Quantitative measurements of the distance Np-2 shRNA transfected cranial NCCs and DiI-labeled cranial NCCs migrated from the neural tube into ba2 as a percentage of the distance from the neural tube to the distal end of ba2, and the anterior-posterior width cranial NCCs spread out in ba2 as a percentage of the total width of ba2, n=7. (G) Quantitative measurements of the distance Np-1 siRNA/Np-2 shRNA transfected cranial NCCs and DiI-labeled cranial NCCs migrated, and the anterior-posterior width cranial NCCs spread out in ba2, n=11. The scale bars are 100um. The notations are R, rostral, C, caudal, r, rhombomere, ba, branchial arch, *, significantly different, p<0.01.
To determine whether neuropilin-2 was involved in the segmental migration of chick trunk NCCs and confirm the effectiveness of our Np-2 shRNA construct, we electroporated Np-2 shRNA into trunk NCCs. Neuropilin-2 has been shown to be involved in proper mouse trunk NCC migration (Gammill et al., 2006). In the trunk region in control embryos, NCCs migrated through the rostral half-somites in a typical, segmented manner (Fig. 4D). However, 18 hours after transfection with Np-2 shRNA, the initial trunk NCC segmental migratory pattern was disrupted as some NCCs migrated into typical NCC-free zones (the caudal half-somites) (compare Fig. 4D,E). The trunk NCC phenotype was transient and at 42hours after electroporation, the proper segmental migration pattern was recovered (data not shown).
Dual knockdown of neuropilin-1 and neuropilin-2 expression results in a defect in NCC entry into and invasion of the second branchial arch
To determine whether neuropilin-1 and −2 have a level of functional redundancy, we co-electroporated the Np-1 siRNA and Np-2 shRNA constructs into the hindbrain and DiI-labeled premigratory r4 NCCs. Measurements of the r4 NCC migratory stream dimensions revealed that when both neuropilins were knocked down, there was a resulting failure of cranial NCC invasion of ba2 (Fig. 4F). Np-1 siRNA/Np-2 shRNA transfected cranial NCCs migrated 85.5% (±4.2%) of the distance from the neural tube to the distal tip of ba2 and 71.5% (±5.7%) of the width of ba2 (Fig. 4G). DiI-labeled cranial NCCs migrated 98.7% (±1.2%) of the distance from the neural tube to the distal tip of ba2 and 97% (±2.1%) of the width of ba2 (Fig. 4G). Knockdown of the expression levels of both neuropilins had no noticeable affect on the early formation and maintenance of the r4 NCC migratory stream (data not shown), when analyzed 14–24 hours after electroporation.
Discussion
We used the chick cranial neural crest cell (NCC) migratory pattern as a model system to study the signals that regulate NCC entry into and invasion of the second branchial arch (ba2). We addressed specific questions related to our previous result (neuropilin-1 is required for invasion of ba2 (McLennan and Kulesa, 2007)), and discovered 4 main conclusions. First, the failure of a cranial NCC to enter into ba2 was independent of its rhombomere of origin, but required neuropilin-1 expression. Second, cranial NCC-ba2-microenvironment interactions dramatically altered cell migratory behaviors and protrusive activity characteristic of a loss of polarity and motility. The failure of cranial NCCs to invade ba2 was not due to a delay in migration, as cells survived through E3.5, but did not participate in cranial NC-derived ba2 structures. Third, cranial NCC directed migration was rescued by transplantation of stopped cells to a permissive microenvironment, such as the rhombencephalon of younger host embryos. Fourth, reduction of neuropilin-2 expression solely or in combination with reduction of neuropilin-1 expression either did not affect or dramatically affected cranial NCC entry into and invasion of ba2, respectively, but not more severe of the phenotype with loss of neuropilin-1 expression alone.
Transplantation experiments confirmed that failure of cranial NCCs to enter into and invade the second branchial arch was due to a loss of neuropilin-1 direct interactions with the ba2 microenvironment, rather than the rhombomere from which the NCCs originated (Fig. 1). With this data, it was tempting to speculate that our phenotype was due to a specific molecular fingerprint of the ba2 and anterior ba3 microenvironments (Fig. 5), requiring neuropilin-1-ligand interactions for cells to enter into and invade distal positions within the arches. Indeed, our results support previous data that show cranial NCCs may reach the entrance to the branchial arches, but then fail to enter into and/or properly colonize proximal and distal sites within the arches. For example, loss of the endothelin-1-mediated endothelin A receptor (Ednra), expressed by migratory and post-migratory cranial NCCs leads to defects in lower jaw and neck structures (Abe et al., 2007). Absence of Ednra signaling results in increased cranial NCC apoptosis in proximal first branchial arch and distal bone loss in the lower jaw, suggesting Ednra signaling is required after cranial NCCs reach the branchial arches (Abe et al., 2007). Similarly, perturbation of Fgfr1 function leads to a failure of mouse cranial NCC entry into the second branchial arch (Trokovic et al., 2003). It is thought that an Fgfr1 signaling center in the distal ectoderm of ba2 creates a permissive environment for cranial NCC migration into distal portions of the second branchial arch (Trokovic et al., 2005). Thus, cranial NCC entry into and invasion within head targets may be regulated by a combination of factors distinct to particular branchial arches.
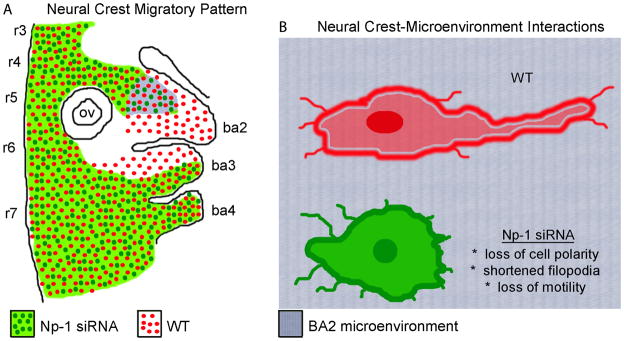
Figure 5. A schematic representation showing the Np-1 siRNA phenotype.
(A) Cranial NCCs transfected with Np-1 siRNA failed to migrate properly into ba2 and the anterior portion of ba3, however invasion of more posterior targets was unaffected. (B) A schematic representation of the cellular phenotypes of Np-1 siRNA transfected cranial NCCs at the entrance to the ba2 microenvironment. Cranial NCCs transfected with Np-1 siRNA failed to completely enter the ba2 microenvironment, lost cell polarity, collapsed filopodia. The notations are r, rhombomere, ba, branchial arch, ov, otic vesicle, WT, wildtype.
Np-1 siRNA transfected r4 NCCs that reached the entrance to the second branchial arch displayed a sequence of cellular events that suggested cells triggered a mechanism to stop, in the absence of a physical barrier. NCCs displayed a rounding-up of cell body and nuclear shapes, severely reduced motility, and shorter filopodial extensions (Fig. 2). Although NCCs stopped, it is not clear why the cells did not re-route their trajectories to other locations, since cranial NCCs can overcome a physical alteration to their migratory pathway (Kulesa et al., 2005). Interestingly, chick cranial NCCs that encounter a physical barrier (that is shorter than the width of the r4 NCC migratory stream) initially stop, but trailing cells are able to reroute around the barrier and enter into the second branchial arch (Kulesa et al., 2005). We have also observed this behavior and result when chick r3 or r5 NCCs wander into NCC-free zones (Kulesa and Fraser, 1998). In this scenario, NCCs either stopped and remained in a location or actively extended filopodia and migrated to join a neighboring migratory stream after contact with cells leaving r2 or r4 (Kulesa and Fraser, 1998). Thus, it appears that when NCCs encounter a local inhibitory signal or physical barrier in their migratory pathway, cells are still able to respond to guidance signals in a manner that is distinct from the behavior when permissive receptor-ligand interactions are altered.
Np-1 siRNA transfected r4 NCCs regained their migratory ability when heterochronically and heterotopically transplanted from the ba2 microenvironment entrance into r4 of host embryos, suggesting that cells could recover directed migration in permissive regions that do not require neuropilin-1 expression (Fig. 2). These data support the hypothesis that in the absence of proper neuropilin-1-ligand interactions, cells failed to interpret local invasion cues and stopped. This proved to be an effective mechanism to limit aberrant cranial NCC migration. Future experiments focused on revealing the sequence of molecular events that cause highly invasive NCCs to prematurely stop may help to better understand how cranial NCCs are programmed to stop in precise locations or wait before responding to further signaling events.
NCC-derived structures were able to form normally in the absence of Np-1 siRNA transfected r4 NCCs, suggesting that other non-transfected r4 NCCs compensated for the loss of missing neighbors (Fig. 3). We previously examined cell death and showed there was no short term (24hrs after electroporation) increase in cell death when NCCs were transfected with Np-1 siRNA (McLennan and Kulesa, 2007). In the cranial region, typical NC-derivatives include neurons and glia of the cranial ganglia as well as bone and cartilage of the face and neck (Le Douarin and Kalchiem, 1999; Le Douarin, 2004; Baker and Bronner-Fraser, 1997). Np-1 siRNA transfected cranial NCCs did not contribute to the cranial ganglia in normal numbers, but the ganglia did form appropriately (Fig. 3B). The neural crest-derived lower jaw and hyoid bone also formed normally when cranial NCCs were transfected with Np-1 siRNA (Fig. 3G, H). Since these tissue structures developed normally, other non-transfected r4 NCCs were able to alter their proliferative activity. Alternatively, NCCs from neighboring migratory streams were able to compensate by altering their trajectories in a similar manner to that observed in chick embryos where subpopulations of premigratory cranial NCCs are ablated (Saldivar et al., 1995; Kulesa et al., 2000). Thus, Np-1 siRNA transfected NCCs that prematurely stopped prior to colonizing the second branchial arch, failed to reach critical survival factors and contribute to NC-derived tissues.
When both neuropilins were knocked down, the r4 NCC migratory stream failed to enter into and invade ba2 (Fig. 4), but the phenotype was not stronger than with loss of neuropilin-1 expression alone. The Np-2 shRNA did successfully knockdown the expression of neuropilin-2 (Fig. 4A). Np-1 siRNA and Np-2 shRNA transfected embryos had quantitative measurements of NCC distance migrated into and throughout the second branchial arch that were dramatic, but not as significant as with reduction of neuropilin-1 expression alone (compare Fig. 4F,G with Fig. S1). This suggests that neuropilins do not compensate for each other in regulating the entry into and invasion of cranial NCCs into the second branchial arch. Alternatively, since we were unable to determine the efficiency of whether NCCs were transfected with both neuropilins, we may not have been able to assess a stronger phenotype. Surprisingly, we did not observe any severe affects on the ability of r4 NCCs to invade the second branchial arch when neuropilin-2 expression was knocked down (Fig. 4A-C). However, we did recapitulate the neuropilin-2 mouse knockout trunk NCC migratory defect (Gammill et al., 2006), as we observed trunk NCCs to invade caudal somite-halves (Fig. 4D, E). Although our Np-2 shRNA phenotype did not appear to affect the early cranial NCC migratory pattern similar to observations in mouse (Gammill et al., 2007), this may have been due to knockdown methodology differences in chick (reduction of neuropilin-2 expression in NCCs) versus mouse (whole embryo knockout), or species differences.
In summary, our findings implicate an intimate interaction between the neuropilin-1 expressing cranial NCCs and the microenvironment that ensures the proper entry into and invasion of the second branchial arch (Fig. 5). This specificity may be a means for the embryo to control which cells enter the arches and prevent exposure of survival and patterning signals to unwanted neighbors. We interpret the characteristic loss of cell polarity and motility to be due to an inability of these cranial NCCs to interpret navigational signals. Interestingly, the inhibition of an invasive cell type, such as the neural crest, by precise knockdown of molecular signaling suggests a means to regulate the position of where a cell stops. This is an important goal for the embryo to properly position migratory cells, yet control the invasion of misguided neighbors. Further molecular details of neuropilin-1-ligand interactions in the migratory neural crest may yield important insights for mechanisms of embryonic cell migration.
Experimental Procedures
Embryos
Fertilized white leghorn chicken eggs (supplied by Placid Acre Poultry, Jasper, MO) were incubated at 38°C in a humidified incubator until the desired HH stages of development, and prepared for experiments as previously described (McLennan and Kulesa, 2007).
In ovo Electroporation and Cell Labeling
Embryos were incubated until Hamburger and Hamilton (HH) Stage 9 (Hamburger and Hamilton, 1951), when 6–8 somite pairs were visible for cranial electroporations, and HH Stage 10–12 for trunk electroporations. Cranial NCCs were electroporated and labeled in vivo as previously described (McLennan and Kulesa, 2007). The constructs we used were a control EGFP empty vector named pMES (a kind gift from Cathy Krull, University of Michigan), siRNA against neuropilin-1 (a kind gift from Frances Lefcort, Montana State University, made in the J. Cohen laboratory, MRC Centre for Developmental Neurobiology, London, UK; Bron et al., 2004), and neuropilin-2 shRNA constructed in our laboratory (see below for details). In some experiments, a lipophilic dye, DiI (C-7000, Invitrogen, Carlsbad, CA, USA) was mixed with the plasmid DNA and co-injected into the lumen of the neural tube. After electroporation, eggs were re-incubated for 4–72 hours, depending on the experiment. After re-incubation, eggs were screened and selected for quality of cell labeling and overall embryo health using a fluorescence dissecting microscope (SV11, Carl Zeiss MicroImaging, Thornwood, NY, USA) before being harvested. For static imaging, embryos were fixed in 4% paraformaldehyde for 2 hours at room temperature, or 4°C overnight.
Construction and testing of Np-2 shRNA-EGFP
The shRNA was designed towards neuropilin-2 using various online shRNA design programs. This portion of the construct was ordered as two separate oligonucleotides, 5′-CCAGAAGATTATCCTCAACTTCAAGAGA GTTGAGGATAATCTTCTGGTTTTTT-3′ (top) and 5′-AATTAAAAAACCAGAAG ATTATCCTCAACTCTCTTGAAGTTGAGGATAATCTTCTGGGGC -3′ (bottom) from Invitrogen. The oligonucleotides were annealed to one another and cloned into the pSilencer 1.0 U6 siRNA expression vector (AM7207, Ambion Biosystems, Foster City, CA, USA) by following the instructions provided with the vector. The mouse U6 promoter (which drives expression of the shRNA) and the shRNA sequences were excised from pSilencer and ligated into the multiple cloning site of pMES. This resulted in a vector in which EGFP and the neuropilin-2 shRNA were transcribed separately. To test the efficiency of the Np-2 shRNA construct, neural tubes were electroporated with Np-2 shRNA. After approximately 18 hours of re-incubation, embryos were harvested, and highly transfected neural tube regions were isolated in cold Ringers and RT-PCR was performed (described below).
Tissue Transplantations
For r7 to ba2 transplants, the dorsal third of the r7 neural tube were removed from each donor (re-incubated for 1–4 hours after electroporation) with a sharpened tungsten needle. These regions were then cut into 2–4 small subpopulations of r7 NCCs which were individually heterochronically and heterotopically transplanted into the r4 NCC migratory stream pathway (HH Stages 10–12) of unlabeled host embryos, using a glass needle. For ba2 to r4 transplants, donors where harvested 18 hours after re-incubation. The region containing the most distal portion of the r4 NCC migratory stream isolated using a tungsten needle and heterochronically and heterotopically transplanted into the dorsal mid-r4 (HH Stages 8–9) of unlabeled host embryos. After transplantation, host embryos were re-incubated for 24–40 hours before being harvested and imaged.
RT-PCR analysis
RNA was isolated from transfected neural tubes using the RNeasy Mini Kit (74104, Qiagen, Valencia, CA). Primers were purchased from Invitrogen. Primers for Neuropilin-2 were 5′-CAACGGCTGGACCCCCAATG-3′ (forward) and 5′-CTTTGGGACGCCCAGGTCCA-3′ (reverse). Primers for beta-actin were 5′-CGGTTTCGCCGGGGACGATG-3 ′ (forward) and 5 ′-CGTCAGGTCACGGCCAGCCAGA-3′ (reverse). All resulting DNA products were approximately 500bp in length. Touchdown PCR was performed using the following conditions: 94°C for 5 minutes followed by 34 cycles of 94°C for 1 minute, annealing temperature decreasing every 2 cycles from 78°C to 62°C for 1 minute and 72°C for 1.5 minutes, then 15 cycles of 94°C for 1 minute, 50°C for 1 minute and 72°C for 1.5 minutes. The resulting PCR products were analyzed by gel electrophoresis.
3D Confocal Imaging
For static analysis, cryostat sections or half hindbrain mounts were prepared and imaged as previously described (McLennan and Kulesa, 2007). 3D image z-stacks were collected on an inverted laser scanning confocal microscope (LSM5 Pascal, Zeiss) using either a Plan-Neofluar 10X/0.3 (Zeiss), Plan-Neofluar 40X/0.75 (Zeiss) or C-Apochromat 40X/1.2W objective (Zeiss). Images were visualized using AIM software (Zeiss). Time-lapse confocal imaging of whole embryo cultures were prepared and imaged as previously described (Kulesa and Fraser, 1998).
Bone and Cartilage Stains
E9.5 embryos were harvested, rinsed with PBS and placed in 95% ethanol for 1 hour. The ethanol was replaced with fresh 95% ethanol and the embryos were mildly agitation overnight. The embryos were then placed into an Alcian Blue solution (0.02% Alcian Blue 8GX (A-5268, Sigma-Aldrich, St Louis, MO, USA), 70% ethanol, 5% glacial acetic acid, 24.98% water) overnight before being rinsed with water. Unwanted tissue layers were dissolved for 30 minutes to 1 hour by 1% KOH. After a wash in 0.25% KOH for 30 minutes, embryos were cleared through glycerol gradients with 0.25% KOH up to 50% glycerol with 0.25% KOH for storage and imaging.
Immunohistochemistry
DAPI staining to label nuclei was performed on 10um cryostat sagittal tissue sections through the r4 NCC migratory stream, 18 hours after re-incubation. The slides were rehydrated in Tris-buffered saline (TBS) for 20 minutes before being mounted with Vectashield Hard Set Mounting Medium with DAPI (H-1500, Vector Laboratories, Inc, Burlingame, CA, USA).
BEN (an Ig superfamily cell adhesion molecule implicated in axon guidance and target recognition; Developmental Studies Hybridoma Bank, University of Iowa, IA, USA) staining, to label cranial ganglia, was performed on 15um cryostat sagittal tissue sections of E4.5 embryos. The slides were rehydrated in Tris-buffered saline (TBS) for 20 minutes and washed 4 times in TBS plus 0.5% Triton-X-100, 10 minutes each wash. The slides were then blocked with NGS block for 1 hour. BEN, diluted 1:10 in NGS block was added to slides and left overnight at 4°C. The slides were then washed 4 times in TBS plus 0.5% Triton-X-100, 10 minutes each wash before being blocked for 1 hours in NGS block. Alexa Fluor 546 goat anti-mouse IgG (A11003, Invitrogen), diluted 1:1000 in NGS block was added to slides and left 1 hour at room temperature. The slides were then washed three times, 10 minutes each in TBS before being mounted with Vectashield Hard Set Mounting Medium with DAPI (Vector Laboratories, Inc, Burlingame, CA, USA).
Quantitative Measurements
We analyzed details of the cranial NCC migratory streams using AIM software (Zeiss) in wildtype and experimental embryos. Briefly, collected confocal 3D-stacks were collapsed into 2D projections and the length and width of each migratory stream was measured. For the r7 to ba2 transplants, the furthest distance the cranial NCCs migrated was measured. Brightfield images confirmed the tissue boundaries of the neural tube and branchial arches. To analyze cranial NCC morphologies, we first calculated the length and orientation of cellular extensions. Individual cranial NCCs were chosen for analysis based on their quality of brightness and resolution. In each region of interest we selected 10 cranial NCCs (in n=7 control EGFP and n=11 Np-1 siRNA transfected embryos). In the AIM software (Zeiss), the line tool was overlaid along each cellular extension to measure its length. Orientation was scored as being either directed towards ba2 or directed backwards to the r4. Nuclear and cytoplasmic distribution characteristics were measured on cryostat tissue sections labeled with DAPI throughout the ba2 microenvironment. The aspect ratio (length/width) of each nucleus and cytoplasmic distribution was calculated using the line measurement tool. Statistical analyses were performed using the standard student’s t-test.
Supplementary Material
(A) Static confocal image of a typical embryo in which post-otic cranial NCCs were transfected with a control EGFP construct, n=8 for 24 hours and n=5 for 36 hours after electroporation. (B) Static confocal image of an embryo in which post-otic cranial NCCs were transfected with Np-1 siRNA, 36 hours after electroporation, n=10 for 24 hours and n=6 for 36 hours after electroporation. The Np-1 siRNA transfected cranial NCCs (green) migrated out of the neural tube, however, the cranial NCCs failed to invade the anterior portion of ba3. Untransfected cranial NCCs (red) were seen throughout the developing arches. Dotted lines represent how quantitative measurements were calculated. (C) Quantitative measurements of the distance cranial NCCs migrated from the neural tube into the ba3 microenvironment, as a percentage of the distance from the neural tube to the distal end of ba3. (D) Quantitative measurements of the anterior-posterior width of the ba3 microenvironment that cranial NCCs populated, as a percentage of the total ba3 width. (E) Quantitative measurements of the distance cranial NCCs migrated from the neural tube into the ba4 microenvironment, as a percentage of the distance from the neural tube to the distal end of ba4. (F) Quantitative measurements of the anterior-posterior width of the ba4 microenvironment that cranial NCCs populated, as a percentage of the total ba4 width. The scale bars are 100um. The notations are r, rhombomere, ba, branchial arch, *, significantly different, p<0.005. r7 NCCs transfected with Np-1 siRNA migrate when transplanted into r4. (G) A schematic representation of the ba2 to r4 transplantation technique. (H) A static confocal image of a host embryo, 24 hours after a subpopulation of DiI-labeled (red), EGFP-transfected (green) migratory r4 NCCs from the ba2 microenvironment entrance were transplanted heterochronically into r4, n=8. Both the transfected (green) and untransfected (red) cranial NCCs were able to migrate from the transplant site towards the ba2 microenvironment. (I) A static confocal image of a host embryo, 24 hours after a subpopulation of DiI-labeled (red), Np-1 siRNA-transfected (green) migratory r4 NCCs from the ba2 microenvironment entrance were transplanted heterochronically into r4, n=10. Both the transfected (green) and untransfected (red) cranial NCCs were able to migrate from the transplant site towards the ba2 microenvironment. The scale bars are 100um. The notations are r, rhombomere, ba, branchial arch.
Acknowledgments
Grant Sponsor: National Institute of Health; Grant Number: 1R01HD057922
The authors would like to kindly thank Teri Johnson and her colleagues for their help with sectioning and members of the Trainor laboratory for their help with cartilage stains, Hua Li for help with statistics. R.M. is very grateful to Jennifer Kasemeier-Kulesa for her help and guidance in building the Np-2 shRNA construct. This work was funded by NIH grant 1R01HD057922 and the Stowers Institute for Medical Research.
References
- Abe M, Ruest LB, Clouthier DE. Fate of cranial neural crest cells during craniofacial development in endothelin-A receptor-deficient mice. Intl J Dev Biol. 2007;51:97–105. doi: 10.1387/ijdb.062237ma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M. The origins of the neural crest. Part I: embryonic induction. Mech Dev. 1997;69:3–11. doi: 10.1016/s0925-4773(97)00132-9. [DOI] [PubMed] [Google Scholar]
- Berndt JD, Clay MR, Langenberg T, Halloran MC. Rho-kinase and myosin II affect dynamic neural crest cell behaviors during epithelial to mesenchymal transition in vivo. Dev Biol. 2008;324:236–244. doi: 10.1016/j.ydbio.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron R, Eickholt BJ, Vermeren M, Fragale N, Cohen J. Functional knockdown of neuropilin-1 in the developing chick nervous system by siRNA hairpins phenocopies genetic ablation in the mouse. Dev Dyn. 2004;230:299–308. doi: 10.1002/dvdy.20043. [DOI] [PubMed] [Google Scholar]
- Carmona-Fontaine C, Matthews HK, Kuriyama S, Moreno M, Dunn GA, Parsons M, Stern CD, Mayor R. Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature. 2008;456:957–961. doi: 10.1038/nature07441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton JK, Guthrie S. Cranial expression of class 3 secreted semaphorins and their neuropilin receptors. Dev Dyn. 2003;228:726–733. doi: 10.1002/dvdy.10396. [DOI] [PubMed] [Google Scholar]
- Couly G, Grapin-Botton A, Coltey P, Le Douarin NM. The regeneration of the cephalic neural crest, a problem revisited: the regenerating cells originate from the contralateral or from the anterior and posterior neural fold. Dev. 1996;122:3393–3407. doi: 10.1242/dev.122.11.3393. [DOI] [PubMed] [Google Scholar]
- Eickholt BJ, Mackenzie SL, Graham A, Walsh FS, Doherty P. Evidence for collapsin-1 functioning in the control of neural crest migration in both trunk and hindbrain regions. Dev. 1999;126:2181–2189. doi: 10.1242/dev.126.10.2181. [DOI] [PubMed] [Google Scholar]
- Farlie PG, Kerr R, Thomas P, Symes T, Minichiello J, Hearn CJ, Newgreen D. A paraxial exclusion zone creates patterned cranial neural crest cell outgrowth adjacent to rhombomeres 3 and 5. Dev Biol. 1999;213:70–84. doi: 10.1006/dbio.1999.9332. [DOI] [PubMed] [Google Scholar]
- Gammill LS, Gonzalez C, Bronner-Fraser M. Neuropilin 2/semaphorin 3F signaling is essential for cranial neural crest migration and trigeminal ganglion condensation. Dev Neurobiol. 2007;67:47–56. doi: 10.1002/dneu.20326. [DOI] [PubMed] [Google Scholar]
- Gammill LS, Gonzalez C, Gu C, Bronner-Fraser M. Guidance of trunk neural crest migration requires neuropilin 2/semaphorin 3F signaling. Dev. 2006;133:99–106. doi: 10.1242/dev.02187. [DOI] [PubMed] [Google Scholar]
- Graham A, Begbie J, McGonnell I. Significance of the cranial neural crest. Dev Dyn. 2004;229:5–13. doi: 10.1002/dvdy.10442. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morph. 1951;88:49–92. [PubMed] [Google Scholar]
- Kulesa PM, Fraser SE. Neural crest cell dynamics revealed by time-lapse video microscopy of whole embryo chick explant cultures. Dev Biol. 1998;204:327–344. doi: 10.1006/dbio.1998.9082. [DOI] [PubMed] [Google Scholar]
- Kulesa PM, Bronner-Fraser M, Fraser SE. In ovo time-lapse analysis after dorsal neural tube ablation shows rerouting of chick hindbrain neural crest. Dev. 2000;127:2843–2852. doi: 10.1242/dev.127.13.2843. [DOI] [PubMed] [Google Scholar]
- Kulesa PM, Ellies DL, Trainor PA. Comparative analysis of neural crest cell death, migration, and function during vertebrate embryogenesis. Dev Dyn. 2004;299:14–29. doi: 10.1002/dvdy.10485. [DOI] [PubMed] [Google Scholar]
- Kulesa PM, Lu CC, Fraser SE. Time-lapse analysis reveals a series of events by which cranial neural crest cells reroute around physical barriers. Brain Behav Evol. 2005;66:255–265. doi: 10.1159/000088129. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM. The avian embryo as a model to study the development of the neural crest: a long and still ongoing story. Mech Dev. 2004;121:1089–1102. doi: 10.1016/j.mod.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Kalcheim C. The Neural Crest. 2. Cambridge Univ. Press; Cambridge: 1999. [Google Scholar]
- McCabe KL, Bronner-Fraser MB. Molecular and tissue interactions governing induction of cranial ectodermal placodes. Dev Biol. 2009;332:189–195. doi: 10.1016/j.ydbio.2009.05.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan R, Kulesa PM. In vivo analysis reveals a critical role for neuropilin-1 in cranial neural crest cell migration in chick. Dev Biol. 2007;301:227–239. doi: 10.1016/j.ydbio.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Olesnicky-Killian EC, Birkholz DA, Artinger KB. A role for chemokine signaling in neural crest migration and craniofacial development. Dev Biol. 2009;333:161–172. doi: 10.1016/j.ydbio.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne NJ, Begbie J, Chilton JK, Schmidt H, Eickholt BJ. Semaphorin/neuropilin signaling influences the positioning of migratory neural crest cells within the hindbrain region of the chick. Dev Dyn. 2005;232:939–949. doi: 10.1002/dvdy.20258. [DOI] [PubMed] [Google Scholar]
- Pourquie O, Coltey M, Thomas JL, Le Douarin NM. A widely distributed antigen developmentally regulated in the nervous system. Dev. 1990;109:743–752. doi: 10.1242/dev.109.4.743. [DOI] [PubMed] [Google Scholar]
- Saldivar JR, Sechrist JW, Krull CE, Ruffins S, Bronner-Fraser M. Dorsal hindbrain ablation results in rerouting of neural crest migration and changes in gene expression by normal hyoid development. Dev. 1997;124:2729–2739. doi: 10.1242/dev.124.14.2729. [DOI] [PubMed] [Google Scholar]
- Santagati F, Rijli FM. Cranial neural crest and the building of the vertebrate head. Nat Rev Neurosci. 2003;4:806–818. doi: 10.1038/nrn1221. [DOI] [PubMed] [Google Scholar]
- Schneider RA, Helms JA. The cellular and molecular origins of beak morphology. Science. 2003;299:565–568. doi: 10.1126/science.1077827. [DOI] [PubMed] [Google Scholar]
- Schwarz Q, Vieira JM, Howard B, Eickholt BJ, Ruhrberg C. Neuropilin 1 and 2 control cranial gangliogenesis and axon guidance through neural crest cells. Dev. 2008;135:1605–1613. doi: 10.1242/dev.015412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneyhill LA. To adhere or not to adhere: the role of Cadherins in neural crest development. Cell Adh Migr. 2008;2:223–230. doi: 10.4161/cam.2.4.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teddy JM, Kulesa PM. In vivo evidence for short- and long-range cell communication in cranial neural crest cells. Dev. 2004;131:6141–6151. doi: 10.1242/dev.01534. [DOI] [PubMed] [Google Scholar]
- Trainor PA, Krumlauf R. Hox genes, neural crest cells and branchial arch patterning. Curr Opin Cell Biol. 2001;13:698–705. doi: 10.1016/s0955-0674(00)00273-8. [DOI] [PubMed] [Google Scholar]
- Trokovic N, Trokovic R, Mai P, Partanen J. Fgfr1 regulates patterning of the pharyngeal region. Genes Dev. 2003;17:141–153. doi: 10.1101/gad.250703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trokovic N, Trokovic R, Partanen J. Fibroblast growth factor signalling and regional specification of the pharyngeal ectoderm. Intl J Dev Biol. 2005;49:797–805. doi: 10.1387/ijdb.051976nt. [DOI] [PubMed] [Google Scholar]
- Yu HH, Moens CB. Semaphorin signaling guides cranial neural crest cell migration in zebrafish. Dev Biol. 2005;280:373–385. doi: 10.1016/j.ydbio.2005.01.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Static confocal image of a typical embryo in which post-otic cranial NCCs were transfected with a control EGFP construct, n=8 for 24 hours and n=5 for 36 hours after electroporation. (B) Static confocal image of an embryo in which post-otic cranial NCCs were transfected with Np-1 siRNA, 36 hours after electroporation, n=10 for 24 hours and n=6 for 36 hours after electroporation. The Np-1 siRNA transfected cranial NCCs (green) migrated out of the neural tube, however, the cranial NCCs failed to invade the anterior portion of ba3. Untransfected cranial NCCs (red) were seen throughout the developing arches. Dotted lines represent how quantitative measurements were calculated. (C) Quantitative measurements of the distance cranial NCCs migrated from the neural tube into the ba3 microenvironment, as a percentage of the distance from the neural tube to the distal end of ba3. (D) Quantitative measurements of the anterior-posterior width of the ba3 microenvironment that cranial NCCs populated, as a percentage of the total ba3 width. (E) Quantitative measurements of the distance cranial NCCs migrated from the neural tube into the ba4 microenvironment, as a percentage of the distance from the neural tube to the distal end of ba4. (F) Quantitative measurements of the anterior-posterior width of the ba4 microenvironment that cranial NCCs populated, as a percentage of the total ba4 width. The scale bars are 100um. The notations are r, rhombomere, ba, branchial arch, *, significantly different, p<0.005. r7 NCCs transfected with Np-1 siRNA migrate when transplanted into r4. (G) A schematic representation of the ba2 to r4 transplantation technique. (H) A static confocal image of a host embryo, 24 hours after a subpopulation of DiI-labeled (red), EGFP-transfected (green) migratory r4 NCCs from the ba2 microenvironment entrance were transplanted heterochronically into r4, n=8. Both the transfected (green) and untransfected (red) cranial NCCs were able to migrate from the transplant site towards the ba2 microenvironment. (I) A static confocal image of a host embryo, 24 hours after a subpopulation of DiI-labeled (red), Np-1 siRNA-transfected (green) migratory r4 NCCs from the ba2 microenvironment entrance were transplanted heterochronically into r4, n=10. Both the transfected (green) and untransfected (red) cranial NCCs were able to migrate from the transplant site towards the ba2 microenvironment. The scale bars are 100um. The notations are r, rhombomere, ba, branchial arch.