Abstract
Background
The purpose of this study was to develop a multi-harmonic phase analysis method to measure diastolic dyssynchrony from conventional gated SPECT myocardial perfusion imaging(MPI) data and to compare it with systolic dyssynchrony in normal subjects and in patients with end-stage renal disease (ESRD) and normal left-ventricular ejection fraction (LVEF).
Methods
121 consecutive patients with ESRD and normal LVEF and 30 consecutive normal controls were enrolled. Diastolic dyssynchrony parameters were calculated using 3-harmonic phase analysis. Systolic dyssynchrony parameters were calculated using the established 1-harmonic phase analysis.
Results
The systolic and diastolic dyssynchrony parameters were correlated, but significantly different in both control and ESRD groups, indicating they were physiologically related but measured different LV mechanisms. The systolic and diastolic dyssynchrony parameters were each significantly different between the control and the ESRD groups. Significant systolic and diastolic dyssynchrony were found in 47% and 65% of the entire ESRD group.
Conclusion
Multi-harmonic phase analysis has been developed to assess diastolic dyssynchrony, which measured a new LV mechanism of regional function from gated SPECT MPI and showed a significantly higher prevalence rate than systolic dyssynchrony in patients with ESRD and normal LVEF.
Keywords: Systolic dyssynchrony, diastolic dyssynchrony, gated SPECT, myocardial perfusion imaging, end-stage renal disease
INTRODUCTION
Cardiovascular mortality is 10 to 30 times higher in patients with end-stage renal disease (ESRD) as compared to the general population and approaches 50% at 2 years after myocardial infarction.1 Only 16% of new dialysis patients has normal cardiac morphology and function.2,3 ESRD is associated with a variety of cardiac alterations including left-ventricular (LV) hypertrophy, LV dilation, and reduction in systolic and diastolic functions.4 Such cardiac alterations may by themselves induce or be also worsened by LV mechanical dyssynchrony. In addition, LV mechanical dyssynchrony can be caused by abnormal loading conditions.5 For example, excessive volume overload can intensify imbalances in the regional stretching and shortening of myocardial fibers following abnormal stress to myocardial tissue, especially in patients with ESRD.6
LV mechanical dyssynchrony can be systolic and/or diastolic. Systolic dyssynchrony has been extensively studied in patients with systolic heart failure after the introduction of cardiac resynchronization therapy. It has been shown by echocardiography that systolic dyssynchrony is a relatively common finding in patients with systolic heart failure7–10 and prognostically independent of QRS duration.11,12 Recently, echocardiography has been shown to measure LV diastolic dyssynchrony.13
Phase analysis has been developed for measuring LV systolic dyssynchrony from gated single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI).14 Phase analysis has been shown to correlate well with 2D and 3D echocardiography in measuring systolic dyssynchrony in heart failure patients.15–17 Importantly, a recent study has shown that systolic dyssynchrony as assessed by phase analysis was an independent predictor of mortality in patients with ESRD18 and can provide incremental prognostic information to other parameters measured from gated SPECT MPI such as perfusion19 and LV ejection fraction (LVEF).20
Phase analysis using multiple Fourier harmonic functions can better approximate the variation of myocardial wall thickness over the cardiac cycle to calculate the onset of mechanical relaxation (OMR) as a measure of LV diastolic dyssynchrony. The purpose of this study is to develop a multi-harmonic phase analysis tool to measure diastolic dyssynchrony from conventional gated SPECT MPI data and to compare it in normal subjects and in patients with ESRD and normal LVEF.
MATERIALS AND METHODS
Patient Studies
The study population was selected from the Emory Clinical Data Warehouse provided by Emory Healthcare Information Services. Emory Clinical Data Warehouse is a central repository of historical data for all entities of Emory Healthcare from 1994 to the present. The data is generally extracted from Emory operational systems nightly then transformed into a usable format. Patients with ESRD, who had gated SPECT MPI between June 2009 and June 2010 and had echocardiography within 3 months showing normal LVEF (>50%), were included. A total of 121 consecutive patient studies were enrolled. Table 1 shows the patient characteristics. In addition to the ESRD patients, 30 consecutive normal controls, who had gated SPECT MPI scans and echocardiography within 3 months during the same period of time, were enrolled to derive the normal limits of LV systolic and diastolic synchrony and to compare with those measured in the ESRD patients. The characteristics of the control group are shown in Table 1. The normal controls had no functional abnormalities such as LV systolic or diastolic dysfunction according to echocardiography, heart failure, cardiomyopathy, left-bundle branch block, or any risk factors indicated on their medical records and had a low likelihood of coronary artery disease defined based on age, sex, pretest symptom, and electrocardiographic response to treadmill stress test.21
Table 1.
Characteristics of the ESRD patients and normal controls
| Variables | ESRD (N = 121) | Controls (N = 30) |
|---|---|---|
| Age (years) | 52.5 ± 10.8 | 58.5 ± 13.0 |
| Female gender | 49 (41%) | 19 (63%) |
| Diabetes mellitus | 57 (47%) | 3 (10%) |
| Hypertension | 121 (100%) | 2 (6%) |
| Dialysis type | ||
| Hemodialysis | 76 (63%) | N/A |
| Peritoneal dialysis | 16 (13%) | N/A |
| Not yet dialyzed | 29 (24%) | N/A |
| Coronary artery disease by angiogram | 21 (17%) | None |
| Prior myocardial infarction | 3 (3%) | None |
| LV hypertrophy by echo | 58 (48%) | None |
| LVEF by echo | 59.6 ± 5.3% | 61.0 ± 5.4% |
| Diastolic dysfunction by echo* | 73 (60%) | None |
| QRS duration (ms) | 91.0 ± 12.2 (N = 98) | 86.8 ± 11.3 (N = 23) |
| QRS > 110 ms | 3 (3%) (N = 98) | None |
Diastolic dysfunction was assessed by echocardiographers at Emory, who were blinded from this study, as their standard clinical practice. The major parameters used by echocardiographers at Emory to classify diastolic dysfunction were the mitral inflow E/A velocity ratio and the mitral deceleration time.
All patients had standard same-day Tc-99m stress/rest MPI. Only the resting gated MPI data were analyzed in this study. In the resting protocol, radiopharmaceutical was injected at rest at time zero. The radiopharmaceutical dose scheme was 370 MBq (10 mCi) for patients up to 200 lbs, 444 MBq (12 mCi) for patients between 200 and 300 lbs, and 555 MBq (15 mCi) for patients heavier than 300 lbs. After 60 minutes, the patient was positioned supine for SPECT acquisition. The images were acquired over an 180° collection arc beginning at 45° right anterior oblique and ending at 45° left posterior oblique with 64 projections, 30 seconds per projection. Matrix size was 64 × 64 with 8 frames/cycle. The scan was acquired using a dual-head SPECT system equipped with a low-energy high-resolution parallel-hole collimator.
All patient data were reconstructed by ordered subsets expectation maximization with 3 iterations and 10 subsets. A Butterworth filter with a cutoff frequency of 0.35 cycles/cm and a power of 10 was used to filter the gated images. The reconstructed images were reoriented manually to generate gated short-axis images, and then submitted to the multi-harmonic phase analysis tool.
Multi-Harmonic Phase Analysis
According to Fourier’s theorem, any periodic function F(t) with a frequency f can be decomposed into the sum of cosine functions with frequencies of f, 2f, 3f…, etc., as follows:
| (1) |
Each term in the above equation is called a harmonic. For example, the term A0 is called the zero harmonic, the term A1cos(2πft + P1) is called the first harmonic, and the term A2cos(4πft + P2) is called the second harmonic, etc. For each of the harmonic, the A value represents its amplitude and the P value represents its phase. Figure 1 demonstrates how to use Fourier harmonic functions to approximate a typical discrete wall-thickening curve into a continuous curve. As shown in Figure 1, better approximation can be achieved by incorporating higher harmonics. The Nyquist-Shannon sampling theorem states that if a continuous-time signal, whose spectral content is limited to frequencies smaller than f, it can be recovered from its sampled version (discrete-time) if the sampling rate is higher than 2f. In other words, the sampling rate must be high enough to provide more than two points over one cycle to recover the signal. As gated SPECT MPI usually acquires 8 frames per cardiac cycle, the highest harmonic function that can be used in the approximation is the third harmonic function. Thus, in this study 3-harmonic phase analysis was used to approximate LV wall thickening curve for diastolic dyssynchrony.
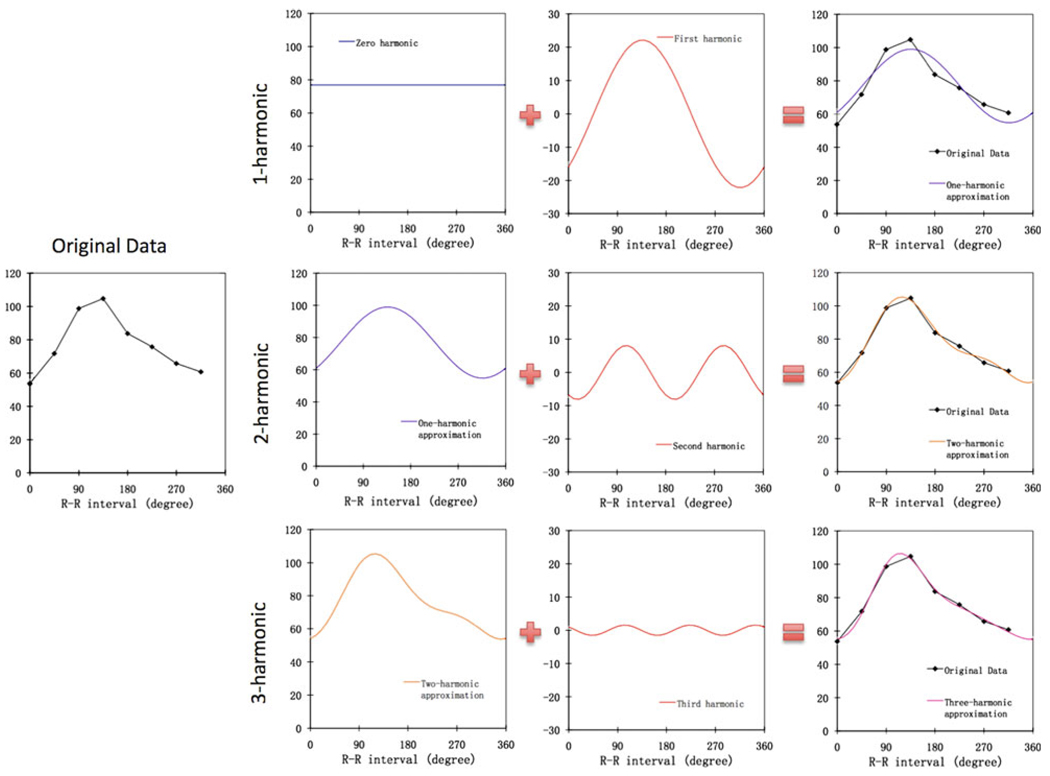
Figure 1.
The use of the Fourier harmonics to approximate a typical discrete wall-thickening curve. The harmonic functions (zero-, first-, second-, and third-harmonic) were generated from the original data by the fast Fourier transform. The 1-harmonic approximation includes the zero- and first-harmonic functions. Adding the second- and third-harmonic functions to the approximation yields the 2- and 3-harmonic approximation, respectively. As shown in this figure, the lower harmonics determine the basic shape of the curve, whereas higher harmonics refine the approximation and improve its accuracy.
Figure 2 shows the processing steps in this study to measure LV systolic and diastolic dyssynchrony. The input was the standard gated SPECT short-axis image. At first, regional maximal count detection was performed in 3D for each temporal frame to generate wall-thickening curves for over 600 LV regions. Based on the partial volume effect,22 the variation of the regional maximal counts was proportional to the regional wall thickening over the cardiac cycle. The linear relationship was demonstrated in a phantom study.23 The wall-thickening curve for each region was approximated by the 1-harmonic function for systolic dyssynchrony. It underwent a count drop correction and then was approximated by the 3-harmonic function for diastolic dyssynchrony. The count drop correction scaled every pixel in the last frame to make the total myocardial counts in the last frame equal to the total myocardial counts in the first frame. The phase angles derived by the systolic and diastolic approximation represented the onset of mechanical contraction (OMC) and onset of mechanical relaxation (OMR), respectively. Once the OMC and OMR phase angles of all regions were obtained, OMC and OMR phase distributions were generated that provided information on the degree of systolic and diastolic dyssynchrony for the entire left ventricle, respectively. The quantitative parameters for LV diastolic dyssynchrony were calculated as OMR phase standard deviation (PSD) (standard deviation of the OMR phase distribution) and OMR phase histogram bandwidth (PHB) (the width of the band that includes 95% of the OMR phase angles), similar to the definitions of OMC PSD and OMC PHB in the reported phase analysis tool for measuring systolic dyssynchrony.14
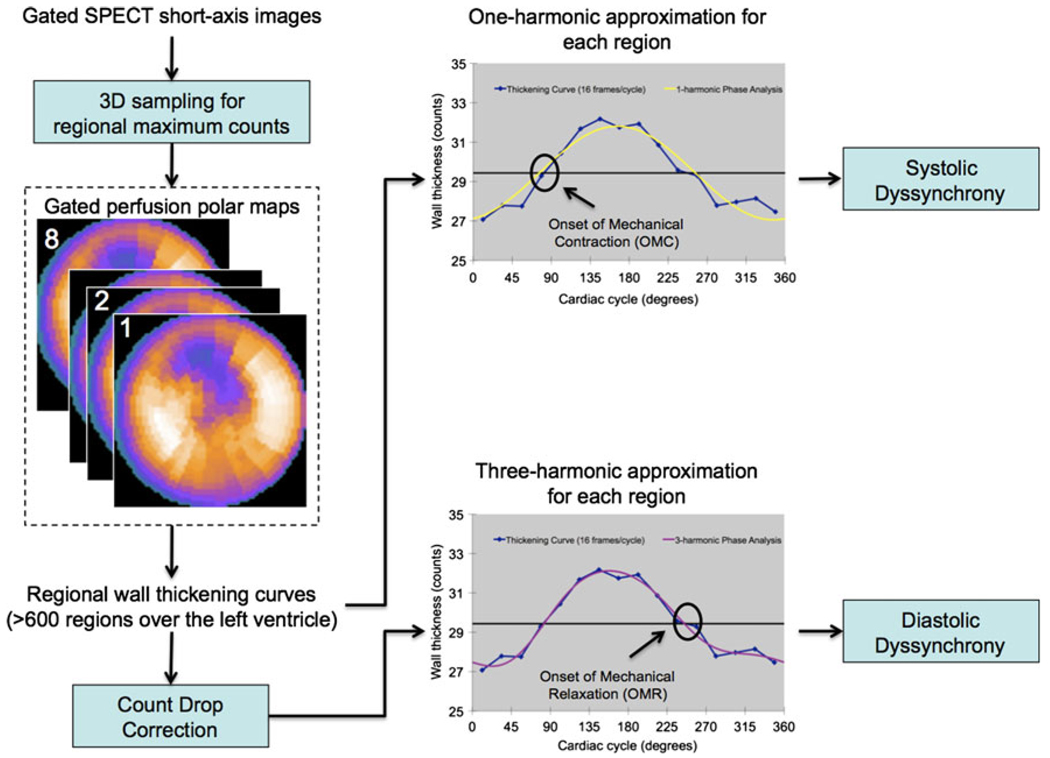
Figure 2.
Processing steps of the multi-harmonic phase analysis tool. The input was the standard gated SPECT short-axis image. At first, regional maximal count detection was performed in 3D for each temporal frame to generate wall-thickening curves for over 600 LV regions. The wall-thickening curve for each region was approximated by the 1-harmonic function for systolic dyssynchrony and by the 3-harmonic function after a count drop correction for diastolic dyssynchrony. The count drop correction scaled every pixel in the last frame to make the total myocardial counts in the last frame equal to the total myocardial counts in the first frame. The phase angles derived by the systolic and diastolic approximation represented the onset of mechanical contraction (OMC) and onset of mechanical relaxation (OMR) respectively. Once the OMC and OMR phase angles of all regions were obtained, OMC and OMR phase distributions were generated that provided information on the degree of systolic and diastolic dyssynchrony for the entire LV, respectively.
Statistical Analysis
The systolic and diastolic parameters within the normal controls and in the ESRD patients were compared by paired t test. The normal limits of the systolic and diastolic dyssynchrony parameters were determined as the mean plus 2 standard deviations of these parameters in the normal controls. The systolic and diastolic dyssynchrony parameters were compared between the control and the ESRD groups by unpaired t test with unequal variance. Each patient, who had systolic and/or diastolic dyssynchrony parameters beyond the normal limits, was considered having significant systolic and/or diastolic dyssynchrony, respectively. The prevalence rates of systolic and diastolic dyssynchrony were calculated, and then compared by Chi-square test, in the entire ESRD group, and in several subsets such as patients with diabetes mellitus, LV hypertrophy and LV diastolic dysfunction according to echocardiography, respectively.
RESULTS
Table 2 shows the systolic and diastolic dyssynchrony parameters in the control group. The normal limits, calculated as the mean plus 2 standard deviations in the normal controls, were 11.7° and 40.2° for systolic PSD and PHB, and 18.3° and 53.1° for diastolic PSD and PHB, respectively. The correlation coefficients between the systolic and diastolic dyssynchrony parameters were 0.53 and 0.61 for PSD and PHB, respectively. Table 3 shows the systolic and diastolic dyssynchrony parameters in the ESRD group. The correlation coefficients between the systolic and diastolic dyssynchrony parameters were 0.78 and 0.79 for PSD and PHB, respectively. The diastolic dyssynchrony parameters were significantly different from the systolic dyssynchrony parameters by paired t test in both control and ESRD groups. These findings support that systolic and diastolic dyssynchrony were physiologically related, but measured different LV mechanisms.
Table 2.
LV systolic and diastolic dyssynchrony in the normal controls (N = 30)
| Systolic (°) | Diastolic (°) | |||
|---|---|---|---|---|
| PSD | PHB | PSD | PHB | |
| Range | 4.7–13.1 | 17–42 | 5.0–19.4 | 19–57 |
| Mean | 7.6 | 26.1 | 11.0 | 34.6 |
| SD | 2.0 | 7.0 | 3.7 | 9.3 |
| Cutoff | 11.7 | 40.2 | 18.3 | 53.1 |
| P | <.0001 | <.0001 | ||
| R | 0.53 | 0.61 | ||
PSD, Phase standard deviation; PHB, phase histogram bandwidth; SD, standard deviation; Cutoff, mean plus 2 standard deviations; P value by paired t test between the systolic and diastolic parameters; R, correlation coefficient.
Table 3.
LV systolic and diastolic dyssynchrony in the ESRD patients (N = 121)
| Systolic (1-harmonic) (°) | Systolic (3-harmonic) (°) | Diastolic (3-harmonic) (°) | ||||
|---|---|---|---|---|---|---|
| PSD | PHB | PSD | PHB | PSD | PHB | |
| Range | 6.6–46.8 | 22–154 | 7.7–48.3 | 25–167 | 7.6–54.1 | 26–192 |
| Mean | 15.9 | 50.0 | 16.4 | 52.5 | 23.2 | 70.0 |
| SD | 7.8 | 22.8 | 8.6 | 24.6 | 11.1 | 35.1 |
| P | <.0001 | .0011 | <.0001 | <.0001 | ||
| R | 0.78 | 0.79 | ||||
PSD, Phase standard deviation; PHB, phase histogram bandwidth; SD, standard deviation; P value by paired t test between the systolic and diastolic parameters; R, correlation coefficient.
Table 3 also shows the systolic PSD and PHB based on 3-harmonic approximation. They were slightly, but systematically larger than those based on 1-harmonic approximation, as demonstrated by the small differences in the mean values and significant P values by paired t test between the two methods. The differences between the 3-harmonic systolic and diastolic values were much greater than the differences between the 1- and 3-harmonic systolic values, indicating that the diastolic values represented a different LV mechanism. Note that the diastolic PSD and PHB with 1-harmonic approximation were exactly the same as the systolic PSD and PHB with 1-harmonic approximation. Therefore, they are not shown in Table 3. For each region, the OMC and OMR phase angles were distinct, but by exactly 180° with 1-harmonic approximation. Thus, the systolic and diastolic dyssynchrony parameters, describing the heterogeneity of the OMC and OMR phase distributions over the entire ventricle, were the same.
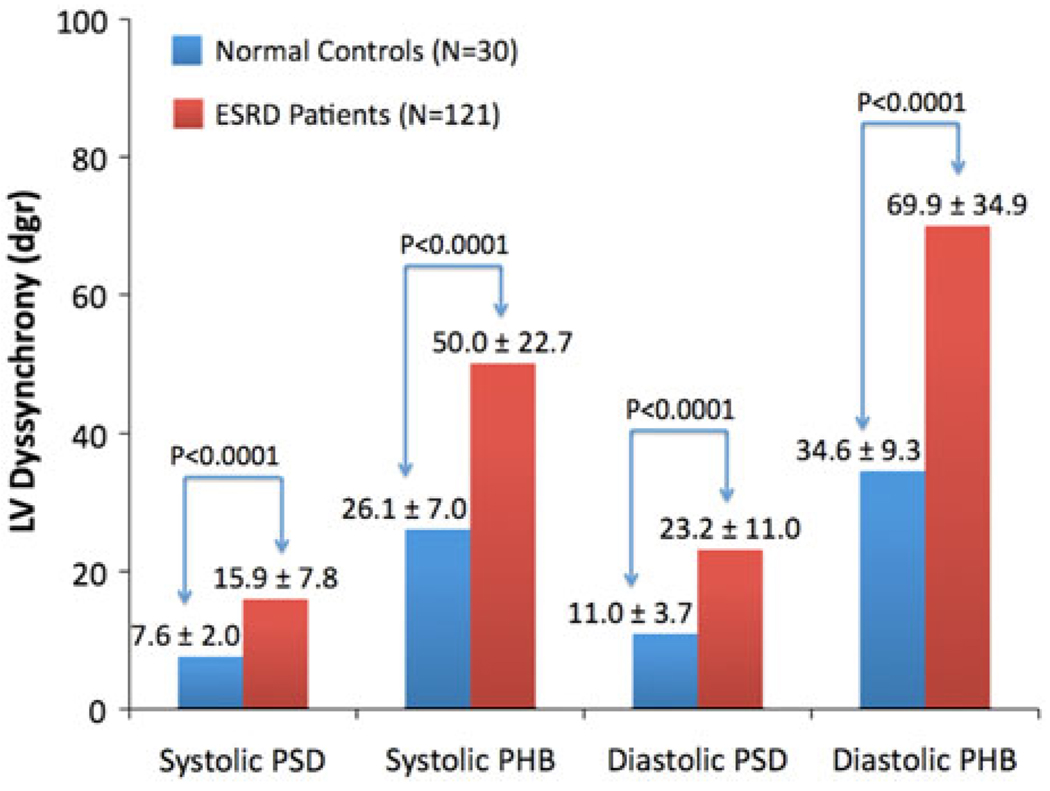
Figure 3 compares the systolic and diastolic dyssynchrony parameters between the control and the ESRD groups. All parameters were significantly different (P < .0001) between the two groups by unpaired t test with unequal variance. Based on the normal limits, given in Table 2, 57 ESRD patients (47%) had systolic dyssynchrony and 78 ESRD patients (65%) had diastolic dyssynchrony. These two prevalence rates were significantly different by Chi-square test (P = .0071). Importantly, 21 of the 78 ESRD patients had diastolic dyssynchrony without systolic dyssynchrony, and none had systolic dyssynchrony without diastolic dyssynchrony.
Figure 3.
Left-ventricular (LV) systolic and diastolic dyssynchrony parameters (PSD, phase standard deviation; PHB, phase histogram bandwidth) in normal controls vs patients with end-stage renal disease (ESRD) and normal LV ejection fraction (LVEF). All parameters were significantly different between the normal controls and ESRD patients by unpaired t test with unequal variance.
Figure 4 compares the prevalence rates of systolic and diastolic dyssynchrony in the entire ESRD group and in the subsets of patients with diabetes mellitus (N = 57), and LV hypertrophy (N = 58) and LV diastolic dysfunction according to echocardiography (N = 73), respectively, by Chi-square test. Systolic and diastolic dyssynchrony were found in 53% and 70% (P = .0944) of the patients with diabetes mellitus, 43% and 62% (P = .0625) of the patients with echocardiographic evidence of LV hypertrophy, and 53% and 69% (P = .0700) of the patients with echocardiographic evidence of diastolic dysfunction, respectively. There were no significant differences in the prevalence rates of systolic and diastolic dyssynchrony between the entire ESRD group and each subset, indicating that diabetes mellitus, and LV hypertrophy and LV diastolic dysfunction according to echocardiography did not result in higher prevalence rates of LV systolic and diastolic dyssynchrony in the ESRD group than ESRD alone.
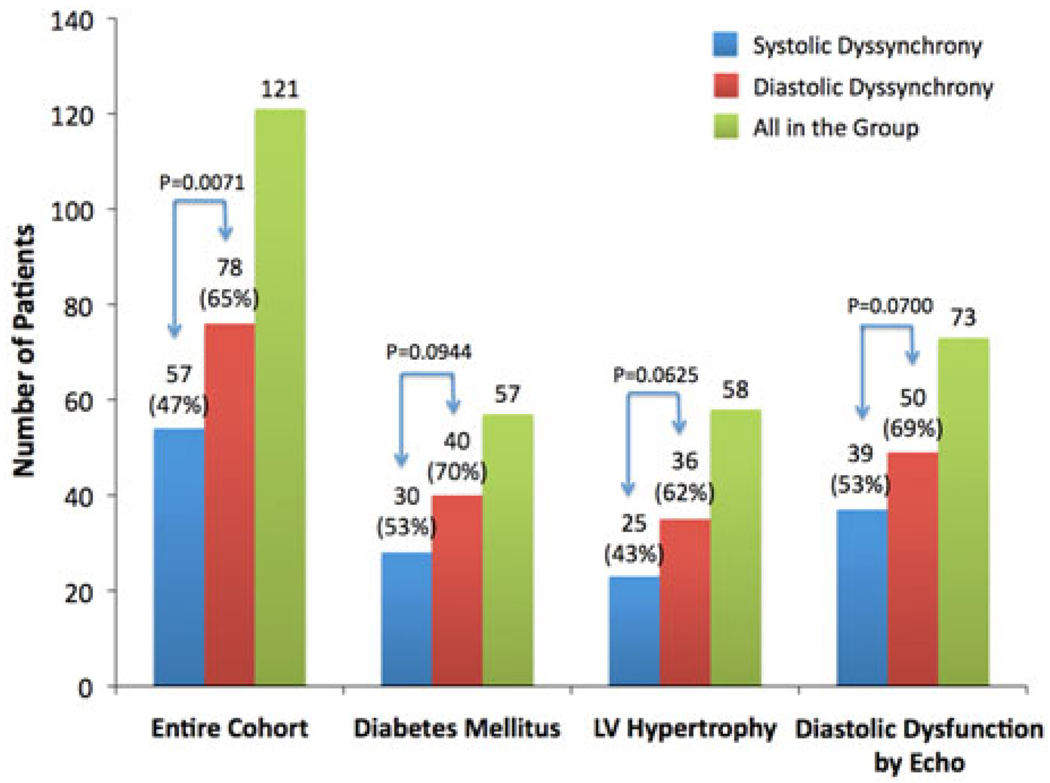
Figure 4.
Prevalence rates of systolic and diastolic dyssynchrony in the entire ESRD group (N = 121) and the subsets of patients with diabetes mellitus (N = 57), and LV hypertrophy (N = 58) and LV diastolic dysfunction according to echocardiography (N = 73), respectively. Significant LV systolic and diastolic dyssynchrony were found in 47% and 65% the entire ESRD group, 53% and 70% of the patients with diabetes mellitus, 43% and 62% of the patients with echocardiographic evidence of LV hypertrophy, and 53% and 69% of the patients with echocardiographic evidence of diastolic dysfunction, respectively. There were no significant differences in prevalence rates of systolic and diastolic dyssynchrony between the entire ESRD group and each subset, indicating that these conditions did not result in higher prevalence of LV dyssynchrony in the ESRD patients than ESRD alone.
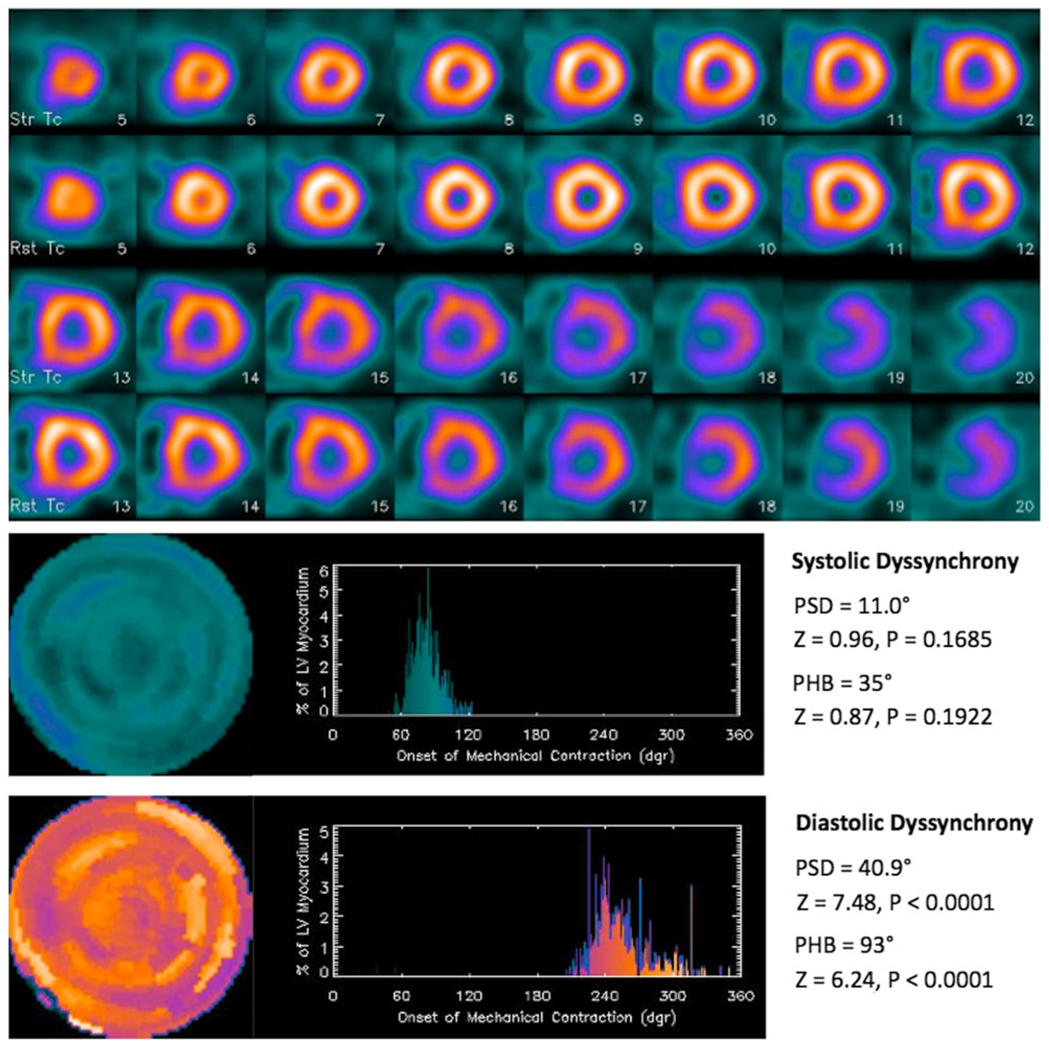
Figure 5 shows an ESRD patient example with significant diastolic dyssynchrony, but no significant systolic dyssynchrony. A 53-year-old male with ESRD, hypertension, diabetes mellitus, and hemodialysis had no coronary artery disease or myocardial infarction. Echocardiography showed that he had LV hypertrophy and LVEF of 55%. His QRS duration was 94 ms. Comparing his LV dyssynchrony parameters to the normal limits showed that he had no significant systolic dyssynchrony, but significant diastolic dyssynchrony by one-sample z test.
Figure 5.
A patient example with significant diastolic dyssynchrony, but no significant systolic dyssynchrony. A 53-year-old male had end-stage renal disease, hypertension, diabetes mellitus, hemodialysis, but no coronary artery disease. He had LV hypertrophy and LVEF of 55% according to echocardiography. His QRS duration was 94 ms. Comparing his LV dyssynchrony parameters to the normal limits, he had no significant systolic dyssynchrony, but significant diastolic dyssynchrony by one-sample z test.
DISCUSSION
A multi-harmonic phase analysis tool has been developed to measure LV diastolic dyssynchrony from gated SPECT MPI studies. This tool approximated the regional myocardial wall thickening/thinning curve over the cardiac cycle with multiple Fourier harmonic functions. With three harmonics, this tool can assess regional OMR phases as a measure of diastolic dyssynchrony. This study applied this tool to 30 normal controls and 121 patients with ESRD and normal LVEF. Comparing to the normal limits generated from the 30 normal controls, 57 ESRD patients (47%) had significant systolic dyssynchrony, and 78 ESRD patients (65%) had significant diastolic dyssynchrony. Importantly, all the 57 ESRD patients with systolic dyssynchrony had evidence of diastolic dyssynchrony. This finding is consistent with the tight curvilinear relationship between the absolute peak systolic and early diastolic velocities seen by echocardiography, which explains dyssynchronous systole causes dyssynchronous diastole, in a range of cardiac diseases and normal subjects.24
There are limited data on LV mechanical dyssynchrony in patients with ESRD. One study using the 1-harmonic phase analysis of gated SPECT MPI showed that patients with ESRD and normal LVEF had a higher systolic PHB and PSD than normal subjects.25 Another study using the 1-harmonic phase analysis of gated SPECT MPI showed that LV systolic dyssynchrony is associated with all-cause mortality in patients with ESRD.18 One echocardiography study showed that LV systolic dyssynchrony presented frequently and improved after a single session of hemodialysis in a small group of ESRD patients.5 A recent study using echocardiography with speckle tracking strain imaging showed that the presence of ESRD was associated with longitudinal and radial LV systolic dyssynchrony that was significantly reduced after haemodialytic therapy.6 All the above studies were focused on LV systolic dyssynchrony in ESRD patients.
This is the first study to investigate both systolic and diastolic dyssynchrony in patients with ESRD by gated SPECT MPI. The systolic PHB and PSD values in the ESRD patients in this study were 50.0 ± 22.7° and 15.9 ± 7.8°, which were consistent with those (56 ± 26° and 19 ± 8°) reported in a previous study.25 This study showed that the magnitude of the diastolic dyssynchrony parameters, measured by the novel multi-harmonic phase analysis tool, were significantly different than that of the systolic dyssynchrony parameters in both the control and the ESRD groups, indicating that they measured a new LV mechanism. 17% (21 out of 121) of the ESRD patients had significant diastolic dyssynchrony without significant systolic dyssynchrony.
Hypertension, diabetes mellitus, and LV hypertrophy are major cardiac risk factors in patients with ESRD, and can be intrinsic mechanisms for LV mechanical dyssynchrony. Echocardiography studies have shown that there is evidence of both systolic and diastolic dyssynchrony in a significant number in patients with hypertension,26 diabetes mellitus,27 and LV hypertrophy.28 Our study, using gated SPECT MPI, showed a consistent finding that LV systolic and diastolic dyssynchrony presented frequently in ESRD patients with these cardiac risk factors. However, the prevalence rates were not statistically different between the entire ESRD group and each subset. This finding indicated that those conditions did not result in higher prevalence rates of LV systolic and diastolic dyssynchrony in ESRD patients than ESRD alone, confirming the well-accepted clinical concept.
Another important finding of this study was that 53% and 69% of the 73 patients, who had echocardiographic evidence of diastolic dysfunction, had significant systolic and diastolic dyssynchrony, respectively. The concept of diastolic dyssynchrony and its contribution to diastolic dysfunction has been reported in severe hypertrophic conditions including aortic stenosis and hypertrophic obstructive cardiomyopathy.29,30 In an echocardiography study, tissue Doppler imaging was used to assess LV systolic and diastolic dyssynchrony in 60 patients with diastolic heart failure (LVEF > 50%).31 It showed that 20 patients (33%) had systolic dyssynchrony, and 35 patients (58%) had diastolic dyssynchrony. Importantly, all the 20 patients with systolic dyssynchrony had evidence of diastolic dyssynchrony. In another echocardiography study, the prevalence rates of systolic and diastolic dyssynchrony were similar in 92 patients with diastolic heart failure (39% and 56%, respectively).13 These echocardiography studies concluded that there was evidence of both systolic and diastolic dyssynchrony in a significant number of patients with diastolic dysfunction and normal LVEF. This study showed consistent findings using multi-harmonic phase analysis of gated SPECT MPI.
One limitation of this study was that it was designed to develop the novel technique of measuring diastolic dyssynchrony as the variation of regional OMR phases over the entire left ventricle and characterizing it in normal controls and ESRD patients, not to validate the technique. The novel diastolic dyssynchrony parameters measured from gated SPECT MPI need to be validated prospectively against a reference standard, such as echocardiography parameters of diastolic dysfunction and dyssynchrony, or to other clinical variables such as B-type natriuretic peptide level in heart failure patients. Another limitation of this study was related to the count-drop phenomenon. In practice, gated SPECT MPI studies are acquired as forward gating. The time bin of each gate is pre-calculated based on the patient’s heart rate. When an R wave is triggered, the system records events in each time bin and assigns them into each frame. Since the patient’s heart rate varies during the acquisition, it is possible that another R wave is triggered before the acquisition for the last frame is finished. Thus, it is commonly observed in clinical data that the last frame contains fewer myocardial counts than the other frames. One solution to this effect is the count-drop correction, as done in this study. It assumed that the last frame should be very close, in total myocardial counts, to the first frame. Therefore, it scaled every pixel in the last frame to make the total myocardial counts in the last frame equal to the total myocardial counts in the first frame. Another potential and more accurate solution to this effect is backward gating. In backward gating, the data are acquired by list mode, and then rebinned into each frame. The rebinning process starts from the last frame to the first frame, therefore, there will be no count drop for the diastolic frames. However, this solution requires list-mode acquisition and is beyond the scope of this study.
CONCLUSION
A tool based on multi-harmonic phase analysis of conventional gated SPECT MPI has been developed to assess the variation of regional OMR phases over the entire left ventricle as a measure of LV diastolic dyssynchrony—a new LV mechanism measured from gated SPECT MPI. LV diastolic dyssynchrony as assessed by the multi-harmonic phase analysis tool was more prevalent than the established LV systolic dyssynchrony measured from gated SPECT MPI in patients with ESRD and normal LVEF.
Acknowledgments
This study was supported in part by an NIH Grant (1R01HL094438; PI: Ji Chen, PhD). Drs. Chen and Garcia receives royalties from the sale of the Emory Cardiac Toolbox.
Footnotes
The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict-of-interest practice.
References
- 1.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 2.Verma A, Anavekar NS, Meris A, et al. The relationship between renal function and cardiac structure, function and prognosis after myocardial infarction. The VALIANT Echo Study. J Am Coll Cardiol. 2007;50:1238–1245. doi: 10.1016/j.jacc.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Foley RN, Parfrey PS, Harnett JD, et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47:186–192. doi: 10.1038/ki.1995.22. [DOI] [PubMed] [Google Scholar]
- 4.Curtis BM, Parfrey PS. Congestive heart failure in chronic kidney disease: Disease-specific mechanisms of systolic and diastolic heart failure and management. Cardiol Clin. 2005;23:275–284. doi: 10.1016/j.ccl.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi SY, Seeberger A, Lind B, Nowak J, do Nascimento MM, Lindholm B, et al. A single session of haemodialysis improves left ventricular synchronicity in patients with end-stage renal disease: A pilot tissue synchronization imaging study. Nephrol Dial Transplant. 2008;23:3622–3628. doi: 10.1093/ndt/gfn311. [DOI] [PubMed] [Google Scholar]
- 6.Murata T, Dohi K, Onishi K, Sugiura E, Fujimoto N, Ichikawa K, et al. Role of haemodialytic therapy on left ventricular mechanical dyssynchrony in patients with end-stage renal disease quantified by speckle-tracking strain imaging. Nephrol Dial Transplant. 2010 doi: 10.1093/ndt/gfq590. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Yu CM, Lin H, Zhang Q, Sanderson JE. High prevalence of left ventricular systolic and diastolic asynchrony in patients with congestive heart failure and normal QRS duration. Heart. 2003;89:54–60. doi: 10.1136/heart.89.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghio S, Constantin C, Klersy C, et al. Interventricular and intraventricular dyssynchrony are common in heart failure patients, regardless of QRS duration. Eur Heart J. 2004;25:571–578. doi: 10.1016/j.ehj.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 9.Bleeker GB, Schalij MJ, Molhoek SG, et al. Relationship between QRS duration and left ventricular dyssynchrony in patients with end-stage heart failure. J Cardiovasc Electrophysiol. 2004;15:544–549. doi: 10.1046/j.1540-8167.2004.03604.x. [DOI] [PubMed] [Google Scholar]
- 10.Sade LE, Kanzaki H, Severyn D, Dohi K, Gorcsan J., III Quantification of radial mechanical dyssynchrony in patients with left bundle branch block and idiopathic dilated cardiomyopathy without conduction delay by tissue displacement imaging. Am J Cardiol. 2004;94:514–518. doi: 10.1016/j.amjcard.2004.04.071. [DOI] [PubMed] [Google Scholar]
- 11.Bader H, Garrigue S, Lafitte S, et al. Intra-left ventricular electromechanical asynchrony. A new independent predictor of severe cardiac events in heart failure patients. J Am Coll Cardiol. 2004;43:248–256. doi: 10.1016/j.jacc.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 12.Cho GY, Song JK, Park WJ, et al. Mechanical dyssynchrony assessed by tissue Doppler imaging is a powerful predictor of mortality in congestive heart failure with normal QRS duration. J Am Coll Cardiol. 2005;46:2237–2243. doi: 10.1016/j.jacc.2004.11.074. [DOI] [PubMed] [Google Scholar]
- 13.Yu CM, Zhang Q, Yip GW, Lee PW, Kum LC, Lam YY, et al. Diastolic and systolic asynchrony in patients with diastolic heart failure: A common but ignored condition. J Am Coll Cardiol. 2007;49:97–105. doi: 10.1016/j.jacc.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Garcia EV, Folks RD, Cooke CD, Faber TL, Tauxe EL, et al. Onset of left ventricular mechanical contraction as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging: Development of a diagnostic tool for assessment of cardiac mechanical dyssynchrony. J Nucl Cardiol. 2005;12:687–695. doi: 10.1016/j.nuclcard.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 15.Henneman MM, Chen J, Ypenburg C, Dibbets P, Stokkel M, van der Wall EE, et al. Phase analysis of gated myocardial perfusion SPECT compared to tissue Doppler imaging for the assessment of left ventricular dyssynchrony. J Am Coll Cardiol. 2007;49:1708–1714. doi: 10.1016/j.jacc.2007.01.063. [DOI] [PubMed] [Google Scholar]
- 16.Marsan NA, Henneman MM, Chen J, Ypenburg C, Dibbets P, Ghio S, et al. Real-time 3-dimensional echocardiography as a novel approach to quantify left ventricular dyssynchrony: A comparison study with phase analysis of gated myocardial perfusion single photon emission computed tomography. J Am Soc Echocardiogr. 2008;21:801–807. doi: 10.1016/j.echo.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsan NA, Henneman MM, Chen J, Ypenburg C, Dibbets P, Ghio S, et al. Left ventricular dyssynchrony assessed by two 3-dimensional imaging modalities: Phase analysis of gated myocardial perfusion SPECT and tri-plane tissue Doppler imaging. Eur J Nucl Med Mol Imaging. 2008;35:166–173. doi: 10.1007/s00259-007-0539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aljaroudi W, Aggarwal H, Venkataraman R, Heo J, Iskandrian AE, Hage FG. Impact of left ventricular dyssynchrony by phase analysis on cardiovascular outcomes in patients with end-stage renal disease. J Nucl Cardiol. 2010;17:1058–1064. doi: 10.1007/s12350-010-9271-x. [DOI] [PubMed] [Google Scholar]
- 19.Hage FG, Smalheiser S, Zoghbi GJ, Perry GJ, Deierhoi M, Warnock D, et al. Predictors of survival in patients with end-stage renal disease evaluated for kidney transplantation. Am J Cardiol. 2007;100:1020–1025. doi: 10.1016/j.amjcard.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 20.Hage FG, de Mattos AM, Khamash H, Mehta S, Warnock D, Iskandrian AE. QT prolongation is an independent predictor of mortality in end-stage renal disease. Clin Cardiol. 2010;33:361–366. doi: 10.1002/clc.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diamond GA, Forrester JS, Hirsch M, et al. Application of conditional probability analysis to the clinical diagnosis of coronary artery disease. J Clin Invest. 1980;65:1210–1221. doi: 10.1172/JCI109776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman EJ, Huang SC, Phelps ME. Quantitation in positron emission computed tomography: 1. Effect of object size. J Comput Assist Tomogr. 1979;3:299–308. doi: 10.1097/00004728-197906000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Galt JR, Garcia EV, Robbins WL. Effects of myocardial wall thickness on SPECT quantification. IEEE Trans Med Imaging. 1990;9:144–150. doi: 10.1109/42.56338. [DOI] [PubMed] [Google Scholar]
- 24.Yip GW, Zhang Y, Tan PY, Wang M, Ho PY, Brodin LA, et al. Left ventricular long-axis changes in early diastole and systole: Impact of systolic function on diastole. Clin Sci (Lond) 2002;102:512–522. [PubMed] [Google Scholar]
- 25.Trimble MA, Borges-Neto S, Smallheiser S, Chen J, Honeycutt EF, Shaw LK, et al. Evaluation of left ventricular mechanical dyssynchrony as determined by phase analysis of ECG-gated SPECT myocardial perfusion imaging in patients with left ventricular dysfunction and conduction disturbances. J Nucl Cardiol. 2007;14:298–307. doi: 10.1016/j.nuclcard.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 26.Chang SA, Kim HK, Kim YJ, Sohn DW, Oh BH, Park YB. Left ventricular systolic and diastolic dyssynchrony in asymptomatic hypertensive patients. J Am Soc Echocardiogr. 2009;22:337–342. doi: 10.1016/j.echo.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 27.Burgess MI, Fang ZY, Marwick TH. Role of diastolic dyssynchrony in the delayed relaxation pattern of left ventricular filling. J Am Soc Echocardiogr. 2007;20:63–69. doi: 10.1016/j.echo.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Lee AP, Song JK, Yip GW, Zhang Q, Zhu TG, Li C, Chang A, Yu CM. Importance of dynamic dyssynchrony in the occurrence of hypertensive heart failure with normal ejection fraction. Eur Heart J. 2010;31:2642–2649. doi: 10.1093/eurheartj/ehq248. [DOI] [PubMed] [Google Scholar]
- 29.Villari B, Vassalli G, Betocchi S, Briguori C, Chiariello M, Hess OM. Normalization of left ventricular nonuniformity late after valve replacement for aortic stenosis. Am J Cardiol. 1996;78:66–71. doi: 10.1016/s0002-9149(96)00229-9. [DOI] [PubMed] [Google Scholar]
- 30.Park TH, Lakkis NM, Middleton KJ, Franklin J, Zoghbi WA, Quinones MA, et al. Acute effect of nonsurgical septal reduction therapy on regional left ventricular asynchrony in patients with hypertrophic obstructive cardiomyopathy. Circulation. 2002;106:412–415. doi: 10.1161/01.cir.0000025418.96995.ed. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Kurrelmeyer KM, Torre-Amione G, Nagueh SF. Systolic and diastolic dyssynchrony in patients with diastolic heart failure and the effect of medical therapy. J Am Coll Cardiol. 2007;49:88–96. doi: 10.1016/j.jacc.2006.10.023. [DOI] [PubMed] [Google Scholar]