Abstract
Background
A novel method to quantify dyssynchrony using phase analysis of single-photon emission computed tomography (SPECT) myocardial perfusion imaging has been developed. We sought to determine the prevalence of SPECT-derived mechanical dyssynchrony, and we report clinical variables which predict mechanical dyssynchrony in patients with left ventricular dysfunction.
Methods
We used a count-based Fourier analysis method to convert the regional myocardial counts from discrete frames per cardiac cycle into a continuous thickening function which allows resolution of the phase of the onset of myocardial contraction. The standard deviation of left ventricular phases (Phase SD) describes the regional phase dispersion as a measure of dyssynchrony. Significant dyssynchrony was defined as Phase SD ≥ 43°. 260 patients with left ventricular ejection fraction ≤35% were examined.
Results
The prevalence of mechanical dyssynchrony in the entire cohort of patients studied was 52%. Univariate predictors of Phase SD were age (P = .03), black race (P = .0005), QRS duration, EF, EDV, summed stress score (SSS), and summed rest score (SRS) (all P = <.0001). Black race, male gender, QRS EF, and SRS were independent predictors of SPECT-based mechanical dyssynchrony.
Conclusions
Significant SPECT-based mechanical dyssynchrony is relatively common among patients with left ventricular dysfunction. In a population of patients with predominantly ischemic heart disease referred for SPECT, a reduced EF, increasing QRS duration, severity and extent of myocardial scar on SPECT imaging are independent predictors of mechanical dyssynchrony and may serve to identify patients for dyssynchrony screening.
Keywords: Myocardial perfusion imaging: SPECT, coronary artery disease, gated SPECT, heart failure, diagnostic and prognostic applications
INTRODUCTION
Cardiac resynchronization therapy (CRT) is an increasingly important consideration in the treatment of patients with heart failure (HF). It is approved for the treatment of patients with advanced HF symptoms, a left ventricular (LV) ejection fraction (EF) ≤35%, and prolonged QRS duration. Several studies have documented improvements in quality of life, functional class, exercise capacity, EF, and mortality for patients who received CRT in addition to optimal medical therapy.1–4 Unfortunately, current patient selection parameters lead to implantation in an estimated third of patients for whom improvement is not demonstrable. Alternatively, using QRS duration as a surrogate for electrical dyssynchrony may lead to the inappropriate exclusion of large segments of the HF population that may benefit from CRT.
Efforts have been directed towards precisely defining LV mechanical dyssynchrony to improve patient selection for CRT. Patients with LV dysfunction form the population that is generally targeted for dyssynchrony screening. Although dyssynchrony is common in patients with LV dysfunction, its exact prevalence has not been thoroughly described. Also, less known are predictive factors the presence of which would prompt further testing for dyssynchrony.
The use of phase analysis of gated single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) for quantifying mechanical dyssynchrony has been well described in literature.5–10 The study presented here is, to date, the largest study utilizing the phase analysis technique on gated perfusion SPECT imaging studies, and is the first to describe the prevalence and the predictors of mechanical dyssynchrony in patients with significantly impaired LV function.
METHODS
Study Population
The study population composed of a retrospective cohort of consecutive patients who were referred for routine gated SPECT MPI to the Duke Nuclear Cardiology Lab between January 2001 and December 2006. Enrollment was based on the following inclusion criteria: (1) age > 18 years, (2) weight < 440 pounds, (3) EF ≤ 35% as determined by gated SPECT MPI, (4) diagnostic image quality as determined by reading physician blinded to this study, (5) diagnostic electrocardiogram done within 24 hours of the SPECT MPI available for review in the electronic medical record system, (6) SPECT MPI images acquired both at rest and post-stress, (7) regular R-R interval allowing gated image acquisition. Two hundred and sixty patients met our inclusion criteria.
SPECT Myocardial Perfusion Imaging Protocol and Image Interpretation
The protocol for performing SPECT MPI studies has been previously described.11 Briefly, SPECT data were obtained with multi-head detectors using a step-and-shoot protocol. Images at rest were obtained for 30 second/projection and those during stress were obtained for 20 seconds/projection. A single isotope (technetium-99m) protocol was used for most patients. The degree of perfusion abnormalities on the resting SPECT MPI was assessed visually and quantified using the summed rest perfusion score (SRS) and the degree of perfusion abnormalities on the post-stress SPECT perfusion imaging was quantified using the summed stress perfusion score (SSS). The summed difference score (SDS) was also calculated (SSS-SRS).
Dyssynchrony Evaluation Using Phase Analysis of Gated SPECT Perfusion Imaging
Data were acquired at eight frames per cardiac cycle as it has been demonstrated that with enough counts (>10 counts/pixel), this technique provides adequate temporal resolution.12 The short-axis data sets were generated by Butterworth reorientation using filtering followed by filtered back projection reconstruction and oblique standard protocols. Only the post-stress imaging was gated and therefore used in the phase analysis. A method to extract amplitude (representing the degree of systolic wall thickening) and phase (representing the timing of the onset of mechanical contraction) from regional LV count changes obtained during gated SPECT MPI has been previously reported and the method has been incorporated into a clinical application to quantify dyssynchrony.13 Briefly, three-dimensional count distributions are extracted from each of the eight LV short-axis data sets and submitted to a one-dimensional fast Fourier transform to the count variation over time of each voxel to calculate the phase of the first Fourier harmonics. Then, the analysis generates a three-dimensional phase distribution describing the timing of the LV regional onset of mechanical contraction as a function of degrees, with the 360° range representing the entire length of the R-R interval. Once the phase distribution is generated, it is displayed on the polar map as well as in histogram format. A representative phase histogram is shown in Figure 1. The X-axis represents the timing of one cardiac cycle (R-R interval) in degrees, and the Y-axis represents the percent of myocardium which demonstrated the onset of mechanical contraction.
Figure 1.
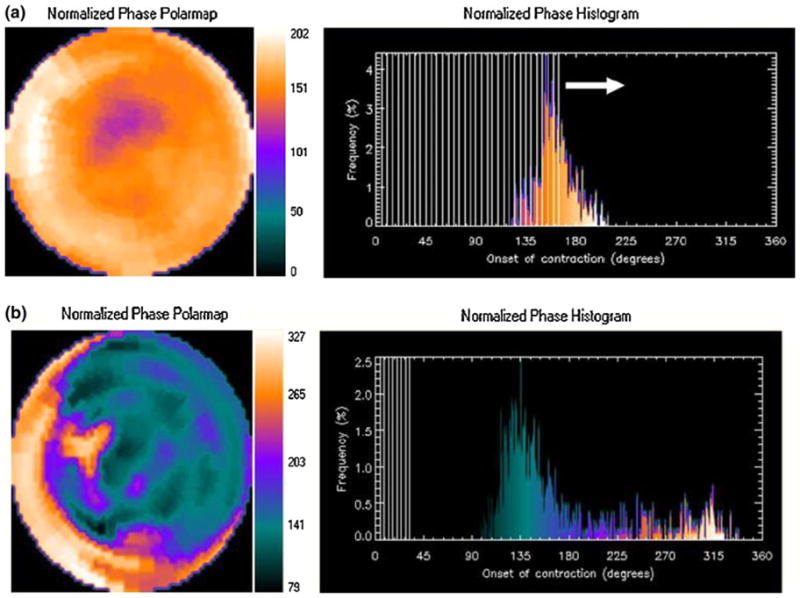
Representative phase histogram. The X-axis represents the timing of one cardiac cycle (R-R interval) in degrees. The Y-axis represents the percent of myocardium demonstrating the onset of mechanical contraction during any particular phase of the cardiac cycle. The color maps have 256 levels with the minimum level corresponding to black and the maximum level corresponding to white. The normalized phase polar map and histogram are displayed with 0° as black and the maximum phase as white. A is an example of a normal study and B representative of dyssynchrony.
The standard deviation of the left ventricular phases (Phase SD) is a quantitative index which describes the dispersion of the LV regional phases of onset of myocardial contraction as a measure of dyssynchrony. A higher phase SD corresponds to increased dyssynchrony. Significant dyssynchrony is defined as phase SD ≥ 43° .7 The software has been implemented in the Emory Cardiac Toolbox (Emory University/Syntermed, Atlanta, Ga) for analysis of gated SPECT MPI.
Statistical Analysis
At first a descriptive analysis was performed examining the age, gender, and risk burden distribution in the entire cohort. The mean, median, and standard deviations were calculated for continuous variables and categorical variables were described as percentages. We examined the prevalence significant mechanical dyssynchrony in the entire cohort, in those with normal (<120 ms) and prolonged (>120 ms) QRS duration, and in the cohorts with normal (SSS = 0) or abnormal myocardial perfusion (SSS > 0) demonstrated on SPECT imaging.14 Furthermore, we also described the cohort demonstrating significant dyssynchrony by varying QRS duration.
Following descriptive analysis, unadjusted association of age, gender, race, history of myocardial infarction, hypertension, diabetes mellitus, SSS, SRS, SDS, QRS duration, end-diastolic volume, with mechanical dyssynchrony defined by increasing Phase SD was examined using simple linear regression models. Phase SD as a measure of dyssynchrony was treated as a continuous variable. Next, a multiple linear regression model was created with Phase SD as a single explanatory variable. Significant and clinically relevant univariate predictors were incorporated in the multivariable model. The level of statistical significance was a priori set at 0.05 and a two-sided P value was used for all analyses. All statistical analyses were performed using SAS E-guide version 4 for Windows (SAS Institute, Cary NC)
This study was approved by the institutional review board at Duke University Medical Center. The authors had access to and take full responsibility for the integrity of the data.
RESULTS
The cohort studied consisted of 260 patients of which 76% were male and 64% were Caucasian. The mean age was 65 ± 13 years, mean EF was 26.8% ± 6.5%, and the mean QRS duration was 119 ± 13 milliseconds. Eighty five percent of patients had a known history of coronary artery disease. Likewise, there was a significant burden of cardiac risk factors as demonstrated in Table 1.
Table 1.
Baseline characteristics
| Characteristic | Total |
|---|---|
| Number of patients | 260 |
| Age | 65 ± 13 |
| Male | 199/260 (76) |
| Caucasian | 166/260 (64) |
| Black | 67/260 (26) |
| CAD | 222/260 (85) |
| DM | 118/260 (45) |
| HTN | 215/260 (83) |
| CRI | 95/260 (36) |
| A. Fib | 77/260 (30) |
| QRS | 119 ± 34 |
| EF | 27 ± 7 |
| EDV | 217 ± 71 |
| ESV | 152 ± 60 |
| LV mass | 201 ± 40 |
| SSS | 14 ± 7 |
| SRS | 11 ± 7 |
| SDS | 3 ± 3 |
CAD, History of coronary artery disease; DM, diabetes mellitus; HTN, hypertension; CRI, chronic renal insufficiency; A. Fib, atrial fibrillation; EF, ejection fraction; EDV, end-diastolic volume; SSS, summed stress score; SRS, summed rest score; SDS, summed difference.
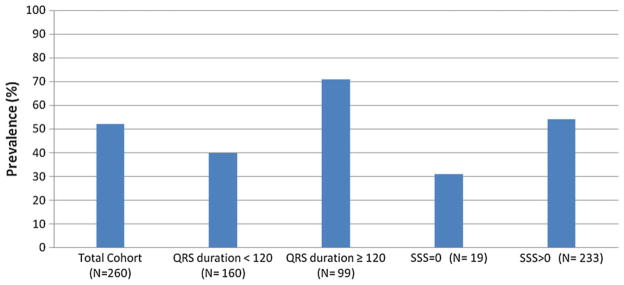
Figure 2 shows the prevalence of significant dyssynchrony in the cohorts examined. The prevalence of significant mechanical dyssynchrony, defined by a Phase SD ≥ 43°, in the entire cohort of patients was 52%. The prevalence of significant dyssynchrony in patients with QRS duration <120 milliseconds was 39% as compared to 71% in those with prolonged QRS duration. The prevalence of significant dyssynchrony was 56% in patients with perfusion abnormalities and 31% in those with normal perfusion.
Figure 2.
Prevalence of significant mechanical dyssynchrony (Phase SD ≥ 43°) in the total cohort and various sub-groups.
Among patients with QRS duration <100 milliseconds, 39% had dyssynchrony and this group as a whole had less dyssynchrony, a higher EF, lower SRS, lower SSS, lower end diastolic volume and lower end systolic volumes than those with QRS duration 100–119 milliseconds (41% with dyssynchrony), QRS duration 120–149 milliseconds (61% dyssynchrony), QRS duration ≥ 150 milliseconds (80% dyssynchrony). The patients with significant dyssynchrony and QRS duration <100 milliseconds had a high prevalence of coronary artery disease (91%).
Table 2 presents the significant univariate predictors of mechanical dyssynchrony. Advancing age, black race, hypertension, increasing QRS duration, declining EF, enlarging left ventricular volume, increasing SSS, and SRS were associated with increasing Phase SD.
Table 2.
Univariate predictors of mechanical dyssynchrony
| Variable | Parameter estimate | Standard error | P value |
|---|---|---|---|
| Age | 2.2 | 1.05 | .033 |
| Black Race | −10.4 | 2.95 | .0005 |
| HTN | −10.9 | 3.4 | .001 |
| QRS | 0.23 | 0.04 | <.0001 |
| EF | −1.4 | 0.19 | <.0001 |
| EDV | 0.10 | 0.02 | <.0001 |
| SSS | 1.4 | 0.16 | <.0001 |
| SRS | 1.7 | 0.15 | <.0001 |
EF, Ejection fraction; EDV, end-diastolic volume; SSS, summed stress score; SRS, summed rest score.
Four multivariable models were created to examine the incremental value of SPECT derived variables over historical data on prediction of dyssynchrony. The first model only included QRS, EF, SSS, and SRS (F value 37, R2 0.43). The second model added demographic variables of age, gender, and race (F value 21.4, R2 0.43) to the previous variables. The third model added historical variables such as diabetes, history of myocardial infarction, and hypertension (F value 15.4, R2 0.43), and the fourth model was generated without SRS. We found that the first model was statistically more robust than the second model (f stat 3.7; P = .01) with the second and third models not being statistically different (f stat 0.13; P = .93) and model 3 (containing SRS) was significantly more robust than the fourth model (f stat 20.2; P = <.0001).
Table 3 presents the multivariate predictors of Phase SD. The parameter estimate of the regression slope provides the change in average Phase SD for every unit change in the predictor variable. In the multi-variable linear regression model, black race, male gender, QRS duration (P = .0005), declining EF (P < .0001), and summed rest score (P = .0002) were found to be independent predictors of Phase SD. EDV (P = .13), SSS (P = .88), age (P = .96) did not reach significance in the multivariable model. There was strong correlation between SSS and SRS with phase SD (Pearson correlation coefficient = 0.85, P < .001). These variables were still included together in the multivariable model because of known impact on outcomes in heart failure patients.
Table 3.
Significant multivariate predictors of dyssynchrony
| Variable | Parameter estimate | Standard error | P value |
|---|---|---|---|
| Black | −7.76 | 2.75 | .005 |
| Male | −5.6 | 2.53 | .02 |
| QRS | 0.105 | 0.03 | .004 |
| EF | −0.666 | 0.20 | .001 |
| SRS | 1.28 | 0.28 | <.0001 |
F statistic = 21.43, R2 = 0.43.
EF, Ejection fraction; SRS, summed rest score.
DISCUSSION
To our knowledge this is the largest study utilizing phase analysis in patients with severe LV dysfunction and the first to describe the prevalence and predictors of SPECT defined mechanical dyssynchrony in such a cohort. This study presents several important findings. First, approximately half the patients with LV EF <35% have significant dyssynchrony as defined by Phase SD ≥43°. Second, approximately 40% of patients with normal QRS duration demonstrate evidence of significant SPECT-based mechanical dyssynchrony. Third, a declining EF, increasing extent and severity of scar as defined by SRS and, increasing QRS duration are independent predictors of dyssynchrony.
Traditionally, a wide QRS complex has served as the dyssynchrony criteria to identify appropriate patients for CRT. However 30–40% of patients with a wide QRS duration fail to respond to CRT15 suggesting that QRS duration when used alone, may not be the best marker for mechanical dyssynchrony. Echocardiographic techniques such as tissue Doppler imaging (TDI) and strain rate imaging have been used to identify dyssynchrony, but these methods lack reproducibility and have not consistently been able to discriminate between responders and non-responders to CRT.15–17 Phase analysis of gated myocardial SPECT perfusion imaging has emerged as a potential tool to evaluate dyssynchrony. The dyssynchrony measures obtained by phase analysis are reproducible, comparable to TDI, and appear to predict response to CRT.8,10
The exact prevalence of dyssynchrony in the left ventricular dysfunction population is debated.18 We used phase analysis of gated SPECT MPI to assess dyssynchrony with a cutoff of Phase SD ≥ 43° used to define significant dyssynchrony.7 It should be noted that Phase SD is a continuous variable and while it correlates well with increasing measures of dyssynchrony such as QRS duration, its use to dichotomize populations into normal versus dyssynchronous using a single cut point is still based on single center data that included only patients with wide QRS duration.7 In contrast, most of our cohort had patients with QRS duration <120. It is quite possible that a higher or lower phase SD cut point is more predictive of CRT response or other clinically relevant outcomes such as mortality. A larger prospective study is needed to answer this question.
Nevertheless, using the cut point of Phase SD > 43°, we determined that approximately half of the study population had significant SPECT-based mechanical dyssynchrony. Since ischemic burden and ventricular dimensions are known to affect the prevalence of dyssynchrony,19 a high prevalence of coronary artery disease and an average EDV 217 ± 71 mL in our study population, may in part, explain the given prevalence of dyssynchrony.
In concordance with earlier studies,20 we found a high prevalence of dyssynchrony in patients with QRS duration ≥120 milliseconds. In patients with QRS duration <120 milliseconds, the prevalence was still significant. This high prevalence of SPECT-based mechanical dyssynchrony in patients with very narrow QRS complexes is interesting and should be further explored. It is possible that the cut point of Phase SD > 43° in narrow QRS patients may or may not indicate true mechanical dyssynchrony, and could be a surrogate for left ventricular physiology related to prior myocardial infarction or ischemia. Furthermore, there is no prior evidence that phase-SD is predictive of CRT response in patients with narrow QRS complexes.
This investigation highlights the significant prevalence of dyssynchrony in patients with LV dysfunction and points to the potential for more expansive but better targeted delivery of CRT in HF patients.
A reduced EF was predictive of increasing mechanical dyssynchrony in our study. Left ventricular remodeling is accompanied by substantial geometric distortion and the shape of the left ventricle changes from an ellipsoid to an almost spherical geometric shape.21 This is often accompanied by conduction disturbances, alteration of the normal cardiac contraction pattern, and subsequent dyssynchrony which impairs ventricular filling, worsens HF, and leads to more adverse remodeling. Reduced mechanical dyssynchrony could potentially halt or reverse this process as demonstrated in a recent observational study where reverse LV remodeling was seen in patients after 3 months of CRT.22
Although a reduced EF predicts dyssynchrony, the etiology of LV dysfunction is also important. Both SSS and SRS were predictors of dyssynchrony in univariate analyses. SRS was an independent predictor of mechanical dyssynchrony. This is plausible since infarcted and ischemic segments being hypokinetic, akinetic, or dyskinetic lead to abnormal contraction patterns and dyssynchrony.23 Even though a higher burden of scar tends to correlate with more dyssynchrony it is important to note, however, that increasing scar may also be predictive of a lower likelihood to benefit from CRT.24 For example, studies with delayed enhancement cardiac magnetic resonance imaging have demonstrated that both the presence of posterolateral scar and the total scar burden may predict non-response to CRT.25 Thus, dyssynchrony due to scar may be inherently different from that due to delayed activation of a non-infarcted myocardial segment which is more likely to respond to CRT. This brings to light an important point for future research where the presence/absence as well as the extent and location of scar and their collective effects on response to CRT should be evaluated.
Among host factors, we found that blacks had less dyssynchrony as compared to non-blacks, and we postulate that this was due to lower prevalence of ischemic cardiomyopathy in this sub-group of patients. As such, there is no published literature suggesting this association but this observation may warrant further study.
Phase analysis using SPECT MPI is unique in that it not only evaluates dyssynchrony in patients with or without implanted devices, but also allows simultaneous assessment of LV function, and location/extent of scar. In short, several factors that may be important in appropriate patient selection for CRT can be assessed during a single diagnostic test.
LIMITATIONS
This was an observational cohort in a single center and hence subject to the limitations inherent to such studies. Lack of symptom status precluded correlation between heart failure class and dyssynchrony. This is an important limitation since CRT selection is primarily based on heart failure symptom status. Furthermore, the use of SPECT MPI in patients with left ventricular dysfunction may select for a patient population with higher prevalence of CAD and this fact may have some immeasurable influence on the reported prevalence of dyssynchrony in our study population. Additionally, in this study phase analysis was performed on post-stress studies due to the gating protocol in our institution. This may have had unintended effects on the measurement of dyssynchrony in those with severe ischemia and persistent LV dysfunction (i.e., myocardial stunning). However, this is not thought to contribute significantly to our results due to the time delay between stress and image acquisition allowing for resolution of ischemia and recovery of function.
CONCLUSIONS
SPECT-based mechanical dyssynchrony is fairly common in patients with left ventricular dysfunction. Similarly, the prevalence of SPECT-based mechanical dyssynchrony is notable in patients with normal QRS duration. In a population of patients with predominantly ischemic heart disease, a reduced EF, increasing QRS duration, and increasing SRS independently predict mechanical dyssynchrony. There exists an urgent need for prospective, randomized studies to evaluate the feasibility and clinical applicability of phase analysis in improving patient selection for CRT.
Acknowledgments
Grant support: This study was funded by a research grant from the Medtronic-Duke Strategic Alliance, of which Dr Mark Trimble was the primary investigator. Dr Garcia has ownership interest in and serves as a consultant/advisory board member to Syntermed Inc., and receives royalties from the sale of clinical software used as part of this research. Dr Ji Chen receives royalties from the sale of this software. This study was supported in part by funding from NIH (1R01HL094438-01A1, PI: Ji Chen, PhD, Co-Investigator: Ernest Garcia, PhD). Dr Zainab Samad was supported by a Duke/GE Imaging Fellowship training grant. Dr Allen Atchley was supported by a National Institutes of Health T32 grant.
References
- 1.Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 2.Higgins SL, Hummel JD, Niazi IK, et al. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol. 2003;42:1454–9. doi: 10.1016/s0735-1097(03)01042-8. [DOI] [PubMed] [Google Scholar]
- 3.McAlister F, Ezekowitz J, Wiebe N, et al. Cardiac resynchronization therapy for congestive heart failure. Evid Rep Technol Assess. 2004;106:1–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Young JB, Abraham WT, Smith AL, et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: The MIRACLE ICD Trial. JAMA. 2003;289:2685–94. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 5.Ajmone Marsan N, Henneman MM, Chen J, et al. Left ventricular dyssynchrony assessed by two three-dimensional imaging modalities: Phase analysis of gated myocardial perfusion SPECT and tri-plane tissue Doppler imaging. Eur J Nucl Med Mol Imaging. 2008;35:166–73. doi: 10.1007/s00259-007-0539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Garcia EV, Henneman MM, et al. Measuring left ventricular mechanical dyssynchrony from ECG-gated SPECT myocardial perfusion imaging. Minerva Cardioangiol. 2008;56:227–35. [PubMed] [Google Scholar]
- 7.Henneman MM, Chen J, Dibbets-Schneider P, et al. Can LV dyssynchrony as assessed with phase analysis on gated myocardial perfusion SPECT predict response to CRT? J Nucl Med. 2007;48:1104–11. doi: 10.2967/jnumed.107.039925. [DOI] [PubMed] [Google Scholar]
- 8.Henneman MM, Chen J, Ypenburg C, et al. Phase analysis of gated myocardial perfusion single-photon emission computed tomography compared with tissue Doppler imaging for the assessment of left ventricular dyssynchrony. J Am Coll Cardiol. 2007;49:1708–14. doi: 10.1016/j.jacc.2007.01.063. [DOI] [PubMed] [Google Scholar]
- 9.Trimble MA, Borges-Neto S, Smallheiser S, et al. Evaluation of left ventricular mechanical dyssynchrony as determined by phase analysis of ECG-gated SPECT myocardial perfusion imaging in patients with left ventricular dysfunction and conduction disturbances. J Nucl Cardiol. 2007;14:298–307. doi: 10.1016/j.nuclcard.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 10.Trimble MA, Velazquez EJ, Adams GL, et al. Repeatability and reproducibility of phase analysis of gated single-photon emission computed tomography myocardial perfusion imaging used to quantify cardiac dyssynchrony. Nucl Med Commun. 2008;29:374–81. doi: 10.1097/MNM.0b013e3282f81380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borges-Neto S. Perfusion and function assessment by nuclear cardiology techniques. Curr Opin Cardiol. 1997;12:581–6. doi: 10.1097/00001573-199711000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Faber TL, Cooke CD, Garcia EV. Temporal resolution of multiharmonic phase analysis of ECG-gated myocardial perfusion SPECT studies. J Nucl Cardiol. 2008;15:383–91. doi: 10.1016/j.nuclcard.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, Garcia EV, Folks RD, et al. Onset of left ventricular mechanical contraction as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging: Development of a diagnostic tool for assessment of cardiac mechanical dyssynchrony. J Nucl Cardiol. 2005;12:687–95. doi: 10.1016/j.nuclcard.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 14.Borges-Neto S, Shaw LK, Tuttle RH, et al. Incremental prognostic power of single-photon emission computed tomographic myocardial perfusion imaging in patients with known or suspected coronary artery disease. Am J Cardiol. 2005;95:182–8. doi: 10.1016/j.amjcard.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Bax JJ, Abraham T, Barold SS, et al. Cardiac resynchronization therapy: Part 1—issues before device implantation. J Am Coll Cardiol. 2005;46:2153–67. doi: 10.1016/j.jacc.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Anderson LJ, Miyazaki C, Sutherland GR, Oh JK. Patient selection and echocardiographic assessment of dyssynchrony in cardiac resynchronization therapy. Circulation. 2008;117:2009–23. doi: 10.1161/CIRCULATIONAHA.107.721332. [DOI] [PubMed] [Google Scholar]
- 17.Chung ES, Leon AR, Tavazzi L, et al. Results of the predictors of response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–16. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 18.Hawkins NM, Petrie MC, MacDonald MR, Hogg KJ, McMurray JJ. Selecting patients for cardiac resynchronization therapy: Electrical or mechanical dyssynchrony? Eur Heart J. 2006;27:1270–81. doi: 10.1093/eurheartj/ehi826. [DOI] [PubMed] [Google Scholar]
- 19.Nagueh SF. Mechanical dyssynchrony in congestive heart failure: Diagnostic and therapeutic implications. J Am Coll Cardiol. 2008;51:18–22. doi: 10.1016/j.jacc.2007.08.052. [DOI] [PubMed] [Google Scholar]
- 20.Yu CM, Lin H, Zhang Q, Sanderson JE. High prevalence of left ventricular systolic and diastolic asynchrony in patients with congestive heart failure and normal QRS duration. Heart. 2003;89:54–60. doi: 10.1136/heart.89.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Q, Fung JW, Auricchio A, et al. Differential change in left ventricular mass and regional wall thickness after cardiac resynchronization therapy for heart failure. Eur Heart J. 2006;27:1423–30. doi: 10.1093/eurheartj/ehi885. [DOI] [PubMed] [Google Scholar]
- 22.Soliman OI, Geleijnse ML, Theuns DA, et al. Reverse of left ventricular volumetric and structural remodeling in heart failure patients treated with cardiac resynchronization therapy. Am J Cardiol. 2008;101:651–7. doi: 10.1016/j.amjcard.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 23.Forrester JS, Wyatt HL, Da Luz PL, Tyberg JV, Diamond GA, Swan HJ. Functional significance of regional ischemic contraction abnormalities. Circulation. 1976;54:64–70. doi: 10.1161/01.cir.54.1.64. [DOI] [PubMed] [Google Scholar]
- 24.Jansen AH, Bracke F, Dantzig JM, et al. The influence of myocardial scar and dyssynchrony on reverse remodeling in cardiac resynchronization therapy. Eur J Echocardiogr. 2008;9:483–8. doi: 10.1016/j.euje.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Bleeker GB, Kaandorp TA, Lamb HJ, et al. Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation. 2006;113:969–76. doi: 10.1161/CIRCULATIONAHA.105.543678. [DOI] [PubMed] [Google Scholar]