Abstract
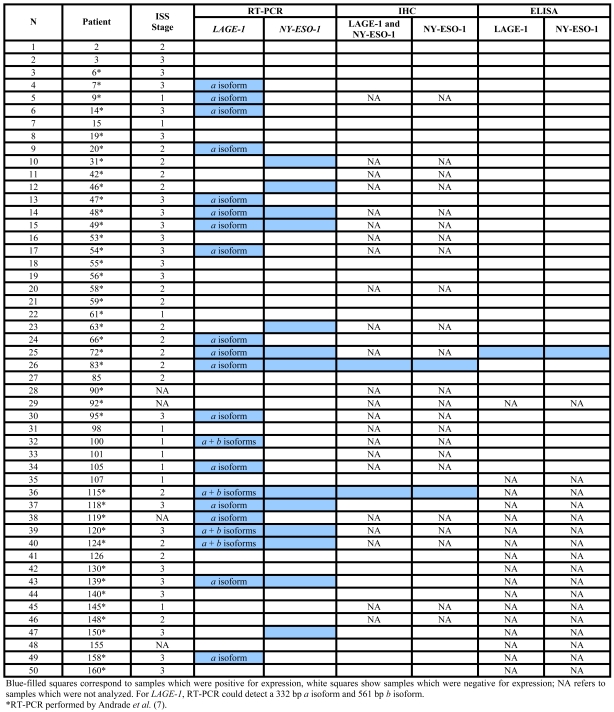
Due to the high homology between the LAGE-1 and NY-ESO-1 proteins, we hypothesized that an anti-NY-ESO-1 vaccine might elicit LAGE-1 immunity and hence may be effective in multiple myeloma (MM) patients with LAGE-1-positive/NY-ESO-1-negative tumors. Therefore, we set out to evaluate LAGE-1 and NY-ESO-1 mRNA and protein expression in MM patients in a bid to evaluate possible benefits of their homology for immunotherapy. LAGE-1 (a and b isoforms) and NY-ESO-1 mRNA expression was studied in 18 normal tissues and 50 bone marrow MM samples by RT-PCR. LAGE-1 and NY-ESO-1 protein expression was analyzed by immunohistochemistry (IHC) in 27 MM specimens using mAbs 219-510-23 and E978. Spontaneous serological immune response against both antigens was analyzed by ELISA in sera from 33 MM patients. LAGE-1 (a and b isoforms) was positive in 42% and NY-ESO-1 in 26% of the MM samples analyzed by RT-PCR. Both genes were found to be expressed in 18% of the cases, while at least one of the genes was found to be expressed in 50% of the cases. In LAGE-1 positive samples, 81% were positive for LAGE-1a and 19% were positive for both LAGE-1a and -1b. LAGE-1 and NY-ESO-1 protein expression could only be detected in two cases by IHC and there was a clear strong spontaneous antibody response to LAGE-1 and NY-ESO-1 in only one MM patient. In conclusion, LAGE-1a and NY-ESO-1 homology cannot be easily exploited in an anti-NY-ESO-1 vaccine given the low frequency of protein expression detected by IHC or serum analysis.
Keywords: human, multiple myeloma, CT antigens, RT-PCR, immunohistochemistry, ELISA
Introduction
In recent years, the aberrant activation and overexpression of cancer/testis antigens (CTAs) has been studied in various types of human malignant tumors (1-3). CTAs are not expressed or expressed at low levels in normal tissues, except for germline cells (and, sometimes, placenta). Based on their tumor-associated expression and due to their ability to elicit an immune response in the autologous host, CTAs are potential targets for vaccine-based cancer immunotherapy (4-6). Since different CTAs can be expressed simultaneously in the same tumor, they are possible targets for polyvalent vaccines.
Currently, an immunotherapy trial targeting the CTAs MAGE-A3 and NY ESO 1 in multiple myeloma (MM) patients is in progress (NCT00090493). Based on our previous findings, MAGE-C1/CT7, MAGE-A3/6 and LAGE-1 were considered good candidates for immunotherapy in MM, with 85% of the 39 cases analyzed expressing at least one of these genes. In particular, LAGE-1 mRNA was detected in 49% of MM patients (7).
LAGE-1, located to the q28 band of chromosome X, is not expressed at significant levels in normal adult tissues (with the exception of testis). LAGE-1 encodes for two alternative splicing isoforms (a and b). The proteins encoded by the two transcripts are largely identical with 95% similarity between the two isoforms in the first 134 amino acids (LAGE-1a [180 amino acids, NP_758965.1] and LAGE-1b [210 amino acids, NP_066274.1]), with the remaining sequence being unique to each isoform (8). However, while both LAGE mRNA sequences have high similarity to the NY-ESO-1 mRNA sequence, at the protein level NY-ESO-1 has high similarity with LAGE-1a but shares only the initial 134 amino acids with LAGE-1b. Considering the high homology between proteins, we speculate that an anti-NY-ESO-1 vaccine might elicit immunity in LAGE-1-expressing tumors and may hence be beneficial to patients with LAGE-1a-positive/NY-ESO-1-negative tumors. The aims of this study were to evaluate the expression of LAGE-1 (a and b isoforms) and NY-ESO-1 at both the mRNA and protein levels in MM samples, as well as the occurrence of spontaneous antibody response against these two proteins, in order to explore the possibility of extending the use of an anti-NY-ESO-1 vaccine to MM patients with LAGE-1a positive tumors.
Results
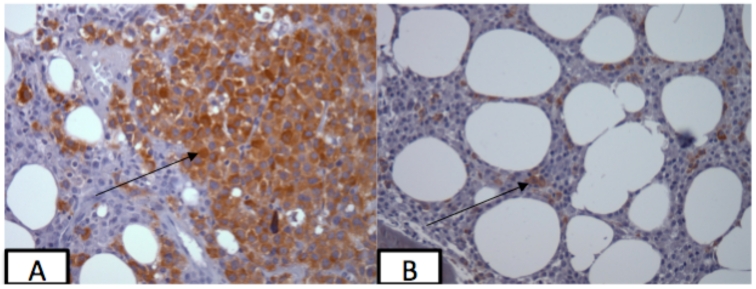
We did not find an association between the International Staging System (ISS) stage and the percentage of LAGE-1 and NY-ESO-1 CTAs expressed by RT-PCR in the MM cases studied (Table 1), either when analyzed individually or when both genes were concordant (both positive or negative) (data not shown). Normal brain was positive for LAGE-1 expression, while normal mammary gland was positive for NY-ESO-1 expression. All other normal tissues, monoclonal gammopathy of undetermined significance (MGUS) and solitary plasmocytoma bone marrow samples were negative for both CTAs evaluated. For the 50 MM analyzed samples, LAGE-1 was positive in 42% (21/50) and NY-ESO-1 in 26% (13/50) of the cases. In 9 out of 50 cases (18%), both genes were positive and in 25 out of 50 cases (50%) the expression of at least one of the genes could be detected by RT-PCR. Of the 21 LAGE-1 positive samples, 17 (81%) were positive for the a isoform and 4 (19%) were positive for both a and b isoforms. However, LAGE-1 and NY-ESO-1 protein expression was detected in only 2 of the 27 cases analyzed by IHC (#83 and #115; both cases were also positive by RT-PCR for both genes) (Table 1 and Figure 1). A strong spontaneous antibody response to LAGE-1 and NY-ESO-1 was detected in only 1 (3%) of the 33 MM patient serum samples analyzed (#72; titers to LAGE-1 and NY-ESO-1 were 2,532 and 2,175, respectively). However, due to the lack of available slides for IHC analysis for this patient, only RT-PCR could be performed which, as expected, was positive for both genes (Table 1).
Table 1.
International Staging System (ISS) stage and LAGE-1 and NY-ESO-1 expression in multiple myeloma patients.
Figure 1.
NY-ESO-1 and LAGE-1 expression as determined by immunohistochemistry. (A) Focal expression of NY-ESO-1 in the bone marrow biopsy of patient 83 using monoclonal antibody E978. (B) Expression of both LAGE-1 and NY-ESO-1 in the bone marrow biopsy of patient 115 using monoclonal antibody 219-510-23. Magnification 400x.
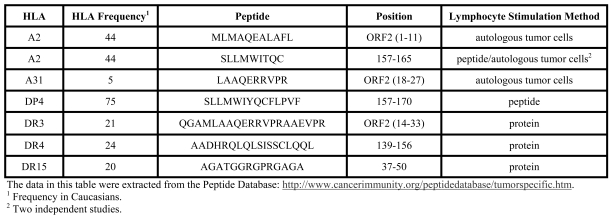
The in silico analysis of peptides recognized by T lymphocytes of cancer patients identified 11 peptides for LAGE-1 and 22 peptides for NY-ESO-1. A comparison between these peptides reveals 7 peptides in common between the two CTAs, confirming the high similarity between the proteins, especially between the LAGE1a isoform and NY-ESO-1, corroborating our hypothesis of probable cross-reactivity to the two proteins (Table 2).
Table 2.
Peptides common to the LAGE-1 and NY-ESO-1 proteins recognized by CD4+ or CD8+ T cells.
Discussion
Using conventional RT-PCR, we found LAGE-1 and NY-ESO-1 mRNA expression in 42% and 26% of the 50 MM analyzed patients, respectively. At least one of the genes was expressed in 50% of cases, but according to IHC analysis, LAGE-1 and/or NY-ESO-1 protein were expressed in only 7.4% of the MM analyzed cases.
In a previous study (7), we showed that both antigens were not expressed in many normal tissues, except for brain (positive for LAGE-1) and mammary gland (positive for NY-ESO-1). Hofmann et al. (9) showed that some CTAs can be expressed in normal brain samples and Sugita et al. (10) showed expression of NY-ESO-1 mRNA in benign breast lesions.
In this study, we observed that LAGE-1a mRNA was more frequent than LAGE-1b expression in MM cases (Table 1). LAGE-1a protein has 84% similarity with NY-ESO-1 protein and, using in silico analysis, we identified seven peptides present in both CTAs that were recognized by T lymphocytes in different tumors (Table 2). Therefore the LAGE-1a isoform and NY-ESO-1 could be considered as one "single" CTA for immunotherapy purposes.
We also found that 18% of MM patients expressed LAGE-1 and NY-ESO-1. Vaughan et al. (11) showed that both antigens (LAGE-1 and NY-ESO-1) were expressed in 31% (37/120) of melanoma patients, but they suggested that both CTAs were not consistently co-expressed.
In the present study, we found a high discrepancy between mRNA and protein expression for both LAGE-1 and NY-ESO-1. Breast cancer studies have also found divergences when NY-ESO-1 RT-PCR data was compared to IHC results (10, 12, 13). We believe that the discordance in our study could be explained by the low sensitivity of the antibodies used or the quality of bone marrow tumor tissues present in the slides (routinely submitted to bone decalcification).
Spontaneous humoral response at diagnosis was detected in 1 out of 33 of MM samples analyzed. Curioni-Fontecedro et al. (14) found NY-ESO-1 expression in only 1 out of 24 MM tumors and also a low incidence of antibodies to NY-ESO-1 in patient sera (2/60 cases of previously treated MM). Moreover, Atanackovic et al. (15) analyzed the humoral response against MAGEA3, SSX2 and NY-ESO-1 in 66 patients with MM before and after allogeneic hematopoietic stem cell transplantation. Spontaneous humoral immunity against NY-ESO-1 was not detected before the allogeneic transplant. Hence a low frequency of spontaneous humoral response against CTAs at MM diagnosis could be common, possibly due to humoral immune compromise in such patients.
The question of LAGE-1 immunogenicity is very interesting but poorly understood, since most studies usually focus on NY-ESO-1. Although in general both antigens tend to be co-expressed, it is still an open question whether they are both equally immunogenic. There are reports of LAGE-1-specific T cell responses that are distinct from NY-ESO-1, as well as possible epitopes for regulatory T cells. The correlation with protein expression data is therefore quite interesting, and in the absence of purely LAGE-1-specific reagents, extrapolations could be done from assays comparing antibodies reacting exclusively with NY-ESO-1. Worth mentioning, while this report may shed some light on the potential controversy regarding LAGE-1 expression and immunogenicity, it depends on the reliability of the reagents used to assess expression at the protein level. This study could be more valuable if we could show that MM cells (especially those that are RT-PCR positive but IHC negative) can be recognized by specific T cell clones. However, our MM patients were not HLA-typed and we do not have bone marrow cell samples in DMSO (frozen cells) available.
To continue and improve this work, we could extend the study to a larger number of MM samples obtained from patients at different stages of their disease to explore whether the CTA antigen expression is related to disease stage. A possible correlation would further substantiate the data presented here, when established in a higher number of cases.
In conclusion, for the first time the frequency of LAGE-1 isoforms is shown in MM patients. Because of the high similarity between the LAGE-1a and NY-ESO-1 proteins, we hypothesize that an anti-NY-ESO-1 vaccine could benefit MM patients with tumors that express LAGE-1a but not NY-ESO-1.
Abbreviations
- CTA
cancer/testis antigen
- IHC
immunohistochemistry
- MM
multiple myeloma
Acknowledgements
We thank Dr. Otavia L. Caballero for critically revising the manuscript. FC was supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and BK was supported by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo), Brazil.
References
- 1.Jungbluth AA, Chen YT, Stockert E, Busam KJ, Kolb D, Iversen K, Coplan K, Williamson B, Altorki N, Old LJ. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer. 2001;92:856–860. doi: 10.1002/ijc.1282. [DOI] [PubMed] [Google Scholar]
- 2.Wadle A, Kubuschock B, Imig J, Wuellner B, Wittig C, Zwick C, Mischo A, Waetzig K, Romeike BFM, Lindemann W, Schilling M, Pfreundschuh M, Renner C. Serological immune response to cancer testis antigens in patients with pancreatic cancer. Int J Cancer. 2006;119:117–125. doi: 10.1002/ijc.21744. [DOI] [PubMed] [Google Scholar]
- 3.Purbhoo MA, Sutton D, Brewer JE, Mullings RE, Hill ME, Mahon TM, Karbach J, Jäger E, Cameron BJ, Lissin N, Vyas P, Chen JL, Cerundolo V, Jakobsen BK. Quantifying and Imaging NY-ESO-1/LAGE-1-derived epitopes on tumor cells using high affinity T cells receptors. J Immunol. 2006;176:7308–7316. doi: 10.4049/jimmunol.176.12.7308. [DOI] [PubMed] [Google Scholar]
- 4.Eichmüller S, Usener D, Jochim A, Schadendorf D. mRNA expression of tumor-associated antigens in melanoma tissues and cell lines. Exp Dermatol. 2002;11:292–301. doi: 10.1034/j.1600-0625.2002.110402.x. [DOI] [PubMed] [Google Scholar]
- 5.Jungbluth AA, Ely S, Diliberto M, Niesvizky R, Williamson B, Frosina D, Chen YT, Bhardwaj N, Chen-Kiang S, Old LJ, Cho HJ. The cancer-testis antigens CT7 (MAGEC1) and MAGE-A3/6 are commonly expressed in multiple myeloma and correlate with plasma-cell proliferation. Blood. 2005;106:167–174. doi: 10.1182/blood-2004-12-4931. [DOI] [PubMed] [Google Scholar]
- 6.Gnjatic S, Old LJ, Chen YT. Autoantibodies against cancer antigens. Methods Mol Biol. 2009;520:11–19. doi: 10.1007/978-1-60327-811-9_2. [DOI] [PubMed] [Google Scholar]
- 7.Andrade VCC, Vettore AL, Felix RS, Almeida MSS, Carvalho F, Oliveira JSR, Chauffaille MLLF, Andriolo A, Caballero OL, Zago MA, Colleoni GWB. Prognostic impact of cancer/testis antigen expression in advanced stage multiple myeloma patients. Cancer Immun. 2008;8:2. http://www.cancerimmunity.org/v8p2/071018.htm [PMC free article] [PubMed] [Google Scholar]
- 8.Lethé B, Lucas S, Michaux L, De Smet C, Godelaine D, Serrano A, De Plaen E, Boon T. LAGE-1, a new gene with tumor specificity. Int J Cancer. 1998;76:903–908. doi: 10.1002/(sici)1097-0215(19980610)76:6<903::aid-ijc22>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Hofmann O, Caballero OL, Stevenson BJ, Chen YT, Cohen T, Chua R, Maher CA, Panji S, Schaefer U, Kruger A, Lehvaslaiho M, Carninci P, Hayashizaki Y, Jongeneel CV, Simpson AJG, Old LJ, Hide W. Genome-wide analysis of cancer/testis gene expression. Proc Natl Acad Sci USA. 2008;105:20422–20427. doi: 10.1073/pnas.0810777105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugita Y, Wada H, Fujita S, Nakata T, Sato S, Noguchi Y, Jungbluth AA, Yamaguchi M, Chen YT, Stockert E, Gnjatic S, Williamson B, Scanlan M, Ono T, Sakita I, Yasui M, Miyoshi Y, Tamaki Y, Matsuura N, Noguchi S, Old LJ, Nakayama E, Monden M. NY-ESO-1 expression and immunogenicity in malignant and benign breast tumors. Cancer Res. 2004;64:2199–2204. doi: 10.1158/0008-5472.can-03-3070. [DOI] [PubMed] [Google Scholar]
- 11.Vaughan HA, Svobodova S, MacGregor D, Sturrock S, Jungbluth AA, Browning J, Davis ID, Parente P, Chen YT, Stockert E, Clair FS, Old LJ, Cebon J. Immunohistochemical and molecular analysis of human melanomas for expression of the human cancer-testis antigens NY-ESO-1 and LAGE-1. Clin Cancer Res. 2004;10:8396–8404. doi: 10.1158/1078-0432.CCR-04-0809. [DOI] [PubMed] [Google Scholar]
- 12.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 13.Oba-Shinjo SM, Caballero OL, Jungbluth AA, Rosemberg S, Old LJ, Simpson AJ, Marie SK. Cancer-testis (CT) antigen expression in medulloblastoma. Cancer Immun. 2008;8:7. http://www.cancerimmunity.org/v8p7/080307.htm [PMC free article] [PubMed] [Google Scholar]
- 14.Curioni-Fontecedro A, Knights AJ, Tinguely M, Nuber N, Schneider C, Thomson CW, von Boehmer L, Bossart W, Pahlich S, Gehring H, Moch H, Renner C, Knuth A, Zippelius A. MAGE-C1/CT7 is the dominant cancer-testis antigen targeted by humoral immune responses in patients with multiple myeloma. Leukemia. 2008;22:1646–1648. doi: 10.1038/leu.2008.43. [DOI] [PubMed] [Google Scholar]
- 15.Atanackovic D, Arfsten J, Cao Y, Gnjatic S, Schnieders F, Bartels K, Schilling G, Faltz C, Wolschke C, Dierlamm J, Ritter G, Eirmann T, Hossfeld DK, Zander AR, Jungbluth AA, Old LJ, Bokemeyer C, Kröger N. Cancer-testis antigens are commonly expressed in multiple myeloma and induce systemic immunity following allogeneic stem cell transplantation. Blood. 2007;109:1103–1112. doi: 10.1182/blood-2006-04-014480. [DOI] [PubMed] [Google Scholar]
- 16.Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Bladé J, Boccadoro M, Child JA, Avet-Loiseau H, Kyle RA, Lahuerta JJ, Ludwig H, Morgan G, Powles R, Shimizu K, Shustik C, Sonneveld P, Tosi P, Turesson I, Westin J. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 17.Peptide database: T-cell defined tumor antigens. http://www.cancerimmunity.org/peptidedatabase/tumorspecific.htm [Last updated: July 12, 2010]
Materials and methods
Patients
This study was approved by the Ethical Committee of the Universidade Federal de São Paulo (São Paulo, Brazil) and informed consent was obtained from all patients. Disease stage was classified according to the International Staging System (ISS) (16). Of the 50 MM patients studied, 18% (9) were stage I, 32% (16) were stage II and 42% (21) were stage III. In four (8%) MM cases ISS staging was unavailable.
RT-PCR
The expression of NY-ESO-1 and LAGE-1 was previously studied by our group using RT-PCR in different normal tissues (including normal bone marrow aspirates from allogeneic stem-cell transplant donors), bone marrow aspirates of monoclonal gammopathy of undetermined significance (MGUS) and of solitary plasmacytomas, and bone marrow aspirates from MM patients obtained at diagnosis, without any previous treatment (7). Here, we analyzed 11 additional bone marrow MM samples for both LAGE-1 and NY-ESO-1 expression by RT-PCR. RNA extraction and RT-PCR reactions were conducted as described by Andrade et al. (7). The primers used for evaluation of the LAGE-1 gene expression were able to amplify both mRNA isoforms (a 332 bp product is seen for the a isoform and a 561 bp product for the b isoform).
Immunohistochemistry
LAGE-1 and NY-ESO-1 protein expression were analyzed by immunohistochemistry in 27 MM bone marrow specimens using monoclonal antibodies (mAbs) 219-510-23 and E978, respectively (1, 11). The mAb 219-510-23 detects both NY-ESO-1 and LAGE-1 antigens and it is the first time that this monoclonal antibody is reported (a manuscript describing mAb 219-510-23 in more detail is in preparation).
ELISA
The spontaneous serological immune response against both LAGE-1 and NY-ESO-1 antigens was analyzed by ELISA with recombinant CTA or CTA fragments (6). Sera from 33 MM patients (obtained at diagnosis without any previous treatment) were tested in serial dilutions: 1/100, 1/400, 1/1600 and, in some cases, 1/6400. From the curve, a titer was extrapolated based on cutoffs from negative control serum pools for each antigen. Positive control sera were present on each plate to validate the assay. A reciprocal titer >100 was considered significant. RT-PCR data for all 33 MM samples analyzed was available and immunohistochemistry data was obtained for 16 of the samples.
Peptides recognized by T lymphocytes
LAGE-1 and NY-ESO-1 peptides recognized by T lymphocytes in tumors were taken from the Cancer Immunity Peptide Database (17).