Abstract
We have created a transgenic mouse with tissue-specific expression of the human papilloma virus (HPV) 16 E6 and E7 oncoproteins in the thyroid as a model of HPV transformed cancer. The expression of the transgenes results in the formation of palpable thyroid tumors. E7 is not expressed in other tissues but is expressed in medullary thymic epithelial cells, which have been implicated in the control of negative selection. We show that Listeria-based vaccines against E7 can induce the regression of solid implanted tumors in the transgenic mice, although at a lower frequency than in wild type (WT) mice. E7-specific CD8+ T cells induced in transgenic mice are of both lower avidity and lower frequency when compared to the WT mice. In this model, Listeria-based vaccines against E7 appear to be overcoming central tolerance by expanding low avidity CD8+ T cells specific for E7 that are not deleted during thymopoesis and can eliminate solid tumors.
Keywords: transgenic mice, vaccination, cytotoxic T cells, tumor immunity, tolerance
Introduction
Cancer vaccines directed against self-antigens must be able to overcome host tolerance in order to elicit a productive cell-mediated immune response capable of completely killing the tumor. In mouse models of transplantable cancer, non-tolerant animals are relatively easy to cure. Indeed there are many examples of the induction of CD8+ T cell responses against tumor associated antigens (TAAs) expressed by implanted tumors in mice that are not tolerant to the TAA (1). There have also been reports demonstrating that self-tolerance can be surmounted in several different tumor models (2, 3, 4). In this study, we ask whether Listeria-based vaccines can elicit a productive immune response to a syngeneic transplanted tumor in a transgenic mouse that overexpresses a tissue-specific TAA and that displays tolerance to the TAA. A major advantage of using a transgenic mouse to a "self" tumor antigen is that it allows an examination of the immune response to this antigen when it is a "self" antigen in the transgenic mouse contrasted with it being a "foreign" antigen in the wild type mouse with the same genetic background.
We created a mouse model of cancer on the C57BL/6 background that expresses the HPV-16 tumor associated antigens, E6 and E7, under the control of the bovine thyroglobulin promoter similar to a previously described E7 transgenic mouse (5). We chose to use E6 and E7 as we and others have developed a number of reagents over the years that allow us to carefully measure the immune responses induced in this transgenic mouse (6, 7, 8). More importantly however, HPV is the primary causative agent of ano-genital cancer and has been strongly associated with certain forms of head and neck cancer. In fact, over 50% of both cervical cancer and squamous cell carcinoma of the tonsil are associated with HPV-16 (9, 10).
One of the immortalizing proteins in HPV-induced cancers is the Rb-binding protein, E7, which has been extensively studied as a potential tumor antigen for cytotoxic T lymphocyte (CTL) killing (11). A well-characterized transplantable mouse tumor which expresses E7 is the TC-1 tumor cell line. This cell line is a C57BL/6 immortalized primary lung epithelial cell line that was transformed with the E6, E7, and c-Ha-ras oncogenes (8). TC-1 cells are poorly immunogenic in vivo and form solid tumors when injected s.c. into syngeneic host mice which cannot control the growth of the tumor without some type of intervention (8). This model has been used to evaluate the efficacy of HPV-16 induced cancer vaccines prior to clinical use and was used in this study to examine whether there is a difference in the immune response mounted against the TC-1 tumors in the E6/E7 transgenic mice as opposed to TC-1 tumors implanted in WT C57BL/6 mice.
For many cancers, immunotherapeutic approaches are focused on inducing a CTL response that can kill tumor cells that consistently express a TAA required for the maintenance of the malignant phenotype (1). The generation of CD8+ tumor infiltrating lymphocytes (TILs) can be used as a surrogate marker for vaccine efficacy (12). As we have shown previously, Listeria monocytogenes (LM) can be used to eliminate palpable, vascularized tumors in several mouse models due to the vaccine’s ability to generate TILs (12, 13, 14, 15). Not only does the Listeria bacterium act as a natural adjuvant capable of eliciting a powerful cell-mediated immune response, but in our approach to creating Listeria immunotherapeutics, the bacterium is genetically engineered to secrete the TAA fused to a molecular adjuvant that enhances the overall immune response. In previous studies we have created two vaccines LM-LLO-E7 and LM-ActA-E7. A truncated listeriolysin O (LLO) is fused to E7 in LM-LLO-E7, whereas LM-ActA-E7 has E7 fused to a fragment of the ActA protein (6, 7). Both the truncated LLO and ActA have PEST-like domains within their amino acid sequences which have been postulated to result in the rapid degradation of the fusion protein when secreted into the host cell cytosol (16, 17, 18). These two vaccines have been previously shown to induce CD8+ T cells that can home to, penetrate and kill the solid tumor formed by TC-1 cells in a normal C57BL/6 mouse (6, 12, 13).
We sought to answer several questions in this study. Can we create a mouse model of cancer that displays tolerance to the TAAs E6 and E7? What is the efficacy of Listeria vaccines in this E6/E7 transgenic mouse as compared to the C57BL/6 mouse? Can these differences, should they be observed, be attributed to differences in the avidity of the CD8+ T cell populations induced?
Results
The E6/E7 transgenic mouse phenotype
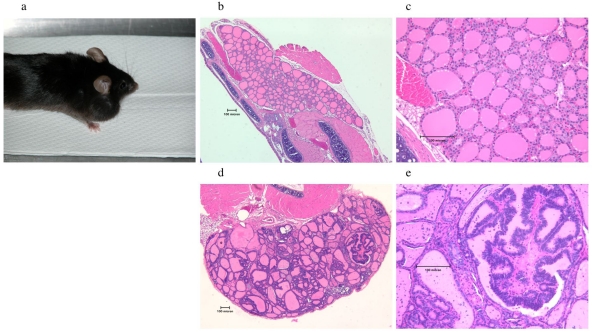
The phenotype of the E6/E7 transgenic mouse appears to be very similar to the E7 transgenic mouse described by Ledent et al. (5). Thyroid hyperplasia begins at 2 months (data not shown); capsular invasion and palpable thyroids appear in many mice at around 6 months. Although some thyroid hyperplasia begins to become apparent at 2 months of age, the overall size of the thyroid remains very small and comparable in size to that of a WT mouse (data not shown). A large, palpable goiter from an 18-month-old mouse is shown in Figure 1a. WT C57BL/6 and E6/E7 transgenic mice were sacrificed at 6 months, and their thyroids removed and examined by hematoxylin/eosin staining after sectioning. Normal thyroids from a six month old C57BL/6 mouse are shown in Figure 1 (panels b and c) at 5x and 20x magnification. We found that the transgenic thyroid demonstrated typical de-differentiation of the normal thyroid architecture, indicative of an early stage of cancer (Figure 1, panels d and e). We also observed the formation of a papillary carcinoma as clearly seen in Figure 1e. The elongated follicular cells at 5x (Figure 1d) and 20x (Figure 1e) magnification contain colloid, where thyroid hormones accumulate. Their enlargement is also seen in the E7 transgenic mouse (5).
Figure 1.
E6/E7 transgenic mouse phenotype. (a) Gross photograph of an 18 month old E6/E7 transgenic mouse with an enlarged thyroid visible externally. (b and c) Photomicrograph of a thyroid gland from a 6 month old WT mouse at 5x (b) and 20x (c) magnification. (d and e) Photomicrograph of a thyroid gland from a 6 month old transgenic mouse at 5x (d) and 20x (e) magnification. The thyroid follicles are engorged with colloid and are irregular in shape. Also found are solid masses of cells with little or no follicular organization. A papillary carcinoma is evident in (e).
E7 message is not detected in intact thymic tissue but is detected in medullary thymic epithelial cells
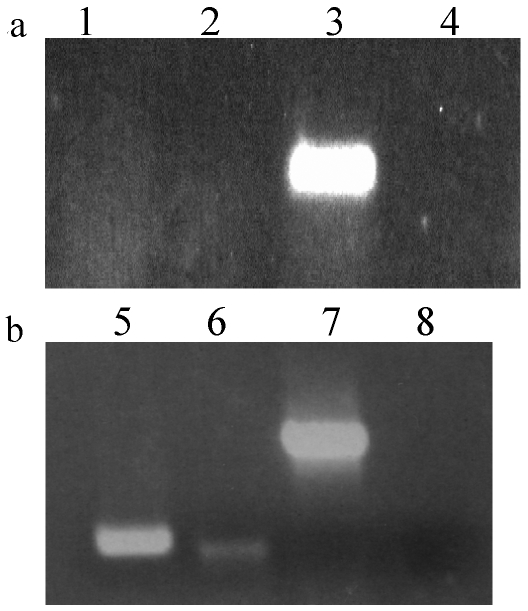
We next examined whether or not E7 was expressed in the thymus, as this organ controls both the positive and negative selection of T cell development. We examined various tissues, liver, spleen, thymus and thyroid, for the expression of the transgene in 5 to 6 week old mice. As Figure 2a shows, abundant E7 message was found in the thyroid but not in other tissues, including the thymus. However, the absence of E7 message in whole thymus preparations was unsurprising since the level of message of a peripherally expressed, organ-specific antigen, including thyroglobulin, has been shown to be too low to detect in whole thymocyte preparations (19).
Figure 2.
E7 message is expressed in the thyroid and medullary thymic epithelial cells of the E6/E7 transgenic mouse. (a) Tissue-specific expression of the E7 transgene is detected in the thyroid only but not in the liver, spleen or whole thymus. Organs were isolated and RT-PCR was performed as described in Materials and Methods. Lanes: 1, liver; 2, spleen; 3, thyroid; 4, whole thymus. (b) Medullary thymic epithelial cells (mTECs) express E7. mTECs were isolated by cell sorting into an RNA protection solution as detailed in Materials and Methods. RT-PCR results are shown for equivalent amounts of cDNA loaded for 40 cycles. Lanes: 5, cathepsin S; 6, E7; 7, actin; 8, negative control (water).
It has been shown that tolerance to peripheral antigens in the thymus, including thyroglobulin, is mediated by the transient expression of these genes by the autoimmune regulator (AIRE) in medullary thymic epithelial cells (mTECs), with peak expression occurring prior to birth (19). AIRE is a transcription factor critical for maintaining tolerance to self as it is a key regulator of negative selection in the thymus (20, 21). Since E7 expression is driven by the thyroglobulin promoter, it seemed likely that tolerance to E7 would be mediated by the same mechanism. Thyroglobulin mRNA is detectable in the embryonic thymus on day 15 of gestation, and peaks at day 18. After birth it can no longer be detected by week 8 (19). We thus examined the expression of E7 in mTECs isolated from thymi from E6/E7 transgenic mice of 3 to 5 weeks in age, and found that they expressed E7 message (Figure 2b). Additionally, as a positive control, we also looked for mRNA expression for the protease cathepsin S, which is known to be expressed in mTECs, and found it expressed in the mTECs of the E6/E7 transgenic mouse (19). For both panels of Figure 2, RT negative controls were used to ensure that no genomic DNA contamination was present. No contaminants were observed (data not shown). Although the E7 band in Figure 2b is of lower intensity compared to that of cathepsin S, we believe that the E7 DNA band does represent E7 mRNA because it is similar to the thyroglobulin cDNA band in mTECs isolated by Derbinski et al. (19). In that study, the intensity of the thyroglobulin band is similarly attenuated compared to cathepsin S (19). Thus, this difference in intensity between E7 and cathepsin S in Figure 2b is unsurprising since E7 is expressed under the control of the thyroglobulin promoter. As E7 is expressed in the thymus, we can infer a certain level of central tolerance to the E7 antigen in this mouse and would expect that E7-specific T cells with a high avidity would be deleted from the T cell repertoire of the E6/E7 transgenic mouse.
Listeria E7 vaccines are less effective at inducing complete tumor regression in the E6/E7 transgenic mouse compared to the wild-type mouse
The Listeria vaccines, LM-LLO-E7 and LM-ActA-E7, are known to induce a therapeutic immune response against the E7 oncoprotein capable of eradicating the TC-1 tumor in the WT syngeneic C57BL/6 mouse (6). We thus asked whether expressing the E6/E7 proteins as a tissue-specific self antigen would affect the efficacy of these vaccines.
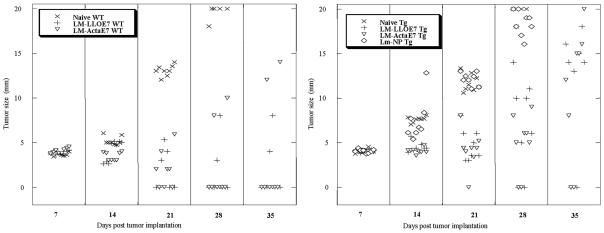
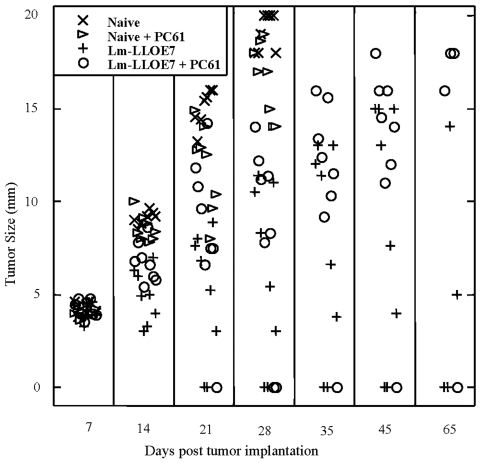
TC-1 cells were implanted s.c. and allowed to form solid tumors in 6 to 8 week old WT and transgenic mice (n = 8). Seven and fourteen days later, the mice were immunized with LM-LLO-E7, LM-ActA-E7, a control vector LM-NP, or left unimmunized. Tumor size was monitored and by day 35 the naive mice had a large tumor burden, as anticipated, and were sacrificed. LM-NP also failed to slow tumor growth. Tumors regressed in the C57BL/6 mice immunized with either LM-LLO-E7 or LM-ActA-E7 in 6 out of 8 mice. However, in contrast, only 2 out of 8 E6/E7 transgenic mice showed complete tumor regression for either vaccine. These data are shown in Figure 3. This experiment has been repeated two more times but we did not observe cure rates of more than 3 out of 8 E6/E7 transgenic mice with either vaccine. A statistically significant difference by the Wilcoxon test was observed for the average tumor size in vaccinated transgenic mice when compared to naive (P = 0.001) and WT (P = 0.05) mice for either Listeria vaccine at day 28. Thus, in the E6/E7 transgenic mouse, both Listeria vaccines are impaired as measured by the corresponding tumor regression, but the vaccines are competent enough to cure some of the animals and slow the growth of the tumors in the other animals. This reinforces the notion that the E6/E7 transgenic mouse displays immune tolerance to E7 and that this can be partially overcome by Listeria vaccines.
Figure 3.
Listeria-based anti-E7 vaccines induce regression of solid tumors in the E6/E7 transgenic mice, albeit at a lower frequency than in the WT C57BL/6 mice. 1 x 105 TC-1 tumor cells were implanted s.c. at day 0 in six to eight week old mice. When palpable tumors formed seven days later, mice were i.p. immunized with 1 x 108 LM-LLO-E7, 2.5 x 108 LM-ActA-E7, left naive, or treated with LM-NP as a control. Mice were boosted with the same vaccine on day 14. Every seven days, tumors were measured using a caliper in two dimensions, and the average of those values was plotted against days post tumor implantations for each mouse. Values shown are only for surviving mice. Depicted is one of 3 experiments.
The CTL response in the E6/E7 transgenic mouse is less potent compared to that in the WT mouse
We next inquired as to whether there was an appreciable difference in the induction of CD8+ cytotoxic T lymphocytes (CTLs) in the E6/E7 transgenic mouse, on vaccination, as opposed to the WT C57BL/6 mouse. A difference here could potentially indicate some disparity in the functional avidity between C57BL/6 mice and E6/E7 transgenic mice. Avidity is the sensitivity of a CD8+ T cell to respond to a peptide/MHC I complex, and in this particular instance, we used killing of a target cell as one marker of functional avidity (22).
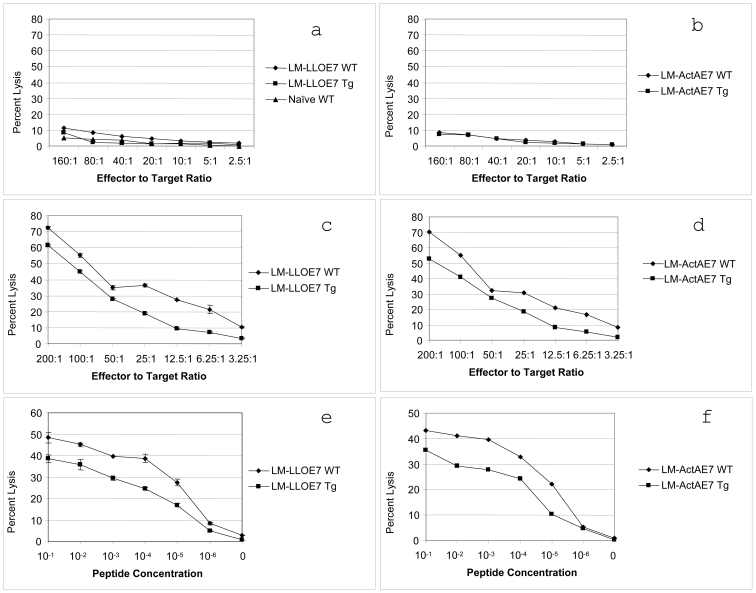
Splenocytes were isolated from immunized transgenic or age matched WT mice and expanded in culture for 4 days in the presence of TC-1 feeder cells and IL-2. Their cytolytic activity was then measured in a standard 51Cr release assay using peptide-pulsed syngeneic EL4 cells as targets. We demonstrated that both strains of mice can elicit a CTL response capable of killing their target cells, but that the CTLs produced in the E6/E7 transgenic mouse kill less effectively at any given effector to target ratio (Figure 4, panels c and d). When the cells are not in the presence of the RAHYNIVTF peptide, a small amount of nonspecific killing is observed in both strains of mice (Figure 4, panels a and b). When EL4 cells are pulsed with differing quantities of peptide, at a constant effector to target ratio, the CTLs from the E6/E7 transgenic mice require a log more peptide to display equivalent % specific lysis to the CTLs from the C57BL/6 mouse (Figure 4, panels e and f). The SC50 value for the WT mouse was 0.83 x 10-5 µM for the LM-LLO-E7 vaccine and 0.95 x 10-5 µM for the LM-ActA-E7 vaccine. The SC50 value for the E6/E7 transgenic mouse was 0.3 x 10-4 µM for the LM-LLO-E7 vaccine and 0.79 x 10-4 µM for the LM-ActA-E7 vaccine. Thus, splenic CTLs isolated from the transgenic mouse have a lower functional avidity in vitro when compared to the CTL cells from the WT mouse.
Figure 4.
CTLs generated in the E6/E7 transgenic mouse have reduced cytotoxicity as compared to CTLs generated from the C57BL/6 mouse. Either six- to eight-week-old WT or transgenic mice were immunized with 1 x 108 LM-LLO-E7 or 2.5 x 108 LM-ActA-E7 on days zero and seven. On day 14, splenocytes were removed and cultured with irradiated TC-1 cells for 4 days. Following this culture step, splenocytes were used in a four-hour 51Cr release assay. (a and b) EL4 cells alone. (c and d) EL4 cells pulsed with 1 µM of the RAHYNIVTF peptide with differing concentrations of effector cells to target cells. (e and f) EL4 cells plus differing concentrations of peptide with an effector to target cell ratio of 50:1. Assays were preformed in triplicate with the mean number plotted and standard deviation present as error bars. Depicted is one of 2 experiments.
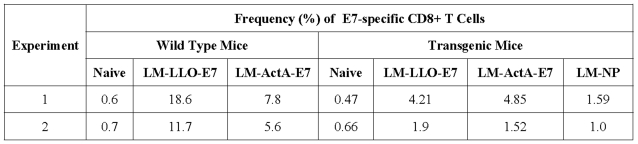
In order to measure the cytolytic function shown in Figure 4, it was necessary, as is customary for this type of assay, to culture the splenocytes for 4 days to expand the E7-specific CTLs. While this provides useful information about the overall quality of the induced CD8+ T cell response, it does not address the issue of the frequency of the response. We thus used ex vivo tetramer staining to examine the number of E7-specific CD8+ T cells induced in the spleen of E6/E7 transgenic mice on vaccination compared to WT mice. Table 1 shows that the transgenic mice generated a lower frequency of E7 tetramer specific CD8+ T cells as compared to the WT mice.
Table 1.
E7-specific CD8+ T cells are detected at different frequencies in the spleens of tumor-free mice.
To establish that this reduced frequency was caused by E7 expressed as a self-antigen in the E6/E7 transgenic mouse, we examined the immune response to a non-self antigen, OVA. We found that both WT mice and E6/E7 transgenic mice elicited approximately the same number of OVA-specific CD8+ effector cells in the spleen (data not shown). This would suggest that the E6/E7 transgenic mice are capable of mounting normal CD8+ T cell responses.
Tumor infiltrating lymphocytes in the E6/E7 mice are of lower avidity than in the C57BL/6 mice
Although the induction of CD8+ T cells is a necessary condition for effective tumor immunotherapy, it is not a sufficient one (12). The activated CD8+ T cells that home to and penetrate the tumor are the cell population that dictates vaccine efficacy, as measured by tumor regression. Additionally, the relative T cell avidities of a given population to a specific peptide can be measured by titrating in varying amounts of tetramer and analyzing the data using flow cytometry (23). We thus used this technique to determine if the two strains of mice elicited different amounts of TILs, and if those TILs had differing avidities.
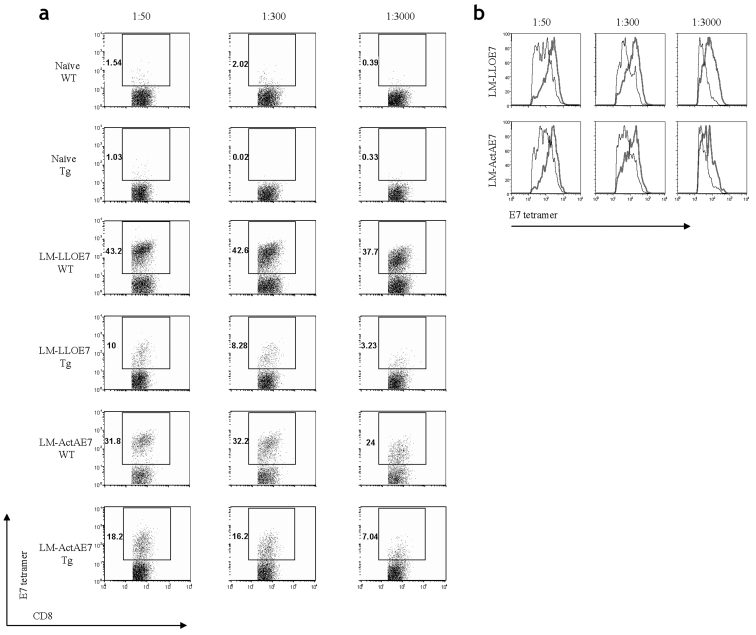
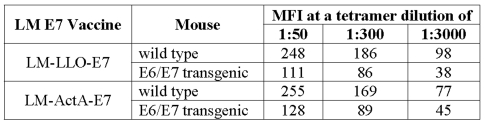
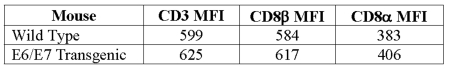
Upon examination of the TILs by flow cytometry, we observed that the effector CD8+ T cells were found at a higher frequency in the WT mice as opposed to the transgenic mice for any given concentration of tetramer. In Figure 5a, TILs from naive mice have only background staining. These data correlate directly with the observations in the tumor load study (Figure 3), in which we observed different levels of tumor regression. The frequencies of effector CD8+ T cells in both the WT and E6/E7 transgenic mice that are immunized with LM-LLO-E7 or LM-ActA-E7 are also shown. We observe very different frequencies of TILs present at each concentration of tetramer for either mouse. Upon closer analysis, we found that the two different strains of mice induce TIL populations of different avidities. Displaying the CD8 positive, CD62L negative, tetramer positive T cells as a histogram allowed a comparison of the mean channel fluorescence intensity (MFI) of tetramer binding at different titrations (Figure 5b). Table 2 shows that for either vaccine, LM-LLO-E7 or LM-ActA-E7, at the highest concentration of tetramer (1:50), TILs from the WT mice bound the tetramer with an approximately two-fold higher MFI compared with those from the E6/E7 transgenic. These results are concomitantly observed for both the 1:300 and 1:3000 dilutions of the RAHYNIVTF tetramer. To confirm that the difference in tetramer MFI between the TILs is due to differences in TCR avidity and not to the level of TCR expression, we examined CD3 and CD8 levels on the surface of these populations and found them to be identical (Table 3) (24). Thus, the lower tetramer staining of the TILs generated in the E6/E7 mouse confirm that they are of lower avidity compared with TILs isolated from tumors from the C57BL/6 mouse.
Figure 5.
A titration of the RAHYNIVTF-loaded tetramer demonstrates that the TILS induced in the C57BL/6 mice have a higher avidity than those in the E6/E7 transgenic mice. 1 x 105 TC-1 tumor cells in Matrigel were implanted s.c. in both six- to eight-week-old C57BL/6 and six-to eight-week old E6/E7 transgenic mice. Seven days later, when palpable tumors about 5 mm in size form, the mice were immunized i.p. with 1 x 108 LM-LLO-E7, 2.5 x 108 LM-ActA-E7, or left untreated. Mice were boosted on day 14 with the same vaccine dose. On day 21, mice were sacrificed, and each group had tumors pooled together, digested with collagenase, crushed, filtered, and made into a single cell suspension as described in Materials and Methods. Cells were stained for CD62L, CD8α, CD11b, and the H-2Db tetramer loaded with the RAHYNIVTF peptide at dilutions of 1:50, 1:300, and 1:3000. 7AAD was used as a viability dye. Cells shown in (a) were positive for CD8α and the E7 tetramer, were negative for 7AAD and CD11b, and were low for CD62L. Histograms shown in (b) were gated from the data in (a) and overlayed. (a) Naive mice fail to accumulate any TILs. Mice immunized with LM-LLO-E7 or LM-ActA-E7 have an increased frequency of TILs at any given concentration of tetramer in the WT mice as opposed to those in the transgenic mice. (b) The MFI was also higher for the overall CD8+ effector T cells found in the tumor for the C57BL/6 mice (thick line) as opposed to the transgenic mice (thin line). Data shown are representative of one of two experiments.
Table 2.
Mean fluorescent intensity (MFI) of tetramer staining of CD8+ TILs from normal and transgenic mice vaccinated with LM-LLO-E7 or LM-ActA-E7.
Table 3.
Mean fluorescent intensity (MFI) of CD8 and CD3 staining of tetramer positive TILs from normal and transgenic mice.
Depletion of CD4+ CD25+ regulatory T cells does not increase vaccine efficacy
In one transgenic mouse model, it has been proposed that high avidity T cells are crucial in controlling the growth of transplantable tumors. There, tumor-specific T cells were only detected by tetramer titration after removal of CD4+ CD25+ regulatory T cells (25). To determine whether CD4+ CD25+ regulatory T cells play a role in the CD8+ T cell mediated anti-tumor efficacy of the Listeria-based E7 vaccine, tumors were implanted in 6 to 8 week old E6/E7 transgenic mice (Figure 6). Mice were depleted of CD4+ CD25+ regulatory T cells, vaccinated and boosted, or mice were left naive and depleted of CD4+ CD25+ regulatory T cells. Two control groups were included – the first was simply naive mice that were administered TC-1 and the second was mice that were implanted with TC-1 then vaccinated but not depleted of CD4+ CD25+ regulatory T cells. At 65 days post TC-1 implantation, there is no difference between depleted versus non-depleted groups (Figure 6).
Figure 6.
Depletion of CD4+ CD25+ regulatory T cells has no effect on vaccine efficacy. 1 x 105 TC-1 tumor cells were implanted s.c. at day 0 in six- to eight-week-old E6/E7 transgenic mice. For experimental groups, mice were depleted of regulatory T cells using i.p. injections of 0.5 mg of PC61 anti-CD25 mAb on days 4 and 6, which was then followed either by i.p. immunization with 1 x 108 LM-LLO-E7 or the mice were left naive. In control groups, when palpable tumors formed seven days later, mice were i.p. immunized with 1 x 108 LM-LLO-E7 or left naive. Mice were boosted with the same vaccine on day 14. Tumors were measured in two dimensions using a caliper, and the average of those values was plotted against days post tumor implantation for each mouse. Depicted is one of 2 experiments.
Discussion
Here we have described a new mouse model with which to examine immune tolerance to the tissue-specific tumor-associated antigens E6 and E7. We also show that Listeria-based protein secreting vaccines elicit a therapeutic immune response in this mouse. Several insights into this new model have been made, and the questions that we originally posed were answered. First, we created a mouse tolerant to a tissue-specific TAA and furthermore isolated E7 message from the medullary thymic epithelium of the transgenic mice to verify its expression in the thymus. Second, we showed that there is a pronounced difference in the efficacy of Listeria-based E7 vaccines in the E6/E7 transgenic mouse versus the parental C57BL/6 strain. Third, the effector CD8+ T cells produced by Listeria vaccination have lower avidity in the E6/E7 transgenic mice when they are contrasted with those effector CD8+ T cells in WT mice. Finally, despite the lower avidity of these T cells, they do appear to be competent to infiltrate and control tumor growth in the E6/E7 transgenic mouse, albeit at lower levels than in the WT mouse.
The genetic background of the E6/E7 transgenic mouse is identical to C57BL/6 except for the tissue-specific expression of the E6/E7 transgenes. Thus our data strongly suggest that the E7-specific CD8+ T cells present in the E6/E7 transgenic mouse have been modulated by the expression of the E7 transgene when compared with the CD8+ T cells from the WT mouse. T cells of high avidity to self antigen are believed to be deleted by negative selection during the formation of the T cell repertoire in the thymus. We hypothesize that E7, as it is expressed in thymic stromal tissue, results in a loss of the high avidity, RAHYNIVTF-specific CD8+ T cells present in the C57BL/6 WT mouse.
In the thymus, the medullary thymic epithelial cells have been shown to play a critical role during the negative selection stage of T cell development by inducing the removal of the subset of high avidity T cells that are autoreactive to peripherally expressed self antigens (19). mTECs can delete autoreactive T cells either directly or indirectly by thymic DC cross-presentation of the peripheral antigen to the autoreactive T cell (26). mTECs express the autoimmune regulator (AIRE) gene, which codes for a transcription factor critical for maintaining tolerance to self (20, 21). AIRE controls the expression of peripheral antigens, the antigen processing/presentation of those antigens, and has other functions in the thymus, although all of these have yet to be elucidated (27). When AIRE is knocked out, the mice rapidly succumb to a variety of autoimmune disorders that are tissue-specific in the periphery. Most importantly, for our studies, mTECs have been shown to play a critical role in inducing the deletion of T cells reactive to thyroid antigens during repertoire selection in the thymus in an AIRE-dependent manner (28). In this study we show that the E7 transgene is expressed in mTECs, consistent with the reduction of the average avidity of the peripheral T cell repertoire in the E6/E7 transgenic mouse.
The CD8+ T cell response induced by the Listeria vaccines that we observe in the transgenic mice is probably due to the activation of low avidity T cells that are not deleted during thymic selection. However, although LM-LLO-E7 and LM-ActA-E7 still showed therapeutic efficacy in the E6/E7 transgenic mice, as measured by tumor regression, the WT mice mounted a more robust immune response presumably due to a lack of tolerance to the E7 antigen in the TC-1 tumor. This lack of tolerance to E7 in the WT mice allows for a robust, high avidity anti-E7 CTL response. The lower avidity of the T cell population from transgenic mice compared with WT mice suggests a loss of the high avidity CD8+ T cells specific for the RAHYNIVTF peptide by negative selection. If this is the case, what is the role of peripheral tolerance in this model? In most cervical cancer patients, tolerance to viral antigens prevents clearance and allows infected epithelial cells to transform into neoplastic lesions. In these patients, peripheral tolerance mechanisms are most likely at work (29). It was recently shown in a transgenic mouse model of breast cancer that the CD4+ CD25+ regulatory T cell subset controls the outcome of the immune response observed (25). We have examined whether this specific T cell subset plays a role in maintaining tolerance to E7 in the E6/E7 transgenic mouse and have concluded that CD4+ CD25+ regulatory T cells do not play a significant role in maintaining tolerance to E7 in young transgenic mice.
There are a number of transgenic mice that express the E6 and E7 oncogenes of HPV-16 from various promoters. In one study, a novel Semliki Forest virus vector’s ability to overcome tolerance against HPV-16 E6 and E7 in a transgenic mouse was measured (30). In this model, E6 and E7 are expressed in the suprabasal layers of the epidermis under the control of the keratin 10 promoter (30, 31) Although the mechanism of surmounting host tolerance was not elucidated, the recombinant Semliki Forest virus vector expressing E6 and E7 could overcome host tolerance and induce an HPV-specific immune response (30). The α-crystallin promoter has been used to target HPV 16 E6 and E7 to the ocular lens (32). Interestingly, expression of E6 and E7 occurred in a number of non-lens tissue in some lines of these mice. In particular a line (line 19) was developed that expressed E6/E7 in the skin. Skin dysplasia occurred in these mice and about 20% progressed to squamous cell tumors. However, these transgenic mice do not appear to be tolerant to E7 and both humoral and cell mediated immunity could be readily induced to E7 in the line 19 mice at levels comparable to those in normal mice (33). This is curious as it was clearly shown by Derbinski et al. (19) that crystallin is expressed in the thymus, specifically, but not limited to mTECs. When E6 and E7 oncoproteins are expressed under the control of the keratin 14 promoter, E7 was shown to be expressed in the thymus in addition to epithelia (34, 35). These mice develop progressive papillomatosis but practically no mice develop tumors spontaneously in the absence of the application of carcinogens to the skin. In addition, there is some controversy as to the level of tolerance of T cells in K14-Tg mice to E7 (33, 35, 36). However, despite the earlier studies, immune tolerance has been observed in this mouse and was presumed to be due to mostly peripheral mechanisms (37). In the K14-Tg mouse, the E7 transgene is highly expressed in the cortex of the thymus, in addition to being expressed in the epithelium (34). Moreover, this mouse shows a downregulation of all T cell-mediated immune responses to a variety of antigens, including E7. There is no difference in the avidities of E7 specific CD8+ cells in these mice as contrasted with WT mice (37). Thus, our observations in the E6/E7 transgenic mice, which have low avidity E7-specific CD8+ T cells capable of killing solid tumors, can be considered a novel finding, when contrasted with the other E7 transgenic mice.
The ability of Listeria-based vaccines to break tolerance has not been extensively examined. One study used Listeria that secretes the melanoma self antigen, Trp-2, treated in conjunction with imiquimod (2). However, unlike our study, the level of tolerance to this antigen could not be examined because there is no syngeneic mouse that lacks this antigen. Another group has claimed to break tolerance to a self antigen using Listeria targeting the gp70 retroviral gene in the CT26 mouse tumor model; however, the epitope that their strain secreted did not have complete homology with the target TAA sequence and therefore cannot be stringently considered to be a self antigen (38).
We chose to develop the E6/E7 thyroglobulin model as the transgenic mouse model for these studies due to conflicting reports on the degree of tolerance displayed by other E7 expressing transgenic mice. In this E6/E7 transgenic mouse E7 expression is restricted to two locations, the thyroid and medullary thymic epithelium. This model, therefore, appears to be physiologically relevant to studying the immune response to tissue-specific antigens. Although we have not directly shown deletion of high avidity T cells in the thymus, it seems likely that central tolerance contributes to the diminished immune responses we have observed in this E6/E7 transgenic mouse. Future studies will primarily focus on examining the contributions of peripheral tolerance to the immune response in this model.
Abbreviations
- 7AAD
7-amino-actinomycin D
- LD50
the lethal dose for 50% of the mice
- LLO
listeriolysin O
- LM
Listeria monocytogenes
- mTEC
medullary thymic epithelial cell
- TAA
tumor associated antigen
- WT
wild type
Acknowledgements
We would like to thank Cristina Picca and Andrew Caton for their mTEC protocol. Additionally, the authors would like to thank Hank Pletcher and the University of Pennsylvania School of Medicine Flow Core Sorting Facility for their assistance in sorting the mTECs. We are grateful to Bin Wang for assistance in developing the transgenic mouse colony and are also grateful to Jeff Tsai for his expert assistance in isolating murine thyroids.
This work was supported by grant number CA69632 from the National Institutes of Health and American Cancer Society grant number TURSG LIB-01-168-01. Nicholas Souders is supported in part by NIH/NCI sponsored program T32 CA 09140, Training program in Immunobiology of Normal and Neoplastic Lymphocytes.
Yvonne Paterson wishes to disclose that she has a financial interest in Advaxis, Inc., a vaccine and therapeutic company that has licensed or has an option to license all patents from the University of Pennsylvania that concern the use of Listeria or listerial products as vaccines.
References
- 1.Stevanovic S. Identification of tumour-associated T-cell epitopes for vaccine development. Nat Rev Cancer. 2002;2:514–520. doi: 10.1038/nrc841. [DOI] [PubMed] [Google Scholar]
- 2.Craft N, Bruhn KW, Nguyen BD, Prins R, Lin JW, Liau LM, Miller JF. The TLR7 agonist imiquimod enhances the anti-melanoma effects of a recombinant Listeria monocytogenes vaccine. J Immunol. 2005;175:1983–1990. doi: 10.4049/jimmunol.175.3.1983. [DOI] [PubMed] [Google Scholar]
- 3.Nair S, Boczkowski D, Moeller B, Dewhirst M, Vieweg J, Gilboa E. Synergy between tumor immunotherapy and antiangiogenic therapy. Blood. 2003;102:964–971. doi: 10.1182/blood-2002-12-3738. [DOI] [PubMed] [Google Scholar]
- 4.Engelhard VH, Bullock TN, Colella TA, Sheasley SL, Mullins DW. Antigens derived from melanocyte differentiation proteins: self-tolerance, autoimmunity, and use for cancer immunotherapy. Immunol Rev. 2002;188:136–146. doi: 10.1034/j.1600-065x.2002.18812.x. [DOI] [PubMed] [Google Scholar]
- 5.Ledent C, Marcotte A, Dumont JE, Vassart G, Parmentier M. Differentiated carcinomas develop as a consequence of the thyroid specific expression of a thyroglobulin-human papillomavirus type 16 E7 transgene. Oncogene. 1995;10:1789–1797. [PubMed] [Google Scholar]
- 6.Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol. 2001;167:6471–6479. doi: 10.4049/jimmunol.167.11.6471. [DOI] [PubMed] [Google Scholar]
- 7.Sewell DA, Douven D, Pan ZK, Rodriguez A, Paterson Y. Regression of HPV-positive tumors treated with a new Listeria monocytogenes vaccine. Arch Otolaryngol Head Neck Surg. 2004;130:92–97. doi: 10.1001/archotol.130.1.92. [DOI] [PubMed] [Google Scholar]
- 8.Lin KY, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM, Wu TC. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–26. [PubMed] [Google Scholar]
- 9.Lowy DR, Kirnbauer R, Schiller JT. Genital human papillomavirus infection. Proc Natl Acad Sci U S A. 1994;91:2436–2440. doi: 10.1073/pnas.91.7.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, Shah KV, Sidransky D. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 11.Feltkamp MC, Smits HL, Vierboom MP, Minnaar RP, de Jongh BM, Drijfhout JW, ter Schegget J, Melief CJ, Kast WM. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993;23:2242–2249. doi: 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- 12.Hussain SF, Paterson Y. What is needed for effective antitumor immunotherapy? Lessons learned using Listeria monocytogenes as a live vector for HPV-associated tumors. Cancer Immunol Immunother. 2005;54:577–586. doi: 10.1007/s00262-004-0600-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sewell DA, Shahabi V, Gunn GR 3rd, Pan ZK, Dominiecki ME, Paterson Y. Recombinant Listeria vaccines containing PEST sequences are potent immune adjuvants for the tumor-associated antigen human papillomavirus-16 E7. Cancer Res. 2004;64:8821–8825. doi: 10.1158/0008-5472.CAN-04-1958. [DOI] [PubMed] [Google Scholar]
- 14.Pan ZK, Ikonomidis G, Lazenby A, Pardoll D, Paterson Y. A recombinant Listeria monocytogenes vaccine expressing a model tumour antigen protects mice against lethal tumour cell challenge and causes regression of established tumours. Nat Med. 1995;1:471–477. doi: 10.1038/nm0595-471. [DOI] [PubMed] [Google Scholar]
- 15.Singh R, Dominiecki ME, Jaffee EM, Paterson Y. Fusion to Listeriolysin O and delivery by Listeria monocytogenes enhances the immunogenicity of HER-2/neu and reveals subdominant epitopes in the FVB/N mouse. J Immunol. 2005;175:3663–3673. doi: 10.4049/jimmunol.175.6.3663. [DOI] [PubMed] [Google Scholar]
- 16.Decatur AL, Portnoy DA. A PEST-like sequence in listeriolysin O essential for Listeria monocytogenes pathogenicity. Science. 2000;290:992–995. doi: 10.1126/science.290.5493.992. [DOI] [PubMed] [Google Scholar]
- 17.Rogers S, Wells R, Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986;234:364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- 18.Rechsteiner M, Rogers SW. PEST sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21:267–271. [PubMed] [Google Scholar]
- 19.Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–1039. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- 20.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 21.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura MI, Roszkowski JJ, Moore TV, Brasic N, McKee MD, Clay TM. Antigen recognition and T-cell biology. Cancer Treat Res. 2005;123:37–59. doi: 10.1007/0-387-27545-2_2. [DOI] [PubMed] [Google Scholar]
- 23.Lustgarten J, Dominguez AL, Cuadros C. The CD8+ T cell repertoire against Her-2/neu antigens in neu transgenic mice is of low avidity with antitumor activity. Eur J Immunol. 2004;34:752–761. doi: 10.1002/eji.200324427. [DOI] [PubMed] [Google Scholar]
- 24.Cawthon AG, Lu H, Alexander-Miller MA. Peptide requirement for CTL activation reflects the sensitivity to CD3 engagement: correlation with CD8alphabeta versus CD8alphaalpha expression. J Immunol. 2001;167:2577–2584. doi: 10.4049/jimmunol.167.5.2577. [DOI] [PubMed] [Google Scholar]
- 25.Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels JP, Bieler JG, Emens LA, Reilly RT, Jaffee EM. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J Exp Med. 2005;201:1591–1602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med. 2004;200:1039–1049. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Liston A, Gray DH, Lesage S, Fletcher AL, Wilson J, Webster KE, Scott HS, Boyd RL, Peltonen L, Goodnow CC. Gene dosage--limiting role of Aire in thymic expression, clonal deletion, and organ-specific autoimmunity. J Exp Med. 2004;200:1015–1026. doi: 10.1084/jem.20040581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tindle RW. Immune evasion in human papillomavirus-associated cervical cancer. Nat Rev Cancer. 2002;2:59–65. doi: 10.1038/nrc700. [DOI] [PubMed] [Google Scholar]
- 30.Riezebos-Brilman A, Regts J, Freyschmidt EJ, Dontje B, Wilschut J, Daemen T. Induction of human papilloma virus E6/E7-specific cytotoxic T-lymphocyte activity in immune-tolerant, E6/E7-transgenic mice. Gene Ther. 2005;12:1410–1414. doi: 10.1038/sj.gt.3302536. [DOI] [PubMed] [Google Scholar]
- 31.Auewarakul P, Gissmann L, Cid-Arregui A. Targeted expression of the E6 and E7 oncogenes of human papillomavirus type 16 in the epidermis of transgenic mice elicits generalized epidermal hyperplasia involving autocrine factors. Mol Cell Biol. 1994;14:8250–8258. doi: 10.1128/mcb.14.12.8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambert PF, Pan H, Pitot HC, Liem A, Jackson M, Griep AE. Epidermal cancer associated with expression of human papillomavirus type 16 E6 and E7 oncogenes in the skin of transgenic mice. Proc Natl Acad Sci U S A. 1993;90:5583–5587. doi: 10.1073/pnas.90.12.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herd K, Fernando GJ, Dunn LA, Frazer IH, Lambert P, Tindle RW. E7 oncoprotein of human papillomavirus type 16 expressed constitutively in the epidermis has no effect on E7-specific B- or Th-repertoires or on the immune response induced or sustained after immunization with E7 protein. Virology. 1997;231:155–165. doi: 10.1006/viro.1997.8491. [DOI] [PubMed] [Google Scholar]
- 34.Doan T, Chambers M, Street M, Fernando GJ, Herd K, Lambert P, Tindle R. Mice expressing the E7 oncogene of HPV16 in epithelium show central tolerance, and evidence of peripheral anergising tolerance, to E7-encoded cytotoxic T-lymphocyte epitopes. Virology. 1998;244:352–364. doi: 10.1006/viro.1998.9128. [DOI] [PubMed] [Google Scholar]
- 35.Melero I, Singhal MC, McGowan P, Haugen HS, Blake J, Hellstrom KE, Yang G, Clegg CH, Chen L. Immunological ignorance of an E7-encoded cytolytic T-lymphocyte epitope in transgenic mice expressing the E7 and E6 oncogenes of human papillomavirus type 16. J Virol. 1997;71:3998–4004. doi: 10.1128/jvi.71.5.3998-4004.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frazer IH, Fernando GJ, Fowler N, Leggatt GR, Lambert PF, Liem A, Malcolm K, Tindle RW. Split tolerance to a viral antigen expressed in thymic epithelium and keratinocytes. Eur J Immunol. 1998;28:2791–2800. doi: 10.1002/(SICI)1521-4141(199809)28:09<2791::AID-IMMU2791>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 37.Tindle RW, Herd K, Doan T, Bryson G, Leggatt GR, Lambert P, Frazer IH, Street M. Nonspecific down-regulation of CD8+ T-cell responses in mice expressing human papillomavirus type 16 E7 oncoprotein from the keratin-14 promoter. J Virol. 2001;75:5985–5997. doi: 10.1128/JVI.75.13.5985-5997.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brockstedt DG, Giedlin MA, Leong ML, Bahjat KS, Gao Y, Luckett W, Liu W, Cook DN, Portnoy DA, Dubensky TW Jr. Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc Natl Acad Sci U S A. 2004;101:13832–13837. doi: 10.1073/pnas.0406035101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ledent C, Parmentier M, Vassart G. Tissue-specific expression and methylation of a thyroglobulin-chloramphenicol acetyltransferase fusion gene in transgenic mice. Proc Natl Acad Sci U S A. 1990;87:6176–6180. doi: 10.1073/pnas.87.16.6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmiter RD, Sandgren EP, Avarbock MR, Allen DD, Brinster RL. Heterologous introns can enhance expression of transgenes in mice. Proc Natl Acad Sci U S A. 1991;88:478–482. doi: 10.1073/pnas.88.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tesson L, Heslan JM, Menoret S, Anegon I. Rapid and accurate determination of zygosity in transgenic animals by real-time quantitative PCR. Transgenic Res. 2002;11:43–48. doi: 10.1023/a:1013928600442. [DOI] [PubMed] [Google Scholar]
- 42.Gray DH, Chidgey AP, Boyd RL. Analysis of thymic stromal cell populations using flow cytometry. J Immunol Methods. 2002;260:15–28. doi: 10.1016/s0022-1759(01)00493-8. [DOI] [PubMed] [Google Scholar]
Materials and methods
Mice
Six- to eight-week-old C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA). Animals were cared for and utilized in accordance with protocols approved by the Animal Care and Use Committee of The University of Pennsylvania (Philadelphia, PA).
Creation of the E6/E7 transgenic mouse
The E6/E7 transgenic mouse was based on a similar mouse where E7 is expressed in the thymus under the control of the thyroglobulin promoter (5). Although this study is limited to examining E7-based vaccines, we chose to include the E6 gene to extend the utility of the mouse to testing E6 as a TAA. The plasmid used to create the E6/E7 mouse was a very generous gift from Dr. Catherine Ledent, Université Libre de Bruxelles. The plasmid, constructed in the pSG5 vector, contained the bovine thyroglobulin promoter, a rabbit β-intron, the E6, E7 genes, and a polyadenylation signal in a gene cassette. The bovine thyroglobulin promoter was used as it has been shown to be tightly regulated and expressed in thyrocytes (39). The rabbit β-intron was used to increase the expression of the transgenes (40). This cassette was removed and purified by gel electrophoresis followed by a Geneclean kit (Q-Biogen, Morgan Irvine, CA). DNA was concentrated by ethanol precipitation. The cut DNA was then microinjected by the University of Pennsylvania School of Medicine Transgenic Facility under the direction of Dr. Jean Richa. The founder mice strain was C57BL/6. The founder mice were thus mated to WT C57BL/6, and the progeny back crossed and screened by the ΔCT real time PCR method until a homozygous, genetically pure population was obtained as described (41).
Detection of E7 expression in whole tissue
E7 gene expression was examined by RT-PCR in various tissues harvested from five to six week old E6/E7 transgenic mice. Tissue from the liver, spleen, thyroid and thymus was treated with RNAlater (Qiagen, Valencia, CA) according to the manufacturer’s recommendations and stored at –80˚C. After thawing on ice, RNA was isolated from each tissue sample using the RNAeasy Mini Kit (Qiagen). cDNA was synthesized using E7 primers and a Superscript III platinum RT PCR kit (Invitrogen Corporation, Carlsbad, CA). The sequences of the E7 primers used were 5’ cat gga gat aca cct aca ttg (forward) and 3’ tta tgg ttt ctg aga aca g (backward). The expression of mouse β-actin was examined for comparative purposes (5’ cct aag gcc aac cgt gaa aag and 3’ tct tca tgg tgc tag gag cca).
Detection of E7 message in mTECs
Five thymi were harvested from E6/E7 transgenic mice 3 to 5 weeks of age. Thymi were gently crushed in RPMI 1640 to loosen thymocytes from stroma. Media was removed and the remaining thymus tissue underwent a series of enzymatic digestions. First, thymi underwent three collagenase digestions (RPMI 1640, 20 mM HEPES, 2% FCS, and 0.2 mg/ml collagenase type IV; Worthington Biochemical Corp, Lakewood, NJ) for 15 minutes at 30˚C each time removing and discarding the supernatant, keeping the thymus tissue. Second, thymi were digested by a collagenase/dispase mixture (RPMI 1640, 20 mM HEPES, 2% FCS, 0.2 mg/ml collagenase type IV, 25 µg/ml dispase grade I, and DNase I, the latter two from Roche Diagnostics, Indianapolis, IN) for 25 minutes at 30˚C, each time keeping and decanting the supernatant. The supernatant was immediately filtered with a 100 µm filter, diluted with media, and kept on ice. The remaining thymic tissue underwent two digestions with trypsin-EDTA solution (Gibco, Invitrogen Corporation, Carlsbad, CA) supplemented with 0.3% BSA (Sigma, St. Louis, MO) for 25 minutes at 37˚C. The supernatant was filtered with a 100 µm filter, diluted with media, and kept on ice. All cells were centrifuged down, pooled together in media, and Fc-blocked with the antibody 2.4G2. They were next stained with anti-CD45 PE (clone 30-F11, BD-Pharmingen, Franklin Lakes, NJ) and then with anti-PE MicroBeads (Miltenyi Biotec, Auburn, CA). CD45+ cells were depleted using an LD column (Miltenyi Biotec) allowing the selection of a mixed population of thymic epithelial cells. In order to cell sort the mTECs from other cell populations, all cells were stained sequentially with anti-CD326 (clone G8.8, BD-Pharmigen), anti-rat IgG2a-FITC (clone RG7/1.30 BD-Pharmigen), anti-Ly51-biotin (clone 6C3/BP-1, BD-Pharmigen), streptavidin-APC (BD-Pharmigen), and 7-amino-actinomycin D (7AAD) as a viability dye (42). Cells were sorted by the Flow Cytometry and Cell Sorting Facility at the University of Pennsylvania into an RNA protection solution (RNAlater, Qiagen, Valencia, CA) and stored at -80˚C. mTECs were isolated based upon being viable, CD45 negative, CD326 positive, and Ly51 negative/intermediate. After thawing on ice, RNA was isolated using the RNAeasy Mini Kit using Qiashredders (Qiagen) and the RNAse-Free DNAse Set (Qiagen) as directed by the manufacturer’s instructions. RNA was reverse transcribed into cDNA using the First-Strand for cDNA Synthesis Kit (Roche Applied Sciences). PolyA primers included in the kit were used to synthesize cDNA, and this cDNA was pooled together and ethanol precipitated to concentrate DNA. PCR reactions to amplify cDNA to detect message of cathepsin S, E7, or β-actin were performed using Expand High-Fidelity Polymerase System from Roche Applied Sciences for 40 cycles. Primers were described previously (19).
Histology and histochemistry of thyroid tissue
Thyroid sections were taken from 6-month-old C57BL/6 WT and E6/E7 transgenic mice. Thyroids were fixed in paraformaldehyde and then imbedded in paraffin. They were sectioned and stained with hematoxylin/eosin by the Pathology Core Facility at the School of Medicine, University of Pennsylvania.
Listeria E7 vaccines
Listeria-based anti-E7 vaccines, LM-LLO-E7 and LM-ActA-E7, were used and maintained as previously described (6, 7). LM-NP was used as a control vaccine as described (14).
TC-1 tumor cell line
The TC-1 cell line, a generous gift from Dr. T. C. Wu (Johns Hopkins University School of Medicine, Baltimore, MD) is a lung epithelial cell immortalized with HPV-16 E6 and E7 and transformed with the c-Ha-ras oncogene (8). TC-1 was grown in RPMI 1640 medium supplemented with 10% FCS, 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 100 µM nonessential amino acids, 1 mM sodium pyruvate, and 50 µM 2-ME at 37˚C with 10% CO2 (8).
Tumor regression study
Six- to eight-week-old C57BL/6 or E6/E7 transgenic mice received 1 x 105 TC-1 cells s.c. on the left flank. Seven and fourteen days post tumor inoculation, groups of eight mice were then immunized i.p. with 0.1 LD50 LM-LLO-E7, LM-ActA-E7, LM-NP, or left naive. The tumors were measured in two dimensions with calipers. The mean of these two measurements was plotted as the mean tumor diameter in millimeters against various time points. Mice were sacrificed when the tumor diameter reached 2.0 cm in compliance with our IACUC protocols. Tumor measurements for each time point are shown only for surviving mice. For comparison of tumor diameters of each treatment group, individual tumor sizes were examined statistically at day 28 using the non-parametric Wilcoxon test. Mice that had been sacrificed were considered to have a value of 20 mm for the statistical analysis.
To examine the role of regulatory T cells in the E6/E7 transgenic mice, four groups of 6- to 8-week-old E6/E7 transgenic mice received 1 x 105 TC-1 cells s.c. on the left flank. For the first two groups, seven and fourteen days post tumor inoculations, groups of eight mice were then immunized i.p. with 0.1 LD50 LM-LLO-E7 or left naive. For the third and fourth groups, mice were depleted of all CD25+ cells by injecting each mouse i.p. with 0.5 mg of PC61 mAb on days 4 and 6 after tumor challenge (6). Mice depleted of CD25+ cells were then immunized i.p. with 0.1 LD50 LM-LLO-E7 seven and fourteen days post tumor inoculation or left naive. The tumors were measured in two dimensions with calipers. The mean of these two measurements was plotted as the mean tumor diameter in millimeters against various time points. Mice were sacrificed when the tumor diameter reached 2.0 cm in compliance with our IACUC protocols. Tumor measurements for each time point are shown only for surviving mice.
Splenic analysis of E7-specific CD8+ T cells
Three 6-8 week-old C57BL/6 mice (Charles River, Wilmington, MA) and three 6-8 week-old E6/E7 transgenic mice were immunized i.p. with 0.1 LD50 (1 x 108) LM-LLO-E7, (2.5 x 108) LM-ActA-E7, (1 x 107) LM-NP, or left untreated. Mice were boosted with the same vaccine dose on day 7. On day 14, the mice were sacrificed and the spleens were harvested and pooled together. Next, the spleens were minced in DMEM plus 10% FCS. The cells were then filtered using a 100 µm cell strainer (BD Biosciences Pharmingen, San Diego, CA). Red blood cells (RBCs) were lysed using ACK Lysing Buffer (BioSource, Rockville, MD). Splenocytes were analyzed by four color flow cytometry on a FACScalibur for CD8α (FITC), CD11b (PerCP Cy5.5), CD62L (APC) (BD Biosciences Pharmingen, San Diego, CA), 7AAD (Immunotech, Beckman-Coulter, Marseilles, FR), and tetramer (PE) of the H-2Db restricted E7 epitope in the C57BL/6 mouse (RAHYNIVTF). The E7/Db tetramer was supplied by the NIAID Tetramer Core Facility at Emory University through the NIH AIDS Research and Reference Reagent Program. Cells were analyzed by comparing tetramer positive, CD8 positive, CD11b negative, 7AAD negative, and CD62L low cells within the spleens of the different groups. The data was analyzed with Cell Quest software (Becton Dickenson, Mountain View, CA).
51Chromium release assay
Three 6-8 week old C57BL/6 mice or E6/E7 transgenic mice were immunized i.p. with 0.1 LD50 LM-LLO-E7 or LM-ActA-E7 twice, seven days apart. The mice were then sacrificed seven days after both immunizations and the spleens were harvested. Splenocytes were then cultured with irradiated TC-1 cells as feeder cells and 20 U/ml IL-2 (Roche, Indianapolis, IN). Following four days of culture, the splenocytes were used as effector cells in a standard 51Cr release assay. Two types of experiment were performed. In the first, target cells (EL4) were labeled with chromium and were then cultured with the splenocyte culture at effector to target cell ratios of 200:1, 100:1, 50:1, 25:1, 12.25:1, 6.125: 1 and 3:1 in triplicate for four hours in the presence of 1 µM of RAHYNIVTF peptide. In the second experiment, splenocytes were washed and cultured with EL4 cells at an effector:target cell ratio of 50:1 and RAHYNIVTF peptide at concentrations ranging from 10-1 µM to 10-5 µM for four hours. Following these incubations, 100 µl of supernatant was assayed for 51Cr release. The percentage specific lysis was determined as [(experimental counts per minute – spontaneous counts per minute)/(total counts per minute – spontaneous counts per minute)] x 100. SC50 values were calculated as the peptide concentration required for 50% of the value of maximum peptide concentration used (10-1 µM) minus the background level at 0 µM.
Tetramer titration to measure CD8+ T cell avidity
Six- to eight-week-old C57BL/6 mice (Charles River, Wilmington, MA) and six- to eight-week-old E6/E7 transgenic mice received 1 x 105 TC-1 cells suspended in Matrigel Basement Membrane (BD Biosciences, Bedford, MA) s.c. on the left flank. When tumors became palpable on day 7, groups of four mice were then treated i.p. with 0.1 LD50 LM-LLO-E7 or LM-ActA-E7, or left untreated. Mice were boosted with the same vaccine dose on day 14. On day 21, the mice were sacrificed and tumors were harvested, pooled together, digested with collagenase (RPMI 1640, 20 mM HEPES, 2% FCS, and 0.2 mg/ml collagenase type IV; Worthington, Lakewood, NJ) for 30 minutes, and then crushed. The cells were then filtered using a 100 µm cell strainer (BD Biosciences Pharmingen, San Diego, CA). RBCs were lysed using ACK Lysing Buffer (BioSource, Rockville, MD). TILs were analyzed by four color flow cytometry on a FACScalibur for CD8α (FITC), CD11b (PerCP Cy5.5), CD62L (APC) (BD Biosciences Pharmingen, San Diego, CA), 7AAD (Immunotech, Beckman-Coulter, Marseilles, FR), and three different concentrations (1:50, 1:300, 1:3000) of the tetramer (PE) of the H-2Db restricted E7 epitope in the C57BL/6 mouse (RAHYNIVTF). The E7/Db tetramer was supplied by the NIAID Tetramer Core Facility at Emory University through the NIH AIDS Research and Reference Reagent Program. The data was analyzed with FlowJo software (Tree Star Inc, Ashland, OR).
Analyses of CD3 and CD8 levels were performed as described above but with the following changes. TILs were stained with CD8α (FITC), CD11b (PerCP Cy5.5), and 7AAD. One concentration (1:300) of the tetramer (PE) of the H-2Db restricted E7 epitope in the C57BL/6 mouse (RAHYNIVTF) was used. On the FL4 channel, cells were stained with either CD3 (APC) or CD8β (APC). Positive events were tetramer positive, CD8α positive, CD11b negative, and 7AAD negative. Those cells were then examined for the MFI of their CD3, CD8α, or CD8β population.