Abstract
During the consolidation of fear memory, it has been shown that GABAA receptors (GABAAR) are rapidly downregulated in amygdala. This rapid decrease in GABAAR functioning may permit transient hyperexcitablity, contributing to cellular mechanisms of memory consolidation. Memory consolidation also requires BDNF activation of TrkB receptors in the amygdala and hippocampus. We hypothesized that rapid internalization of GABAARα1 is mediated via TrkB activation of PKA and PKC-dependent processes. Primary neuronal cell cultures, from postnatal day 14–21 mouse amygdala and hippocampus, were analyzed with immunofluorescence using cell-surface, whole-cell permeabilization, and antibody internalization techniques, as well as with 3H-muscimol binding assays. In both hippocampal and amygdala cultures, we found a >60% reduction in surface GABAARα1 within 5 minutes of BDNF treatment. Notably, the rapid decrease in surface GABAARα1 was confirmed biochemically using surface biotinylation assays followed by western blotting. This rapid effect was accompanied by TrkB phosphorylation and increased internal GABAARα1 immunofluorescence, and was blocked by k252a, a broad-spectrum tyrosine kinase antagonist. To further demonstrate TrkB specificity, we used previously characterized TrkBF616A mice, in which the highly selective TrkB-mutant specific antagonist, 1NMPP1, prevented the BDNF-dependent GABAARα1 internalization. In hippocampus, we found both PKA and PKC inhibition, using Rp-8-Br-cAMP and Calphostin C, respectively, blocked GABAARα1 internalization, whereas inhibition of MAPK (U0126) and PI3K (LY294002) did not prevent rapid internalization. By contrast in amygdala cultures, Rp-8-Br-cAMP had no effect. Together, these data suggest that rapid GABAAR internalization during memory consolidation is BDNF-TrkB dependent. Further, it appears that hippocampal GABAAR internalization is PKA and PKC dependent, while it may be primarily PKC dependent in amygdala, implying differential roles for TrkB-dependent kinase activation in BDNF-dependent memory formation.
Keywords: GABAA receptor, BDNF, internalization, Phosphorylation, PKC, PKA, Hippocampus, Amygdala
Introduction
The majority of fast inhibitory neurotransmission in the central nervous system (CNS) is mediated by the activation of γ-aminobutyric acid A receptors (GABAARs). These receptors are pentameric structures assembled mostly from of alpha (α) and beta (β) subunits along with either gamma (γ) or delta (δ) subunits. The assembly of different subunit combinations produces a large family of subtypes, each of which confers distinct temporal and pharmacological profiles. Of the at least 16 GABAAR subtypes identified, the most abundant subtype in brain is composed of α1β2γ2 subunits, representing over half of all GABAARs (McKernan and Whiting, 1996; Gao and Fritschy, 1994; Sperk et al., 1997; Olsen and Sieghart, 2009). In the hippocampus and other brain regions including amygdala, the α1-(GABAARα1) subtypes are located on both pyramidal and parvalbumin-positive interneurons (Freund and Gulyas, 1997; McDonald and Mascagni, 2004; McDonald et al., 2004; Muller et al., 2007) and are known to be involved in feedforward, feedback, and tonic inhibition in addition to mediating the synchronized rhythmic activity of pyramidal cells important for proper functioning (Mann et al., 2005).
GABAARs undergo dynamic changes in composition on the cell surface of neurons. The trafficking to and from the synapse is regulated by activation of several cell-signaling pathways that have profound effects on GABAAR function and the efficacy of synaptic inhibition. Past studies have revealed that intracellular signaling pathways activated by brain-derived neurotrophic factor (BDNF) influences GABAergic transmission. In hippocampal neurons, Brunig et al. (2001) found that BDNF decreased mIPSC amplitude after a 5 minute application. In cerebellar granule cells, BDNF application caused the internalization of GABAAR β2/3 subunits and a depression of GABA-induced currents (Cheng and Yeh, 2003).
It remains unclear how the downstream signaling effects of BDNF induce changes in GABAAR function. BDNF activation of its tyrosine receptor kinase B receptor (TrkB) triggers several protein kinases, and may alter GABAergic inhibition via cAMP-dependent protein kinase (PKA) and/or Ca2+/phospholipid-dependent protein kinase (PKC) signaling pathways (Huang and Reichardt, 2001; Cai et al., 1999; Patapoutian and Reichardt, 2001; Gallo et al., 2002). For example, in hippocampal neurons, BDNF induced PKC activation mediated phosphorylation of β3-GABAAR subunits (Jovanovic, et al. 2004). In cerebellum, the effect of BDNF was mediated by PKA but not PKC (Cheng Q. and Yeh HH., 2005). In oocytes expressing heterologous GABAAR subunits, the effect of BDNF was PKC, but not PKA dependent (Palma E., et al. 2005). Although the contribution of these pathways remains poorly characterized, the currently available results indicate that BDNF-induced changes in GABAergic transmission may differ across brain regions and cell types. In addition, a number of additional variables may account for outcome discrepancies of across studies, including the type of neurons used (Cheng and Yeh, 2005), duration of BDNF activation (Henneberger et al., 2005) and the maturation of cells (Baldelli et al., 2002; Mizoguchi et al., 2003; Yamada et al., 2002).
We have previously shown that GABAA subunits, including GABAARα1, are rapidly and dynamically downregulated following emotional learning within the amygdala (Chhatwal et al., 2005; Heldt and Ressler, 2007). Furthermore we have found that fear conditioning is BDNF and TrkB dependent (Rattiner et al., 2004a, b, 2005; Choi et al., 2010). However, the mechanism of learning-dependent rapid GABAA downregulation is unknown. Specifically, no studies have investigated the regulation of GABAARα1 subunits following BDNF activation or the effects of BDNF on GABAAR trafficking in cultured amygdala neurons. In the present study, we investigated the BDNF-TrkB mediated signaling processes leading to rapid internalization of GABAARα1 in cultured mouse hippocampal and amygdala neurons.
Materials and Methods
BDNF peptide and inhibitors
Recombinant human BDNF was purchased from Cell Sciences (Canton, MA, USA) and reconstituted in sterile PBS as 100 mg/mL stock. The aliquots of stock were stored at −30°C and final concentration of application on neurons was 100 ng/mL. Other drugs and final concentrations for cell culture experiments were as following: K252a (Sigma, 200 nM), Calphostin C (Enzo Life Sciences, Plymouth Meeting, PA, USA, 200 nM), Rp-8-Br-cAMP (Biolog, Bremen, Germany, 200 µM), 1NMPP1 (Cayman Chemical, Ann Arbor, MI, USA, 100 nM), U0126 (Tocris biosciences, Ellisville, MO, USA, 10 µM), and LY294002 (Cayman, Ann Arbor, MI, USA, 20 µM).
Antibodies
The following antibodies were used in the described experiments: polyclonal rabbit antisera against α1-GABAA receptor subunits (epitope region: N-terminus, Millipore, Temecula, CA, USA); goat anti-rabbit IgG conjugated with Alexa Fluor 488 (Invitrogen); goat anti-rabbit IgG conjugated with Alexa Fluor 568 (Invitrogen); goat polyclonal antibody against mouse TrkB (0.2 mg/mL; R&D Systems Inc., Minneapolis, MN, USA); peroxidase-conjugated horse anti-goat secondary (Vector Laboratories, Burlingame, CA, USA); rabbit polyclonal antibody for p-TrkB (phosphorylated Tyr 706, 0.1 mg/mL; Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Hippocampus and amygdala neuronal cell culture
All procedures involving animals were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Primary cultures of postnatal hippocampal neurons were described previously (Brewer, 1997) with modifications. C57BL/6J mice (21 days postnatal) and TrkBF616A mice (14 days postnatal) were decapitated, and the hippocampus and amygdala were removed and immersed in ice-cold dissection buffer consisting of Hibernate-A medium (BrainBits, Springfield, IL, USA), B27 supplement (Invitrogen, Carlsbad, CA, USA), 2 mM Glutamax (invitrogen), and gentamycin (invitrogen) (12 µg/mL) for the preparation of separate hippocampal and amygdala neuronal cell cultures. The hippocampus and amygdala tissues were sliced and then enzymatically digested with papain (Worthington, Lakewood, NJ, USA) in Hibernate-A medium at 32°C for 30 minutes. Cells were dissociated by triturating with pasteur pipettes fired on tips to narrow openings. Neurons were purified in a density gradient media including Hibernate-A and OptiPrep (Sigma, St. Louis, MO, USA) by centrifugation. The density gradient media consisted of four layers. The first was 1 ml dissection buffer containing 35% OptiPrep; the second 1 ml dissection buffer contained 25% OptiPrep; the third 1 ml dissection buffer contained 20% OptiPrep; and the fourth 1 ml dissection buffer contained 15% OptiPrep. They were added on the top of each other carefully, resulting in clear layer separation. Then, cells were added on the top of density gradient media. After centrifugation, the most dense layer with a cream color, located at the middle of tube, could be seen. This layer of neurons was taken out by using a sterile transfer pipette and put into a new tube. After washing with dissection buffer, neuronal cells were plated onto Poly-D-Lysine (Sigma) coated plates or glass coverslips at the density of 2.5 × 105 cells / cm2 in culture media consisting of Neurobasal A medium (Invitrogen) with 2% B27 supplement, 2 mM glutamax and gentamycin (5 µg/mL). Thereafter, the cultures were kept in a humidified incubator at 37°C and 5% CO2, and media were changed every 5 days until used for experiments. After 2–3 weeks in vitro, the cells were used for the experiments reported in the present study.
Viability of Neuronal Cultures
Neurons were kept in the incubator for 2 weeks post-dissection, at which point 4% Trypan blue solution (Mediatech Inc., Herndon, VA, USA) was added onto cells to test the cell viability. Trypan blue positive dead cells were counted relative to the total number of cells. There were very few (<1%) dead cells, suggesting a >99% viability of cells at the 2-week timepoint. To determine ratio of neurons to total plated cells at the time of isolation, cells were incubated for 12 hours to let them attach the well, then fixed with methanol at −20°C for 20 minutes. For the 2-week timepoint, cells were grown in vitro for two weeks, then fixed and stained in a similar manner. Following fixation, cells were stained with neuronal specific, mouse anti-NeuN and subsequently with goat anti-mouse Alexa Fluor 488. At the time of isolation (12 hrs post isolation) we found that 90% of the DAPI+ cells were NeuN positive. After 2 weeks in culture, we found that 73% of the DAPI+ cells were NeuN positive. Thus, we can assume that approximately 75% of the cells in most of the studies outlined within this manuscript were neuronal.
Immunocytochemistry and analysis of immunofluorescence
Antibody feeding protocol
The surface GABAARs were tagged in living cultured hippocampus or amygdala neurons with the primary antibody against α1-GABAAR subunits. The tagged α1 subunits were allowed to be endocytosis at 37°C, before fixation and permeabilization of cells, followed by subsequent secondary antibody labeling of internalized α1 subunits. This protocol began with changing half the culture media with fresh media and incubating cultures with polyclonal rabbit antisera against α1-GABAA receptor subunits (diluted 1:100; epitope region: N-terminus, Millipore, Temecula, CA, USA). Cells were incubated for 30 minutes at 37°C. After washing three times with dissection buffer, culture media was returned to cells with half fresh media. In indicated wells, cells were then treated with BDNF, K252a, Calphostin C, Rp-8-Br-cAMP or different experimental combinations for 5, 10, and 20 minutes. Treatments were stopped by removing media and rinsing cells three times with dissection buffer. To label the surface α1 subunits, cells were incubated with goat anti-rabbit IgG conjugated with Alexa Fluor 488 (Invitrogen, 1:2000) diluted in culture media for 20 minutes in incubator. Cells were then rinsed three times with ice-cold PBS on ice and fixed with methanol at −20°C for 20 minutes. Following washing with PBS, cells were incubated with blocking buffer (1% BSA and 3% normal goat serum in PBS) at room temperature for 1 hour. All subsequent antibodies were diluted in the blocking buffer. To detect the internalized α1 subunits, the goat anti-rabbit IgG conjugated with Alexa Fluor 568 (Invitrogen, 1:2000) was applied to cells for additional 1 hour at room temperature. Cells without primary antibody treatment and only the above secondary were used as negative controls.
Distinction of cell-surface and intracellular α1-GABAAR subunits
Cells were fixed for 20 minutes at room temperature in 4% paraformaldehyde in PBS. After rinsing with PBS they were incubated in blocking buffer at room temperature for 1 hour. Then, the rabbit antisera against α1 subunits (1:500) in blocking buffer was added to cells and incubated overnight at 4°C. Following washing with PBS, the cells were incubated with goat anti-rabbit IgG conjugated with Alexa Fluor 488 (1:2000) in blocking buffer at room temperature for 2 hours to stain surface receptors. Cells were then rinsed again with PBS and permeabilized with methanol at −20°C for 20 minutes. The same primary antibody against α1 subunits (1:500) diluted in blocking buffer was again added to cells overnight at 4°C. Following washing with PBS cells were treated with the goat anti-rabbit IgG conjugated with Alexa Fluor 568 (1:2000) for 2 hours at room temperature to detect intracellular α1 subunits. Finally, cells were rinsed with PBS and ready for microscopy.
Analysis of immunofluorescence
Immunofluoresence images were visualized and captured using Nikon eclipse TE300 microscope with a high resolution digital camera (Nikon, Melville, NY, USA). The relative immnofluorescence intensity was analyzed using software of NIS-Elements BR2.30 (Nikon). Measured immunofluorescence intensity levels were subtracted from non-fluorescence background. The maximum intensities were normalized to controls. For quantitative analysis in each experiment, imaged cells were chosen on the presence of fluorescence signals and randomly selected neuritis or somas from 20 neurons were measured. Images were also acquired with a laser scanning confocal microscope (LSM500, Carl Zeiss, Thornwood, NY, USA) with 100X objective to visualize the surface and internalized α1-GABAAR subunits.
Surface Biotinylation Assays
Primary mouse hippocampal neurons were kept in a humidified incubator at 37°C with 5% CO2 before being used for the experiment. BDNF or vehicle was added into indicated wells for 10 and 20 minutes, respectively. Cells were then washed with ice-cold PBS on ice. Biotin (1mg, EZ-Link Sulfo-NHS-LC-Biotin, Thermo Scientific, Pittsburgh, PA, USA) in PBS (pH 8.0) was added into each well of 6-well plate. Plates were incubated on ice for 30 minutes with gentle shaking. Cells were washed with PBS + 100 mM glycine on ice three times, followed by two more times with PBS alone. Cells were harvested with RIPA buffer (50 mM Tris HCl, pH8, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS) containing proteinase inhibitors (proteinase inhibitor cocktail tablet, Roche diagnostics, Indianapolis, IN, USA). After centrifugation, supernatant was transferred and divided into two samples. 40 µl Streptavidin beads (Thermo Scientific) were added to supernatant of part 1 containing 50 µg protein, and incubated at 4°C overnight. The beads were then centrifuged and washed with washing buffer (0.1% SDS, 50 mM NaF, 1 mM EDTA) four times. Protein was eluted from beads with loading buffer, and used for SDS-PAGE and western blotting. For total α1-GABAAR subunit expression, 40 µg of protein from the supernatant of part 2 was used. For receptor detection with western blot, we used a rabbit @ α1-GABAAR (Millipore, Billerica, MA, USA), 1:500, as the primary antibody. Goat @ Rabbit HRP-conjugated (Vector Laboratories, Burlingame, CA, USA) 1:2000 was used as the secondary antibody, and was visualized by West Pico chemiuminescent substrate (Pierce, Rockford, IL, USA). Bands were detected and quantified using Fluorchem Sp (Alpha Innotech, San Leandro, CA, USA).
[3H]muscimol binding assay
Saturation binding analysis
Tritiated muscimol ([methylene-3H(N)]-Muscimol; 25.5 Ci/mmol specific activity) was purchased from PerkinElmer (Boston, MA, USA). The binding of [3H]muscimol to GABAARs was performed as previously described by Strac et al. (2008), with slight modifications. Neurons were harvested and homogenized for crude membranes in 50 mM tris-citrate buffer (pH 7.4). The membrane homogenates were incubated with 0.05% Triton X-100 diluted in 50 mM tris-citrate buffer (pH 7.4) for 30 min on ice. Homogenates were then centrifuged and pellet was washed with 50 mM tris-citrate buffer before assay. The assay mixture contained 20 µg of membrane homogenate protein. Protein concentrations were determined by BCA protein assay kit (Pierce, Rockford, IL, USA) using bovine serum albumin as a standard. [3H]muscimol (0.5–32 nM) was in a final volume of 1 mL buffer containing 20 mM HEPES (pH 7.5), 100 mM NaCl and 10 mM MgCl2. Incubation with [3H]muscimol was carried out at room temperature overnight. Membranes were captured using FP-200 Whatman GF/C filters (Whatman Paper Ltd, Gaithersburg, MD, USA) on a Brandell cell harvester (Brandell, Gaithersburg, MD, USA). Each sample was rinsed three times with 2 mL ice-cold 50 mM NaH2PO4 buffer (pH 7.0). Filters were placed in scintillation vials containing 5 mL scintillation fluid. Non-specific binding was determined as amount of [3H]muscimol binding in the presence of 5 µM bicuculline (Tocris Bioscience, Ellisville, MO, USA).
[3H]muscimol binding assay with intact neurons
The binding of [3H]muscimol to surface GABAARs was performed on intact amygdala neuronal monolayers in 24-well plates. The drug treatments were stopped by aspiration of the media, followed by washes with ice-cold PBS. From this point, the plates were kept on ice. Ice-cold PBS (1 mL) was added to each well. The addition of bicuculline at a final concentration of 5 µM was added to indicated wells for non-specific binding. [3H]muscimol was then added to each well at a final and saturated concentration of 8 nM. The binding was stopped by removing [3H]muscimol solution followed by PBS rinse. Triton (0.5 mL of 1%) was added to each well and cells were harvested by scrapping them into scintillation vials. After the addition of 5 mL scintillation fluid, each vial was vigorously vortexed and allowed to equilibrate at room temperature overnight.
Immunoblot analysis of TrkB and p-TrkB expression on hippocampus neurons
Drug treatment of cultures was terminated by aspiration of media and wash with ice-cold PBS on ice. The cells were lysed with RIPA buffer supplemented with protease inhibitor cocktail tablet (Roche Diagnostics) at 4°C for 30 minutes with constant agitation. Protein lysates (20 µg) were subjected to 4–15% sodium dodecyl sulfate-polyacylamide gel electrophoresis (Bio-Rad Laboratories, Hercules, CA, USA). After transfer to nitrocellulose membrane (Bio-Rad), blots were incubated with goat polyclonal antibody against mouse TrkB (0.2 mg/mL; R&D Systems Inc., Minneapolis, MN, USA). Immunocomplexes were visualized by using a horseradish peroxidase (HRP) -conjugated horse anti-goat secondary (Vector Laboratories). After stripping membranes, blots were probed again with rabbit polyclonal antibody for p-TrkB (phosphorylated Tyr 706, 0.1 mg/mL; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Then a HRP-conjugated goat anti-rabbit IgG (Vector) was applied for viewing p-TrkB expression. SuperSignal west pico chemiluminescent substrate (Pierce, Rockford, IL, USA) was used for detecting HRP.
Results
Rapid internalization of surface GABAARα1 subunits following BDNF treatment of hippocampal neuronal cultures
We first assessed whether BDNF administration led to an alteration of surface expression of GABAARα1 subunits on cultured mouse hippocampal neurons. For this experiment, cell cultures were treated with 100 ng/mL of BDNF for 5, 10 or 20 minutes. The cells were immediately fixed with 4% paraformaldehyde, without permeabilization, prior to staining. Overall, BDNF treatment decreased α1 subunits from the cell surface for all three time points. As seen in Figure 1A, this treatment resulted in a remarkable decrease of surface α1 subunits within 5 minutes. Quantitative measurements of staining intensity were conducted on neurite segments and part of soma membranes of at least 20 BDNF-immunostained neurons for each treatment. After acute BDNF exposure for only 5 minutes, a significant reduction of staining intensity of control cultures was observed (fig. 1B, p<0.01). This reduction, which was more than 60% of control cultures, was most pronounced at the 5 min timepoint, but was also quite robust after 10 and 20 minute BDNF incubation periods (fig. 1B). We next examined recovery of surface α1 subunits by performing the same 10 minute BDNF incubation, followed by fixation and staining, or removal of BDNF and continued in vitro culture for an additional 24 or 48 hours (fig. 1C). In the 10 minute incubation group, we fully replicate the prior experiment, showing a rapid decrease in surface GABAARα1 subunits. However, we find a complete recovery / normalization of surface level staining by 24 and 48 hours post-BDNF treatment.
Figure 1. Surface GABAARα1 subunits are decreased by BDNF on cultured mouse hippocampal neurons.
Panel A illustrates green-fluorescent labeled surface GABAARα1 subunits. Decreased surface expression of GABAARα1 subunit expression is seen following BDNF treatment for 5, 10, and 20 minutes with light microscopy (40X), compared with no treatment controls. Panel B demonstrates quantification of fluorescence intensity of surface alpha 1, normalized with controls. Panel C illustrates recovery of GABAARα1 surface levels, with similar rapid decrease in signal following 10 min BDNF treatment, but with complete normalization at 24 or 48 hours following BDNF removal (Mean ± SEM of normalized intensity, * p < 0.01, ** p<0.05, relative to no treatment).
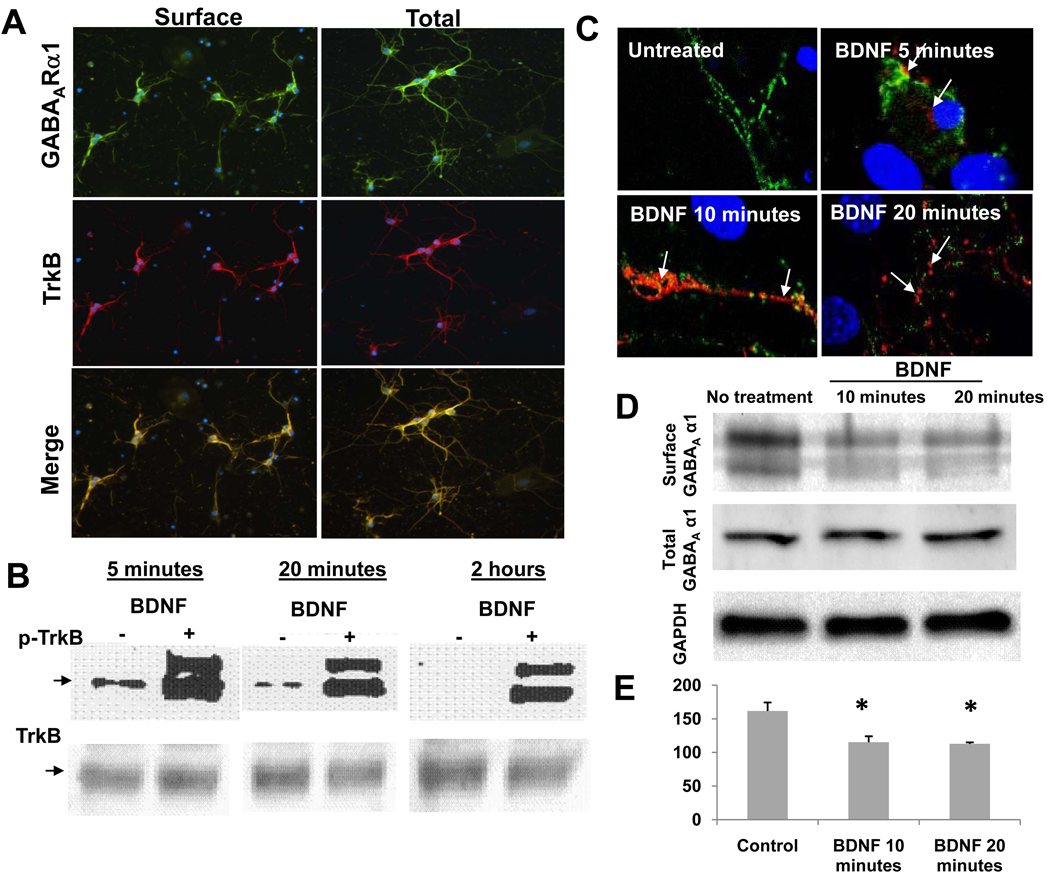
We next wished to assess if the BDNF-mediated decrease in surface GABAARα1 subunits was mediated via the TrkB receptor. First, we confirmed that TrkB was expressed on hippocampal neurons and that TrkB immunoreactivity was overlapping with that of GABAARα1 expression (fig. 2A). Further, we tested the phosphorylation level of TrkB by BDNF with western blot. The treatment of cultures with 100 ng/mL BDNF for 5 min, 20 min, and 2 hrs all induced phosphorylation of TrkB. No group differences were observed for total TrkB in treated cultures and control cultures (fig. 2B).
Figure 2. TrkB activation, co-localization with GABAARα1, and GABAARα1 internalization.
Panel A: surface (left) or total (right) GABAARα1 expression (green), TrkB expression (red), or the merge between the two (yellow). Panel B: an immunoblot, with phospho-TrkB (top, arrow is p-TrkB) and total TrkB (bottom), with vehicle vs. BDNF treatment at 5 min, 20min, and 2hrs after BDNF addition. Panel C: Confocal microscopy analysis of surface (green) and internalized (red) GABAARα1 subunits. Untreated neurons have abundant surface, but undetectable internal GABAARα1. BDNF (5, 10, or 20 minutes) treated cultures show internalized alpha 1 (red) in neuronal soma (5 min) and dendrites (10 and 20 min) after BDNF treatment. Arrows indicate internalized GABAARα1 subunits. Panel D: Biotinylation studies showing that the apparent internalization of GABAARα1 seen with antibody feeding techniques is confirmed with biochemical analyses of surface receptor localization. Western blot analyses is shown of biotin-precipitated, surface GABAARα1, total GABAARα1 from cell homogenate, and total GADPH as a loading control. Panel E: Quantification of surface:total GABAARα1 ratio following vehicle (control) or 10–20 minutes of BDNF treatment using the biotinylation surface-labeling technique. (Mean ± SEM, * p < 0.01, relative to control).
A previous study reported that 30-minute and 60-minute exposure to BDNF induced internalization of GABAA receptors in mouse cerebellar granule cells as detected with a β2/3 subunit antibody (Cheng and Yeh, 2003). Thus, we examined whether the observed reduction of surface α1 could be accounted for by its internalization. To distinguish surface from internalized α1 subunits, BDNF-treated and untreated hippocampal cultures were first surface labeled with the α1 primary antibody in live cultures. Then cells were treated with BDNF (100 ng/mL) for 5, 10, or 20 minutes. After stopping BDNF treatment, live cells were incubated with goat anti-mouse Alexa Fluor 488 to label the surface α1. Cells were then fixed and permeabilized with methanol followed by incubation with goat anti-mouse Alexa Fluor 568 to label the internalized GABAARα1. In untreated control cultures, surface GABAARα1 clusters were clearly seen on neurite and soma surfaces. For this short period of 5 to 20 minutes, the constitutive internalization of surface α1 was almost undetectable. In contrast, BDNF treated cells showed significantly less surface α1 expression and a more reliable intracellular signal, suggesting a BDNF-induced internalization of GABAARα1 from the surface membrane to intracellular cytosolic compartments, as seen with both confocal (Fig. 2C) and light microscopy (Fig. 3).
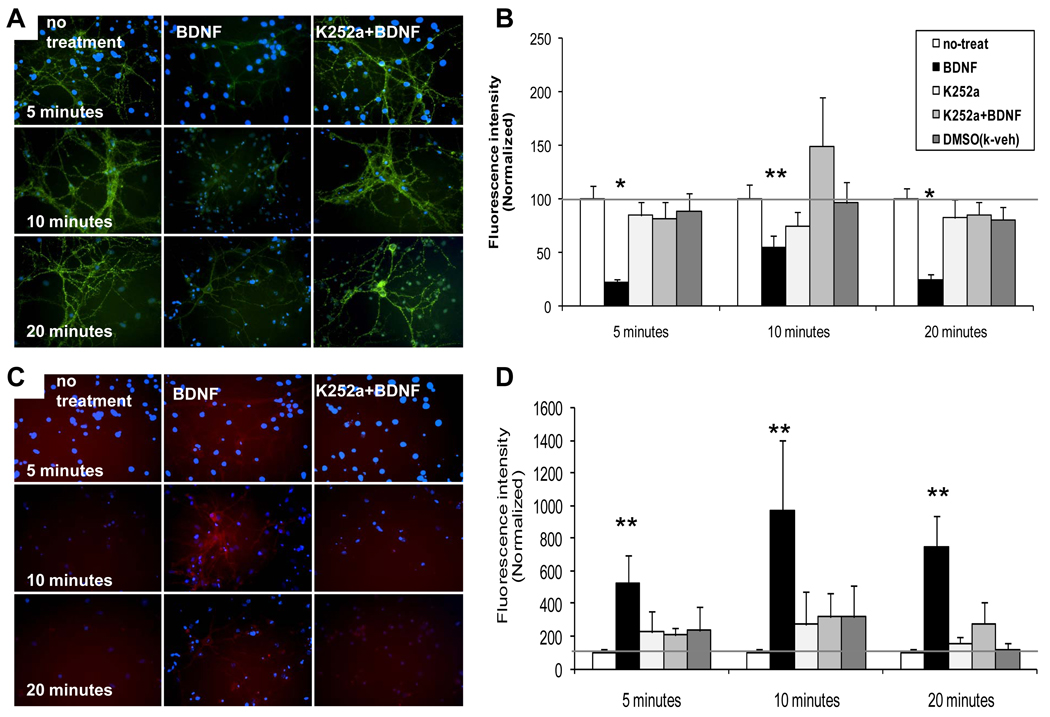
Figure 3. BDNF-dependent internalization of surface GABAARα1 subunits is attenuated by K252a treatment in hippocampal neurons.
Panel A illustrates light microscopic images of surface (green) GABAARα1 subunits following vehicle, BDNF, or BDNF + k252a treatment for 5, 10, or 20 minutes. Panel B demonstrates quantification of fluorescence intensity of surface alpha 1, normalized with controls. Panel C illustrates images of internalized (red) GABAARα1 subunits following vehicle, BDNF, or BDNF + k252a treatment for 5, 10, or 20 minutes. Panel D demonstrates quantification of fluorescence intensity of internalized α1, normalized with controls. (Mean ± SEM of normalized intensity, * p <0.001, ** p<0.05, relative to no treatment).
To confirm with additional biochemical methods, that the labeled GABAARα1 was indeed located at the surface, we performed surface biotinylation (Mammen et al., 1997; van Rijnsoever et al., 2005) to assess internalization of GABAAR independently of antibody tagging. Cells underwent surface biotinylation, precipitation of biotinylated protein with streptavidin beads, and western blotting for this surface, biotinylated GABAARα1 compared to total GABAARα1 (fig. 2D). We found that the surface:total ratio was significantly decreased by both 10 and 20min treatment with BDNF using this biochemical biotinylation assay (fig. 2E).
Effect of tyrosine kinase inhibition on BDNF-induced GABAARα1 internalization in hippocampal neurons
To determine whether the observed effect of BDNF on GABAARα1 subunits was mediated by its TrkB receptor, BDNF was applied in combination with 200 nM K252a, which is a membrane permeable tyrosine kinase inhibitor with relatively high affinity for TrkB receptors (Tapley et al., 1992). When BDNF alone was added, we replicated the rapid loss of surface labeling and increased cytoplasmic signal, as described above. Quantitative measurements of fluorescence intensity indicated that BDNF treatment lowered the GABAARα1 surface expression to at least 21 ± 3.3% of control cultures (Figs 3 A, B; p<0.001) and increased internalized GABAARα1 to more than 5 times that of control (Figs 3C, D; p<0.05) for all three treatment time points.
The addition of 200 nM K252a prior to BDNF treatment on cultures fully blocked the effect of BDNF-dependent α1 internalization. The decreased surface α1 expression by BDNF was reversed by K252a at the time points of 5, 10 and 20 minutes and the internalization of surface α1 was altered by K252a. Figures 3B and 3D show that the relative fluorescence intensity of K252a + BDNF treated cells were similar to control cultures, suggesting that the activation of TrkB receptors was required for the effects of BDNF on GABAARα1 internalization. Together, these findings suggest TrkB activation by BDNF mediates the internalization of GABAARα1 subunits.
Effect of BDNF and the involvement of PKC and PKA on hippocampal neurons
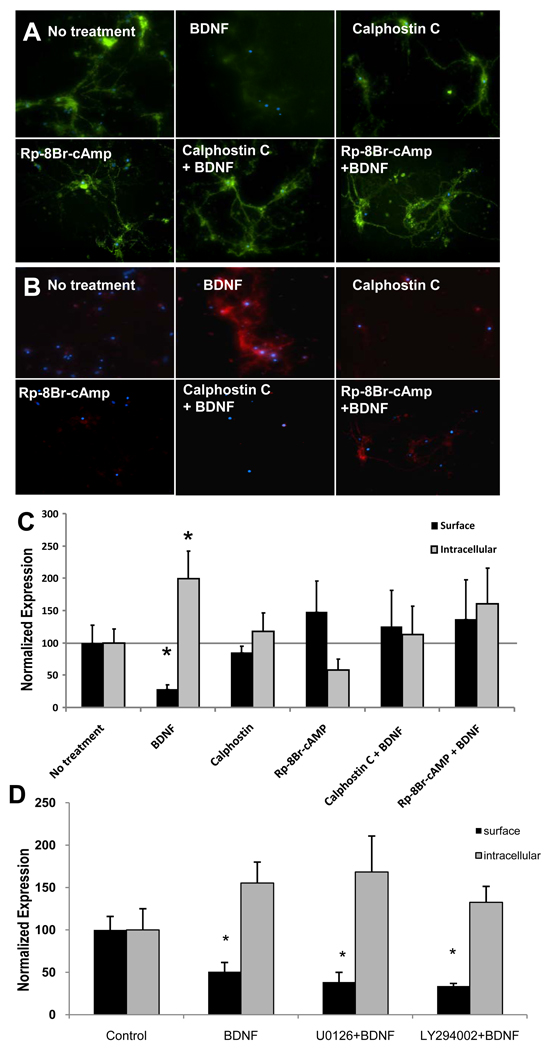
Previous studies reported that activation of PKC induced GABAAR internalization (Chapell et al., 1998) and decreased GABAAR function, as measured by lowered GABA currents (Brandon et al., 2000). Thus, we asked whether the BDNF effects on GABAARα1 surface expression were related to level of PKC activation. To test this, we disrupted PKC activation with Calphostin C, a cell permeable and highly selective inhibitor of protein kinase C (Bruns et al., 1991). We observed that the application of 200 nM Calphostin C on cultures for 10 minutes prior to adding BDNF for 20 minutes completely prevented α1 internalization induced by BDNF (Fig. 4A – surface, Fig 4B – internal, Fig 4C - quantification).
Figure 4. BDNF-dependent decrease in surface GABAARα1 subunits is reversed by treatment with PKA and PKC inhibitors.
Panel A illustrates light microscopic images of surface (green) GABAARα1 subunits following treatment with vehicle, BDNF, Calphostin C (PKC inhibitor), Rp-8Br-cAMP (PKA inhibitor), or these agents combined with BDNF. Panel B illustrates images of internalized (red) GABAARα1 subunits following the same conditions as in (A). Panel C demonstrates quantification of fluorescence intensity of surface (black) or intracellular (gray) GABAARα1, normalized with controls. Panel D demonstrates quantification of fluorescence intensity of surface (black) or intracellular (gray) GABAARα1, normalized with controls following inhibitors of MAPK (U0126) or PI3K (LY294002), neither of which had an effect on BDNF-induced GABAARα1 internalization. (Mean ± SEM of normalized intensity, * p<0.05, relative to no treatment).
Next we examined whether PKA played a role in GABAARα1 internalization. Rp-8-Br-cAMP, a potent membrane-permeant inhibitor of cAMP-dependent-protein kinases (Poppe et al., 2008), was added to cultures for 10 minutes followed by treatment of BDNF for an additional 20 minutes. As seen in Figure 4, the effect of BDNF on the internalization of GABAARα1 was abolished by Rp-8-Br-cAMP. We found no changes in relative fluorescence intensities of surface or intracellular GABAARα1 immunofluorescence between combined treatment of Calphostin C + BDNF, Rp-8-Br-cAMP + BDNF, or control cultures, suggesting that blockade of either PKA- or PKC-dependent signaling pathways disrupts BDNF-induced GABAARα1 internalization within hippocampal neurons.
TrkB is also known to activate MAPK and PI3K pathways. Thus we examined whether inhibitors of MAPK (U0126) or PI3K (LY294002) would disrupt BDNF-induced GABAARα1 internalization within hippocampal neurons. Following standard culture conditions as described above, cells were treated with 10 µM U0126 or 20 µM LY294002 for 20 minutes before a 10-minute BDNF treatment. BDNF alone significantly decreased surface GABAARα1 as shown above. However, in contrast to the PKA- and PKC-dependent effects, we found no effect of MAPK or PI3K inhibition on BDNF-dependent internalization of GABAARα1 (fig. 4D).
Effect of BDNF on amygdala GABAAR internalization using radiometric binding assays
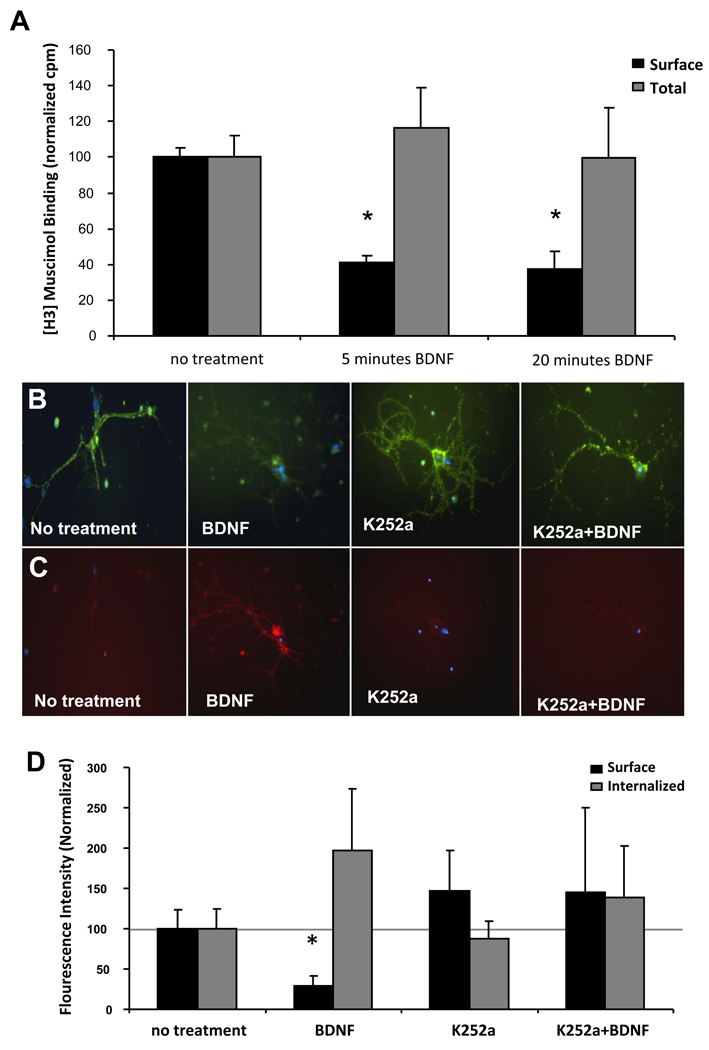
To date there have been no published studies on the effect of BDNF on GABAARα1 subunits in cultured amygdala neurons. To determine whether or not BDNF caused internalization of surface GABAARα1, we performed radiometric binding assays with intact neurons from amygdala of C57BL/6J mice by using the hydrophilic GABAAR agonist [3H]muscimol (Svob Strac et al., 2008). [3H]Muscimol has been previously shown to only label cell surface GABA binding sites and to possibly be membrane impermeable based on intact (vs. homogenate) cell binding of [3H]Muscimol (Mizokami A, et al., 2007; Jazvinsćak Jembrek M, et al., 2008). Although its level of membrane penetration remains somewhat unclear, it is clearly very hydrophilic based on previous use of [3H]Muscimol in cell-surface assays (Primus et al., 1996). After BDNF treatment for 5 and 20 minutes, respectively, surface receptors were reduced to 41% and 37 %, compared to non-treated control cells (Fig. 5A). On the basis of prior in vitro saturation analyses, we examined saturating concentrations of [3H]muscimol to calculate total GABAARs. [3H]muscimol binding on total GABAARs from crude amygdala membranes revealed no significant differences observed among BDNF treatments and controls. These data suggest that as in hippocampal neurons, a variety of measures can reveal a rapid (<5 min) internalization of amygdale GABAARs.
Figure 5. Amygdala GABAA Receptor and GABAARα1 subunit BDNF-dependent internalization.
Panel A: Surface (black bars) and total (gray bars) [3H]-Muscimol binding to dissociated neurons from mouse amygdala after no treatment or 5 and 20 minute treatments with BDNF. (Mean ± SEM of normalized counts per minute, * p<0.02, relative to no treatment). Panel B: light microscopic images of surface (green) GABAARα1 subunits following vehicle, BDNF, k252a, or BDNF + k252a treatment for 10min. Panel C: images of internalized (red) GABAARα1 subunits following the same treatments as in (B). Panel D: quantification of fluorescence intensity of surface (black) or intracellular (gray) GABAARα1, normalized with controls. (Mean ± SEM of normalized intensity, * p<0.05, relative to no treatment).
Effect of BDNF and activation of TrkB on amygdala neurons
To test whether BDNF, via activation of TrkB, had a similar effect in amygdala as in hippocampal cultures on the sequestration of membrane α1 subunits, 200 nM K252a was added to cells for 10 minutes before BDNF application. Similar to above, we found that there was a rapid and robust decrease in surface labeling of GABAARα1 (Fig. 5B–D). The decrease of membrane α1 subunits by BDNF was totally reversed by TrkB blockade with K252a, which had no effect by itself. As with the hippocampal cultures, we next examined recovery of surface α1 subunits by performing the same 10 minute BDNF incubation, followed by fixation and staining, or removal of BDNF and continued in vitro culture for an additional 24 or 48 hours. In the 10 minute incubation group, we fully replicate the prior experiment, showing a rapid decrease in surface GABAARα1 subunits (p<.05). However, we find a complete recovery / normalization of surface level staining by 24 and 48 hours post-BDNF treatment (data not shown).
Effect of PKC and PKA manipulation on BDNF-dependent internalization in amygdala neuron cultures
In hippocampal cultures, we have demonstrated that activation of either PKC or PKA was required for the effect of BDNF on internalization of GABAARα1 subunits. Thus, we next examined whether or not this was the case on cultured mouse amygdala neurons. In a separate series of amygdala culture experiments, we first replicated the robust BDNF-dependent internalization (Fig. 6). We also found that application of Calphostin C (PKC inhibitor) treatment prior to BDNF increased membrane α1 subunit density by 150% compared to BDNF treated neurons (p=0.02), however, membrane α1 subunits were still lowered relative to untreated control cells (p<0.05). These data suggested that PKC activation induced by BDNF could only partially inhibit the sequestration of membrane surface α1 subunits (Fig. 6). In contrast to the hippocampal culture results, application of Rp-8-Br-cAMP before BDNF treatment of amygdala cultures failed to have any effect on BDNF-dependent internalization. Together these data suggest that, unlike within the hippocampal cultures, the amygdala neuronal cultures do not depend on PKA activation for BDNF-dependent internalization of GABAARα1.
Figure 6. BDNF-dependent decrease in surface GABAARα1 subunits is partially reversed by PKC inhibition, but not by PKA inhibition in amygdala cultures.
Panel A illustrates light microscopic images of surface (green) GABAARα1 subunits following treatment with vehicle, BDNF, Calphostin C (PKC inhibitor), Rp-8Br-cAMP (PKA inhibitor), or these agents combined with BDNF. Panel B illustrates images of intracellular (red) GABAARα1 subunits following the same conditions as in (A). Panel C demonstrates quantification of fluorescence intensity of surface (black) or intracellular (gray) GABAARα1, normalized with controls. (Mean ± SEM of normalized intensity, * p < 0.05, ** p < 0.01, relative to no treatment.)
BDNF-dependent internalization of GABAARα1 in TrkBF616A amygdala neurons
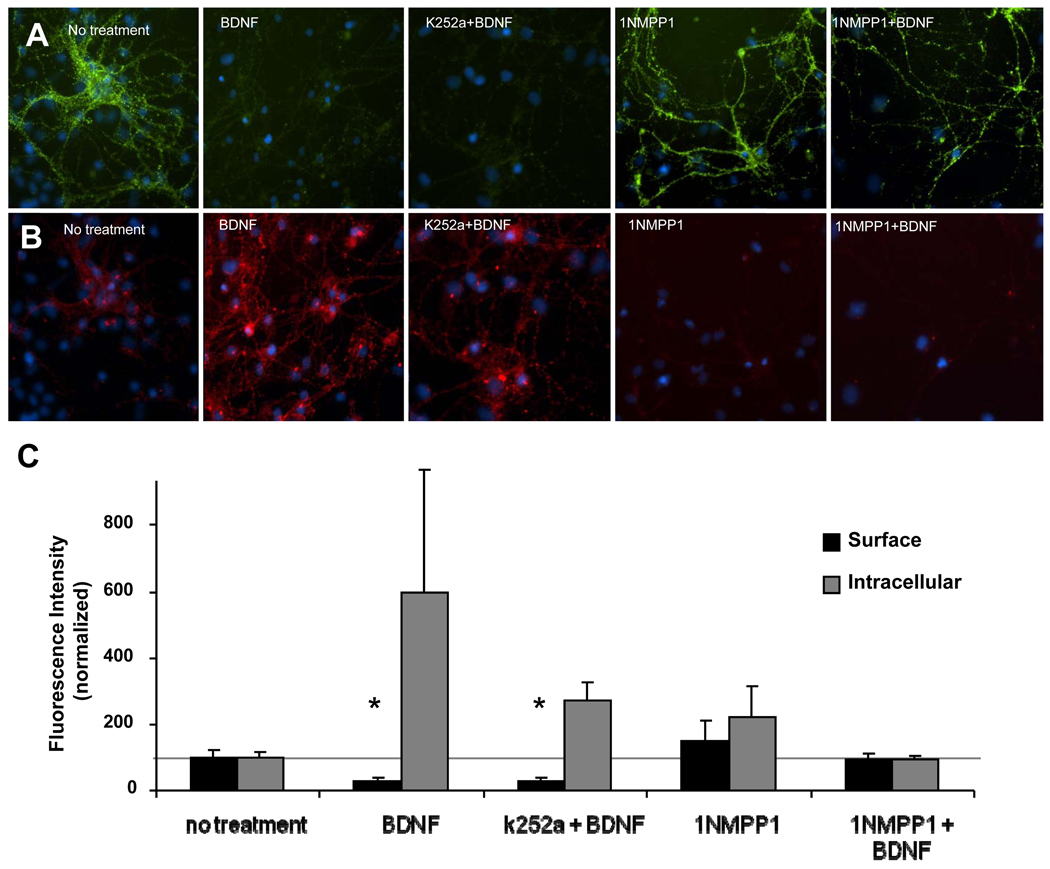
Chen et al. (2005) generated TrkBF616A knockin mice in which the TrkB phenalanine (F616) was replaced by alanine. This mutation has little or no effect on BDNF-mediated TrkB signaling. However, TrkB function can be selectively inhibited by nanomolar concentrations of 1NMPP1 which is a derivative of the general kinase inhibitor, PP1. Conversely, the less specific inhibitor, k252a, does not block mutant TrkBF616A activation. We took advantage of this chemical-genetic approach to examine the effect of 1NMPP1 on BDNF-dependent GABAARα1 internalization on amygdala neurons (Fig. 7). We first observed that BDNF induced the decrease of surface GABAARα1 subunits by more than 70% compared untreated neurons. Pre-treatment of k252a failed to inhibit the effect of BDNF due to the insensitivity of TrkBF616A for k252a. However, the specific inhibitor, 1NMPP1, significantly reversed the decrease of surface α1 subunits. This further demonstrates with a genetic-pharmacological approach, that the BDNF-dependent internalization of GABAARα1 subunits is TrkB dependent.
Figure 7. BDNF-dependent decrease in surface GABAARα1 in TrkBF616A amygdala cultures.
Panel A illustrates light microscopic images of surface (green) GABAARα1 subunits following treatment with vehicle, BDNF, K252a + BDNF, 1NMPP1, or 1NMPP1 + BDNF. Panel B illustrates images of intracellular (red) GABAARα1 subunits following the same conditions as in (A). Panel C demonstrates quantification of fluorescence intensity of surface (black) or intracellular (gray) GABAARα1, normalized with controls. (Mean ± SEM of normalized intensity, * p<0.02, relative to no treatment).
Discussion
In this study we found: 1) That BDNF application resulted in the rapid (< 5min) sequestration of membrane α1 subunits in cultured mouse hippocampal and amygdala neurons, demonstrated via immunohistochemistry and biochemically using surface biotinylation. 2) This BDNF-dependent internalization of GABAARα1 subunits is dependent on activation of TrkB, as shown with both the nonspecific kinase inhibitor k252a, and the specific inhibitor 1NMPP1 in TrkBF616A transgenic mouse cultures. 3) BDNF-dependent internalization of GABAARα1 is PKC and PKA dependent in hippocampal neurons. 4) In contrast, in amygdala neurons, this internalization is only partially PKC-dependent and is not PKA dependent; and 5) that the decreased surface GABAARα1 levels have returned to normal 24hrs post-treatment. Together, these data suggest that BDNF activation of TrkB receptors mediates the sequestration of membrane α1 subunits via differential phosphorylation pathways in hippocampal and amygdala neurons. These data lead to specific implications regarding the differential roles of PKA and PKC mediators in different types of memory formation as a function of BDNF and TrkB dependent intracellular pathways.
The current study demonstrates that acute in vitro application of BDNF on mouse hippocampal and amygdala neurons results in the quick sequestration of membrane GABAARα1 subunits. Our results are consistent with conclusions of Brunig et al (2001) who reported that a 5 minute application of BDNF caused the decrease of mIPSCs as well as decreases in GABAAR α2 and β2/3 subunits in hippocampal slices. Our results are also consistent with the findings of Mizoguchi et al (2003) who demonstrated that postnatal day 14 GABAAR function in rat hippocampal neurons was decreased following BDNF treatment. In contrast to our data, Boxall (2000) reported that BDNF had no effects on mIPSCs on Purkinje cells of cerebellum, and Jovanovic et al (2004) found that BDNF induced a transient enhancement of GABAAR function in embryonic hippocampal and cortical neurons. These different effects might be explained by alternate composition of GABAAR subunits in different brain regions, different developmental stages of CNS in that developmental GABAA Rs are excitatory due to differential cellular chloride concentration, or different signaling cascades and thus different modulations of the receptors.
In our study, we find that the BDNF-dependent decrease in cell-surface expression of GABAARα1 subunits occurs via the activation of TrkB receptors. This is consistent with the report of Hewitt et al (2006) who demonstrated that application of the tyrosine kinase inhibitor k252a, in hypothalamus could block the BDNF effects on decreased of GABAA receptor function. Similarly, Brunig et al (2001) had previously shown that BDNF effects on GABAA receptor function were only seen in TrkB positive neurons. Taken together, these data suggest that TrkB activation via BDNF leads to a rapid decrease in numbers of GABAARα1 subunits from the cell membrane.
The most likely fate of GABAARα1 removed from the cell surface is via their internalization into cytoplasmic compartments. This mechanism has been tested in a recombinant model following phosphorylation of GABAA Rs by activation of Protein Kinase C (Chapell et al, 1998; Herring, et al, 2005). However, Brandon et al (2000) reported that phosphorylation of GABAAR β3 subunits by PKC caused decreased function of receptors without changing numbers of surface receptors in cortical neurons. Jovanovic et al (2004) reported that PKC mediated phosphorylation of β3 subunits induced by BDNF caused a transiently enhanced GABAAR function followed by a lasting depression in hippocampal and cortical neurons. They also found that the increased surface GABAAR number was correlated with BDNF modulation.
In the current study, our data show that on mouse hippocampal neurons, the internalization of membrane-surface GABAARα1 subunits induced by BDNF is mediated by the activation of both PKC and PKA. In contrast, on mouse amygdala neurons the PKC activation is only partially responsible, and PKA is not involved, in the BDNF-dependent sequestration of surface GABAARα1. This suggests that there may be different mechanisms of cellular signaling downstream of TrkB activation between hippocampus and amygdala. The intracellular domains of GABAAR β and γ subunits can be phosphorylated by many kinases, including PKA, PKC, PKG, Ca2++/calmodulin-dependent protein kinase II (CaMKII), Akt/PKB, and Src. Among these protein kinases, it has been demonstrated that the β1 subunits could be phosphorylated by CaMKII (McDonald and Moss, 1994). Moreover, CaMKII could be a modulator on GABAAR function by regulating β2 and β3 subunits (Houston, et al, 2008). Our data suggest that the down-regulation of surface membrane GABAARα1 subunits induced by BDNF in mouse amygdala neurons may be, in part, mediated by kinases other than PKC and PKA, and that differential modulation occurs between hippocampal and amygdala neurons.
Our data also suggest that there may be different fates of internalized α1 subunits between hippocampal neurons and amygdala neurons. We found that internalized GABAARα1 subunits in amygdala neurons were not detectable by immunofluorescence, whereas the hippocampal GABAARα1 subunits were. It has been clarified that the endocytosis of GABAA Rs involves a clathrin-mediated dynamin-dependent mechanism in the recombinant expression system in heterologous cells (Herring et al, 2005; Kittler et al 2000). The surface derived receptors are either recycled to membrane or degraded. These processes are modulated by a variety of proteins, such as Phospholipase C-related inactive protein (PRIP) which modulates the phosphorylation level of GABAARs (Kanematsu, et al., 2007). These data imply that different receptor trafficking processes, mediated by TrkB-dependent phosphorylation may occur in different brain regions.
BDNF activation of TrkB plays an important role not only in the development of CNS, but also at mature synapses and during learning and memory (McAllister et al, 1999). We have previously found that activation of TrkB by BDNF is required for fear memory formation and, separately, that GABAA receptor subunits are dynamically regulated during fear memory consolidation. Specifically, we found that GABAARα1 subunits were among the most markedly regulated within the amygdala with fear conditioning. We hypothesized that TrkB-dependent internalization of GABAA receptors may partially underlie a transient period of amygdala hyperactivation during fear memory consolidation. Such hyperexcitability would allow for many of the activity-dependent and calcium-dependent molecular mechanisms of synaptic plasticity to mediate memory consolidation. For fear conditioning, associated changes in protein levels would likely decrease the level of phasic and/or tonic inhibition resulting in a state of heightened excitatory drive. Such a shift in the excitation–inhibition balance would likely support long-term potentiation (LTP) of excitatory CS-US (conditioned stimulus – unconditioned stimulus) associations formed during fear acquisition (Maren, 1999). Alternatively, such excitation may represent LTP-induced alterations associated with the consolidation or retention of CS-US associations.
It is well established that fear conditioning is dependent on the basolateral amygdala (BLA) (Davis, 1992; Fanselow and Ledoux, 1999), and that contextual fear conditioning also involves plasticity within the hippocampus (Anagnostaras et al., 2001). Previous findings using in situ hybridization have revealed transient increases in BDNF mRNA in the basolateral amygdala after associative fear conditioning but not after exposure to an equal number of CS-alone or US alone presentations (Rattiner et al., 2004a, b, 2005). Fear conditioning also results in activation of Trk receptors in the amygdala, as indicated by increased receptor phosphorylation during consolidation period (Rattiner et al., 2004a).
The molecular and cellular events underlying synaptic plasticity that mediates memory consolidation remain largely unknown. During the consolidation of fear memory, it has been shown that GABAA receptors (GABAAR) are rapidly downregulated in amygdala, leading to transient hyperexcitability, contributing to cellular mechanisms of memory formation. In these studies, we find that BDNF-dependent TrkB activation, which is also required for memory consolidation, may underlie the rapid internalization of GABAARα1, and that this process is differentially dependent on PKA and PKC-dependent processes in amygdala and hippocampal neurons. Further understanding of the molecular and cellular events which mediate memory formation, as well as differential mechanisms in brain regions that support different forms of memory (e.g. emotional vs. declarative), have broad implications in the identification of neural mechanisms and therapeutic approaches to disorders of memory.
Acknowledgments
Support was provided by NIH (DA019624), the Burroughs Wellcome Fund, and the National Primate Research Center base grant #RR-00165.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11(1):8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Baldelli P, Novara M, Carabelli V, Hernandez-Guijo JM, Carbone E. BDNF up-regulates evoked GABAergic transmission in developing hippocampus by potentiating presynaptic N- and P/Q-type Ca2+ channels signalling. Eur J Neurosci. 2002;16:2297–2310. doi: 10.1046/j.1460-9568.2002.02313.x. [DOI] [PubMed] [Google Scholar]
- Bolton MM, Pittman AJ, Lo DC. Brain-derived neurotrophic factor differentially regulates excitatory and inhibitory synaptic transmission in hippocampal cultures. J Neurosci. 2000;20:3221–3232. doi: 10.1523/JNEUROSCI.20-09-03221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon NJ, Delmas P, Kittler JT, McDonald BJ, Sieghart W, Brown DA, Smart TG, Moss SJ. GABAA receptor phosphorylation and functional modulation in cortical neurons by a protein kinase C-dependent pathway. J Biol Chem. 2000;275:38856–38862. doi: 10.1074/jbc.M004910200. [DOI] [PubMed] [Google Scholar]
- Brewer GJ. Isolation and culture of adult rat hippocampal neurons. J Neurosci Methods. 1997;71:143–155. doi: 10.1016/s0165-0270(96)00136-7. [DOI] [PubMed] [Google Scholar]
- Brunig I, Penschuck S, Berninger B, Benson J, Fritschy JM. BDNF reduces miniature inhibitory postsynaptic currents by rapid downregulation of GABA(A) receptor surface expression. Eur J Neurosci. 2001;13:1320–1328. doi: 10.1046/j.0953-816x.2001.01506.x. [DOI] [PubMed] [Google Scholar]
- Brunig I, Scotti E, Sidler C, Fritschy JM. Intact sorting, targeting, and clustering of gammaaminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J Comp Neurol. 2002;443:43–55. doi: 10.1002/cne.10102. [DOI] [PubMed] [Google Scholar]
- Bruns RF, Miller FD, Merriman RL, Howbert JJ, Heath WF, Kobayashi E, Takahashi I, Tamaoki T, Nakano H. Inhibition of protein kinase C by calphostin C is light-dependent. Biochem Biophys Res Commun. 1991;176:288–293. doi: 10.1016/0006-291x(91)90922-t. [DOI] [PubMed] [Google Scholar]
- Cai D, Shen Y, De Bellard M, Tang S, Filbin MT. Prior Exposure to Neurotrophins Blocks Inhibition of Axonal Regeneration by MAG and Myelin via a cAMP-Dependent Mechanism. Neuron. 1999;22:89–101. doi: 10.1016/s0896-6273(00)80681-9. [DOI] [PubMed] [Google Scholar]
- Chapell R, Bueno OF, Alvarez-Hernandez X, Robinson LC, Leidenheimer NJ. Activation of protein kinase C induces gamma-aminobutyric acid type A receptor internalization in Xenopus oocytes. J Biol Chem. 1998;273:32595–32601. doi: 10.1074/jbc.273.49.32595. [DOI] [PubMed] [Google Scholar]
- Chen X, Ye H, Kuruvilla R, Ramanan N, Scangos KW, Zhang C, Johnson NM, England PM, Shokat KM, Ginty DD. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 2005;46:13–21. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Yeh HH. Brain-derived neurotrophic factor attenuates mouse cerebellar granule cell GABA(A) receptor-mediated responses via postsynaptic mechanisms. J Physiol. 2003;548:711–721. doi: 10.1113/jphysiol.2002.037846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Yeh HH. PLCgamma signaling underlies BDNF potentiation of Purkinje cell responses to GABA. J Neurosci Res. 2005;79:616–627. doi: 10.1002/jnr.20397. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Myers KM, Ressler KJ, Davis M. Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J Neurosci. 2005;25(2):502–506. doi: 10.1523/JNEUROSCI.3301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Maguschak KA, Ye K, Jang SW, Myers KM, Ressler KJ. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. Proc Natl Acad Sci U S A. 2010;107(6):2675–2680. doi: 10.1073/pnas.0909359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23(2):229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Freund TF, Gulyas AI. Inhibitory control of GABAergic interneurons in the hippocampus. Can J Physiol Pharmacol. 1997;75:479–487. [PubMed] [Google Scholar]
- Gallo G, Ernst AF, McLoon SC, Letourneau PC. Transient PKA activity is required for initiation but not maintenance of BDNF-mediated protection from nitric oxide-induced growth-cone collapse. J Neurosci. 2002;22:5016–5023. doi: 10.1523/JNEUROSCI.22-12-05016.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Fritschy JM. Selective allocation of GABAA receptors containing the alpha 1 subunit to neurochemically distinct subpopulations of rat hippocampal interneurons. Eur J Neurosci. 1994;6:837–853. doi: 10.1111/j.1460-9568.1994.tb00994.x. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Ressler KJ. Training-induced changes in the expression of GABAA-associated genes in the amygdala after the acquisition and extinction of Pavlovian fear. Eur J Neurosci. 2007;26(12):3631–3644. doi: 10.1111/j.1460-9568.2007.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberger C, Kirischuk S, Grantyn R. Brain-derived neurotrophic factor modulates GABAergic synaptic transmission by enhancing presynaptic glutamic acid decarboxylase 65 levels, promoting asynchronous release and reducing the number of activated postsynaptic receptors. Neuroscience. 2005;135:749–763. doi: 10.1016/j.neuroscience.2005.06.044. [DOI] [PubMed] [Google Scholar]
- Herring D, Huang R, Singh M, Dillon GH, Leidenheimer NJ. PKC modulation of GABAA receptor endocytosis and function is inhibited by mutation of a dileuci motif within the receptor beta 2 subunit. Neuropharmacology. 2005;48(2):181–194. doi: 10.1016/j.neuropharm.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Hewitt SA, Bains JS. Brain-derived neurotrophic factor silences GABA synapses onto hypothalamic neuroendocrine cells through a postsynaptic dynamin-mediated mechanism. J Neurophysiol. 2006;95(4):2193–2198. doi: 10.1152/jn.01135.2005. [DOI] [PubMed] [Google Scholar]
- Houston CM, Hosie AM, Smart TG. Distinct regulation of beta2 and beta3 subunit-containing cerebellar synaptic GABAA receptors by calcium/calmodulin-dependent protein kinase II. J Neurosci. 2008;28(30):7574–7584. doi: 10.1523/JNEUROSCI.5531-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. NEUROTROPHINS: Roles in Neuronal Development and Function1. Annual Review of Neuroscience. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazvinsćak Jembrek M, Jazvinsćak Jembrek M, Svob Strac D, Vlainić J, Pericić D. The role of transcriptional and translational mechanisms in flumazenil-induced up-regulation of recombinant GABA(A) receptors. Neurosci Res. 2008;61(3):234–241. doi: 10.1016/j.neures.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Thomas P, Kittler JT, Smart TG, Moss SJ. Brain-derived neurotrophic factor modulates fast synaptic inhibition by regulating GABA(A) receptor phosphorylation, activity, and cell-surface stability. J Neurosci. 2004;24:522–530. doi: 10.1523/JNEUROSCI.3606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanematsu T, Mizokami A, Watanabe K, Hirata M. Regulation of GABA(A)-receptor surface expression with special reference to the involvement of GABARAP (GABA(A) receptor-associated protein) and PRIP (phospholipase C-related, but catalytically inactive protein) J Pharmacol Sci. 2007;104(4):285–292. doi: 10.1254/jphs.cp0070063. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Delmas P, Jovanovic JN, Brown DA, Smart TG, Moss SJ. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci. 2000;20(21):7972–7977. doi: 10.1523/JNEUROSCI.20-21-07972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly SM, Zeng XJ, Tietz EI. Role of protein kinase A in GABAA receptor dysfunction in CA1 pyramidal cells following chronic benzodiazepine treatment. J Neurochem. 2003;85:988–998. doi: 10.1046/j.1471-4159.2003.01746.x. [DOI] [PubMed] [Google Scholar]
- Mammen AL, Huganir RL, O’Brien RJ. Redistribution and stabilization of cell surface glutamate receptors during synapse formation. J. Neurosci. 1997;17:7351–7358. doi: 10.1523/JNEUROSCI.17-19-07351.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EO, Radcliffe CA, Paulsen O. Hippocampal gamma-frequency oscillations: from interneurones to pyramidal cells, and back. J Physiol. 2005;562:55–63. doi: 10.1113/jphysiol.2004.078758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Long-term potentiation in the amygdala: a mechanism for emotional learning and memory. Trends Neurosci. 1999;22(12):561–567. doi: 10.1016/s0166-2236(99)01465-4. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Parvalbumin-containing interneurons in the basolateral amygdala express high levels of the alpha1 subunit of the GABAA receptor. J Comp Neurol. 2004;473:137–146. doi: 10.1002/cne.20101. [DOI] [PubMed] [Google Scholar]
- McDonald BJ, Moss SJ. Differential phosphorylation of intracellular domains of gamma-aminobutyric acid type A receptor subunits by calcium/calmodulin type 2-dependent protein kinase and cGMP-dependent protein kinase. J Biol Chem. 1994;269(27):18111–18117. [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Mizoguchi Y, Ishibashi H, Nabekura J. The action of BDNF on GABA(A) currents changes from potentiating to suppressing during maturation of rat hippocampal CA1 pyramidal neurons. J Physiol. 2003;548:703–709. doi: 10.1113/jphysiol.2003.038935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizokami A, Mizokami A, Kanematsu T, Ishibashi H, Yamaguchi T, Tanida I, Takenaka K, Nakayama KI, Fukami K, Takenawa T, Kominami E, Moss SJ, Yamamoto T, Nabekura J, Hirata M. Phospholipase C-related inactive protein is involved in trafficking of gamma2 subunit-containing GABA(A) receptors to the cell surface. J Neurosci. 2007;27(7):1692–1701. doi: 10.1523/JNEUROSCI.3155-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Postsynaptic targets of somatostatin-containing interneurons in the rat basolateral amygdala. J Comp Neurol. 2007;500:513–529. doi: 10.1002/cne.21185. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma E, Torchia G, Limatola C, Trettel F, Arcella A, Cantore G, Di Gennaro G, Manfredi M, Esposito V, Quarato PP, Miledi R, Eusebi F. BDNF modulates GABAA receptors microtransplanted from the human epileptic brain to Xenopus oocytes. Proc Natl Acad Sci U S A. 2005;102:1667–1672. doi: 10.1073/pnas.0409442102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol. 2001;11:272–280. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- Poppe H, Rybalkin SD, Rehmann H, Hinds TR, Tang XB, Christensen AE, Schwede F, Genieser HG, Bos JL, Doskeland SO, Beavo JA, Butt E. Cyclic nucleotide analogs as probes of signaling pathways. Nat Methods. 2008;5:277–278. doi: 10.1038/nmeth0408-277. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Yu J, Xu J, Hartnett C, Meyyappan M, Kostas C, Ramabhadran TV, Gallager DW. Allosteric uncoupling after chronic benzodiazepine exposure of recombinant gamma-aminobutyric acid(A) receptors expressed in Sf9 cells: ligand efficacy and subtype selectivity. J Pharmacol Exp Ther. 1996;276(3):882–890. [PubMed] [Google Scholar]
- Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 19. 2004a;24(20):4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, Ressler KJ. Differential regulation of brain-derived neurotrophic factor transcripts during the consolidation of fear learning. Learn Mem. 2004b;11(6):727–731. doi: 10.1101/lm.83304. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, Ressler KJ. Brain-derived neurotrophic factor in amygdala-dependent learning. Neuroscientist. 2005;11(4):323–333. doi: 10.1177/1073858404272255. [DOI] [PubMed] [Google Scholar]
- Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. GABA(A) receptor subunits in the rat hippocampus I: immunocytochemical distribution of 13 subunits. Neuroscience. 1997;80:987–1000. doi: 10.1016/s0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]
- Svob Strac D, Vlainic J, Jazvinscak Jembrek M, Pericic D. Differential effects of diazepam treatment and withdrawal on recombinant GABAA receptor expression and functional coupling. Brain Res. 2008;1246:29–40. doi: 10.1016/j.brainres.2008.09.093. [DOI] [PubMed] [Google Scholar]
- Tapley P, Lamballe F, Barbacid M. K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors. Oncogene. 1992;7:371–381. [PubMed] [Google Scholar]
- van Rijnsoever C, Sidler C, Fritschy JM. Internalized GABA-receptor subunits are transferred to an intracellular pool associated with the postsynaptic density. Eur J Neurosci. 2005;21(2):327–338. doi: 10.1111/j.1460-9568.2005.03884.x. [DOI] [PubMed] [Google Scholar]
- Whiting PJ, Bonnert TP, McKernan RM, Farrar S, Le Bourdelles B, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ, Thompson SA, Wafford KA. Molecular and functional diversity of the expanding GABA-A receptor gene family. Ann N Y Acad Sci. 1999;868:645–653. doi: 10.1111/j.1749-6632.1999.tb11341.x. [DOI] [PubMed] [Google Scholar]
- Yamada MK, Nakanishi K, Ohba S, Nakamura T, Ikegaya Y, Nishiyama N, Matsuki N. Brain-derived neurotrophic factor promotes the maturation of GABAergic mechanisms in cultured hippocampal neurons. J Neurosci. 2002;22:7580–7585. doi: 10.1523/JNEUROSCI.22-17-07580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]