Abstract
BACKGROUND:
Severe neonatal hyperbilirubinemia continues to occur in healthy newborns. Recent guidelines have supported using transcutaneous devices in estimating bilirubin levels. Previous studies using these devices are limited.
METHODS:
Newborns requiring serum bilirubin level measurements before hospital discharge were recruited prospectively. The agreement between a transcutaneous bilirubin (TCB) and total serum bilirubin (TSB) level was assessed. Sensitivity analysis was conducted.
RESULTS:
A total of 430 infants were enrolled. Correlation between the values was high (Pearson’s correlation coefficient 0.83; Lin’s concordance coefficient 0.81 [95% CI 0.77 to 0.84]; P<0.001). The mean (± SD) TSB level was 194±60 μmol/L. The TCB measurement tended to overestimate the value (mean difference 12.7), with wide 95% limits of agreement (−52 μmol/L to 77 μmol/L). Sensitivity and specificity analysis of TCB values allowed estimation of clinically important TSB levels.
CONCLUSIONS:
The TCB correlated, but was imprecise in predicting TSB. TCB values can be used at the time of discharge to safely plan care for jaundiced infants if the limits of agreement are considered and clinical judgment is maintained.
Keywords: Bilirubin, Hyperbilirubinemia, Jaundice, Newborn, Transcutaneous bilirubin measurement
Abstract
HISTORIQUE :
On continue d’observer des cas d’hyperbilirubinémie néonatale grave chez les nouveau-nés en santé. De récentes lignes directrices préconisent l’utilisation de dispositifs transcutanés pour évaluer les taux de bilirubine. Peu d’études ont fait appel à ces dispositifs.
MÉTHODOLOGIE :
Les chercheurs ont procédé au recrutement prospectif des nouveau-nés chez qui il fallait mesurer le taux de bilirubine sérique avant leur congé de l’hôpital. Ils ont évalué la concordance entre la bilirubine transcutanée (BTC) et la bilirubine sérique totale (BST) et effectué une analyse de sensibilité.
RÉSULTATS :
Au total, 430 nourrissons ont été inscrits à l’étude. La corrélation entre les valeurs était élevée (coefficient de corrélation de Pearson de 0,83; coefficient de concordance de Lin de 0,81 [95 % IC 0,77 à 0,84]; P<0,001). Le taux de BST moyen (± ÉT) était de 194±60 μmol/L. La mesure de BTC tendait à surestimer la valeur (différence moyenne de 12,7), les limites de concordance étant importantes, à 95 % (−52 μmol/L à 77 μmol/L). L’analyse de sensibilité et de spécificité des valeurs de BTC a permis d’évaluer les taux de BST importants sur le plan clinique.
CONCLUSIONS :
Le calcul de la BTC était corrélé, mais imprécis pour prévoir la BST. Les valeurs de BTC peuvent être utilisées au moment du congé pour planifier en toute sécurité les soins aux nourrissons atteints de jaunisse, pourvu que les limites de concordance soient respectées et que le jugement clinique soit maintenu.
Hyperbilirubinemia continues to be the most common cause of early neonatal readmission in North America despite existing guidelines and nomograms that identify at-risk newborns before they are discharged from hospital (1–8). As a result, severe neonatal hyperbilirubinemia and bilirubin encephalopathy continue to be reported in healthy term and near-term infants worldwide (8–13).
Both the American Academy of Paediatrics (AAP [6]) and the Canadian Paediatric Society (CPS [7]) have published guidelines regarding the management of neonatal hyperbilirubinemia in term infants. Both groups have endorsed the use of the total serum bilirubin (TSB) or a transcutaneous bilirubin (TCB) measurement to identify infants with significant hyperbilirubinemia requiring repeat testing or phototherapy. These guidelines have been published following previous work suggesting the accuracy of the TCB measurement as a predictor of TSB (1,14–23).
TCB measurement devices offer an instantaneous, noninvasive method for estimating serum bilirubin levels. Previous studies (1,14–23) have shown their accuracy in estimating serum bilirubin levels at relatively low bilirubin levels (lower than 250 μmol/L [15 mg/dL]), in ethnically homogeneous populations, and/or in studies using a single observer. If TCB measurements are performed routinely in a healthy newborn population, however, they would be done by multiple health care providers, and need to be reliable in a multiethnic population.
Our study compared the accuracy of the TSB measurement with the TCB measurement using a BiliChek meter (Respironics Inc, USA) in an ethnically diverse population of term and near-term infants, when used by various health care personnel just before discharge. This represents more realistic use in a health care setting at a time in which follow-up or treatment decisions are being made with regard to jaundice (21).
METHODS
Term and near-term (35 to 37 weeks’ gestational age) jaundiced neonates born at an academic hospital in Toronto, Ontario, from July 1, 2005, to March 1, 2007, were eligible to participate in the study. Informed written consent was obtained from a parent by nursing staff or a study investigator before bilirubin measurements. Local research ethics board approval was obtained before commencement of the study. Neonates older than 35 weeks’ completed gestational age who were deemed jaundiced by medical staff and cared for in the post-partum ward of the hospital were eligible to participate in the study before initial discharge. Infants were excluded if they had undergone phototherapy, were admitted to the neonatal intensive care unit, had major congenital anomalies or birth marks, or were under the care of child protective services. Parents were not approached for study consideration if language barriers existed.
At the time of the study, neonates underwent a routine bilirubin assessment only if they appeared jaundiced to the nurse and/or physician during their newborn stay. Nursing staff had become familiar with use of the BiliChek meter before initiation of the study for a practice period of one year, and its use was becoming routine in healthy newborns close to the time of discharge. Nevertheless, the nursing staff participated in a 1 h training session organized by the authors (DC and SC) before study initiation. For patients enrolled in the study, a TCB measurement was performed by the patient’s postpartum nurse using the BiliChek meter within 30 min of obtaining the TSB. The date and time of both measurements were recorded. Each BiliChek TCB measurement was taken from the infant’s forehead, which is the site recommended by the device’s manufacturer. The BiliChek device displays a calculated average of five measurements for each bilirubin estimate. A new disposable tip was used for each BiliChek measurement. Blood samples (to measure TSB) were collected via a standard heel prick by nursing staff and analyzed by spectrophotometry for total and direct bilirubin levels using a diazo method with the Synchron LX20 Clinical Chemistry System (Beckman Coulter Inc, USA), which is standard in the hospital.
Phototherapy was initiated on TSB values according to the AAP guidelines (6). The bilirubin level was ascribed a risk level at this time, as per the AAP predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia guidelines (low, low-intermediate, high-intermediate and high risk) (21). The decision to discharge the neonate home, continue to observe in hospital or admit to the neonatal intensive care unit was made by the attending physician. Pertinent demographic information such as the patient’s gestational age, birth date and time, birth weight, maternal ethnicity and health history, labour and delivery, and hospital course including discharge weight and need for phototherapy were collected by the principal investigator and/or co-investigator(s) from chart review. Maternal ethnicity was used as a surrogate measure for infant ethnicity.
Statistical analysis
In the present prospective cohort study, complete information on 430 newborns was collected. The sample size calculation was based on the 2000 Bhutani et al (20) study that reported a correlation of r=0.91 between the TCB and TSB measures in a sample of 490 babies. Assuming under the null hypothesis that a correlation of lower than 0.80 is not acceptable and under the alternative hypothesis that a correlation of at least 0.85 is acceptable with a two-sided test, a significance level of 0.05 and power of 80%, 318 babies were needed for the study.
Descriptive data were summarized using Stata/SE 8.2 (Stata Corp, USA). Means ± SDs were reported for normative data, and when appropriate, medians with interquartile ranges were reported. The magnitude of difference between the means was assessed using paired t tests. The agreement between the two measures of TSB and TCB was assessed using Pearson’s correlation and Lin’s concordance coefficients. Because these coefficients alone can be a poor indicator for estimating the agreement between two diagnostic tests, a modified Bland-Altman technique was used to assess TCB and TSB variability (24). In this analysis, the difference between TCB and TSB was plotted against the average of TCB and TSB, and displayed with 95% confidence limits, to compare the variability across a range of bilirubin results (24).
Specificity and sensitivity analyses were estimated at two outcomes of interest (200 μmol/L and 250 μmol/L) because they are clinically important values at 24 h and 48 h of age for healthy term infants ready for discharge home. Separate logistic regression models using TCB as a predictor were created for each outcome, and sensitivity and specificity values were calculated. These were then plotted as ROC curves. As the sensitivity and the specificity of a test increases, the ROC curve will move toward the upper left-hand corner of the plot with a corresponding higher AUC. AUC values of 0.5 lack any diagnostic ability, whereas AUC values of 1.0 correspond to a perfect screening test (25).
RESULTS
The study population consisted of 430 newborns from which paired measurements of TCB and TSB were collected. Demographic data are summarized in Table 1. The study population was representative of the ethnic diversity of the inner city population.
Table 1.
Demographics of the study population (n=430)
| Demographic | |
|---|---|
| Male sex | 236 (55) |
| Gestational age, weeks (mean ± SD) | 38.8±1.4 |
| Birth weight, g (mean ± SD) | 3289±458 |
| TSB, μmol/L (mean ± SD) | 194±60 |
| TCB, μmol/L (mean ± SD) | 206±55 |
| Time of TSB, h (mean ± SD) | 55±27 |
| Exclusive breastfeeding | 280 (65) |
| Caesarean section | 146 (34) |
| Ethnicity | |
| Asian | 146 (34) |
| Caucasian | 140 (33) |
| Latino | 43 (10) |
| Indian | 36 (8) |
| Black | 34 (8) |
| Middle Eastern | 17 (4) |
| Other or unknown | 14 (3) |
| TSB in phototherapy range | 86 (23) |
| High-intermediate zone or high-risk zone | 237 (55) |
| TSB >200 μmol/L (>11 mg/dL) | 164 (38) |
| TSB >220 μmol/L (>12.5 mg/dL) | 111 (26) |
| TSB >250 μmol/L (>15 mg/dL) | 68 (16) |
| Neonatal intensive care unit stay | 44 (10) |
Data presented as n (%) unless otherwise indicated. TCB Transcutaneous bilirubin; TSB Total serum bilirubin
Of the 430 paired measurements taken, the mean and median times of TSB measurement were 55 h and 50 h, respectively. The mean TSB was 194±60 μmol/L (11.4±3.5 mg/dL) and the median TSB was 183 μmol/L (10.7 mg/dL), with an interquartile range of 99 μmol/L to 267 μmol/L (5.8 mg/dL to 15.6 mg/dL). The mean TCB measurement using the BiliChek device was 206±55 μmol/L (12.3±3.2 mg/dL) and the median was 201 μmol/L (11.8 mg/dL), with an interquartile range of 131 μmol/L to 276 μmol/L (7.4 mg/dL to 16.2 mg/dL).
Based on the TSB predictive nomogram from the AAP guidelines (21), 237 newborns (55%) from the present study population were in the high-intermediate (n=140) or high-risk zone (n=97) and, therefore, likely to develop clinically significant jaundice necessitating close follow-up and/or phototherapy. The breakdown of jaundice severity is provided in Table 1. A total of 86 infants (23%) had TSB values in the phototherapy range, while 44 infants (10%) were ultimately admitted to the neonatal intensive care unit due to hyperbilirubinemia (Table 1).
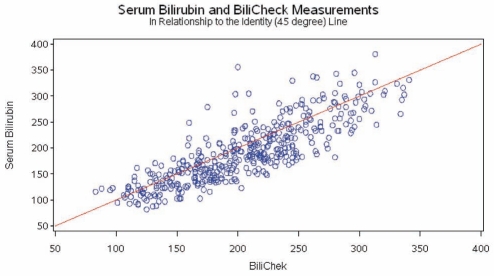
When comparing all 430 paired measurements of TCB and TSB, the Pearson’s correlation coefficient was r=0.83 and Lin’s concordance coefficient was 0.81 (95% CI 0.77 to 0.84; P<0.001). The relationship between TCB and TSB is shown in Figure 1 in reference to the line of identity (45°) for all measurements. The correlation of TCB values to TSB values at different levels of hyperbilirubinemia is outlined in Table 2. The agreement between TCB and TSB did not vary among ethnic groups (Table 3).
Figure 1).
Graphical depiction of total serum bilirubin versus transcutaneous bilirubin measurements. BiliChek (Respironics Inc, USA). All values presented in μmol/L
Table 2.
Accuracy and precision of transcutaneous bilirubin (TCB) measurement based on the total serum bilirubin (TSB) value
| TSB value | Measurements, n | Pearson’s correlation coefficient (r) | Lin’s concordance coefficient (95% CI) | Minimum, maximum difference (TCB–TSB, μmol/L) |
|---|---|---|---|---|
| All | 430 | 0.83 | 0.81 (0.77–0.84) | –156, 98 |
| TSB ≤200 μmol/L | 266 | 0.75 | 0.59 (0.53–0.65) | –45, 98 |
| TSB > 200 μmol/L | 164 | 0.52 | 0.58 (0.48–0.68) | –156, 69 |
| TSB ≤250 μmol/L | 362 | 0.79 | 0.72 (0.68–0.76) | –89, 98 |
| TSB > 250 μmol/L | 68 | 0.23 | 0.20 (−0.01–0.38) | –156, 68 |
Table 3.
Accuracy and precision of transcutaneous bilirubin (TCB) measurement based on ethnicity
| Ethnicity | Measurements, n | Pearson’s correlation coefficient (r) | Lin’s concordance coefficient (95% CI) | Minimum, maximum difference (TCB–TSB, μmol/L) |
|---|---|---|---|---|
| All | 430 | 0.83 | 0.81 (0.77–0.84) | –156, 98 |
| Asian | 146 | 0.84 | 0.81 (0.75–0.86) | –156, 77 |
| Caucasian | 140 | 0.82 | 0.78 (0.72–0.84) | –85, 98 |
| Latino | 43 | 0.86 | 0.85 (0.74–0.92) | –89, 70 |
| Black | 34 | 0.80 | 0.79 (0.62–0.89) | –96, 80 |
| Other | 67 | 0.82 | 0.79 (0.68–0.86) | –104, 70 |
TSB Total serum bilirubin
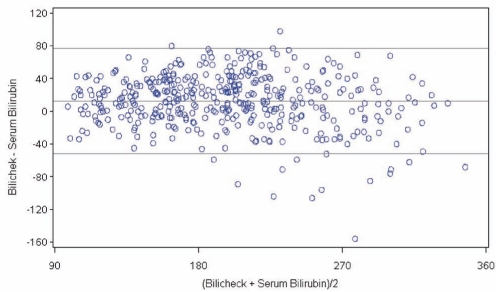
The Bland-Altman error plot demonstrates the level of precision of the BiliChek device by comparing the difference versus the average of measurements between TCB and TSB values (Figure 2). The average difference between these measurements was 12.7±32.9 μmol/L. The 95% limits of agreement were −52 μmol/L to 77 μmol/L. In the 86 infants requiring phototherapy, the TCB overestimated the TSB in the majority of cases (n=49, 57%), but 10 infants (12%) were underestimated by more than 50 μmol/L. In those with TSB in the high-risk zone (n=97), 19 were incorrectly categorized in a lower risk zone.
Figure 2).
Bland-Altman plot of the difference between transcutaneous bilirubin and total serum bilirubin measurements, plotted against the mean bilirubin estimate. BiliChek (Respironics Inc, USA). All values presented in μmol/L
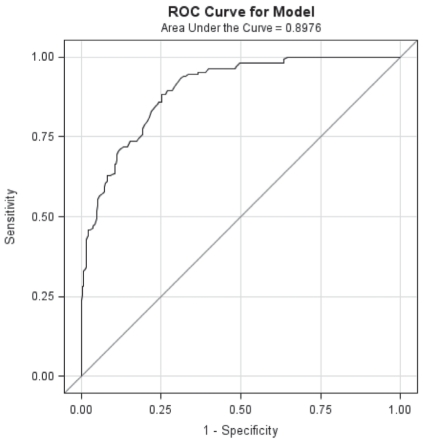
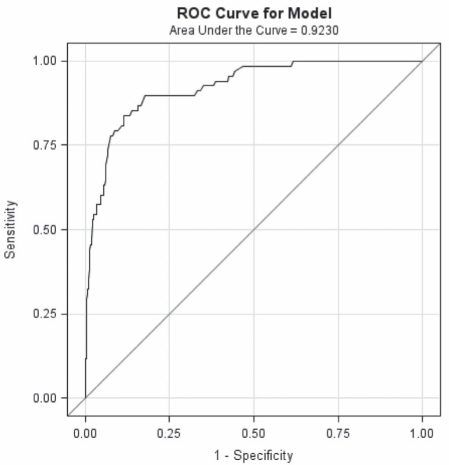
Sensitivity analysis was performed for TSB values of interest at 24 h and 48 h of age, respectively. To detect a TSB value of 200 μmol/L, a TCB value of 180 μmol/L would provide 96% sensitivity and 55% specificity (positive predictive value would be 64% and negative predictive value 96%). Similarly, to detect a TSB value of 250 μmol/L, a TCB of 200 μmol/L would provide 96% sensitivity and 57% specificity (positive predictive value would be 34% and negative predictive value 97%). Refer to Figures 3 and 4, respectively, for a graphical representation. To detect a TSB value of 300 μmol/L, TCB measurements of 200 μmol/L, 220 μmol/L and 250 μmol/L provided decreasing levels of sensitivity (95%, 86% and 81%, respectively).
Figure 3).
ROC curve for transcutaneous bilirubin values predicting a total serum bilirubin value of greater than 200 μmol/L
Figure 4).
ROC curve for transcutaneous bilirubin values predicting a total serum bilirubin value of greater than 250 μmol/L
DISCUSSION
Previous studies examining the accuracy and precision of the TCB using the BiliChek device have demonstrated correlation with the TSB in healthy term and near-term newborns, with wide limits of variability (15–23). These studies were limited by the fact that relatively small or homogeneous populations were examined, TCB measurements were completed by a handful of single users (ie, research nurse or clinical investigator), or TCB and TSB were measured at the time of routine newborn screening rather than when newborns were clinically jaundiced (ie, low levels of jaundice) (1,14–23).
We specifically focused on clinically jaundiced newborns as determined by medical staff at or near the time of discharge from hospital. This is an important time for the newborn because decisions need to be made regarding the need for bloodwork, the type of follow-up required (ie, public health nurse or physician) and/or the timing of follow-up. Second, we reported on a large and ethnically diverse cohort of newborns to address the issue of accuracy of the transcutaneous device among newborns with various degrees of skin pigmentation. Third, the BiliChek device in our study was used by a number of health care professionals looking after newborn infants versus one study nurse or investigator. A previous study (23) using multiple health care providers was restricted by smaller numbers of paired measurements (n=177) and a predominantly Caucasian (80%) cohort.
The results of our study confirm previous reports that TCB values correlate with TSB values in term and near-term neonates. However, the precision of this estimation is poor. The Bland-Altman analysis shows that the agreement between TCB and the TSB can be underestimated by up to 52 μmol/L or overestimated by as much as 77 μmol/L. Confidence limits for the BiliChek device in our study (−52 μmol/L to 77 μmol/L) are comparable with previous reports (15,16,19,20). Each of these studies varies slightly in the population studied and the range of bilirubin levels sampled (15–20). Our study is important due to the large sample size (n=430), the multiethnic population and the bilirubin levels of interest at the time of discharge from hospital (150 μmol/L to 300 μmol/L). If clinicians are using other devices, the confidence limits and/or sensitivity analyses at varying levels of bilirubin for these instruments must be recognized (17–20,26).
The Bland-Altman analysis demonstrates that, on average, the TCB tended to overestimate the TSB, but measurements of TCB could underestimate or overestimate TSB values at both low and high levels of jaundice (Figure 2). It is unlikely to be clinically important if the TCB overestimates the TSB, other than possibly generating unnecessary blood work. However, if the TCB significantly underestimates the TSB, babies requiring close follow-up or phototherapy may be missed. This is especially important at higher bilirubin levels, which are commonly seen at the time of newborn discharge or in primary care practices in the first week of life. In our cohort, near the time of discharge from hospital, of those with TSBs in the high-risk zone (n=97), 19 (19.6%) were incorrectly categorized in a lower risk zone by the BiliChek device. Only five of these infants would have been incorrectly categorized in a lower risk zone if the 95% limits of agreement were used (ie, if we assumed that the TCB estimate was an underestimate of the actual bilirubin level by 52 μmol/L).
We also looked at all jaundiced infants whose TSB measurements were in the phototherapy range based on the AAP guidelines. Of the 86 infants requiring phototherapy, the TCB overestimated the TSB in the majority of cases (n=49; 57%). Therefore, these infants would be identified as appropriately requiring phototherapy based on the BiliChek measurement. However, 10 newborns (11.6%) who required phototherapy had TCB measurements that significantly underestimated the actual TSB level (greater than 50 μmol/L). Were it not for the TSB measurement, these infants would have been missed and falsely deemed ‘safe’ with respect to their level of jaundice. Only three of these infants would have been missed if the 95% limits of agreement of the device had been used (ie, if we assumed that the TCB estimate was an underestimate of the actual bilirubin by 52 μmol/L).
Previous work by Bhutani et al (20,21) showed that the TCB value was most accurate at lower TSB values. Furthermore, a TCB value above the 75th percentile of hour-specific TSB values on the bilirubin nomogram carried risk for subsequent significant hyperbilirubinemia (21,27). In their cohort, a TCB threshold of 220 μmol/L at the time of routine newborn screening (approximately 48 h of life) was used to perform further evaluation (18). In our cohort, an even lower TCB cut-off value was necessary to capture all significantly jaundiced newborns who required phototherapy. For those interested in detecting TSB values of 200 μmol/L or greater, we suggest a TCB threshold of 180 μmol/L when using the BiliChek device, to safely identify at-risk jaundiced newborns. If the clinician is interested in not missing TSB values of 250 μmol/L or greater, we suggest using a TCB cut-off value of 200 μmol/L with this device. The TCB cut-off value used to detect higher TSB values is unclear based on our cohort because a wide range of TCB values generated similar sensitivities and specificities.
Guidelines published by both the AAP (6) and the CPS (7) suggest that the TCB value may be used as a substitute for TSB in evaluating the risk of significant neonatal hyperbilirubinemia in the healthy newborn. The CPS statement qualifies this further: “…the TCB result should be summed with the 95% CI of the device to estimate the maximum probable TSB concentration (recommendation grade C)”. Our study highlights the importance of this statement, especially when bilirubin values are high. Rather than use a 95% limit of agreement for all bilirubin values (as recommended by the CPS), our data suggest that for lower bilirubin values (close to 200 μmol/L), the BiliChek device may prevent a significant amount of bloodwork. As bilirubin levels rise (close to 250 μmol/L), the CPS recommendation becomes more useful and threshold values closer to the confidence limits would be appropriate to assist in clinical decision making.
Based on our data, the BiliChek device can be used as a screening tool for most bilirubin levels of interest. The 95% limits of agreement for this device are wide, and it may not be the most appropriate method to estimate all serum values of interest, especially higher values. For example, to estimate a TSB value of lower than 200 μmol/L, a TCB threshold value of 180 μmol/L would be sufficient. For higher TSB values of interest (eg, greater than 250 μmol/L), a TCB threshold of 200 μmol/L would be prudent.
It should be stressed that clinical judgement needs to be exercised when evaluating the appropriate treatment and/or follow-up needed for jaundiced term and near-term newborns. Risk factors for significant hyperbilirubinemia such as near-term gestational age, low birth weight, Asian ethnicity, male sex, and the presence of ABO incompatibility or glucose-6-phosphate dehydrogenase deficiency may necessitate TSB measurement due to an inherent higher risk of jaundice that these factors present in newborns (7).
Our study has several limitations. The study population size prevents overinterpretation of bilirubin measurements in each ethnic subgroup, or when determining sensitivity analysis of the TCB at bilirubin levels of greater than 300 μmol/L. TSB values used in our cohort were not obtained using high-performance liquid chromatography, which is the gold standard of bilirubin measurement. However, many laboratories across Canada use conventional TSB measuring techniques, which are routinely used in clinical decision making. Finally, maternal ethnicity may not be the best indicator of infant ethnicity or skin pigmentation.
CONCLUSIONS
In the present prospective cohort study of ethnically diverse term and near-term jaundiced newborns, the BiliChek device correlated but was often imprecise in predicting actual TSB levels. However, the use of TCB measurement can be useful if the limits of the device are understood across a range of bilirubin values. Interpretation of the clinical context and knowledge of the limitations of each TCB device are key when estimating clinically relevant TSB bilirubin values in the management of neonatal hyperbilirubinemia.
Acknowledgments
The authors thank Rosane Nisenbaum for her contributions to the statistical analysis of the data.
REFERENCES
- 1.Eggert LD, Wiedmeier SE, Wilson J, Christensen RD. The effect of instituting a prehospital-discharge newborn bilirubin screening program in an 18-hospital health system. Pediatrics. 2006;117:e855–62. doi: 10.1542/peds.2005-1338. [DOI] [PubMed] [Google Scholar]
- 2.Johnson D, Jin Y, Truman C. Early discharge of Alberta mothers post-delivery and the relationship to potentially preventable newborn readmissions. Can J Public Health. 2002;93:276–80. doi: 10.1007/BF03405016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown AK, Damus K, Kim MH, et al. Factors relating to readmission of term and near term neonates in the first two weeks of life. J Perinat Med. 1999;27:263–75. doi: 10.1515/JPM.1999.037. [DOI] [PubMed] [Google Scholar]
- 4.Liu S, Wen SW, McMillan D, Trouton K, Fowler D, McCourt C. Increased neonatal readmission rate associated with decreased length of hospital stay at birth in Canada. Can J Public Health. 1999;91:46–50. doi: 10.1007/BF03404253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee KS, Perlman M, Ballantyne M, Elliott I, To T. Association between duration of neonatal hospital stay and readmission rate. J Pediatr. 1995;127:758–66. doi: 10.1016/s0022-3476(95)70170-2. [DOI] [PubMed] [Google Scholar]
- 6.American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia Management of hyperbilirubinemia in the newborn infants 35 or more weeks of gestation. Pediatrics. 2004;114:297–316. doi: 10.1542/peds.114.1.297. [DOI] [PubMed] [Google Scholar]
- 7.Canadian Paediatric Society, Fetus and Newborn Committee Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants (35 or more weeks’ gestation) Paediatr Child Health. 2007;12(Suppl B):1B–12B. doi: 10.1093/pch/12.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhutani VK, Johnson LH, Maisels MJ, et al. Kernicterus: Epidemiological strategies for its prevention. J Perinatol. 2004;24:650–62. doi: 10.1038/sj.jp.7211152. [DOI] [PubMed] [Google Scholar]
- 9.Sgro M, Campbell D, Shah V. Incidence and causes of severe neonatal hyperbilirubinemia in Canada. CMAJ. 2006;175:587–90. doi: 10.1503/cmaj.060328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Academy of Pediatrics, Subcommittee on Neonatal Hyperbilirubinemia Neonatal jaundice and kernicterus. Pediatrics. 2001;108:763–5. doi: 10.1542/peds.108.3.763. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention Kernicterus in full-term infants – United States, 1994–1998. MMWR. 2001;50:491–4. JAMA 2001;286:299–300. [PubMed] [Google Scholar]
- 12.Joint Commission on Accreditation of Healthcare Organizations Kernicterus threatens healthy newborns. Sentinel event alert. 2001. p. 18. < http://www.jointcommission.org/sentinel_event_alert_issue_18_kernicterus_threatens_healthy_newborns/> (Accessed on February 10, 2011). [PubMed]
- 13.Manning D, Todd P, Maxwell M, Jane Platt M. Prospective surveillance study of severe hyperbilirubinemia in the newborn in the UK and Ireland. Arch Dis Child Fetal Neonatal Ed. 2007;92:F342–6. doi: 10.1136/adc.2006.105361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maisels MJ, Newman TB. Kernicterus in otherwise healthy, breast-fed term newborns. Pediatrics. 1995;96:730–3. [PubMed] [Google Scholar]
- 15.Boo NY, Ishak S. Prediction of severe hyperbilirubinaemia using the Bilicheck transcutaneous bilirubinometer. J Paediatr Child Health. 2007;43:297–302. doi: 10.1111/j.1440-1754.2007.01062.x. [DOI] [PubMed] [Google Scholar]
- 16.Jangaard KA, Curtis H, Goldbloom RB. Estimation of bilirubin using Bilichek, a transcutaneous bilirubin measurement device: Effects of gestational age and use of phototherapy. Paediatr Child Health. 2006;11:79–83. doi: 10.1093/pch/11.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engle WD, Jackson GL, Stehel EK, Sendelbach DM, Manning MD. Evaluation of a transcutaneous jaundice meter following hospital discharge in term and near-term neonates. J Perinatol. 2005;25:486–90. doi: 10.1038/sj.jp.7211333. [DOI] [PubMed] [Google Scholar]
- 18.Slusher TM, Angyo IA, Bode-Thomas F, et al. Transcutaneous bilirubin measurements and serum total bilirubin levels in indigenous African infants. Pediatrics. 2004;113:1636–41. doi: 10.1542/peds.113.6.1636. [DOI] [PubMed] [Google Scholar]
- 19.Engle WD, Jackson GL, Sendelbach D, Manning D, Frawley WH. Assessment of a transcutaneous device in the evaluation of neonatal hyperbilirubinemia in a primarily Hispanic population. Pediatrics. 2002;110:61–7. doi: 10.1542/peds.110.1.61. [DOI] [PubMed] [Google Scholar]
- 20.Bhutani VK, Gourley GR, Adler S, Kreamer B, Dalin C, Johnson LH. Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics. 2000;106:E17. doi: 10.1542/peds.106.2.e17. [DOI] [PubMed] [Google Scholar]
- 21.Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics. 1999;103:6–14. doi: 10.1542/peds.103.1.6. [DOI] [PubMed] [Google Scholar]
- 22.Maisels MJ, Ostrea EM, Jr, Touch S, et al. Evaluation of a new transcutaneous bilirubinometer. Pediatrics. 2004;113:1628–35. doi: 10.1542/peds.113.6.1628. [DOI] [PubMed] [Google Scholar]
- 23.Karon BS, Teske A, Santrach PJ, Cook WJ. Evaluation of the Bilichek non-invasive bilirubin analyzer for prediction of serum bilirubin and risk of hyperbilirubinemia. Am J Clin Pathol. 2008;130:976–82. doi: 10.1309/AJCPRX1E3NWCXHMZ. [DOI] [PubMed] [Google Scholar]
- 24.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–60. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 25.Whiting P, Rutjes AW, Dinnes J, Reitsma J, Bossuyt PM, Kleijnen J. Development and validation of methods for assessing the quality of diagnostic accuracy studies. Health Technol Asses. 2004;8:1–234. doi: 10.3310/hta8250. [DOI] [PubMed] [Google Scholar]
- 26.De Luca D, Zecca E, Corsello M, Tiberi E, Semeraro C, Romagnoli C. Attempt to improve transcutaneous bilirubinometry: A double-blind study of Medick BiliMed versus Respironics BiliCheck. Arch Dis Child Fetal Neonatal Ed. 2008;93:F135–9. doi: 10.1136/adc.2007.121053. [DOI] [PubMed] [Google Scholar]
- 27.Kuzniewicz MW, Escobar GJ, Newman TB, et al. Impact of universal bilirubin screening on severe hyperbilirubinemia and phototherapy use. Pediatrics. 2009;24:1031–9. doi: 10.1542/peds.2008-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]