Abstract
Study Objectives:
To evaluate mouth opening during sleep and the possible correlations between mouth opening and specific patient characteristics.
Methods:
A total of 55 patients consecutively referred to assess snoring and suspected obstructive sleep apnea (OSA) were included. Sensors to record mouth opening were attached to each patient's face and synchronized with a standard polysomnogram. Mouth opening data were evaluated for each sleep stage as a percentage of maximum mouth opening. The patients were divided into 2 groups: patients with REM apnea hypopnea index (AHI) > NREM AHI (REM-dependent group = RD group), and patients with NREM AHI > REM AHI (NREM-dependent group = ND group).
Results:
A total of 42 patients (male 69.0%, mean age 51.4 ± 12.9 years) underwent successful data collection. The amount of mouth opening during stage 1 (18.8% ± 14.6%) was significantly smaller than stage 2 (23.7% ± 16.4%, p < 0.01) and REM (29.2% ± 20.3%, p < 0.01). Age, body mass index (BMI), Epworth Sleepiness Scale (ESS) score, and AHI exhibited no correlation with mouth opening. The RD and the ND groups exhibited similar age, BMI, ESS, and AHI variables, but the ND group opened their mouths significantly more than the RD group during total sleep time (28.3% ± 13.6% vs 17.8% ± 17.3%, p < 0.01), stage 1 (23.2% ± 13.5% vs 12.9% ± 14.3%, p < 0.01), stage 2 (28.1% ± 17.9% vs 17.9% ± 17.4%, p < 0.01), and REM (34.7% ± 19.2% vs 21.9% ± 19.8%, p < 0.05).
Conclusions:
The ND patients opened their mouths wider than the RD patients during most sleep stages. The relationship between REM-dependent AHI and the amount of mouth opening may be a factor in the pathogenesis of OSA.
Citation:
Tsuda H; Lowe AA; Chen H; Fleetham JA; Ayas NT; Almeida FR. The relationship between mouth opening and sleep stage-related sleep disordered breathing. J Clin Sleep Med 2011;7(2):181-186.
Keywords: Obstructive sleep apnea, REM-related obstructive event, mouth opening
A variety of factors contribute to the development of obstructive sleep apnea (OSA). A better understanding of these factors would help future therapeutic advances in OSA.1 Some physiological studies suggest that mouth opening increases upper airway collapsibility.2–4 Miyamoto et al. reported that the vertical mandibular posture is more open during sleep in patients with OSA than in healthy adults. Furthermore, sleep stage was a significant factor in mandibular opening in the supine position but not in the lateral position in patients with OSA, as determined by using magnetic sensors attached inside the subject's mouth.5,6 One study has recommended that jaw opening should be kept to a minimum for better treatment success with oral appliance (OA) therapy in patients with OSA.7 It has also been reported that patients with a large amount of mouth breathing, which is only possible when both the soft palatal seal and mouth are opened, are less adherent to nasal continuous positive airway pressure (nCPAP) therapy than patients with a small amount of mouth breathing.8 Furthermore, some patients with OSA have a higher percentage of obstructive events during REM compared to NREM sleep.9,10 In these studies, REM-dependent subjects were reported as being young and female, and this REM dependency was suggested as a factor in understanding the difference in the mechanism of obstruction between different types of patients with OSA. The clinical meaning of REM dependency has not been fully investigated. However, since it has been reported that sleep disordered breathing (SDB) during NREM sleep, but not REM sleep, is associated with a risk of daytime sleepiness,11 sleep staging should be considered to be a variable that requires understanding.
BRIEF SUMMARY
Current Knowledge/Study Rationale: This study evaluated mouth opening during sleep and correlations between mouth opening and patients' characteristics. Patients with NREM-dependent OSA opened their mouth wider than REM-dependent patients. Disease severity, BMI, age, and body position were not found to affect mouth opening during sleep.
Study Impact: Mouth opening was not a causative factor or associated with sleep apnea. However, considering the physiological mechanism of sleep, NREM-dependent OSA may be partially caused by mouth opening.
Previous physiological studies have investigated the amount of mouth opening and the subsequent collapse of the upper airway; however, REM dependency or supine dependency determined for the entire sleep duration was not assessed in these studies. To better understand the causes of each SDB event and the predictors of treatment success, it may be useful to estimate how often, how long, and how wide patients with OSA open their mouths during sleep, and the correlation between mouth opening and patient characteristics such as age, sex, body mass index (BMI), and apnea hypopnea index (AHI). The aim of this study was to evaluate mouth opening during sleep and the possible correlations between mouth opening and specific patient characteristics.
METHODS
Subjects
Fifty-five consecutive patients referred to the Sleep Disorder Program at the University of British Columbia Hospital to assess snoring and suspected OSA were included in this study. The protocol had the prior approval of the clinical research ethics board, UBC Office of Research Services. The aim of the study was explained to all patients, and written informed consent was obtained.
Sensor for Jaw Movement
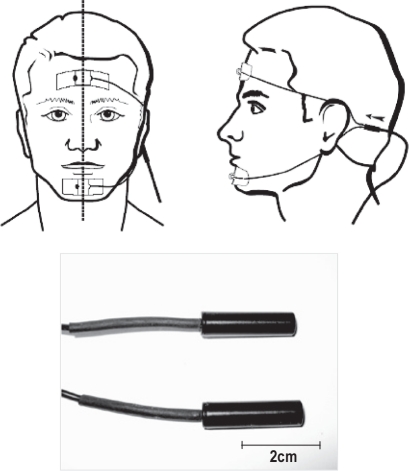
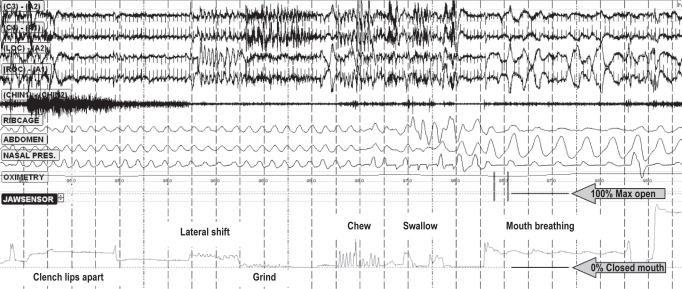
Sensors for recording jaw movement, which use resonating magnetic field transducers (JAWSENS, nomics, Belgium), were attached to each patient's forehead and chin (Figure 1). The data were evaluated simultaneously with a standard polysomnogram (PSG). The data from the magnet sensor were sampled every 0.03 sec throughout the night. Before each patient went to sleep, maximum open, grind, lateral movement, swallow, chew, and mouth breathing were checked and calibrated in a supine position in bed (Figure 2). After this initial data collection, the data, expressed as a percentage of the maximum opening level, were evaluated for each sleep stage. One hundred percent corresponds to a maximum opening of the mouth, and 0% corresponds to a closed mouth position (teeth touching lightly).
Figure 1.
JAWSEN sensors
Picture comes from instruction manual
Figure 2.
Sample of PSG and JAWSENS data at the calibration
Lower line defines 0% as the closed mouth position, upper line defines 100% as the maximum opening position
Polysomnogram (PSG)
PSG data were analyzed manually according to the American Academy of Sleep Medicine (AASM) criteria by polysomnographic technologists blinded to the JAWSEN data. Each stage was defined in 30-sec epochs, and the JAWSEN data were also calculated as the average of each epoch. NREM stages 3 and 4 were combined and referred to as N3 in the analysis.
Data Analysis
Based on the PSG results, patients with an AHI < 5/h were excluded from the analysis in order to exclude normal subjects. Each subject's demographic data such as age, sex, BMI, Epworth Sleepiness Scale (ESS) score, Mallampati score, and medical history were collected from the patient's chart. After scoring the data, the patients were divided into 2 groups: patients with REM AHI > NREM AHI (REM-dependent group: RD group), and patients with NREM AHI > REM AHI (NREM-dependent group: ND group).12 Demographic, sleep-related, and mouth opening data were determined for the 2 groups. The difference between patients according to body position tendency (supine-dependent or non-supine-dependent sleep apnea) was also estimated according to a criteria previously described.13
Methods Error
To assess the methods error, 8 different distances on the ruler (every 1 cm from 9–16 cm) were measured. The methods error (ME) was determined by the formula: ME = ✓Σd2/2(n-1) where d is the difference between measurement pairs and n is the number of pairs. To assess whether and how the measurements from the sensor matched the measurements on the ruler, we also measured 8 different distances (every 1 cm from 9–16 cm) and calculated an intraclass correlation coefficient (ICC).
Statistical Analysis
Statistical analyses were performed, and p values < 0.05 were considered significant. Correlation between measurements from the ruler and the sensor were tested by using the ICC. The Friedman test, followed by the Wilcoxon signed ranks test, was applied between the mouth opening variables and sleep stages. A Pearson correlation test was applied to determine the correlation between demographic variables and mouth opening variables. The independent student t-test, the χ2 test, and the Mann-Whitney test were used for comparisons between the groups.
RESULTS
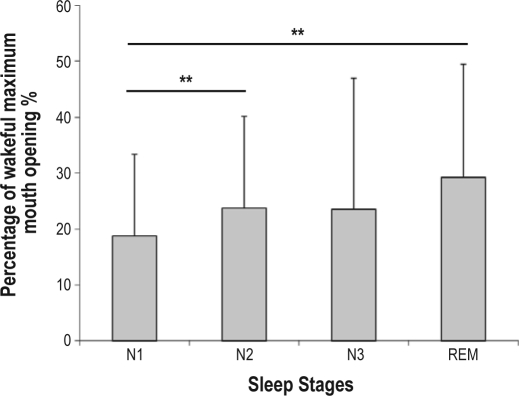
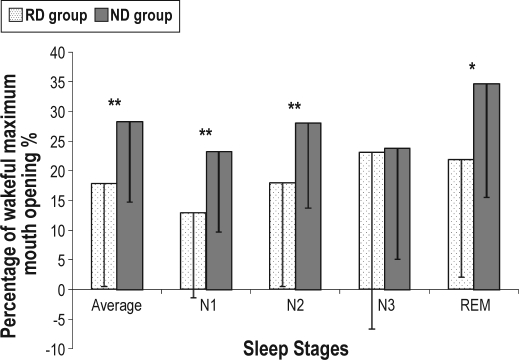
A total of 42 patients (male 69.1%, mean age 51.4 ± 12.9 years) successfully completed both the JAWSENS and PSG data collection (Table 1, 2). Seven patients did not have complete data on JAWSEN, and 6 patients had AHIs < 5/h. The methods error test was performed 10 times for each distance and was 0.17–0.44 according to different measures. The results from the ruler and the measurements from the sensors had a high correlation. The ICC value was 0.9995. Percent averages of maximum mouth opening were calculated for specific sleep stages. Stage 1 (N1) (18.8% ± 14.6%) had the smallest mouth opening when compared to stage 2 (N2) (23.7% ± 16.4%) and REM (29.2% ± 20.3%) (Figure 3). The demographic variables such as age, BMI, neck circumference, Mallampati score, ESS, and AHI were found to have no correlation with the mouth opening variables. A possible concern was whether it is possible that a patient would more likely be assigned to the RD group than the ND group on the basis of chance alone due to potentially small differences between REM AHI and NREM AHI. We excluded patients whose REM AHI and NREM AHI differences were < 5/h and performed the same analysis. There was no difference in the findings, so the results are presented for all patients under the definition of previous criteria.12 The RD group (n = 18) and the ND group (n = 24) exhibited similar age, sex, BMI, ESS, AHI, neck circumference, Mallampati score, previous upper airway surgery, nose congestion, and sleep-related variables (Table 1). The ND group patients opened their mouths significantly wider than the RD group during various sleep stages (Figure 4): total sleep time (RD: 17.8% ± 17.3%, ND: 28.3% ± 13.6%, p < 0.01), N1 (RD: 12.9% ± 14.3%, ND: 23.2% ± 13.5%, p < 0.01), N2 (RD: 17.9% ± 17.4%, ND: 28.1% ± 14.4%, p < 0.01), and REM (RD: 21.9 ± 19.8%, ND: 34.7% ± 19.2%, p < 0.05). Significant mouth opening differences in various body positions (Table 2), including time in the supine and lateral position, REM in the supine position, and NREM in the supine and side positions were identified between ND and RD groups. The supine-dependent group (n = 17) and the non-supine-dependent group (n = 25) also had similar age, sex, BMI, ESS, AHI, neck circumference, Mallampati score, previous upper airway surgery, nasal congestion, sleep-related, and mouth opening variables. In addition, it was difficult to visually detect any specific pattern of the obstructive event from jaw movement data.
Table 1.
Demographic and polysomnographic data of the study patients
| Total | RD group | ND group | p value | |
|---|---|---|---|---|
| N | 42 | 18 | 24 | |
| Male/female | 29 / 13 | 10 / 8 | 19 / 5 | NS |
| Age (years) | 51.4 ± 12.9 | 52.9 ± 11.8 | 50.2 ± 13.7 | NS |
| BMI (kg/m2) | 30.4 ± 6.4 | 31.2 ± 7.3 | 29.7 ± 5.7 | NS |
| ESS | 11.2 ± 4.3 | 11.4 ± 4.8 | 11.2 ± 3.9 | NS |
| Neck circumference (cm) | 40.6 ± 4.5 | 40.2 ± 4.1 | 40.9 ± 4.8 | NS |
| Mallampati score: I | 0 | 0 | 0 | NS |
| II | 14 | 4 | 10 | |
| III | 12 | 7 | 5 | |
| IV | 14 | 7 | 7 | |
| Previous ENT surgery | 9 (21.4%) | 3 (16.7%) | 6 (25.0%) | NS |
| Nasal congestion | 15 (35.7%) | 6 (33.3%) | 9 (37.5%) | NS |
| AHI (/hours) | 26.2 ± 18.8 | 22.3 ± 11.7 | 29.1 ± 22.6 | NS |
| Min O2 (%) | 84.5 ± 8.8 | 82.4 ± 10.5 | 86.0 ± 7.0 | NS |
| Total sleep time (min) | 367.6 ± 64.2 | 353.2 ± 57.2 | 378.4 ± 68.1 | NS |
| Time of stage N1 (%) | 10.8 ± 8.6 | 11.8 ± 9.6 | 9.7 ± 7.8 | NS |
| Time of stage N2 (%) | 70.7 ± 9.2 | 71.7 ± 9.3 | 70.0 ± 9.1 | NS |
| Time of stage N3 (%) | 2.2 ± 4.4 | 1.2 ± 3.0 | 3.0 ± 5.2 | NS |
| Time of stage REM (%) | 16.3 ± 5.5 | 15.3 ± 5.3 | 17.1 ± 5.7 | NS |
NS, no significance (p > 0.05)
Table 2.
Percentage of mouth opening in different body positions and sleep stages
| RD group | ND group | p value | |
|---|---|---|---|
| Mouth opening in | |||
| Supine (%) | 16.0 ± 19.1 | 28.2 ± 11.7 | < 0.01 |
| Side (%) | 18.9 ± 17.9 | 28.1 ± 13.9 | < 0.05 |
| REM-supine (%) | 19.6 ± 20.2 | 35.7 ± 17.7 | < 0.01 |
| NREM-supine (%) | 16.4 ± 18.0 | 28.2 ± 10.9 | < 0.01 |
| REM-side (%) | 24.2 ± 19.9 | 29.6 ± 19.9 | NS |
| NREM-side (%) | 17.5 ± 16.8 | 27.8 ± 16.1 | < 0.05 |
p values mean a significant mouth opening difference between RD group and ND group; NS, no significance (p > 0.05).
Figure 3.
Mouth opening in each sleep stage
Statistical significance **p < 0.01
Figure 4.
Amount of mouth opening in each sleep stage between REM-dependent (RD) and NREM-dependent (ND) groups
Statistical significance *p < 0.05,**p < 0.01
DISCUSSION
In this study, NREM-dependent patients with OSA opened their mouths wider than did REM-dependent patients with OSA in most sleep stages. Severity of disease (AHI), BMI, age, and body position were not found to affect mouth opening. Stage 1 (N1) had the smallest mouth opening when compared to stage 2 (N2) and REM. Koo and colleagues reported that OSA occurring during REM is more frequent in women,9 and younger women may be protected from OSA during NREM sleep, even obese patients.10 Although there was no significant difference between the groups, in this study 44.4% of the RD group were female compared to 20.8% of the ND group, which is similar to what has been reported previously. In the RD group, patients did not open their mouths much in any sleep stages including REM, although most of their obstructive events occurred during REM sleep. As only 20 patients (9 in the RD group and 11 in the ND group) had N3, this may be the reason that no significant difference was found between the two groups in this stage.
ND group subjects opened their mouths wider than did the RD group patients, but the average amount of mouth opening was not greater than 30% of the maximum opening. If it is hypothesized that if a patient can open his or her mouth 50 mm (normal range 40–60 mm),14 then for such patient 30% corresponds to 15 mm. This is almost the same value as the mouth opening used in previous physiological studies.2,3 In this study design, we cannot conclude that mouth opening was a causative factor for sleep apnea. However, considering the physiological mechanism of sleep as previously reported in OSA patients, the ND group's obstructive events may be partially caused by mouth opening.
When comparing our results to the previous study,6 this report is in agreement in that sleep stage was found to be a significant factor in mouth opening. In the previous study, this factor was only significant when patients were in the supine position. The differing results between the two studies may be due to the differing methods of measurement and analysis used. Also, the sensors were attached differently in each study. In the study by Miyamoto and associates,6 the sensors were attached on the molar area in the mouth and were calibrated on an articulated dental cast with vertical opening movement at the incisors. In our study, sensors were attached on the forehead and chin, which results in a greater distance between the sensors than those in the previous study. In our study, the sensors were calibrated with the patient lying on the bed. In addition, an advantage of the sensors used in our study is that sensors do not prevent the mouth from sealing. As patients therefore do not need to put anything in the mouth, they are able to breathe normally.6 In the lateral position, if the direction of the gravity shifted sideways and this was followed by patients opening their mouths with a side shift, the sensor in this study may have been able to detect these movements more accurately than the sensors used in the previous study.
The subjects who were excluded from analysis had similar demographic characteristics to subjects who were included. As the calibration and sensor position are sensitive and important, it is impossible to get correct data without recalibration if the sensor gets displaced. Only four of the seven excluded patients had sensor displacement, while the others wanted to stop because of discomfort of the sensors and nasal cannula.
The limitations of the study may be related to the definition of classification between REM- and NREM-dependent groups, the characteristics of the sensors used, absence of electromyogram (EMG), and lack of information regarding the preferred breathing route of the patient.
In this study, RD and ND groups were divided by using previous criteria12; however, it might have been possible to assign someone to the RD group rather than the ND group on the basis of chance alone due to small difference between REM AHI and NREM AHI. As subjects in the previous report12 had ≥ 5 events/h differences between REM AHI and NREM AHI, we tried to analyze the results excluding the patients whose AHI differences between REM AHI and NREM AHI were < 5 events/h, in order to prevent the effects of these borderline subjects. Both the results with and those without borderline patients exhibited similar significant results; therefore, we have considered that the criteria introduced in the previous report is adequate for grouping REM dependency in this study.
In relation to the type of sensor, mouth opening included the vertical and anterior-posterior movements, and these ratios are different for each patient. Even if the movements were similar for two patients, it would be unlikely that the proportions of vertical/anterior-posterior would be similar, as we just measured the distance between two sensors.
Since we did not collect EMG data in this study, it is also difficult to determine the mechanism of mouth opening. To understand the mechanism or reason for a patient to open his or her mouth, the muscle function and the relationship between the muscle activation and other physiological variables should be investigated.
Breathing route, especially mouth breathing, could be the reason for a patient to open his or her mouth. Although some patients in this study reported nasal congestion, there was no significant correlation between this nasal congestion and mouth opening variables. It has been reported that some patients with OSA use their mouths for breathing while asleep, even if they are able to breathe through their noses when awake,15 and that an older population uses the oronasal route for breathing more often than does a younger population.16 It is impossible to draw conclusions about these relationships without analyzing the breathing route directly. An interesting report related to mouth breathing found that men breathe a significantly higher percentage of total ventilation through their mouths compared to women, and that this increases with age.17 We could speculate that the typical patient who has NREM-dependent characteristics is likely to be a male who suffers from OSA caused by mouth breathing and exhibits wide mouth opening. Patients with REM dependency are more likely to be female with few episodes of mouth opening and mouth breathing. This might be a factor in understanding the pathology of OSA in women or the reason why there is a low percentage of OSA among young women. In addition, it has been reported that female patients and supine-dependent male patients were more likely to experience treatment success with OA therapy.18 We could not find any typical mouth opening followed by an apnea event, as there were many kinds of mouth opening patterns in each subject. Also it was difficult to define the start or end points of mouth opening. This study used the percentage of awake maximal opening as a variable. Further studies are required to explore and compare different definitions for mouth opening during sleep. As mentioned in the limitations of this study, the amount or type of mouth opening could not fully explain specific characteristics of the disease. However, REM dependency was the only variable that exhibited a correlation with mouth opening, and we suggest that REM dependency be explored as an important variable in future studies. Future research is required to determine the relationships between mouth opening, mouth breathing, gender, and REM-dependent OSA. Such studies could further determine the mechanism of the obstructive event in REM-dependent patients and assess whether REM dependency is a useful indicator for the diagnosis or prediction of treatment success with nCPAP, OA, or surgery.
CONCLUSION
For our patients, the amount of mouth opening during N1 sleep was significantly less than during the other sleep stages. The ND patients opened their mouths wider than did the RD patients during most sleep stages. This study demonstrates the relationship between REM dependency and the amount of mouth opening, and this could explain the pathogenesis of REM-dependent OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors would like to thank Mrs. Ingrid Ellis for her editorial assistance in the final preparation of this manuscript. A MITACS Accelerate Internship Grant supported in part the post-doctoral fellowship salary of H. Tsuda.
REFERENCES
- 1.Owens R, Wellman A, Malhotra A. The chicken-or-egg debate in OSA pathogenesis. Sleep. 2009;32:1255–6. doi: 10.1093/sleep/32.10.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meurice JC, Marc I, Carrier G, Series F. Effects of mouth opening on upper airway collapsibility in normal sleeping subjects. Am J Respir Crit Care Med. 1996;153:255–9. doi: 10.1164/ajrccm.153.1.8542125. [DOI] [PubMed] [Google Scholar]
- 3.Isono S, Tanaka A, Tagaito Y, Ishikawa T, Nishino T. Influences of head positions and bite opening on collapsibility of the passive pharynx. J Appl Physiol. 2004;97:339–46. doi: 10.1152/japplphysiol.00907.2003. [DOI] [PubMed] [Google Scholar]
- 4.Ayuse T, Inazawa T, Kurata S, et al. Mouth-opening increases upper-airway collapsibility without changing resistance during midazolam sedation. J Dent Res. 2004;83:718–22. doi: 10.1177/154405910408300912. [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto K, Ozbek MM, Lowe AA, et al. Mandibular posture during sleep in healthy adults. Arch Oral Biol. 1998;43:269–75. doi: 10.1016/s0003-9969(97)00122-2. [DOI] [PubMed] [Google Scholar]
- 6.Miyamoto K, Ozbek MM, Lowe AA, et al. Mandibular posture during sleep in patients with obstructive sleep apnoea. Arch Oral Biol. 1999;44:657–64. doi: 10.1016/s0003-9969(99)00057-6. [DOI] [PubMed] [Google Scholar]
- 7.L'Estrange PR, Battagel JM, Harkness B, Spratley MH, Nolan PJ, Jorgensen GI. A method of studying adaptive changes of the oropharynx to variation in mandibular position in patients with obstructive sleep apnoea. J Oral Rehabil. 1996;23:699–711. doi: 10.1046/j.1365-2842.1996.00416.x. [DOI] [PubMed] [Google Scholar]
- 8.Bachour A, Maasilta P. Mouth breathing compromises adherence to nasal continuous positive airway pressure therapy. Chest. 2004;126:1248–54. doi: 10.1378/chest.126.4.1248. [DOI] [PubMed] [Google Scholar]
- 9.Koo BB, Dostal J, Ioachimescu O, Budur K. The effects of gender and age on REM-related sleep-disordered breathing. Sleep Breath. 2008;12:259–64. doi: 10.1007/s11325-007-0161-7. [DOI] [PubMed] [Google Scholar]
- 10.Koo BB, Patel SR, Strohl K, Hoffstein V. Rapid eye movement-related sleep-disordered breathing: influence of age and gender. Chest. 2008;134:1156–61. doi: 10.1378/chest.08-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Punjabi NM, Bandeen-Roche K, Marx JJ, Neubauer DN, Smith PL, Schwartz AR. The association between daytime sleepiness and sleep-disordered breathing in NREM and REM sleep. Sleep. 2002;25:307–14. [PubMed] [Google Scholar]
- 12.Siddiqui F, Walters AS, Goldstein D, Lahey M, Desai H. Half of patients with obstructive sleep apnea have a higher NREM AHI than REM AHI. Sleep Med. 2006;7:281–5. doi: 10.1016/j.sleep.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Marklund M, Persson M, Franklin KA. Treatment success with a mandibular advancement device is related to supine-dependent sleep apnea. Chest. 1998;114:1630–5. doi: 10.1378/chest.114.6.1630. [DOI] [PubMed] [Google Scholar]
- 14.Okeson JP. Management of temporomandibular disorders and occlusion. 6th ed. St. Louis, MO: Mosby Elsvier; 2008. [Google Scholar]
- 15.Koutsourelakis I, Vagiakis E, Roussos C, Zakynthinos S. Obstructive sleep apnoea and oral breathing in patients free of nasal obstruction. Eur Respir J. 2006;28:1222–8. doi: 10.1183/09031936.00058406. [DOI] [PubMed] [Google Scholar]
- 16.Madronio MR, Di Somma E, Stavrinou R, et al. Older individuals have increased oro-nasal breathing during sleep. Eur Respir J. 2004;24:71–7. doi: 10.1183/09031936.04.00004303. [DOI] [PubMed] [Google Scholar]
- 17.Gleeson K, Zwillich CW, Braier K, White DP. Breathing route during sleep. Am Rev Respir Dis. 1986;134:115–20. doi: 10.1164/arrd.1986.134.1.115. [DOI] [PubMed] [Google Scholar]
- 18.Marklund M, Stenlund H, Franklin KA. Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring: tolerability and predictors of treatment success. Chest. 2004;125:1270–8. doi: 10.1378/chest.125.4.1270. [DOI] [PubMed] [Google Scholar]