Abstract
Study Objectives:
Central sleep apnea can be refractory to traditional positive airway pressure (PAP) therapy (CPAP or bilevel PAP), whether appearing first as a feature of baseline polysomnography or only later once PAP is applied in what is termed “complex sleep apnea” (CompSA). This retrospective study examined the efficacy of adaptive servoventilation (ASV) in 25 consecutive patients with PAP-refractory central sleep apnea, most exhibiting predominantly obstructive apnea during baseline polysomnography.
Methods:
Patient characteristics were: age = 59.8 ± 16.5 yr; BMI = 30.4 ± 6.1 kg/m2; apnea/hypopnea index (AHI) = 48.5 ± 30.2/h; and central apnea index (CAI) = 10.8 ± 16.0/h. Following unsuccessful PAP titrations, patients underwent ASV titration. Eighteen met established criteria for CompSA.
Results:
On traditional PAP, AHI did not improve significantly compared to baseline, whether based on the entire titration (38.5 ± 23.4/h, p = 0.10) or the final PAP pressure(s) (44.4 ± 25.9/h, p = 0.54); CAI tripled across the titration (27.4 ± 23.5/h, p = 0.001) and at the final pressure(s) (34.8 ± 24.2/h, p < 0.001). On ASV, AHI fell to 11.4 ± 8.2/h across the titration (p < 0.001) and decreased further to 3.6 ± 4.2/h at the optimal end expiratory pressure (p < 0.001). AHI was ≤ 5/h in 80% of patients and < 10/h in 92%. ASV virtually eliminated central apneas at optimal end expiratory pressure (0.7 ± 2.2/h, p < 0.001). Respiratory arousals showed parallel improvements on ASV but not PAP.
Conclusions:
ASV proved superior to traditional PAP in reducing the AHI, CAI, and respiratory arousals in a heterogeneous patient group with sleep disordered breathing in whom central apneas emerged or persisted on PAP.
Citation:
Brown SE; Mosko SS; Davis JA; Pierce RA; Godfrey-Pixton TV. A retrospective case series of adaptive servoventilation for complex sleep apnea. J Clin Sleep Med 2011;7(2):187-195.
Keywords: Adaptive servoventilation, complex sleep apnea, central sleep apnea, obstructive sleep apnea, sleep disordered breathing, continuous positive airway pressure therapy, bilevel therapy
Although continuous positive airway pressure (CPAP) is typically effective in patients with obstructive sleep apnea (OSA), optimal treatment strategies for the various central sleep apnea (CSA) syndromes remain less certain.1 Untreated CSA with Cheyne-Stokes respiration (CSR) is associated with negative prognostic consequences in patients with congestive heart failure (CHF).2 A recent review concluded that CSA, like OSA, is linked to important clinical symptoms and risk of adverse cardiovascular outcomes.1 Consequently, further clarification of effective treatment strategies in the various types of CSA is an important goal for sleep medicine.
In patients with OSA, emergence or persistence of central apneas during traditional positive airway therapy (PAP) with CPAP or bilevel PAP is a phenomenon well known to sleep clinicians and technologists. Recently, investigators have suggested that such challenging patients be grouped into a new syndrome characterized as “complex sleep apnea” (CompSA).3,4
There is considerable debate regarding the significance5,6 and prevalence of CompSA, with estimates ranging from 2.5% to 20% of patients undergoing PAP titrations.4,7–12 Uncertainty also exists concerning the eventual fate of PAP-resistant central apneas with some studies documenting improvement or resolution in many patients, but not all, after a few months of CPAP therapy.8,9,11
BRIEF SUMMARY
Current Knowledge/Study Rationale: Prior studies from a few large academic sleep disorders centers have shown that adaptive servoventilation is frequently effective in treating Complex Sleep Apnea and other forms of central apnea that have failed to respond to traditional PAP therapy. This study evaluates ASV effectiveness in the routine practice of sleep medicine in a community hospital-based sleep disorders center.
Study Impact: The findings of this study demonstrate a high success rate with ASV in the clinical setting, comparable to that previously reported from academic centers. Clinicians should consider ASV therapy in patients with central apnea who have failed to stabilize with traditional PAP.
Sleep clinicians are well aware, however, that central apneas sometimes persist even with documentation of regular CPAP use. Effective treatment might not be achieved even after multiple polysomnograms over time using different PAP modalities and/or titration strategies.3,13 We have observed continued CSA in patients with CSA/CHF or CompSA who have used PAP faithfully for over a year. Such clinical impressions have been validated at least once in a large, well-designed Canadian trial of CPAP in CSA/CHF patients after a mean of 2 years on CPAP when the mean AHI had decreased, on average, by only 50%.14
Given the limitations of PAP in the acute (and possibly long-term) treatment of CompSA, investigators have sought alternative approaches considered previously for CSR/CHF, including entrained O2,15 pharmacologic agents that improve periodic breathing or enhance stable NREM sleep,3 avoidance of drugs known to promote central events,16 positional therapy,17 methods to increase PaCO2,13,18 and adaptive servoventilation (ASV). The use of ASV for CSR/CHF was first reported in 2001,19 and subsequent short-term studies in small patient groups appeared to confirm efficacy.20,21 However, most of the above interventions have not been rigorously evaluated in large, randomized controlled studies, even in CSR/CHF patients.
A recent review of treatments for CompSA highlighted studies showing initial success with ASV.22 This bilevel pressure technique relies on variable inspiratory pressure to control fluctuations in tidal volume and minute ventilation to avert transient episodes of hypocapnia. Once the first commercial ASV devices became available in 2006 and the Centers for Medicare and Medicaid Services (CMS) approved their use for various forms of central apnea, interest accelerated in evaluating ASV for CompSA. One early study demonstrated that ASV was equivalent to bilevel PAP in the spontaneous-timed mode (bilevel S-T) in a group of 21 patients with mixed forms of CSA23; in the 9 patients with CompSA, ASV proved most effective. Several other studies in small groups of patients have also found ASV effective in the acute treatment of CompSA. In the largest case series to date, three-quarters of the 63 patients with CompSA exhibited a drop in apnea/hypopnea index to < 10/h on ASV.24
In contrast, Thomas et al. reported, in a preliminary communication, that only a minority of 54 patients with CompSA were best stabilized with ASV alone.25 Over three-quarters responded better when dead space was added to ASV, bilevel PAP, or CPAP. Success relative to traditional CPAP has also been described with a unique PAP circuit employing a non-vented mask together with either addition of dead space26 or entrainment of CO2 via a prototype gas modulator.13 While these novel strategies are intriguing and look promising, the techniques involved are still under investigation in just a few equipped laboratories, and safety for long-term use at home has not been demonstrated. Consequently, ASV may currently represent the best available alternative to traditional PAP for the acute treatment of CompSA and warrants thorough investigation to clarify its efficacy.
Within the setting of a community hospital-based sleep disorders center, the present study is a retrospective case-series comparison of the efficacy of traditional PAP and ASV in patients undergoing evaluation for sleep disordered breathing who exhibited emergence or persistence of CSA on PAP.
METHODS
Participants
The MemorialCare Sleep Disorders Center has been accredited by the American Academy of Sleep Medicine since 1990 and acquired an ASV device in 2006. This case series reflects the first 25 patients undergoing ASV titration, during a 22-month interval, because of emergence or persistence of CSA during in-laboratory CPAP or bilevel titration for sleep apnea. Recommendations for an ASV trial were made by one of the authors (SEB). Patients were included who exhibited both an AHI ≥ 5/h during a baseline polysomnogram (PSGBL) and PAP-refractory CSA during subsequent CPAP or bilevel titration (CAI ≥ 5/h both across the entire titration and at the final PAP pressure(s) as defined below).
Eighteen patients retrospectively met the criteria for CompSA subsequently established by the CMS which specify that OSA predominates at baseline and that residual events on PAP be primarily central with a CAI ≥ 5/h. Seven patients did not fully qualify as CompSA because of a preponderance of CSA at PSGBL (n = 5) or late-night emergence of mixed apneas during PAP titration (n = 2).
The data extracted from patient records included descriptive demographics, polysomnogram derivatives, common coexisting conditions, and use of opioids or hypnotics. The study protocol was approved by the Memorial Health Services Institutional Review Board.
Polysomnogram Techniques
Polysomnography was performed using Sandman version 7.2 (Covidien, Inc., Ottawa, Ontario), in accordance with the standard protocol of the MemorialCare Sleep Disorders Center. During PSGBL, airflow was measured using a nasal air pressure transducer and an oral thermocouple sensor (Pro-Tech Services, Inc., Mukilteo, WA). When CPAP or bilevel PAP was added, the flow signal from the pneumotachometer replaced the nasal pressure transducer. Piezoelectric bands (SleepSense) recorded thoraco-abdominal movements (Scientific Laboratory Products, St. Charles, IL). Apneas were defined as cessation of airflow for ≥ 10 sec. Central and obstructive apneas were distinguished by whether respiratory effort was evident, and mixed apneas were identified when central apnea preceded obstruction within an event. Hypopneas were recognized by a discernable decline in airflow for ≥ 10 sec with ≥ 3% desaturation using oximetry (Oximax N-600x, Nelcor, Boulder, CO). A respiratory arousal index (RAI) was computed from arousals associated with apneas/hypopneas or respiratory effort-related arousals (defined as a sequence of breaths characterized by increasing respiratory effort or snoring leading to an arousal that failed to meet criteria for apnea/hypopnea). Sleep staging27 and arousals28 were scored according to standard methods.
The initial studies were carried out according to a standard “split-night” protocol. After ≥ 2 h of recorded sleep, CPAP (VPAP III, ResMed Ltd., Bella Vista NSW, Australia) was initiated if the estimated AHI was ≥ 15 events/h. When a CMS-qualifying comorbidity was present (excessive daytime sleepiness, insomnia, hypertension, coronary artery disease, history of stroke, mood disorder, impaired cognition), CPAP was considered if the estimated AHI exceeded 10/hr. CPAP was initiated at 5 cm H2O and raised in increments of 1-2 cm H2O at intervals no shorter than 10-15 min with the goal of eliminating apneas, hypopneas and snoring. Patients who did not meet split-night criteria completed both a full-night PSGBL and PAP titration. When time permitted, bilevel titration in the spontaneous (S) mode was also attempted following the failed CPAP trial (n = 8), starting at an expiratory positive airway pressure not exceeding the last applied CPAP pressure. Three individuals intolerant of CPAP went straight to a bilevel S trial, and another was tested also in the S-T mode. The highest PAP pressures applied averaged 8.9 cm H2O for the 16 tested only on CPAP (range 5-14 cm H2O), and for the 11 tried also/or on bilevel PAP the highest applied inspiratory pressure averaged 11.4 cm H2O (range 7–18 cm H2O). Supplemental oxygen was added in one case during PSGBL and in another during the ASV titration.
By virtue of the inclusion criterion, all patients exhibited continued CSA on otherwise best therapeutic PAP pressure. Two approaches were taken to quantify this for later comparison to ASV. First, PSG variables were calculated across the total titration sample (PAPTot). Calculating a reliable AHI and CAI at a “best” or “final” PAP pressure, however, proved problematic because of the persistent central apneas and because some sleep samples at the final PAP were very brief when morning awakening closely followed the last pressure increase. To obtain a good-faith representation of the final AHI and CAI on PAP, the following approach was devised: The indices were derived from the segment at the final PAP pressure if it exceeded 30 min of sleep; otherwise, the sleep samples across the final 2-4 pressures were combined, as needed, to obtain a sleep sample > 30 min (PAPFinal).
ASV titrations took place generally within one month of the PAP studies, using the original ResMed VPAP Adapt SV device at the default settings: end expiratory pressure (EEP) of 5 cm H2O with pressure support minimum of +3 cm H2O and maximum +10 cm H2O. EEP was increased gradually to eliminate apneas, hypopneas and snoring. If snoring or respiratory events persisted at the device's maximum EEP of 10 cm H2O, the minimal pressure support was increased to +4 or +5 cm H2O. All PSG variables were calculated both across the total titration (ASVTot) and at the final ASV pressure (ASVFinal) which always reflected just the last pressure of the night because an optimal setting was consistently achieved well before morning awakening.
To insure that oral or mask interface air leaks did not interfere with either PAP or ASV titrations, mask fitting was performed before bedtime and technicians viewed the leak rate continuously during acquisition on the Sandman display, intervening as necessary to maintain a leak ≤ 10 L/min by either adding a chin strap and/or adjusting or changing masks. Seven patients required a mask change during the PAP titrations, and a chin strap was added in 7 cases, 5 of which were eventually converted to a full face mask because of continued oral leaks. At PAPFinal, 10 patients were using full face masks, five were using chamber style nasal masks, and 10 were using nasal pillows styles. Because of the manufacturer's recommendation that ASV be implemented preferentially with a full face mask (and that nasal pillows styles be avoided), ASV titrations were performed with full face masks in all but 6 cases where a chamber style nasal mask provided the better fit (with chin strap in 3 instances).
Statistics
All PSG-derived variables are listed in Tables 2 and 3. The 3 main variables of interest were AHI, CAI, and RAI—all others were of secondary interest. The Shapiro-Wilk Test was utilized to identify which variables approximated a normal distribution, allowing use of parametric statistical tests. Only 3 met this condition: total arousal index (Table 2) and sleep efficiency and % stage 2 sleep (Table 3). Nonparametric tests were employed for all other variables.
Table 2.
Summary of respiratory variables
| PSGBL | PAPTot | PAPFinal | ASVTot | ASVFinal | |
|---|---|---|---|---|---|
| Total sleep time (min) | 233 ± 121 | 262 ± 100 | 89 ± 72 | 301 ± 85 | 124 ± 92 |
| Apnea/hypopnea index (/h) | 48.5 ± 30.2 (48) | 38.5 ± 23.4 (40) | 44.4 ± 25.9 (45) | 11.4 ± 8.2*** (8) | 3.6 ± 4.2***,b (2) |
| Central apnea index (/h) | 10.8 ± 16.0 (5) | 27.4 ± 23.5** (21) | 34.8 ± 24.2*** (33) | 1.2 ± 2.9** (0) | 0.7 ± 2.2*** (0) |
| Obstructive apnea index (/h) | 20.2 ± 20.5 (16) | 2.6 ± 3.1*** (1) | 2.3 ± 2.9*** (1) | 0.4 ± 0.9*** (0) | 0.0 ± 0.0*** (0) |
| Mixed apnea index (/h) | 8.1 ± 11.0 (2) | 3.0 ± 5.6 (0) | 4.2 ± 8.9 (0) | 0.5 ± 1.4* (0) | 0.1 ± 0.4*** (0) |
| Hypopnea index (/h) | 9.7 ± 7.6 (8) | 5.3 ± 4.1* (5) | 3.3 ± 3.5* (2) | 9.4 ± 7.3 (7) | 2.8 ± 3.2**,b (2) |
| Respiratory arousal index (/h) | 46.4 ± 30.8 (41) | 41.6 ± 28.6 (37) | 51.5 ± 31.7 (49) | 15.2 ± 9.1*** (16) | 5.4 ± 6.0***,b (3.5) |
| Total arousal index (/h) | 56.6 ± 32.6 (50) | 49.5 ± 28.1 (53.5) | 60.7 ± 31.6 (59) | 23.6 ± 8.7*** (25.5) | 15.0 ± 9.6***,a (14) |
| SpO2 nadir (%) | 77.3 ± 11.9 (80) | 83.1 ± 8.4 (83.5) | 86.1 ± 6.5** (87) | 86.0 ± 5.4*** (87) | 89.9 ± 3.7***,b (89) |
| SpO2 < 90% (%) | 12 ± 16 (4) | 8 ± 20* (0.5) | 7 ± 18* (1) | 3 ± 6 ** (1) | 2 ± 4 *** (0) |
Data are presented as mean ± SD with median values given in parentheses. N = 25 except for Respiratory Arousal Index and Total Arousal Index (n = 21), SpO2 Nadir (n = 24) and SpO2 < 90% (n = 22). Comparison to PSGBL: *p < 0.01, **p = 0.001, ***p < 0.001. Comparison to ASVTot: ap = 0.001, bp < 0.001.
Table 3.
Sleep architecture at PSGBL
| Sleep efficiency (%) | 74 ± 18 |
| Stage 1 (%) | 31 ± 24 |
| Stage 2 (%) | 54 ± 22 |
| Stage 3 (%) | 4 ± 6 |
| Stage REM (%) | 12 ± 10 |
| PLMs arousal index (/h) | 3.4 ± 9.5 |
Data are presented as mean ± SD. n = 25. No comparisons of PAPTot or PAPFinal to PSGBL or of ASVFinal to ASVTot reached significance (p > 0.01); Compared to PSGBL, %Stage 1 was reduced for both ASVTot and ASVFinal (p < 0.01 for both).
PSGBL observations were derived from 13 full-night and 12 split-night studies. Because comparisons of the means of these subgroups for AHI, CAI, and RAI all failed to reveal significant differences (Mann-Whitney U test, p > 0.05), the subgroups were merged for subsequent analyses. Similar comparisons between the 9 patients who were using chronic opioid therapy and the 16 who were not (and also between the 10 taking hypnotics during one or more recordings and 15 who were not) uncovered no significant differences, so they too were merged into the single group of 25 subjects.
To evaluate the relative efficacy of ASV to PAP, first the PSGBL segment was compared to both the PAP titration (PAPTot and PAPFinal) and the ASV titration (ASVTot and ASVFinal) using pair-wise comparisons for each dependent variable. Only ASV was associated with significant improvements in the main outcome variables. The relative efficacy of ASVFinal to ASVTot was determined by an additional comparison. Paired t-tests were used for variables found to be normally distributed; otherwise, the Wilcoxon Matched-Pairs Signed Ranks test was employed. The α level for significance was adjusted to 0.01 to reflect the Bonferroni correction for these 5 comparisons. The n for most pair-wise comparisons was 25. However, for 4 variables (respiratory arousal index, total arousal index, SpO2 nadir and SpO2 < 90%) reliable values were missing for at least one sleep study condition in one to 4 patients because of technical issues encountered during data collection. In these cases, the patients were entirely eliminated from analyses involving those variables. The adjusted n's are in the footnote to Table 2.
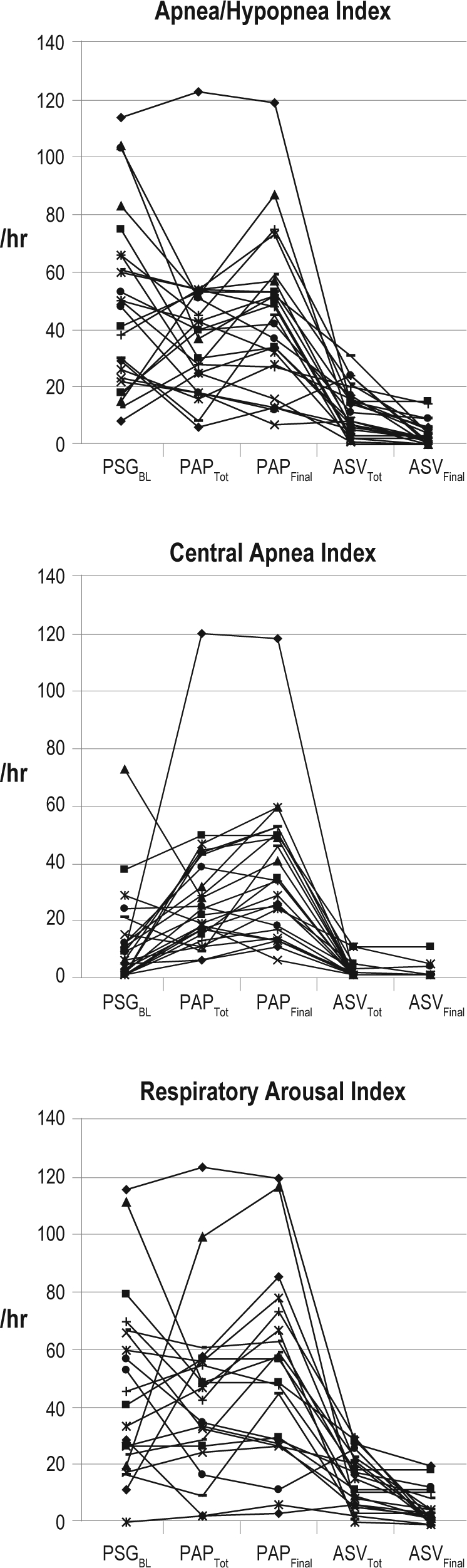
Note that one visual outlier patient for the AHI and CAI at PAPTot and PAPFinal (see Figure 1) was verified statistically (the outlier exceeded the mean of the other 24 patient scores by > 3 SD), so the above paired comparisons were all repeated eliminating this patient's scores. The significance findings were unchanged, indicating that the results were not unduly affected by the outlier. Consequently, we chose not to exclude this patient from the reported findings because he was the most dramatic example of CompSA—i.e., severe OSA at PSGBL with AHI 114/h and CAI of only 8/h, converting to severe CSA on PAP with CAI 120/h.
Figure 1.
Plots of individual patient data for main outcome variables
n = 25 for apnea/hypopnea index and central apnea index; n = 21 for respiratory arousal index. PSG, polysomnogram; BL, baseline; PAP, positive airway pressure (CPAP or bilevel); ASV, adaptive servoventilation; Tot, total.
RESULTS
Demographics and Clinical Findings
Demographic and medical characteristics of the study subjects are summarized in Table 1. Two-thirds of the patients were hypertensive, and about one-third were using opioids. Atrial fibrillation, stable coronary disease, diabetes mellitus, and stroke were also common. Five patients noted a history of CHF, but only 2 listed this condition as a current medical problem. Just one patient exhibited any Cheyne-Stokes respiration (short-lived and non-arousing) at PSGBL. Of the patients reporting current use of hypnotics, only three took a sleep aid during either the PAP or ASV trial, but not both.
Table 1.
Demographic and clinical findings
| Characteristic | Mean or # | ± SD or % |
|---|---|---|
| Age (yr) | 59.8 | ± 16.5 |
| Epworth Sleepiness Scale | 11.7 | ± 5.9 |
| Body mass index (kg/m2) | 30.4 | ± 6.1 |
| Hypertension | 17 | 68% |
| Hypnotics | 10 | 40% |
| Opioid use | 9 | 36% |
| Atrial fibrillation | 5 | 20% |
| Coronary artery disease | 7 | 28% |
| Diabetes mellitus | 6 | 24% |
| Stroke | 4 | 16% |
| Congestive heart failure | 2 | 8% |
| Muscular dystrophy | 1 | 4% |
| Cheyne-Stokes respiration | 1 | 4% |
For the first 3 variables, group means and standard deviations are given; for remaining variables, the number of cases and corresponding percentage of the sample are listed. n = 25.
Baseline PSG Findings
The sleep disordered breathing events observed at PSGBL are summarized in Table 2, and individual patient data are plotted in Figure 1 for AHI, CAI, and RAI. The AHI averaged 48.5 ± 30.2/hr (mean ± SD), ranging from 8/h to 114/h among subjects. Seventeen fell into the severe range (AHI ≥ 30/h), 7 in the moderate range (AHI ≥ 15 < 30/h), and just one in the mild range (AHI < 15/h). Central apneas predominated in only 5 cases (7 had none), with a majority of obstructive and mixed events in the other 20. The mean CAI was 10.8 ± 16.0/h. Sleep was typically highly fragmented, with RAI averaging 46.4 ± 30.8/h. Respiratory events also produced considerable arterial oxygen desaturations.
Other features of sleep architecture are summarized in Table 3. Percent stage 1 sleep was elevated, while stages 3 and REM and sleep efficiency all suffered. Sleep fragmentation associated with periodic limb movements was minimal.
PSG Findings with Traditional PAP
Table 2 and Figure 1 also present the derived respiratory variables for PAPTot and PAPFinal. Compared to PSGBL, the modest decrease in overall AHI for PAPTot (to 38.5 ± 23.4/h) was not significant, although both obstructive apneas and hypopneas were significantly reduced, as expected. The AHI remained above 15/h in 92% of patients, in large part because of central apneas which more than doubled in frequency compared to PSGBL. The CAI exceeded 15/h in 78% of cases. A modest improvement in oxygenation (SpO2 < 90%) was observed. Measures of sleep architecture did not change significantly.
At PAPFinal, the CAI, AHI, and RAI all remained elevated, despite good control of obstructive apneas and hypopneas: 34.8 ± 24.2/h, 44.4 ± 25.9/h, and 51.5 ± 31.7/h, respectively. The CAI remained > 15/h in 76% of individuals, and in 52% it exceeded 30/h. This emergence or persistence of CSA on PAP prompted the subsequent ASV trial.
PSG Findings with ASV
ASV was overall very successful in reducing both the AHI and CAI (Table 2 and Figure 1). Compared to PSGBL, the AHI for ASVTot was significantly reduced to 11.4 ± 8.2/h, and central apneas were all but eliminated (CAI = 1.2 ± 2.9/h). At ASVFinal, the AHI improved further to 3.6 ± 4.2/h. The highest residual AHI at ASVFinal was 15/h, with 80% of patients normalizing to an AHI ≤ 5/h; a CAI of 0/h was achieved for all but 3 patients. All types of breathing events were further improved at ASVFinal compared to ASVTot, although only the changes in AHI and hypopnea index reached statistical significance, perhaps reflecting a floor effect since breathing generally normalized fairly early on during ASV trials.
The RAI was also significantly reduced at ASVTot compared to PSGBL (17.5 ± 12.1/h) and even more so at ASVFinal (5.7 ± 6.1/h). The SpO2 nadir and sleep time with SpO2 < 90% improved significantly on ASV as well. A significant change from PSGBL in sleep architecture was seen only for %Stage 1 sleep which dropped to 18% ± 12% at ASVTot and 15% ± 17% at ASVFinal. The mean final EEP was 7.8 ± 1.6 cm H2O. Only 3 patients were stabilized at the default EEP of 5 cm H2O, and 5 required the maximal EEP of 10 cm H2O.
DISCUSSION
In our experience, ASV proved highly effective in eliminating central apneas as well as obstructive and mixed events in patients with PAP-refractory CSA, most of whom qualified as CompSA. From a baseline AHI of 48.5 ± 30.2/h, the AHI normalized to 3.6 ± 4.2/h with ASV. A successful ASV titration (AHI ≤ 10/h at ASVFinal) was achieved in 92% of patients, and 80% were stabilized using more stringent criteria for success (AHI ≤ 5/h). Central apneas were all but eliminated: The CAI was reduced to < 5/h in all but one patient (96%), with a group median and mode of 0/h. Although obstructive apneas and hypopneas both improved significantly on PAP, the CAI roughly tripled from baseline to PAPFinal (10.8 ± 16.0/hr to 34.8 ± 24.2/hr), undermining the benefit and leaving patients with appreciable sleep disordered breathing (AHI = 44.4 ± 25.9/h). ASV also markedly improved sleep fragmentation: the respiratory arousal and total arousal indices decreased by 88% and 72%, respectively. In contrast, sleep fragmentation remained severe on PAP. Furthermore, measures of oxygenation (SpO2 nadir and SpO2 < 90%) improved more with ASV than PAP. While previous studies have documented the efficacy of ASV in academic, research-oriented sleep disorders centers, our findings demonstrate that the superior response to ASV is sufficiently robust as to be evident even in the routine clinical practice of sleep medicine in a diverse patient population.
The few previous studies comparing ASV to traditional PAP have reported strikingly similar degrees of ASV success in patients meeting criteria for CompSA essentially identical to ours. In a prospective, randomized crossover study of 21 CPAP-refractory patients, the mean AHI of 41.9/h on CPAP decreased to 1.6/h on ASV in the subgroup of nine with CompSA.23 Bilevel S-T was also effective in this patient group, but ASV was even more so. In the largest retrospective case-series study to date, ASV lowered the mean AHI to 4/h compared to 30/h on CPAP in 63 CompSA patients.24 Success (AHI < 10/h) was achieved in 78% of cases, and ASV also proved superior to CPAP + O2, bilevel S, and bilevel S-T in that study. In these two studies, as in ours, there was a far greater reduction in sleep fragmentation from respiratory arousals with ASV than with traditional PAP.
In contrast to these impressive responses to ASV in CompSA, R.J. Thomas and colleagues, in a preliminary report,25 claimed that only 15% of 54 patients “had a complete response” to ASV, whereas over half responded best to ASV when embellished with added dead space, and a third responded best to just CPAP or bilevel PAP with added dead space. The notion that added dead space can improve the response to ASV or even traditional PAP in CompSA is an intriguing idea that has recently been studied in a larger patient group26 and merits continued investigation.
Our study was organized around the common feature of CSA emerging or persisting on PAP (CAI ≥ 5/h). We applied strict CMS criteria to delineate which patients qualified as CompSA. Seven patients did not fully qualify, either because CSA predominated in the diagnostic segment or because of late-night emergence of predominantly mixed apneas on PAP. Given that only one of the two patients who failed to stabilize to an AHI < 10/h did not qualify as CompSA, there was no indication that ASV performed grossly differently among the variants of PAP-refractory CSA represented in our patient sample.
Since CompSA was first described,3,4 discussion has revolved largely around three questions: (1) Is CompSA a distinct clinical entity; (2) how common is it; and (3) what is the best approach to treatment? Although the present study was conceived with just the latter question in mind, it does add pertinent observations to the still sparse literature in which these issues take center stage.
Since all our subjects encountered PAP before ASV, we cannot entirely exclude the possibility that spontaneous sleep-wake transitions or the disruptive effect on sleep of a first encounter with a PAP device explains the acute appearance of CSA on PAP, as some have suggested.8,11 We posit the likelihood that study order played a major role in our findings is small for several reasons. First, the ASV algorithm differs from PAP by attempting to compensate for hypocapnic CSA (the ventilation target is 90% of the trailing 3-min ventilation). The fact that ASV was so much more efficient in eliminating CSA is at least consistent with the view that CO2 is regulated differently in CompSA, and that it is this difference, not order of presentation, which explains the inferior performance of traditional PAP. More direct evidence comes from the Morgenthaler et al. study,23 wherein CompSA patients responded better to ASV than bilevel S-T, even when they received ASV first. Perhaps the strongest evidence has been provided by R.J. Thomas and colleagues who assert that CompSA can be identified before encountering PAP based on a particular pattern of cardiorespiratory coupling revealed by technology currently not employed in most laboratories.3,29 However, recent evidence that chemoreflex sensitivity is increased even in OSA patients not known to have CompSA30 could mean that CompSA reflects the extreme of a continuum of increased ventilatory instability and tendency toward central apnea characteristic of OSA in general.
The retrospective case series of 18 subjects with CompSA reported on here represents 1.4% of the 1,298 patients who underwent a PAP titration for OSA in our sleep disorders center over the time frame of the study. This figure might underestimate the true prevalence of CompSA as there were perhaps other patients who would have demonstrated central apneas had they selected a PAP trial rather than alternative therapy (positional, surgical, or oral appliance) or no treatment. Our prevalence is below the 6.5% to 20% range reported by other investigators.4,7–11 Strict application of CMS criteria in identifying CompSA might have reduced our prevalence estimate relative to others. However, our estimate is close to the 2.5% figure in a recent preliminary report12 by the researchers who, in 2001, published the first description of ASV. Certainly, the prevalence of CompSA in a given patient population would vary depending on the specific diagnostic criteria applied, frequency of CHF and chronic use of opioids, and degree of attention paid to technical issues such as mask leaks and under/over-PAP titration. What emerges clearly from available research is that CompSA presents more than a rare challenge to sleep clinicians who struggle to provide optimal therapy for this “difficult to treat” group.
Given the short interval since CompSA was first described, it is not surprising that the best treatment approach(es) remain subject to investigation and debate. As summarized in Gilmartin et al.,3 earlier approaches to treating CompSA derived largely from experience with CSA/CSR related to CHF or chronic opioid use and included: CPAP or bilevel S or S-T; supplemental O2 alone or in combination with PAP; administration of drugs known to promote stable NREM sleep or act as respiratory stimulants; and avoidance of substances known to precipitate central apnea. None of these modalities has proven consistently effective, and efficacy data for some are virtually nonexistent. The more recent approaches have utilized sophisticated technologies, including ASV algorithms which modulate minute ventilation19 or peak flow,31 added dead space or CO2 entrainment to stabilize CO2 homeostasis,13,18,26 a novel device that combines features of ASV and auto CPAP,32 and simultaneous application of more than one of these treatments. Little is known yet about the relative efficacy of these newer approaches to CompSA. And, while R. J. Thomas and colleagues have provided convincing evidence of improved acute treatment efficacy when CPAP, bilevel PAP, or ASV therapy is used in combination with techniques to minimize hypocapnia and/or enhance oxygenation,3,13,26 the skills and technology required are not available in most sleep centers, and safety for home use has yet to be established. As such, basic ASV emerges perhaps as the most reliable and practical acute approach to treating CompSA in routine clinical practice settings.
However, recent studies suggest that CSA resolves spontaneously in a majority (74% to 89%) of CompSA patients over a 2–3 month period of regular CPAP use.8,9,11 Nevertheless, this leaves between 11% and 25% with persistent sleep disordered breathing. In another study, nearly 50% of CompSA patients still had an AHI > 10/h after six months on CPAP.33 While a majority of patients may do well given enough time on CPAP, the non-responding group is by no means inconsequential.
Presently, it appears that CPAP, bilevel PAP, and ASV remain the mainstream approaches to treating CompSA in clinical settings. Because there are no large, randomized controlled trials as yet comparing these devices, practitioners are left to struggle, case by case, to best match the device to the underlying physiological abnormalities. Each initial treatment choice represents different financial, time and emotional costs to patients. ASV appears to offer greater assurance of rapidly effective treatment, but the initial financial investment is greater because of the additional ASV titration study and the higher cost of the device. Proceeding instead with a home CPAP trial would offer lower costs to patients in whom central events resolve after a period of therapy, although additional costs would be incurred by individuals not stabilized on CPAP who would then need an additional titration study and, likely, conversion to a more expensive PAP device. A bilevel S device is costlier than CPAP but less expensive than bilevel S-T. Not infrequently, however, bilevel S worsens CSA.34 Bilevel S-T is probably the most serious challenger to ASV in terms of acute efficacy but would likely offer minimal if any cost savings because bilevel S-T and ASV share the same billing code. While some have argued against use of expensive “new generation” devices until better proof of clear advantage over traditional PAP is demonstrated,5 the approval of ASV for CompSA by as conservative a source as CMS suggests that these devices should at least be considered when treatment with less expensive devices has failed. Moreover, delay in reaching effective therapy on other PAP devices could be stressful for patients who might perceive that their time, effort, and money are not being well spent, particularly if they remain symptomatic. In some instances, patient dissatisfaction with suboptimal treatment might result in premature discontinuation of initial PAP therapy and unwillingness to proceed to more effective modalities. To expedite identification of the most effective therapy, Kuzniar et al. have described successful implementation of “multi-modality” CPAP-to-bilevel PAP-to-ASV titrations in a single night.35
It is also reasonable to be concerned about possible health risks of the persistent CSA documented in some fraction of CompSA patients on long-term PAP. A large multicenter study of CPAP therapy in patients with CHF and CSA, for example, failed to show any improvement in heart transplant-free survival when CSA remains unresolved.14 As a group, these patients were not optimally stabilized and had a mean on-treatment AHI of 19/h. Conversely, a post hoc analysis, which evaluated only the patients with AHI < 15/h, suggested that both the left ventricular ejection fraction and transplant-free survival might be improved if CSA is suppressed soon after CPAP initiation.36 Whether these observations are relevant to unresolved CSA in CompSA remains an open question. However, one recent study documented greater sleepiness and poorer quality of life in patients with OSA whose AHI remained > 10/h after three months on CPAP in part because of residual central apnea or periodic breathing.37
This study was subject to a number of limitations stemming largely from the retrospective, observational design. Concerns about the non-randomized order of treatments and somewhat heterogeneous study sample with respect to type of sleep disordered breathing have been addressed above. There was also lack of uniformity in medications because patients continued drug regimens as ordered by their physicians. Nine of our patients were taking chronic opioids, and CompSA has previously been associated with opioid use.38 Furthermore, doubt about the applicability of ASV to patients using opioids has been raised by Farney et al., who found ASV ineffective in a retrospective study of 22 patients with chronic opioid-induced sleep disordered breathing.16 However, the patients in our study taking opioids did not differ from those not on opioids in any of the main outcome variables at baseline, and they also responded equally well to ASV: At ASVFinal, AHI = 3.2/h versus 3.7/h, and CAI = 0.3/h versus 0.9/h, respectively. Every opioid user attained an AHI < 10/h, and in all but one CSA was completely eliminated. Farney et al. have acknowledged that in most of their patients the ASV device had not been titrated from the default EEP setting of 5 cm H2O,16 raising the possibility that lack of efficacy in their study reflected inadequate pressure support. Our finding of equal success with ASV in opioid users is more consistent with the results of Javaheri et al.,39 who reported a decrease in AHI from 70/h at baseline to 13/h with ASV in five patients with CompSA using opioids, and suggests that opioid use in a subset of our patients did not bias our assessment of ASV efficacy. Similarly, we do not suspect that hypnotic use, which can impact sleep disordered breathing, significantly altered our comparison of PAP to ASV, because in only three instances did a patient use a hypnotic for one but not the other titration condition.
An additional limitation stemmed from the fact that the methods employed for measuring respiration (piezoelectric bands) and scoring respiratory events preceded newer standards established after data acquisition was complete.28 We cannot be certain that differentiation of central and obstructive breathing events was occasionally impacted by such technical concerns inherent to non-invasive measures of respiration. Furthermore, despite the easily demonstrated superiority of ASV over PAP in improving respiratory and arousal variables, the fact that data were derived from split-night studies in some instances and full-night studies in others might have contributed to our inability to demonstrate parallel improvements in sleep stage architecture. Moreover, our study neither addresses the relative efficacy of CPAP versus bilevel S nor of bilevel S-T versus ASV, and it provides no clues to how often CompSA might have resolved over time on traditional PAP.
Factors inherent to our retrospective study design also limited the number of patients we were able to follow for success on ASV over time, e.g., referring physician preferences, insurance contracting limitations, and patient migration out of the area or to a different insurance plan. However, objective long-term ASV utilization data obtained from the device downloads indicated a high degree of overall compliance. Mean patient use for the 14 patients who were followed for at least 18 months was 90% ± 12% of the nights evaluated with average nightly use of 5.7 ± 1.4 hours. Device downloads for the subset of 11 patients we were able to follow for 2 to 3.5 years revealed that they had maintained ASV use on 89% ± 13% of recorded nights, with an average nightly use of 5.6 ± 1.3 hours. Prospectively designed future studies should be able to compare how patients with CompSA fare long-term on ASV compared to CPAP or bilevel in terms of both compliance and health-related outcomes.
Lastly, we did not have complete physiologic data regarding left ventricular function in most of our patients, which makes it impossible to exclude occasional minor left ventricular dysfunction or subtle evidence of CHF. However, only two patients reported CHF as an active problem, and only one exhibited even minor episodes of Cheyne-Stokes respiration during PSGBL. Therefore, we do not believe that our results were altered significantly by undetected CHF.
In summary, in a community hospital-based sleep disorders center, ASV proved significantly more effective than traditional PAP in acutely normalizing respiratory pattern and arousals in patients with problematic CSA that emerged or persisted on PAP, most of whom met criteria for CompSA. Although the prevalence of CompSA in clinical settings remains somewhat hazy, the need is clear for prospective, randomized studies to establish the most efficacious form(s) of treatment. Reliable methods to match patients to the treatment modality that maximizes outcome, while minimizing costs as measured in time, dollars and emotional distress, would also be welcomed by both patients and physicians.
DISCLOSURE STATEMENT
This was not an industry supported study. An adaptive servoventilation device was provided by the manufacturer. No off-label or investigational use of drugs or medical devices occurred in this study. Dr. Brown has participated in speaking engagements for ResMed. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Performance Site: Long Beach Memorial Medical Center. The authors thank Michelle Cole, RPSGT, and Daniel Brennan, RPSGT, for technical assistance and Kathy Vinton, R.N., COHN-S, and Pamela Chevreaux, M.A., for their administrative support.
ABBREVIATIONS
- PAP
positive airway pressure
- CPAP
continuous positive airway pressure
- Bilevel PAP
bilevel positive airway pressure
- CompSA
complex sleep apnea
- ASV
adaptive servoventilation
- AHI
apnea/hypopnea index
- CAI
central apnea index
- OSA
obstructive sleep apnea
- CSR
Cheyne-Stokes respiration
- CHF
congestive heart failure
- CMS
Centers for Medicare and Medicaid Services
- O2
oxygen
- CO2
carbon dioxide
- PaCO2
partial pressure of arterial carbon dioxide
- RAI
respiratory arousal index
- Bilevel S-T
bilevel PAP in the spontaneous-timed mode
- Bilevel S
bilevel PAP in the spontaneous mode
- EEP
end expiratory pressure
- cm H2O
centimeters of water pressure
- PSGBL
baseline polysomnogram
- PAPTot
total PAP titration
- PAPFinal
final PAP pressure
- ASVTot
total ASV titration
- ASVFinal
final ASV pressure(s)
- SpO2
oxygen saturation measured by pulse oximetry
- SpO2 < 90%
percent time with oxygen saturation below 90%
REFERENCES
- 1.Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea-pathophysiology and treatment. Chest. 2007;131:595–607. doi: 10.1378/chest.06.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanfranchi PA, Braghiroli A, Bosimini E, et al. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation. 1999;99:1435–40. doi: 10.1161/01.cir.99.11.1435. [DOI] [PubMed] [Google Scholar]
- 3.Gilmartin GS, Daly RW, Thomas RJ. Recognition and management of complex sleep-disordered breathing. Curr Opin Pulm Med. 2005;11:485–93. doi: 10.1097/01.mcp.0000183061.98665.b0. [DOI] [PubMed] [Google Scholar]
- 4.Morgenthaler TI, Kagramanov V, Hanak V, Decker PA. Complex sleep apnea syndrome: is it a unique clinical syndrome? Sleep. 2006;29:1203–9. doi: 10.1093/sleep/29.9.1203. [DOI] [PubMed] [Google Scholar]
- 5.Malhotra A, Bertisch S, Wellman A. Complex sleep apnea: it isn't really a disease. J Clin Sleep Med. 2008;4:406–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Gay PC. Complex sleep apnea: it really is a disease. J Clin Sleep Med. 2008;4:403–5. [PMC free article] [PubMed] [Google Scholar]
- 7.Lehman S, Antic NA, Thompson C, Catcheside PG, Mercer J, McEvoy RD. Central sleep apnea on commencement of continuous positive airway pressure in patients with a primary diagnosis of obstructive sleep apnea-hypopnea. J Clin Sleep Med. 2007;3:462–6. [PMC free article] [PubMed] [Google Scholar]
- 8.Cassel W, Leistner S, Becker HF, et al. A prospective polysomnographic study on the natural course of complex sleep apnea in 675 patients treated for obstructive sleep apnea. Sleep. 2009;32(Abstr Suppl):A185. [Google Scholar]
- 9.Javaheri S, Smith J, Chung E. The prevalence and natural history of complex sleep apnea. J Clin Sleep Med. 2009;5:205–11. [PMC free article] [PubMed] [Google Scholar]
- 10.Pusalavidyasagar SS, Olson EJ, Gay PC, Morgenthaler TI. Treatment of complex sleep apnea syndrome: a retrospective comparative review. Sleep Med. 2006;7:474–9. doi: 10.1016/j.sleep.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Dernaika T, Tawk M, Nazir S, Younis W, Kinasewitz GT. The significance and outcome of continuous positive airway pressure-related central sleep apnea during split-night studies. Chest. 2007;132:81–7. doi: 10.1378/chest.06-2562. [DOI] [PubMed] [Google Scholar]
- 12.Toepfer V, Weinreich G, Teschler H. Prevalence and treatment of complex sleep apnea syndrome. Am J Respir Crit Care Med. 2008;(Abstr Suppl):A938. [Google Scholar]
- 13.Thomas RJ, Daly RW, Weiss JW. Low-concentration carbon dioxide is an effective adjunct to positive airway pressure in the treatment of refractory mixed central and obstructive sleep-disordered breathing. Sleep. 2005;28:69–77. doi: 10.1093/sleep/28.1.69. [DOI] [PubMed] [Google Scholar]
- 14.Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–33. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 15.Thalhofer SA, Dorow P, Meissner P. Influence of low-flow oxygen supply on sleep architecture in patients with severe heart failure (NYHA III-IV) and Cheyne-Stokes respiration. Sleep Breath. 2000;4:113–20. doi: 10.1007/s11325-000-0113-y. [DOI] [PubMed] [Google Scholar]
- 16.Farney RJ, Walker JM, Boyle KM, Cloward TV, Shilling KC. Adaptive servoventilation (ASV) in patients with sleep disordered breathing associated with chronic opioid medications for non-malignant pain. J Clin Sleep Med. 2008;4:311–19. [PMC free article] [PubMed] [Google Scholar]
- 17.Szollosi I, Roebuck T, Thompson B, Naughton MT. Lateral sleeping position reduces severity of central sleep apnea/Cheyne-Stokes respiration. Sleep. 2006;29:1045–51. doi: 10.1093/sleep/29.8.1045. [DOI] [PubMed] [Google Scholar]
- 18.Szollosi I, O'Driscoll DM, Dayer MJ, Coats AJ, Morrell MJ, Simonds AK. Adaptive servo-ventilation and deadspace: effects on central sleep apnoea. J Sleep Res. 2006;15:199–205. doi: 10.1111/j.1365-2869.2006.00515.x. [DOI] [PubMed] [Google Scholar]
- 19.Teschler H, Döhring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164:614–19. doi: 10.1164/ajrccm.164.4.9908114. [DOI] [PubMed] [Google Scholar]
- 20.Pepperell JC, Maskell NA, Jones DR, et al. A randomized controlled trial of adaptive ventilation for Cheyne-Stokes breathing in heart failure. Am J Resp Crit Care Med. 2003;168:1109–14. doi: 10.1164/rccm.200212-1476OC. [DOI] [PubMed] [Google Scholar]
- 21.Philippe C, Stoïca-Herman M, Drouot X, et al. Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne-Stokes respiration in heart failure over a six month period. Heart. 2006;92:337–42. doi: 10.1136/hrt.2005.060038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuźniar TJ, Morgenthaler TI. Treatment of complex sleep apnea syndrome. Curr Treat Options Neurol. 2008;10:336–41. doi: 10.1007/s11940-008-0036-7. [DOI] [PubMed] [Google Scholar]
- 23.Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep. 2007;30:468–75. doi: 10.1093/sleep/30.4.468. [DOI] [PubMed] [Google Scholar]
- 24.Allam JS, Olson EJ, Gay PC, Morgenthaler TI. Efficacy of adaptive servoventilation in treatment of complex and central sleep apnea syndromes. Chest. 2007;132:1839–46. doi: 10.1378/chest.07-1715. [DOI] [PubMed] [Google Scholar]
- 25.Rawlins S, Vigneault K, McGeehan B, Gilmartin G, Thomas R. Adaptive servo ventilation and enhanced expiratory rebreathing space for complex sleep apnea treatment. Sleep. 2007;30(Abstr Suppl):A188. [Google Scholar]
- 26.Gilmartin G, McGeehan B, Vigneault K, et al. Treatment of positive airway pressure treatment-associated respiratory instability with enhanced expiratory rebreathing space (EERS) J Clin Sleep Med. 2010;6:529–38. [PMC free article] [PubMed] [Google Scholar]
- 27.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: Brain Information Service/Brain Research Institute UCLA; 1968. [Google Scholar]
- 28.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 29.Thomas RJ, Mietus JE, Peng C-K, et al. Differentiating obstructive from central and complex sleep apnea using an automated electrocardiogram-based method. Sleep. 2007;30:1756–69. doi: 10.1093/sleep/30.12.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salloum A, Rowley JA, Mateika JH, Chowdhuri S, Omran Q, Badr MS. Increased propensity for central apnea in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2010;181:189–93. doi: 10.1164/rccm.200810-1658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Randerath WJ, Galetke W, Stieglitz S, Laumanns C, Schäfer T. Adaptive servo-ventilation in patients with coexisting obstructive sleep apnoea/hypopnoea and Cheyne-Stokes respiration. Sleep Med. 2008;9:823–30. doi: 10.1016/j.sleep.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Randerath WJ, Galetke W, Kenter M, Richter K, Schafer T. Combined adaptive servo-ventilation and automatic positive airway pressure (anticyclic modulated ventilation) in co-existing obstructive and central sleep apnea syndrome and periodic breathing. Sleep Med. 2009;10:898–903. doi: 10.1016/j.sleep.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 33.Kuzniar TJ, Pusalavidyasagar S, Gay PC, Morgenthaler TI. Natural course of complex sleep apnea--a retrospective study. Sleep Breath. 2008;12:135–9. doi: 10.1007/s11325-007-0140-z. [DOI] [PubMed] [Google Scholar]
- 34.Johnson KG, Johnson DC. Bilevel positive airway pressure worsens central apneas during sleep. Chest. 2005;128:2141–50. doi: 10.1378/chest.128.4.2141. [DOI] [PubMed] [Google Scholar]
- 35.Kuzniar TJ, Golbin JM, Morgenthaler TI. Moving beyond empiric continuous positive airway pressure (CPAP) trials for central sleep apnea: a multi-modality titration study. Sleep Breath. 2007;11:259–66. doi: 10.1007/s11325-007-0118-x. [DOI] [PubMed] [Google Scholar]
- 36.Arzt M, Floras JS, Logan AG, et al. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian continuous positive airway pressure for patients with central sleep apnea and heart failure trial (CANPAP) Circulation. 2007;115:3173–80. doi: 10.1161/CIRCULATIONAHA.106.683482. [DOI] [PubMed] [Google Scholar]
- 37.Mulgrew AT, Lawati NA, Ayas NT, et al. Residual sleep apnea on polysomnography after 3 months of CPAP therapy: clinical implications, predictors and patterns. Sleep Med. 2010;11:119–25. doi: 10.1016/j.sleep.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 38.Morgenthaler TI. The quest for stability in an unstable world: adaptive servoventilation in opioid induced complex sleep apnea syndrome. J Clin Sleep Med. 2008;4:321–3. [PMC free article] [PubMed] [Google Scholar]
- 39.Javaheri S, Malik A, Smith J, Chung E. Adaptive pressure support servoventilation: a novel treatment for sleep apnea associated with use of opioids. J Clin Sleep Med. 2008;4:305–10. [PMC free article] [PubMed] [Google Scholar]


