Abstract
Study Objectives:
Patients with postural tachycardia syndrome (POTS) commonly complain of fatigue, unrefreshing sleep, daytime sleepiness, and diminished quality of life. This study's objective was to assess the sleep quality and health-related quality of life in patients with POTS as compared with healthy control subjects.
Methods:
Patients with POTS (n = 44) and healthy control subjects (n = 46) completed a battery of questionnaires including Medical Outcomes Study (MOS) Sleep Survey and the Epworth Sleepiness Scale to assess sleep, fatigue visual analogue scale (VAS) to assess fatigue, and the RAND-36 and EuroQol (EQ-5D) surveys to assess health-related quality of life.
Results:
Compared with healthy control subjects, patients with POTS have more sleep problems (58 ± 18 vs. 20 ± 13; p < 0.0001) and excessive daytime sleepiness (10.2 ± 5.7 vs. 6.2 ± 3.2; p < 0.0001), higher fatigue levels (7.5 ± 2.0 vs. 2.8 ± 2.5; p < 0.0001), and poor health-related quality of life (EQ-5D health thermometer 53 ± 17 vs. 89 ± 7; p < 0.0001). There were strong correlations between MOS Sleep Survey index and the fatigue VAS (Rs = 0.73; R2 = 0.53; p < 0.0001) and the RAND-36 physical health composite scores (Rs = −0.70; R2 = 0.53; p < 0.0001)
Conclusions:
Patients with POTS have higher subjective daytime sleepiness, fatigue, and worse sleep and health related quality of life. The sleep problems contribute significantly to the diminished quality of life: ∼ 50% of the variability in HRQL can be explained by the variability in sleep problems. Further objective studies are needed to delineate the specific nature of the sleep problems in patients with POTS.
Citation:
Bagai K; Song Y; Ling JF; Malow B; Black BK; Biaggioni I; Robertson D; Raj SR. Sleep disturbances and diminished quality of life in postural tachycardia syndrome. J Clin Sleep Med 2011;7(2):204-210.
Keywords: Postural tachycardia syndrome, sleepiness, fatigue, quality of life
Postural tachycardia syndrome (POTS) affects an estimated 500,000 people in the United States alone. POTS is defined as excessive increase in heart rate (≥ 30 beats/min) with upright posture, associated with orthostatic symptoms including palpitation, chest pain syndrome, dyspnea on standing, mental clouding, and difficulties with concentration in the absence of orthostatic hypotension. It can produce substantial disability among otherwise healthy people.1,2 The pathophysiology of POTS is poorly understood, however many patients suffer from either a primary or secondary increase in sympathetic nervous system tone.3
Patients with POTS commonly complain of symptoms including fatigue and difficulty with sleep. One study found that patients with POTS had a diminished quality of life when measured using a standard health status instrument (SF-36).4 There are no published data, however, on the quality of sleep or sleep disturbances in patients with POTS. Conversely, problems with autonomic nervous system regulation have been noted in several primary disorders of sleep. These include relatively common sleep disorders such as obstructive sleep apnea5–7 and less common disorders such as fatal familial insomnia, sleep terrors, and REM sleep behavior disorder.8–10
Recent studies have shown that subjective poor sleep is associated with reduced physical performance, greater functional limitation, increased risk for cardiovascular diseases, and may even predict all-cause mortality.11–14 Therefore poor sleep may be a core factor associated with the low quality of life reported by patients with POTS.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Patients with POTS commonly complain of symptoms including fatigue and difficulty with sleep. Prior to this paper, there were no published data on the quality of sleep or sleep disturbances in patients with POTS.
Study Impact: This study found that patients with POTS have poor sleep quality, excessive sleepiness, excessive fatigue, and a high proportion of diminished quality of life due to sleep problems. Further objective sleep assessments are needed to delineate the specific sleep problems in patients with POTS, which will be a pre-requisite to develop optimal treatments.
The aim of this study was to formally assess sleep quality, fatigue, and health-related quality of life (HRQL) in patients with POTS as compared with healthy control subjects.
METHODS
Study Subjects
Patients with POTS met the conventional criteria.15,16 Briefly, patients developed symptoms of orthostatic intolerance accompanied by a heart rate rise ≥ 30 bpm which occurred within the first 10 min of upright posture, without any evidence of orthostatic hypotension (a fall in blood pressure ≥ 20/10 mm Hg). Patients had at least a 6-month history of symptoms in the absence of another chronic debilitating disorder or prolonged bed rest, and were ≥ 18 years of age. Common symptoms in POTS include rapid palpitation from tachycardia, exercise intolerance, lightheadedness, extreme fatigue, headache, and mental clouding. Patients with POTS were all underwent a posture study to assess their orthostatic tolerance (details below) and an assessment of cardiovagal function (sinus arrhythmia) at the Elliot V. Newman Clinical Research Center at Vanderbilt University. Healthy control subjects (who did not meet criteria for POTS) were similar in age and gender to the POTS patients, but were not matched to individual patients. Control subjects were recruited from healthy volunteers known to the Vanderbilt's Paden Dysautonomia Center, through the Vanderbilt Research Volunteer Database, and through advertisements in the Vanderbilt community. The research protocol was approved by the Vanderbilt Institutional Review Board, and written informed consent was obtained from each subject before the study began.
Supine and Upright Posture Study
Heart rate (HR), blood pressure, and plasma norepinephrine and epinephrine were assessed after overnight rest in the supine position and again after standing for 30 minutes (as tolerated) in patients with POTS. The standing test was performed in order to assess the hemodynamic and biochemical responses to increased central hypovolemia (accentuated by the gravitational stress). For catecholamine measurements, blood was collected in plastic syringes and immediately transferred to chilled vacuum tubes with EGTA and reduced glutathione (Amersham International PLC, Amersham, UK) and immediately put on ice. The plasma was separated by refrigerated centrifugation at −4°C and stored at −70°C until the assay. Concentrations of norepinephrine and epinephrine were measured by batch alumina extraction followed by high performance liquid chromatography for separation with electrochemical detection and quantification.17
Autonomic Testing
Cardiovagal function was assessed in all patients with POTS by testing sinus arrhythmia (SA).18 During continuous electrocardiographic monitoring, patients were asked to breathe deeply at 6 breaths/min, and the maximum and minimum heart rates were measured. Sinus arrhythmia was quantified as the SAdelta (maximum HR-minimum HR) and as SAratio (maximum HR/minimum HR).
Assessment Tools
All subjects completed the Medical Outcomes Study (MOS) Sleep Survey and the Epworth Sleepiness Scale (ESS) to assess sleep quality and sleepiness, a fatigue visual analogue scale (VAS) to assess fatigue, and RAND-36 and EuroQol (EQ-5D) surveys to assess health-related quality of life. Each instrument has been previously validated and was self-administered.
Medical Outcomes Study Sleep Scale
The MOS Sleep Scale quantifies effects of sleep problems on individuals.19 It uses 12 questions to assess the effect of sleep problems through several individual dimensions of sleep, including sleep disturbance, snoring, sleep adequacy, headache, somnolence, and respiratory impairments with sleep. It also allows calculation of a global “sleep problems index.” Each domain is scored from 0-100. For sleep adequacy, a high score indicates better sleep (“more adequate”), but for the remaining parameters, a high score indicates more pathology.
Epworth Sleepiness Scale
The ESS is a widely used self-reported subjective measure of daytime sleepiness.20 It is a brief tool that asks the subjects to rate (on a scale of 0-3) their likelihood of sleeping in 8 specific situations that are commonly met in daily life. It represents the subject's average sleep propensity across these situations of daily life. Total score range from 0-24. Scores < 10 are considered normal. Prior studies have shown that ESS scores are usually > 10 in patients with sleep disorders that cause daytime sleepiness and average 6.5 ± 3.0 in normal controls.20
Fatigue Visual Analogue Scale
The fatigue VAS is a single-item scale (0-10) which measures the severity of fatigue with a specific question, “How much of a problem has fatigue or tiredness been for you in the past week.” It has been compared with longer fatigue scales in patients with rheumatoid arthritis, and was found to perform as well as, or better than, longer scales in respect to sensitivity to change.21 Higher scores indicate more fatigue.
RAND-36
The RAND-36 is a well-validated, commonly used HRQL survey instrument that is composed of 36 items distilled from the larger Medical Outcomes Study.22 These items assess 8 health domains, including physical functioning, role limitations caused by physical health problems, bodily pain, general health, emotional well-being, role limitations due to emotional problems, social functioning, and energy/fatigue. Summary physical health composite and mental health composite scores are also calculated. The questionnaire asks for subjective ratings over the past 4 weeks. Scores range from 0-100, with higher scores indicating a better quality of life.
EuroQol
The EQ-5D is a self-administered general illness assessment tool which is comprised of 5 dimensions of health (mobility, self care, usual activities, pain/discomfort, and anxiety/depression).23 Each health dimension comprises 3 levels (no problems, some/moderate problems, and extreme problems), but were analyzed as dichotomous variables (no problems vs. some/moderate/extreme problems). Each participant is also asked to score their health on a global “health thermometer” in which participants rate their overall health from 0-100. Higher scores represent better global health.
Statistical Methods
In order to avoid the assumptions of normally distributed data, demographic variables between POTS patients and control subjects were compared using Wilcoxon rank sum test for continuous variables and a Fisher exact test for categorical variables. The scores for the ESS, MOS sleep index, fatigue VAS, EQ-5D health thermometer, and the RAND-36 composite and domain scores were compared using Wilcoxon rank sum test between POTS patients and controls. The individual EQ-5D domains were analyzed as categorical variables using the Fisher exact test. Analyses of whether POTS patients have a higher proportion of excessive daytime sleepiness (defined as ESS > 10) was performed with a Fisher exact test.
In order to better understand the relationship between sleep problems and HRQL, fatigue, and age, Spearman correlations were performed.
The null hypothesis (Ho) was that there will be no differences in the sleep quality and HRQL in patients with POTS as compared with healthy control subjects. There is no preliminary data available on sleep in patients with POTS. Therefore, we used data from a recent study on sleep in patients with chronic fatigue syndrome (CFS) in order to estimate the needed samples size. Fossey et al.24 found that 57.7% of subjects with CFS had a diagnosed sleep disorder, compared with only 13.3% of the control subjects. We estimated that 50% of the patients with POTS and 20% of the control subjects would have a diagnosed sleep disorder; a 2 group continuity corrected χ2 test with a 0.05 two-sided significance level had an 80% power to detect this difference with a sample size of 45 in each group (90 subjects in total).
R software version 2.9.1 and SAS version 9.1 (SAS Institute Inc., Cary, NC, USA) were used for data analysis, and a 2-sided 5% significance level was used for all statistical inferences. Data are reported as mean ± standard deviation (SD), unless stated otherwise. Prism for Windows 5 (version 5.03, GraphPad Software Inc.) was used for graphical presentation.
RESULTS
POTS patients (n = 44) and control subjects (n = 46) were of comparable age (36 ± 11 years vs. 36 ± 11 years; p = 0.69), and most subjects in both groups were female (89% vs. 92%; p = 0.74). The baseline autonomic characterization of the patients with POTS is shown in Table 1. The values are similar to prior publications describing our POTS patients, and the standing heart rates and plasma norepinephrine values in these POTS patients are higher than previously reported healthy control subjects.3
Table 1.
Baseline demographics, postural vital signs, catecholamines, and sinus arrhythmia parameters of the subjects with postural tachycardia syndrome (POTS)
| POTS (n = 44) | |
|---|---|
| Female (n) | 39 (89%) |
| Age (years) | 36 ± 11 |
| Height (cm) | 167 ± 8 |
| Weight (kg) | 69.5 ± 20.6 |
| Body mass index (kg/m2) | 24.3 ± 6.0 |
| Supine | |
| Heart rate (/min) | 75 ± 13 |
| Systolic blood pressure (mm Hg) | 112 ± 10 |
| Diastolic blood pressure (mm Hg) | 68 ± 9 |
| Norepinephrine (nmol/L) | 1.77 ± 1.49 |
| Epinephrine (nmol/L) | 0.16 ± 0.20 |
| Standing | |
| Heart rate (/min) | 119 ± 25 |
| Systolic blood pressure (mm Hg) | 116 ± 22 |
| Diastolic blood pressure (mm Hg) | 75 ± 14 |
| Norepinephrine (nmol/L) | 5.21 ± 2.98 |
| Epinephrine (nmol/L) | 0.38 ± 0.51 |
| Change from Supine to Standing | |
| Heart rate (/min) | 43 ± 22 |
| Systolic blood pressure (mm Hg) | 3 ± 18 |
| Diastolic blood pressure (mm Hg) | 6 ± 13 |
| Norepinephrine (nmol/L) | 3.44 ± 2.32 |
| Epinephrine (nmol/L) | 0.22 ± 0.47 |
| Sinus Arrhythmia (SA) | |
| SA Max heart rate (/min) | 95 ± 13 |
| SA Min heart rate (/min) | 70 ± 12 |
| SAdelta heart rate (/min) | 25 ± 10 |
| SAratio | 1.38 ± 0.20 |
Data are presented as the mean ± standard deviation. SAdelta = (SA Max Heart Rate) – (SA Min Heart Rate); SAratio = (SA Max Heart Rate) / (SA Min Heart Rate).
Sleep and Fatigue Assessments
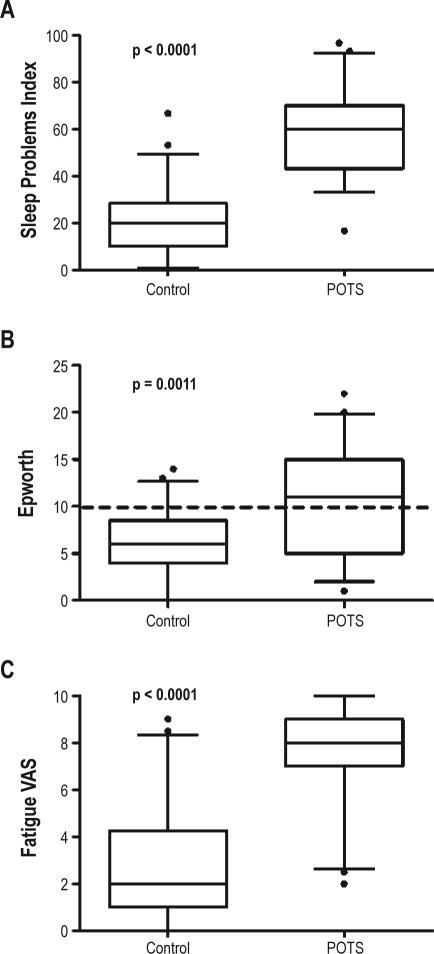
The MOS Sleep Problems Index found significantly more sleep problems in patients with POTS than control subjects (58 ± 18 vs. 20 ± 13; p < 0.0001; Figure 1A). Conversely, “sleep adequacy” was worse in POTS patients (30 ± 27 vs. 62 ± 23; p < 0.0001). Individual domains of the MOS Sleep Survey were worse in patients with POTS, including sleep disturbance, shortness of breath or headache with sleep, and sleep somnolence. The one exception was snoring, which was not different between the 2 groups (p = 0.63).
Figure 1.
Sleep and fatigue in patients with postural tachycardia syndrome (POTS) and healthy control subjects
Box and whisker plots showing the medians, interquartile ranges (IQR), and confidence intervals from 5% to 95% for the MOS Sleep Problems index (A), the Epworth Sleepiness Scale (B), and the fatigue visual analogue scale (C). The horizontal line in Panel B represents the commonly used threshold for “excessive daytime sleepiness.” Outliers are represented with black dots.
POTS patients had a significantly higher ESS scores than control subjects (10.2 ± 5.7 vs. 6.2 ± 3.7; p = 0.001). Excessive daytime somnolence was defined as ESS > 10. A significantly higher proportion of patients with POTS than control subjects had excessive daytime somnolence (51% vs. 16%; p = 0.0004; see Figure 1B).
Using the fatigue VAS, patients with POTS reported significantly greater fatigue than control subjects (7.5 ± 2.0 vs. 2.8 ± 2.5; p < 0.0001; Figure 1C).
Health-Related Quality of Life
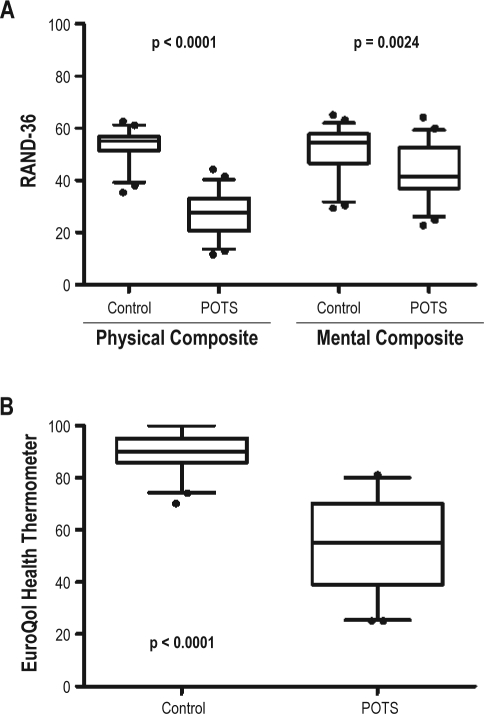
The HRQL data are summarized in Table 3. Patients with POTS had a poorer score in every domain of HRQL assessed in the RAND-36 than the control group. The 8 individual domains in the RAND-36 can be summarized into 2 subscores—a physical health composite score and a mental health composite score. The physical health composite score was significantly lower in patients with POTS than control subjects (26 ± 9 vs. 54 ± 6; p < 0.0001; Figure 2A, left panel). While the mental health composite score was also lower in patients with POTS than control subjects (43 ± 11 vs. 52 ± 10; p = 0.002; Figure 2A, right panel), the difference was not as great as with physical health composite score. While POTS patients had mean score for many of the individual physical domains in the 20-30 range (Table 3), their scores for emotional well-being and role limitation due to emotional problems were both > 40 (indicating less severe deficits in these domains).
Table 3.
Health-related quality of life parameters for patients with POTS and control subjects
| POTS (n = 44) | Control Subjects (n = 46) | p | |
|---|---|---|---|
| RAND-36 | |||
| Physical Health Composite | 26 ± 9 | 54 ± 6 | < 0.0001 |
| Mental Health Composite | 43 ± 11 | 52 ± 10 | 0.0002 |
| Physical Functioning | 28 ± 9 | 55 ± 4 | < 0.0001 |
| RL – Physical Health | 26 ± 2 | 51 ± 9 | < 0.0001 |
| Bodily Pain | 39 ± 9 | 53 ± 6 | < 0.0001 |
| General Health | 30 ± 9 | 56 ± 6 | < 0.0001 |
| Emotional Well-Being | 47 ± 10 | 55 ± 7 | < 0.0001 |
| RL – Emotional Problems | 41 ± 13 | 48 ± 12 | 0.009 |
| Social Functioning | 29 ± 11 | 54 ± 6 | < 0.0001 |
| Energy/Fatigue | 30 ± 7 | 54 ± 10 | < 0.0001 |
| EuroQol | |||
| Global Health Thermometer | 53 ± 17 | 89 ± 7 | < 0.0001 |
| Mobility Problems (%) | 60% | 4% | < 0.0001 |
| Self-Care Problems (%) | 35% | 0% | < 0.0001 |
| Pain/Discomfort Problems (%) | 81% | 9% | < 0.0001 |
| Usual Activites Problems (%) | 95% | 2% | < 0.0001 |
| Anxiety/Depression Problems (%) | 56% | 13% | < 0.0001 |
POTS, postural tachycardia syndrome; RL, role limitation. Data are presented as the mean ± SD. Continuous variables between POTS patients and controls were compared using the Wilcoxon rank sum test. The EuroQol parameters (with the exception of the health thermometer) were reported as percentage of subjects with an abnormal score (> 1), with p values reported using the Fisher exact test.
Figure 2.
Health-related quality of life in patients with postural tachycardia syndrome (POTS) and healthy control subjects
Box and whisker plots show the medians, interquartile ranges (IQR), and confidence intervals from 5% to 95%. Panel A shows the RAND-36 physical health composite score (left) and the mental health composite scores (right). Panel B shows results for the EuroQol health thermometer. Outliers are represented with black dots.
The EQ-5D has a “health thermometer” that provides a global rating of HRQL. Patients with POTS had markedly diminished global HRQL compared with control subjects (53 ± 17 vs. 89 ± 7; p < 0.0001; Figure 2B). Each of the separately assessed EQ-5D dimension, including mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, were significantly worse in patients with POTS than control (Table 3).
Correlation between Sleep Problems and Other Parameters
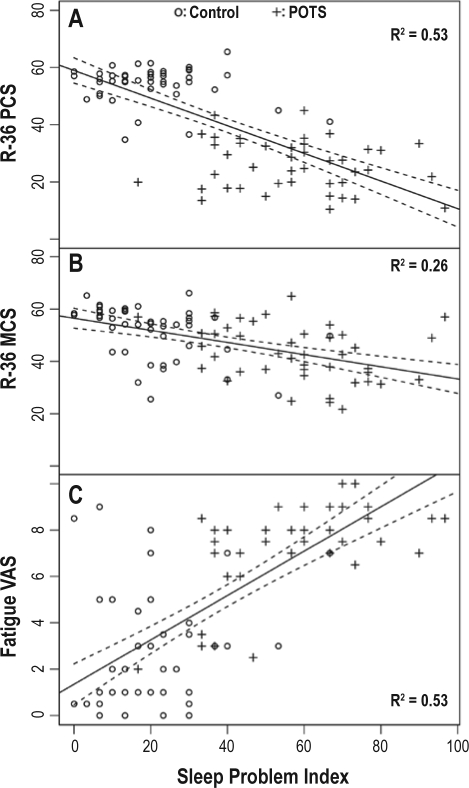
There was no relationship between subject age and the MOS Sleep Problems index (Rs = 0.03; p = 0.75). There was a tight negative correlation between the MOS Sleep Problems index and the RAND-36 physical health composite score (Rs = −0.70; R2 = 0.53; p < 0.0001; Figure 3A). In contrast, there was a weaker relationship between the MOS Sleep Problems index and the RAND-36 mental health composite score (Rs = 0.58; R2 = 0.26; p < 0.0001; Figure 3B). There was also a tight correlation between the MOS Sleep Problems index and the fatigue VAS (Rs = 0.72; R2 = 0.53; p < 0.0001; Figure 3C).
Figure 3.
Scatterplots with linear regression line showing the relationship between the MOS Sleep Problems Index and the RAND-36 physical health composite score (A), the RAND-36 mental health composite score (B), and the fatigue visual analogue scale (VAS) (C)
Each panel shows individual data points for POTS patients (plus signs) and control subjects (open circles).
DISCUSSION
The key findings of this study are that, in comparison to control subjects, patients with POTS: (1) have poor sleep quality and excessive sleepiness, (2) have excessive fatigue, and (3) have a poor health-related quality of life. Further, a high proportion of diminished quality of life is due to sleep problems.
Poor Sleep Quality and Excessive Sleepiness
Although many patients with POTS complain about problems with sleep, to our knowledge, this is the first study comparing measures of sleepiness in POTS patients and control subjects. Compared with healthy control subjects, patients with POTS have inadequate sleep with significantly more sleep problems, including excessive daytime somnolence, sleep related dyspnea, and headache. Interestingly, patients with POTS did not report increased snoring compared with control patients. One possible hypothesis is that, as reported in patients with CFS, patients with POTS may exhibit higher arousals rates at night, resulting in non-restorative sleep.24
The Epworth Sleepiness Scale is an instrument that is commonly used in sleep disorders clinics. The upper limit of normal for the ESS has been set at 10 out of a maximum score of 24.20 The mean ESS score for the entire group of POTS patients was greater than this threshold, as were the individual scores for over 50% of the POTS patients (Figure 1B). These striking findings emphasize that POTS patients do not suffer from fatigue alone, but sleepiness in addition to fatigue.
Health-Related Quality of Life in POTS
One prior study has assessed HRQL in patients with POTS. Benrud-Larson et al.4 administered the Medical Outcomes Study Short-Form 36 (SF-36) to POTS patients from the Mayo Clinic Autonomic Disorders Clinic. They reported that POTS patients had lower HRQL in 6 of the 8 SF-36 domains. The only domains in which the POTS patients did not score poorly were the psychological domains, i.e., mental health and role limitation due to emotional problems.
In the current study, the RAND-36 and EuroQol were used in place of the SF-36. The RAND-36 questions are the same as in the SF-36, but the scoring algorithm is different. Similar to the SF-36, one can derive composite subscores for physical health and mental health, but not a single global score for HRQL.
Our RAND-36 data are consistent with those of Benrud-Larson et al. Both studies document markedly diminished HRQL in POTS patients across many domains compared with healthy controls. In the current study, we found that both physical health and mental health were significantly diminished in POTS patients compared with healthy controls, but the magnitude of deficit was much greater for physical health than mental health (Table 2). This adds to the growing body of evidence that many of the problems in POTS patients have a physiological and not psychological basis.25,26 The magnitudes of RAND-36 scores were comparable to other chronic illness including rheumatoid arthritis and end-stage renal disease.22,27,28
Table 2.
Sleep quality and fatigue parameters for patients with POTS and control subjects
| POTS (n = 44) | Control Subjects (n = 46) | p | |
|---|---|---|---|
| MOS Sleep Survey | |||
| Sleep Problems Index | 58 ± 18 | 20 ± 13 | < 0.0001 |
| Sleep Disturbance | 59 ± 28 | 15 ± 15 | < 0.0001 |
| Sleep SOB/HA | 58 ± 33 | 20 ± 19 | < 0.0001 |
| Sleep Adequacy | 30 ± 27 | 62 ± 23 | < 0.0001 |
| Sleep Somnolence | 59 ± 20 | 20 ± 16 | < 0.0001 |
| Snoring | 21 ± 31 | 17 ± 27 | 0.632 |
| Epworth Sleepiness Scale | 10.2 ± 5.7 | 6.2 ± 3.7 | 0.001 |
| Fatigue VAS | 7.5 ± 2.0 | 2.8 ± 2.5 | < 0.0001 |
POTS, postural tachycardia syndrome; MOS, Medical Outcomes Study; VAS, visual analogue scale. Data are presented as the mean ± SD. Variables between POTS patients and controls were compared using the Wilcoxon rank sum test.
The EuroQol HRQL tool was also used in this study. One key advantage of this tool is that the “EuroQol health thermometer” provides a single global score for HRQL, something that is not provided using the SF-36. Not surprisingly, the health thermometer also found a markedly diminished HRQL in POTS patients.
Fatigue in POTS
Most patients with POTS complain about fatigue. In this study, patients with POTS reported a mean level of fatigue that was almost 3-fold greater than normal. Previous studies have shown an overlap between POTS and CFS. In one study by Bou-Holaigah et al., orthostatic intolerance was present in 22 of 23 patients with well-documented CFS.29 Streeten et al.30 found a 27% incidence of POTS in well-characterized patients with CFS, while Freeman and Komaroff31 reported a significant increase in baseline and standing heart rate in their CFS cohort. These data suggest that there may be incompletely overlapping pathophysiology between POTS and CFS
Relationship between Sleep Problems, Fatigue, and Quality of Life
Using linear regression, associations were determined between sleep problems vs. fatigue and HRQL. There was a strong correlation between the MOS Sleep Problems index and the fatigue VAS (Rs = 0.73; R2 = 0.53). This means that over half of the variance in fatigue across subjects could be explained by differences in sleep problems.
There were similar associations between the Sleep Problems index and HRQL. Over 50% of the variability in the RAND-36 physical health composite score could be explained by differences in the MOS Sleep Problems index, but only ∼25% of the variability in the RAND-36 mental health composite score could be explained in this manner. These data indicate striking effects on individuals in general, and patients with POTS in particular, of sleep problems on their overall lives. Of note, while sleep problems affect both domains of health, physical health is more diminished by sleep problems than mental health.
Limitations
The main limitation of this study is that the data were all based on subjective, self-reported measures of sleep and HRQL. For HRQL, this is actually a standard and well-validated method of assessment. It is noteworthy that not all sleep ratings were worse in the POTS patients. For example, snoring was worse in the control group. This suggests that POTS patients did not globally overreport their symptoms (in which case all scores would be expected to be worse).
This study must be viewed as a starting point in understanding sleep disturbance in POTS, and not the final answer. Formal polysomnography and sleep diaries were not performed; hence the nature of sleep disturbance remains unknown. Follow-up studies including formal objective measures of assessment of sleep disturbances using actigraphy, overnight polysomnography and multiple sleep latency tests will clarify our understanding of the mechanisms underpinning poor sleep and HRQL in POTS patients.
Perspective
Using standardized and previously validated tools, this study documents that patients with POTS have inadequate sleep quality and fatigue, poor health-related quality of life compared with healthy control subjects. These sleep problems contribute significantly to the diminished quality of life of patients with POTS, particularly regarding their physical health. Further studies are needed to further delineate the specific sleep problems in patients with POTS.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Supported in part by NIH grants K23 RR020783 (SRR), R01 HL102387 (SRR), R01 HL071784 (DR), R01 NS055670 (IB), P01 HL56693 (DR), 1 UL1 RR024975 (Clinical and Translational Science Award), and the Paden Dysautonomia Center.
REFERENCES
- 1.Low PA, Opfer-Gehrking TL, Textor SC, et al. Postural tachycardia syndrome (POTS) Neurology. 1995;45:S19–25. [PubMed] [Google Scholar]
- 2.Raj SR. The postural tachycardia syndrome (POTS): pathophysiology, diagnosis – management. Indian Pacing Electrophysiol J. 2006;6:84–99. [PMC free article] [PubMed] [Google Scholar]
- 3.Garland EM, Raj SR, Black BK, et al. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology. 2007;69:790–798. doi: 10.1212/01.wnl.0000267663.05398.40. [DOI] [PubMed] [Google Scholar]
- 4.Benrud-Larson LM, Dewar MS, Sandroni P, et al. Quality of life in patients with postural tachycardia syndrome. Mayo Clin Proc. 2002;77:531–7. doi: 10.4065/77.6.531. [DOI] [PubMed] [Google Scholar]
- 5.Vanninen E, Tuunainen A, Kansanen M, et al. Cardiac sympathovagal balance during sleep apnea episodes. Clin Physiol. 1996;16:209–16. doi: 10.1111/j.1475-097x.1996.tb00569.x. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe T, Mano T, Iwase S, et al. Enhanced muscle sympathetic nerve activity during sleep apnea in the elderly. J Auton Nerv Syst. 1992;37:223–6. doi: 10.1016/0165-1838(92)90044-h. [DOI] [PubMed] [Google Scholar]
- 7.Parati G, Di Rienzo M, Bonsignore MR, et al. Autonomic cardiac regulation in obstructive sleep apnea syndrome: evidence from spontaneous baroreflex analysis during sleep. J Hypertens. 1997;15:1621–6. doi: 10.1097/00004872-199715120-00063. [DOI] [PubMed] [Google Scholar]
- 8.Lugaresi E, Medori R, Montagna P, et al. Fatal familial insomnia and dysautonomia with selective degeneration of thalamic nuclei. N Engl J Med. 1986;315:997–1003. doi: 10.1056/NEJM198610163151605. [DOI] [PubMed] [Google Scholar]
- 9.Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain. 2000;123(Pt 2):331–9. doi: 10.1093/brain/123.2.331. [DOI] [PubMed] [Google Scholar]
- 10.Ferini-Strambi L, Spera A, Oldani A, et al. Cardiac autonomic regulation during sleep in panic disorder. J Neurol Neurosurg Psychiatry. 1996;61:421–2. doi: 10.1136/jnnp.61.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haseli-Mashhadi N, Dadd T, Pan A, et al. Sleep quality in middle-aged and elderly Chinese: distribution, associated factors and associations with cardio-metabolic risk factors. BMC Public Health. 2009;9:130. doi: 10.1186/1471-2458-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldman SE, Stone KL, Ancoli-Israel S, et al. Poor sleep is associated with poorer physical performance and greater functional limitations in older women. Sleep. 2007;30:1317–24. doi: 10.1093/sleep/30.10.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dew MA, Hoch CC, Buysse DJ, et al. Healthy older adults' sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 14.Haseli-Mashhadi N, Pan A, Ye X, et al. Self-Rated Health in middle-aged and elderly Chinese: distribution, determinants and associations with cardio-metabolic risk factors. BMC Public Health. 2009;9:368. doi: 10.1186/1471-2458-9-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43:132–7. doi: 10.1212/wnl.43.1_part_1.132. [DOI] [PubMed] [Google Scholar]
- 16.Raj SR, Biaggioni I, Yamhure PC, et al. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation. 2005;111:1574–82. doi: 10.1161/01.CIR.0000160356.97313.5D. [DOI] [PubMed] [Google Scholar]
- 17.Jacob G, Robertson D, Mosqueda-Garcia R, et al. Hypovolemia in syncope and orthostatic intolerance role of the renin-angiotensin system. Am J Med. 1997;103:128–33. doi: 10.1016/s0002-9343(97)00133-2. [DOI] [PubMed] [Google Scholar]
- 18.Mosqueda-Garcia R. Evaluation of autonomic failure. In: Robertson D, Biaggioni I, editors. Disorders of the autonomic nervous system. Luxembourg: Harwood Academic Publishers GmbH; 1995. pp. 25–59. [Google Scholar]
- 19.Sprintzer KL, Hays RD. Los Angeles: 2003. MOS Sleep Scale: A manual for use and scoring, Version 1.0. [Google Scholar]
- 20.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe F. Fatigue assessments in rheumatoid arthritis: comparative performance of visual analog scales and longer fatigue questionnaires in 7760 patients. J Rheumatol. 2004;31:1896–902. [PubMed] [Google Scholar]
- 22.Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med. 2001;33:350–7. doi: 10.3109/07853890109002089. [DOI] [PubMed] [Google Scholar]
- 23.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–43. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 24.Fossey M, Libman E, Bailes S, et al. Sleep quality and psychological adjustment in chronic fatigue syndrome. J Behav Med. 2004;27:581–605. doi: 10.1007/s10865-004-0004-y. [DOI] [PubMed] [Google Scholar]
- 25.Raj V, Haman KL, Raj SR, et al. Psychiatric profile and attention deficits in postural tachycardia syndrome. J Neurol Neurosurg Psychiatry. 2009;80:339–44. doi: 10.1136/jnnp.2008.144360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masuki S, Eisenach JH, Johnson CP, et al. Excessive heart rate response to orthostatic stress in postural tachycardia syndrome is not caused by anxiety. J Appl Physiol. 2007;102:896–903. doi: 10.1152/japplphysiol.00927.2006. [DOI] [PubMed] [Google Scholar]
- 27.Reginster JY. The prevalence and burden of arthritis. Rheumatology (Oxford) 2002;41(Supp 1):3–6. [PubMed] [Google Scholar]
- 28.Tijhuis GJ, de Jong Z, Zwinderman AH, et al. The validity of the Rheumatoid Arthritis Quality of Life (RAQoL) questionnaire. Rheumatology (Oxford) 2001;40:1112–9. doi: 10.1093/rheumatology/40.10.1112. [DOI] [PubMed] [Google Scholar]
- 29.Bou-Holaigah I, Rowe PC, Kan J, Calkins H. The relationship between neurally mediated hypotension and the chronic fatigue syndrome. JAMA. 1995;274:961–7. [PubMed] [Google Scholar]
- 30.Streeten DH, Thomas D, Bell DS. The roles of orthostatic hypotension, orthostatic tachycardia, and subnormal erythrocyte volume in the pathogenesis of the chronic fatigue syndrome. Am J Med Sci. 2000;320:1–8. doi: 10.1097/00000441-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Freeman R, Komaroff AL. Does the chronic fatigue syndrome involve the autonomic nervous system? Am J Med. 1997;102:357–64. doi: 10.1016/s0002-9343(97)00087-9. [DOI] [PubMed] [Google Scholar]