Abstract
Adolescents are prone to risk-taking behaviors leading to adverse consequences such as substance abuse, accidents, violence, and victimization. However, little is known about the contribution of genetic and environmental factors to individual differences in the propensity for risk-taking. This study investigated developmental changes, longitudinal stability, and heritability of risk-taking using data from 752 adolescent twins including 169 MZ and 203 DZ pairs. The Balloon Analogue Risk Task (BART), an experimental behavioral measure of risk taking, was administered to the twins at age 12 and then re-administered to a part of this sample at age 14. Risk-taking increased with age, but individual differences showed a significant longitudinal stability. Genetic model fitting showed that at age 12, heritability of risk-taking was modest but significant in both sexes, whereas at age 14, heritability increased to 55% in males and became non-significant in females. The findings suggest that propensity for risk-taking as measured by BART can be a useful endophenotype for genetic studies of adolescent externalizing psychopathology, however, the utility of this measure may be limited by sex differences in heritability.
Keywords: risk-taking, adolescents, heritability
INTRODUCTION
Risk taking is a characteristic of behaviors that occur under conditions of uncertainty and involve a tradeoff between beneficial versus detrimental outcomes, perceived or real. It is important that risk taking may or may not include explicit, conscious evaluation of the probability and magnitude of possible outcomes. Developmental research has shown that the propensity for risk-taking is higher during adolescence compared with both childhood and adulthood, which makes adolescence a period of hightened vulnerability to detrimental outcomes such as substance abuse, accidents, violence, and victimization (Kelley et al., 2004; Resnick et al., 1997). Importantly, studies suggest that risk-taking in adolescence cannot be attributed to age differences in risk perception and appraisal, and low self-regulation competence is likely to play a key role (Steinberg, 2004).
Research suggests that this increased risk-taking can be attributed to the relative immaturity of the neural substrates of behavioral regulation and decision making including corticolimbic circuitry in which the prefrontal cortex plays a key regulatory role (Bardo, 2004; Spear, 2000). Both animal and human research indicates substantial developmental changes in the brain during adolescence, and some of these changes such as myelination and the shaping of cortical connectivity continue into late adolescence and young adulthood (Anokhin et al., 1996; Giedd, 2008; Yakovlev & Lecours, 1967). Based on the theory that behavior is guided by two distinct systems, behavior inhibition and activation, Bardo (2004) proposed that high novelty seeking and risk-taking in adolescence can be explained by slower maturation of the behavior inhibition system compared to the reward-based behavioral activation system.
Most human research on risk-taking has relied on self-reports that are subject to a number of biases and limitations (Hunt et al., 2005) and few studies have used objective laboratory-based studies of risk taking. Recently, a novel behavioral measure of risk-taking propensity, The Balloon Analogue Risk Task (BART), was developed to address the limitations of self-report measures (Lejuez et al., 2002). Construct validity of BART has been demonstrated both in adolescent and adult populations by significant associations with various real-world risk-taking behaviors, such as substance use and abuse, gambling, theft, aggression and unprotected sex (reviewed in Hunt et al., 2005). Importantly, BART measures of risk showed significant incremental validity in predicting drug use after other known risk factors were accounted for (Hopko et al., 2006). BART responding also correlated with personality traits previously implicated in risk-taking behaviors, such as sensation seeking and impulsivity (Bornovalova et al., 2008; Lejuez et al., 2002). However, BART measures could explain unique variance in a range or risk taking behaviors above and beyond these personality constructs (Lejuez et al., 2007). Taken together, these data suggest that propensity for risk taking as indicated by BART can serve as an useful endophenotype (Gottesman & Gould, 2003) in genetic studies of the liability to psychopathology in adolescence, particularly substance use disorders, in which risk taking is strongly implicated as one of the key underlying neurobehavioral constructs.
However, little is known about the genetic and environmental influences on behavioral measures of risk-taking propensity in adolescence. The purpose of the present study was three-fold: 1) to characterize developmental changes in risk-taking propensity as measured by the BART, 2) to examine the developmental stability of individual differences, and 3) to estimate the heritability of propensity for risk taking in order to evaluate its potential utility as an endophenotype for genetic studies.
MATERIALS AND METHODS
Sample
Subjects were 745 adolescent twins (48.0% females; age at first assessment: M±SD=12.5±0.21 years) participating in a longitudinal study of the genetics of brain function and behavior. The sample included 169 MZ pairs (82 male 87 female) and 203 DZ pairs (71 male, 49 female, and 83 opposite sex). Twins were recruited from the general population through a twin registry and represented three consecutive birth-year cohorts. The subjects were retested at age 14 (M±SD=14.6±0.24 years; n=448, including 98 MZ pairs (41 male and 57 female) and 125 DZ pairs (41 male, 24 female, and 60 opposite sex). Subjects with a history of serious head trauma or health conditions precluding the laboratory visit or performing experimental tasks (e.g. severe visual impairment or mental retardation) were excluded. The study was approved by the human studies committee of the Washington University School of Medicine. A written informed assent was obtained from all participants, and a written informed consent was obtained from their parents. Zygosity was determined independently using a standard interview administered to twins’ parents, research assistants’ ratings of the twins’ physical similarity, and a set of 7 DNA markers genotyped in 86% of the participants. The reliability of zygosity diagnosis by questionnaire has been demonstrated in previous studies (Kasriel & Eaves, 1976).
Procedure
The BART was presented on the computer in separate testing room according to the protocol described previously (Hunt et al., 2005; Lejuez et al., 2002). At the beginning of each “balloon pumping” trial the computer screen showed a small simulated balloon, a pump button, another button labeled “Collect $$$”, a permanent money-earned display labeled “Total Earned”, and a second display showing the money earned on the current balloon and labeled “Current Balloon”. Participants were instructed to use the computer mouse to click the balloon pump to inflate the balloon to a desired level and told that the total amount of money earned would be paid to them in cash upon the completion of the task. With each pump, balloon size incrementally increased and $0.05 was accrued in a temporary bank (the amount of money in this reserve was never indicated to the participant). At any point during each balloon trial, the participant could stop pumping the balloon and click the “Collect $$$” button. Clicking this button transferred all money from the temporary bank to the permanent bank, and the new total earned was incrementally updated, which was accompanied by a slot machine payoff sound. Then, a new balloon was started. However, if a balloon was pumped past its individual explosion point, an explosive sound effect was generated, and all money in the temporary bank was lost. Importantly, participants were given no detailed information about the probability of a balloon exploding but were told that at some point, each balloon would explode, but the explosion point varied across the balloons and could occur as early as the first pump or as late as the point at which the balloon would expand to fill the entire computer screen (Hunt et al., 2005; Lejuez et al., 2002). The task was thoroughly explained to the participants with visual examples shown on the computer monitor. Following instructions, a total of 30 trials (balloons) were administered to the participants. After the completion of the task, participants were paid the total balance in cash ($6.88 and $8.24 on the average for 12- and 14-years-olds, respectively). The propensity for risk taking was indicated by the number of pumps. Because larger balloon size is associated with a higher probability of explosion, the number of pumps (and, accordingly, the balloon size) indicated the degree of risk the participants were willing to take. The total adjusted number of pumps (ANP) was used as the index of risk taking. The adjusted value did not include the balloons that exploded because the number of pumps is necessarily constrained on these balloons, thereby limiting between-participant variability in the absolute averages (Hunt et al., 2005; Lejuez et al., 2002).
Statistical analyses
The distribution of the ANP variable did not significantly depart from normality (Kolmogorov-Smirnov tests were non-significant at either age), therefore, the raw ANP score was used for statistical analyses. The significance of age differences was assessed using a paired t-test, and possible interaction between age and gender were tested using repeated measures ANOVA.
To estimate heritability, i.e. the relative contribution of genetic and environmental sources to the total phenotypic variance of BART performance, we performed a biometrical genetic analysis using model-fitting, a standard approach in twin genetic research (Neale & Cardon, 1992; Rijsdijk & Sham, 2002). Linear structural equation models were fitted using the Mx package specifically developed to model genetically informative data (Neale et al., 2002). These models assume that phenotypic variance arises from the following factors: additive genetic influences (A), environmental influences shared by family members (C), and individually unique (unshared) environmental influences (E). It is important to note that A and C increase, whereas E decreases, intrapair twin similarity. We did not perform a separate analysis of non-additive genetic influences due to limited power.
Since our sample included both male and female twins and preliminary analyses showed differences between male and female twin correlations, we investigated potential sex differences in genetic and environmental effects on ERP variables by fitting “sex-limitation” models to raw data from five zygosity groups (Neale et al., 2006). The sex-limitation model allows the magnitude of genetic and environmental effects to vary independently in males and females and includes sex-specific genetic influences accounting for the possibility that the set of genes which influences a trait in males is not identical to that which influences a trait in females. Both kinds of sex differences in genetic effects can be tested by fitting sub-models of the general sex-limitation model. The significance of sex-specific influences can be tested by fitting a reduced common effects model that does not include sex-specific genetic effects and assumes that phenotypic variances and covariances are influenced only by the genetic effects that are common to both males and females, but the magnitude of these effects is allowed to differ across sexes.
Path coefficients corresponding to these factors were estimated using a maximum likelihood method, and the goodness of model fit was indicated by a -2LL (log likelihood). Then, different submodels were tested by dropping individual paths from the full model. The significance of individual paths was tested by comparing the goodness of fit of the restricted submodel with the goodness of fit of the more general model using a χ2 test of -2LL difference with degrees of freedom corresponding to the difference in the degrees of freedom between two models (e.g., df=1 if only a one parameter is dropped in the restricted model). If dropping a path significantly reduced the goodness of fit (the χ2 difference was significant), the path was retained in the model, otherwise the more parsimonious restricted model was chosen (i.e. the one that accounted for the variance equally well, but with a fewer number of parameters). Heritability was estimated as the percentage of the total variance of the trait attributed to genetic factors; in addition, 95% confidence intervals of the estimates were computed. A detailed description of the model fitting approach and assessment of heritability can be found elsewhere(Neale & Cardon, 1992; Rijsdijk & Sham, 2002).
RESULTS
The total adjusted number of pumps (ANP), the primary measure of risk taking propensity, significantly increased with age (M±S.D.: 590.7±188.7 and 683.4±175.3 at ages 12 and 14, respectively; paired t-test: t=8.02, df=450, p<.001). There was no significant effect of sex at either age or sex by age interaction. However, a significant effect of ethnicity was observed, with European Americans showing greater propensity for risk-taking compared with ethnic minorities mainly represented by African-Americans (F(1,750)=84.8, p<0.001 and F(1,449)=42.0, p<0.001 at ages 12 and 14, respectively). Because ethnicity can potentially confound intrapair twin correlations, data were adjusted for ethnicity by computing standardized scores separately within ethnic groups. The ANP measure showed a significant correlation between ages 12 and 14 (r= .48, p<.0001), however, the degree of test-retest stability was significantly greater in males than females (r=.56 and .40, respectively, z=2.21, p<.05).
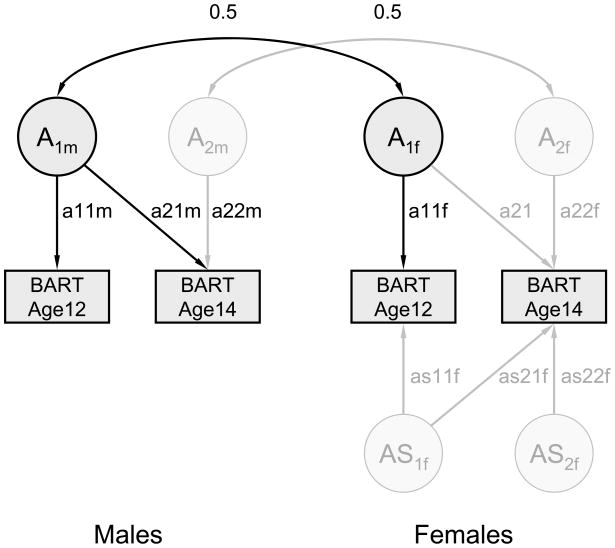
Twin correlations are presented in Table 1. A path diagram showing both the paths retained in the final best-fitting model and the dropped paths is depicted on Fig. 1. The results of genetic model fitting are presented in Table 2, and the estimates of heritability based on the best-fitting model are shown in Table 3.
Table 1.
Intrapair twin correlations for risk-taking propensity by age and sex.
| T1(12) | T2(12) | T1(14) | T2(14) | |
|---|---|---|---|---|
| MZ pairs | ||||
| T1(12) | 1.00 | .30** (.09, .49) | .59*** (.43, .72) | .58*** (.33, .75) |
| T2(12) | .18 (−.03, .38) | 1.00 | .51*** (.24, .71) | .63*** (.40, .79) |
| T1(14) | .26** (.08, .42) | −.04 (−.30, .22) | 1.00 | .60*** (.36, .77) |
| T2(14) | .10(−.16, .35) | .12 (−.15, .37) | −.11 (−.36, 16) | 1.00 |
| DZ same-sex pairs | ||||
| T1(12) | 1.00 | −.01 (−.24, .22) | .41*** (.21, .58) | .02 (−.29, .33) |
| T2(12) | .06 (−.22, .34) | 1.00 | −.03 (.33, .28) | .45** (.17, .67) |
| T1(14) | .43** (.17, .64) | −.36 (−.67, .05) | 1.00 | .19 (−.12, .47) |
| T2(14) | .21 (−.21, .57) | .49** (.11, .75) | .22 (−.20, 57) | 1.00 |
| DZ opposite-sex pairs | ||||
| T1(12) | - | - | - | - |
| T2(12) | .24* (.03, .43) | - | - | - |
| T1(14) | .33*** (.16, .48) | .15 (−.11, .39) | - | - |
| T2(14) | .00 (−.25, .25) | .29* (.04, .50) | .08 (−.18, .33) | - |
For the same-sex MZ and DZ pairs, male and female twin correlations are shown above and below the main diagonal, respectively. T1 and T2 are co-twins of the same pair; age of assessment is shown in brackets. Intrapair twin correlations at ages 12 and 14 are shown in bold face; other coefficients represent cross-age, cross-twin correlations and within-twin, cross-age correlations. Significance levels (two-tailed):
p<0.05;
p<0.01;
p<0.001; lower and upper bounds of the 95% confidence intervals are shown in brackets.
Fig. 1.
Path diagram of the sex-limitation model is depicted for a pair of opposite-sex DZ twins. Paths retained in the best-fitting model are shown in black, and the paths that could be dropped without significant deterioration of fit are shown in grey. Rectangles represent the observed BART phenotype (total adjusted number of pumps) measured at ages 12 and 14; circles represent the corresponding latent genetic factors (A). For simplicity, non-shared environmental influences are not shown. AS stands for female-specific genetic factors. More details can be found in Neale et al. (2006).
Table 2.
Goodness-of-fit statistics for different submodels.
| Model | −2LL | df | Comparison Model | Δχ2 | Δdf | p-value |
|---|---|---|---|---|---|---|
| 1. General effects sex limitation, ACE | 3267.5 | 1171 | - | - | - | - |
| 2. Common effects sex limitation, ACE (drop sex-specific A) | 3267.5 | 1174 | 1 | 0.0 | 3 | n.s |
| 3. AE (drop C) | 3267.5 | 1180 | 2 | 0.0 | 6 | n.s. |
| 4. AE, drop both age-specific and common A in females at age 14 | 3268.2 | 1181 | 3 | .07 | 1 | n.s. |
| 5. AE, drop common A in males at age 14 | 3289.6 | 1182 | 4 | 21.4 | 1 | <.001 |
| 6*. AE, drop age-specific A in males at age 14 | 3268.2 | 1182 | 4 | 0.0 | 1 | n.s |
| 7. AE, drop A in females at age 12 | 3273.9 | 1183 | 5 | 5.7 | 1 | <.05 |
model selected as the best-fitting; −2LL, minus twice the log-likelihood; df, degrees of freedom; Δχ2, Chi-square difference between the submodels; Δdf, difference in the degrees of freedom between the submodels; p-value, significance of differences in the goodness of fit between the compared submodels.
Table 3.
Variance components estimates for risk-taking propensity under the best-fitting model.
| Longitudinal assessment wave | Male Parameters | Female Parameters | ||
|---|---|---|---|---|
| a2 (95% CI) | e2 (95% CI) | a2 (95% CI) | e2 (95% CI) | |
| Age 12 | .28 (.14–.42) | .72 (.58–.86) | .17 (.02–.34) | .83 (.66–.98) |
| Age 14 | .55 (.34–.70) | .45 (.30–.66) | 0 | 1.00 |
a2 is the proportion of total phenotypic variance explained by genetic factors (heritability);
e2 is the proportion of variance explained by non-shared environmental factors including measurement error;
95% confidence intervals of the maximum likelihood estimates of the variance components are shown in brackets.
Sex-specific genetic influences could be dropped without significant deterioration of the goodness of fit. As suggested by twin correlations, shared environmental influences could also be dropped in both sexes. Next, all genetic influences in females at age 14 (i.e. those shared with age 12 and age-specific at age 14) could be dropped. However, dropping genetic influences in females at age 12 led to a significant worsening of fit (Δχ2=5.7, df=1, p<0.05). Finally, we could drop age-specific influences in males at age 14 without significant deterioration of fit, but dropping male genetic influences common to both ages led to a significant worsening of fit (Δχ2=21.4, df=1, p<0.01). Thus, paths retained in the final model included genetic influences in males that were common to both ages, genetic influences in females that were restricted to age 12 only, and non-shared environmental influences (Fig. 1).
DISCUSSION
The first finding of this study was a significant increase in propensity for risk-taking from age 12 to age 14, i.e. the period of transition from early to mid-adolescence. This finding is consistent with the evidence for increased prevalence of high-risk behaviors in adolescents (Bardo, 2004). The second important finding is that, despite these changes, individual differences remain relatively stable as indicated by significant test-retest correlations. Finally, the third and most important finding was significant heritability of individual differences in the propensity for risk-taking. To the best of our knowledge, this is the first demonstration of genetic influences on a direct experimental measure of risk-taking in adolescents.
However, the results also indicate substantial age and gender differences in the genetic/environmental architecture of this trait. At age 12, there are modest but significant influences on risk taking in both genders, with the same genes influencing the trait in males and females, as indicated by the lack of significant sex-specific genetic influences. However, between the ages 12 and 14 this pattern changed considerably: while in males genetic influences increased and accounted for 55% percent of variance, heritability in females became non-significant. It is important to note that in boys the same set of genes influenced the risk-taking phenotype at both ages, and higher heritability at age 14 was due to the increasing relative role of these genetic factors, rather than to additional genetic factors that became active at age 14.
These significant changes in the pattern of genetic and environmental influences over a relatively short period, as well as opposite age-related trends in male and female heritability suggest that the transition from early to mid-adolescence is characterized by substantial changes in the biological substrates of risk-taking behavior. At this time, we can only speculate that these changes can include profound hormonal changes leading to male/female divergence, changes in gene expression, and changes in the role of non-shared, individually unique environmental factors that appear to play a greater role in girls than in boys. Generally, heritability of behavioral phenotypes including externalizing disorders associated with risk taking tends to increase over adolescence (Bergen et al., 2007), however, this was only true for males in the present study. Importantly, we did not find evidence for the role of shared (familial) environment at either age, consistent with extant literature on self-reported personality traits (Bouchard & McGue, 2003). From a behavioral economics perspective, this study suggests that economic decision making is influenced by genetic factors, thus complementing and extending recent reports of significant heritability of economic decision making and risk attitude (Cesarini et al., 2008; Zhong et al., 2009). Finally, the present results suggest that genetic association studies of adolescent risk taking and related traits (e.g. brain activation in “genomic imaging” studies) must account for possible age and sex differences in heritability.
The present study has a number of limitations that indicate the need for further research for a better understanding of the biological underpinnings of risk taking. First, the present report is restricted to a narrow age range of 12–14 years. Although this age period is characterized by intensive changes in adolescents’ biology and behavior, which is likely reflected in different genetic architecture of risk taking at ages 12 and 14, the present results cannot be generalized to the whole period of adolescence, and it remains to be seen how the relative role of genetic and environmental factors in risk-taking will change as the adolescents participating in their study grow older. Second, little is known about specific genetic variants contributing to genetic variability of BART measures. The present study suggests that gene finding efforts must take into account age and sex composition of the sample. Next, the critical role of non-shared (individually unique) environmental factors in shaping individual differences in risk preference in girls raises the question about the nature of individual experiences that affect the propensity to take risks.
In conclusion, this study provides the first evidence for heritability of an experimental measure of risk-taking in adolescence and indicates that the relative role of genetic and environmental factors changes with age, and these changes are different in males and females. These findings suggest that risk-taking propensity as indicated by BART can be a useful endophenotype for genetic studies of psychopathology, but its utility as endophenotype may be restricted to the male gender.
Acknowledgments
Supported by NIH grants DA018899 and DA027096 from the National Institute on Drug Abuse and the Midwest Alcoholism Research Center (P50 AA11998)
References
- Anokhin AP, Birbaumer N, Lutzenberger W, Nikolaev A, Vogel F. Age increases brain complexity. Electroencephalography and Clinical Neurophysiology. 1996;99(1):63–68. doi: 10.1016/0921-884x(96)95573-3. [DOI] [PubMed] [Google Scholar]
- Bardo MT. High-risk behavior during adolescence: comments on part I. Annals of the New York Academy of Sciences. 2004;1021:59–60. doi: 10.1196/annals.1308.006. [DOI] [PubMed] [Google Scholar]
- Bergen SE, Gardner CO, Kendler KS. Age-related changes in heritability of behavioral phenotypes over adolescence and young adulthood: a meta-analysis. Twin Res Hum Genet. 2007;10(3):423–433. doi: 10.1375/twin.10.3.423. [DOI] [PubMed] [Google Scholar]
- Bornovalova MA, Cashman-Rolls A, O’Donnell JM, Ettinger K, Richards JB, Dewit H, Lejuez CW. Risk taking differences on a behavioral task as a function of potential reward/loss magnitude and individual differences in impulsivity and sensation seeking. Pharmacology, Biochemistry and Behavior. 2008 doi: 10.1016/j.pbb.2008.10.023. (in press) [DOI] [PubMed] [Google Scholar]
- Bouchard TJ, Jr, McGue M. Genetic and environmental influences on human psychological differences. Journal of Neurobiology. 2003;54(1):4–45. doi: 10.1002/neu.10160. [DOI] [PubMed] [Google Scholar]
- Cesarini D, Dawes CT, Fowler JH, Johannesson M, Lichtenstein P, Wallace B. Heritability of cooperative behavior in the trust game. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(10):3721–3726. doi: 10.1073/pnas.0710069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. The teen brain: insights from neuroimaging. Journal of Adolescent Health. 2008;42(4):335–343. doi: 10.1016/j.jadohealth.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Hopko DR, Lejuez CW, Daughters SB, Aklin WM, Osborne A, Simmons BL, Strong DR. Construct validity of the balloon analogue risk task (BART): Relationship with MDMA use by inner-city drug users in residential treatment. Journal of Psychopathology and Behavioral Assessment. 2006;28(2):95–101. [Google Scholar]
- Hunt MK, Hopko DR, Bare R, Lejuez CW, Robinson EV. Construct validity of the Balloon Analog Risk Task (BART) - Associations with psychopathy and impulsivity. Assessment. 2005;12(4):416–428. doi: 10.1177/1073191105278740. [DOI] [PubMed] [Google Scholar]
- Kasriel J, Eaves L. The zygosity of twins: further evidence on the agreement between diagnosis by blood groups and written questionnaires. Journal of Biosocial Science. 1976;8(3):263–266. doi: 10.1017/s0021932000010737. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Schochet T, Landry CF. Risk taking and novelty seeking in adolescence: introduction to part I. Annals of the New York Academy of Sciences. 2004;1021:27–32. doi: 10.1196/annals.1308.003. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin W, Daughters S, Zvolensky M, Kahler C, Gwadz M. Reliability and validity of the youth version of the Balloon Analogue Risk Task (BART-Y) in the assessment of risk-taking behavior among inner-city adolescents. Journal of Clinical Child and Adolescent Psychology. 2007;36(1):106–111. doi: 10.1080/15374410709336573. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Strong DR, Brown RA. Evaluation of a behavioral measure of risk taking: The Balloon Analogue Risk Task (BART) Journal of Experimental Psychology-Applied. 2002;8(2):75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx:Statistical Modeling. 6. Richmond, VA: Department of Psychiatry; 2002. [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Vol. 67. Dordrecht: Kluwer Academic Publishers; 1992. [Google Scholar]
- Neale MC, Roysamb E, Jacobson K. Multivariate genetic analysis of sex limitation and G x E interaction. Twin Research and Human Genetics. 2006;9(4):481–489. doi: 10.1375/183242706778024937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick MD, Bearman PS, Blum RW, Bauman KE, Harris KM, Jones J, Tabor J, Beuhring T, Sieving RE, Shew M, Ireland M, Bearinger LH, Udry JR. Protecting adolescents from harm. Findings from the National Longitudinal Study on Adolescent Health. JAMA. 1997;278(10):823–832. doi: 10.1001/jama.278.10.823. [DOI] [PubMed] [Google Scholar]
- Rijsdijk FV, Sham PC. Analytic approaches to twin data using structural equation models. Brief Bioinform. 2002;3(2):119–133. doi: 10.1093/bib/3.2.119. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: what changes, and why? Annals of the New York Academy of Sciences. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR, Minowski A. The myelogenetic cycles of regional maturation of the brain. Regional Development of the Brain in Early life. 1967:3–70. [Google Scholar]
- Zhong S, Chew SH, Set E, Zhang J, Xue H, Sham PC, Ebstein RP, Israel S. The heritability of attitude toward economic risk. Twin Research and Human Genetics. 2009;12(1):103–107. doi: 10.1375/twin.12.1.103. [DOI] [PubMed] [Google Scholar]