Abstract
Background
Inflammatory and hemostasis-related biomarkers may identify women at risk of stroke.
Methods
Hormones and Biomarkers Predicting Stroke is a study of ischemic stroke among postmenopausal women participating in the Women’s Health Initiative Observational Study (n = 972 case-control pairs). A Biomarker Risk Score was derived from levels of seven inflammatory and hemostasis-related biomarkers that appeared individually to predict risk of ischemic stroke: C-reactive protein, interleukin-6, tissue plasminogen activator, D-dimer, white blood cell count, neopterin, and homocysteine. The c index was used to evaluate discrimination.
Results
Of all the individual biomarkers examined, C-reactive protein emerged as the only independent single predictor of ischemic stroke (adjusted odds ratio comparing Q4 versus Q1 = 1.64, 95% confidence interval: 1.15–2.32, p = 0.01) after adjustment for other biomarkers and standard stroke risk factors. The Biomarker Risk Score identified a gradient of increasing stroke risk with a greater number of elevated inflammatory/hemostasis biomarkers, and improved the c index significantly compared with standard stroke risk factors (p = 0.02). Among the subset of individuals who met current criteria for “high risk” levels of C-reactive protein (> 3.0 mg/L), the Biomarker Risk Score defined an approximately two-fold gradient of risk. We found no evidence for a relationship between stroke and levels of E-selectin, fibrinogen, tumor necrosis factor-alpha, vascular cell adhesion molecule-1, prothrombin fragment 1+2, Factor VIIC, or plasminogen activator inhibitor-1 antigen (p >0.15).
Discussion
The findings support the further exploration of multiple-biomarker panels to develop approaches for stratifying an individual’s risk of stroke.
Keywords: stroke, epidemiology, women
A number of prospective studies have reported that risk of incident cardiovascular disease (CVD) among apparently healthy individuals is associated with levels of serum pentraxins [1–3], inflammatory cytokines [4–7], cellular adhesion molecules [8], and markers of coagulation and fibrinolysis activity [9, 10]. In older adults, however, the results of such studies have been inconsistent, and stroke has not been as well-studied as have other types of vascular events [1, 3, 11–16]. In light of the complex interplay of mediators that may contribute to the development of ischemic stroke, panels of several biomarkers related to inflammation, coagulation, and fibrinolysis have the potential to provide additional information about risk as compared with any single biomarker.
The present investigation was designed to evaluate the ability of inflammation and hemostasis-related biomarkers to predict the risk of future acute ischemic stroke among postmenopausal women. We examined biomarkers that had been previously shown to predict risk of ischemic stroke, as well as others that were chosen either based upon known biological mechanisms of stroke, or based upon studies linking them with other vascular outcomes such as coronary events. Biomarkers included seven circulating biomarkers of inflammation: total white blood cell count (WBC) and C-reactive protein (CRP), which are systemic markers of global inflammatory activity; pro-inflammatory cytokines including interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α); neopterin, a well-characterized marker of cell-mediated immunity which is synthesized by macrophages upon stimulation by interferon-γ released by activated T-helper type-1 (Th1) lymphocytes; and cellular adhesion molecules of the selectin (E-selectin) and Ig super-family adhesion molecule type (vascular cell adhesion molecule, VCAM). The panel also included six biomarkers relating to blood coagulation, fibrinolysis, and platelets: the clotting factor Factor VII; prothrombin fragment 1+2, a marker of blood coagulation produced by cleavage of prothrombin by Factor X; tissue plasminogen activator (tPA), the main endothelial cell derived activator of fibrinolysis; plasminogen activator inhibitor type 1 (PAI-1), an inhibitor of fibrinolysis; D-dimer, a marker of fibrin turnover; and fibrinogen, the precursor to fibrin and mediator of platelet aggregation which also behaves as an acute-phase reactant. We also evaluated homocysteine, a marker of folate metabolism that may relate to risk of vascular events through mechanisms relating to atherogenesis, coagulation, or fibrinolysis. Multivariate approaches were used to evaluate whether stroke risk was related to a Biomarker Risk Score based on levels of multiple inflammatory and hemostatic biomarkers.
Methods
Study Population
The present investigation is part of the Hormones and Biomarkers Predicting Stroke (HaBPS) study, a case-control study of incident ischemic stroke nested within the Women’s Health Initiative (WHI) Observational Study. A total of 93,676 postmenopausal 50–79 year old women were recruited from October 1993 through December 1998. Women ineligible or not interested in the WHI clinical trial components, which examined postmenopausal hormone therapy, low-fat diet, and calcium/vitamin D supplementation, were given an opportunity to enroll in the Observational Study, and others were recruited specifically for Observational Study participation. Institutional Review Board approval and informed consent were obtained.
Data Collection
All women who enrolled in WHI completed visits at baseline to determine eligibility and collect data including questionnaires, physical measurements, biological specimens, and laboratory tests. During an initial visit, a physical examination was performed by trained staff using standardized procedures to obtain height and weight and seated blood pressure. Fasting blood samples were collected at study baseline by clinic staff who followed a standardized protocol for venipuncture, centrifugation and separation of blood, freezing of specimens on site at −70C, and shipping of specimens to the central WHI repository for long-term storage. Questionnaires elicited information on many health-related factors including medical history, health behaviors including smoking habits, and demographics. Use of prescription drugs was inventoried.
Variable definition
Hypertension was defined as self-report of hypertension diagnosis with anti-hypertensive medication use, and/or systolic blood pressure >= 140 mm Hg, and/or diastolic blood pressure >=90 mm Hg. Diabetes was defined as being on treatment for diabetes by self-report and/or having a fasting glucose level >=126mg/dl. Body mass index (BMI) was calculated from measured weight and height as kg/m2. A prior diagnosis of high cholesterol requiring pills was determined by self-report.
Follow-up and outcome ascertainment
All incident strokes, other vascular events, and deaths were identified through self report at annual participant contacts and through third-party reports by family members and proxies. Medical records were obtained for potential strokes and other pre-defined health events, and adjudication was performed locally by trained physician adjudicators who assigned a diagnosis according to standard criteria. As part of the HaBPS study, all locally adjudicated strokes were then sent for central adjudication by study neurologists (DMR, AEB, JL). Ischemic stroke was defined as the rapid onset of a persistent neurologic deficit attributed to an obstruction lasting more than 24 hours and without evidence for other causes. Stroke subtype was defined using Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria [17]. Only stroke events that required hospitalization were considered as potential outcomes.
Case and control subject identification
For the HaBPS case-control study, cases included the first 972 confirmed incident ischemic strokes occurring between study baseline and July 1, 2003. All cases not verified during central adjudication as being an ischemic stroke were excluded, including transient ischemic attacks (TIAs) or hemorrhagic strokes. Control subjects were matched individually to the cases according to age (2 years), race/ethnicity, date of enrollment, and follow-up time. Women with a history of myocardial infarction or stroke at baseline were excluded from both the case and control groups.
Biomarker Measurement
Stored blood specimens were sent to the WHI core laboratory for measurement of levels of plasma CRP, IL-6, TNF-α, neopterin, E-selectin, VCAM, Factor VII, prothrombin fragment 1+2, tPA, PAI-1, fibrinogen, and homocysteine, as well as fasting plasma glucose and lipids. These laboratory tests were performed between September 2005 and March 2006, approximately 7 to 12 years since specimen collection (depending on when participants were enrolled). Baseline blood samples had been sent to a local laboratory for analysis of white blood cell count at the time of collection.
Statistical Analyses
To compare characteristics of cases and matched controls, the McNemar’s Chi-square test was used for categorical variables. We examined distributions of biomarkers to assess the need for normalizing transformations and identify outlying values. Due to non-normality of several biomarkers, Wilcoxon Signed Ranks were used to compare levels of median biomarkers between the matched case-control pairs. Spearman correlations between all biomarkers were calculated among the controls. Multivariate unconditional logistic regression, with adjustment for matching factors and confounders, was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) across quartiles of biomarkers. Quartile cut points were defined according to the distribution of biomarkers among controls. Significance tests were computed using two approaches: 1) fitting an ordinal variable for biomarker quartiles as a continuous variable; and 2) examining the OR comparing extreme biomarker quartiles. To examine the joint predictive value of several biomarkers, we derived a Biomarker Risk Score (BRS) by assigning 1 point for each biomarker value that was in the highest quartile of the control group distribution. Biomarkers that were used in computing the BRS were those that were individually associated with stroke at ptrend < 0.15 when modeled as a trend across quartiles; this liberal significance level reflects our hypothesis that a biomarker may contribute importantly to a multiple-biomarker strategy even if it did not achieve the conventional level of statistical significance in its individual association with stroke risk. We examined the association of BRS with risk of stroke using logistic regression models, both overall and in subgroups defined by CRP above and below 3.0 mg/L, which is the cutoff for defining high CRP in currently-available guidelines[18].
Models adjusted for the matching variables age and race/ethnicity, and were additionally adjusted for aspirin use, BMI, diabetes, systolic blood pressure, anti-hypertensive medication use, smoking, lipid-lowering medication use, fasting glucose, low density lipoprotein (LDL) cholesterol and high density lipoprotein (HDL) cholesterol. Additional adjustment for history of atrial fibrillation, diastolic blood pressure, history of revascularization or estrogen and progestin therapy did not affect the results substantially. To assess variation in associations by stroke subtype, we determined the ORs for incident stroke in subgroups of women defined by the TOAST classification. We also examined for effect modification by age, hormone use, hypertension, diabetes, smoking, race, HDL cholesterol, and LDL cholesterol. Adjusted models were based on women for whom complete data were available on all covariates of interest. To assess the ability of models to discriminate between ischemic strokes and controls we calculated the c index, with the use of cross-validation methods to reduce bias introduced by the use of the same population to develop and evaluate models [19]
Results
Subject characteristics
The follow-up time in years was, for stroke cases, mean=4.4, SD=2.3, median=4.5, and for control subjects, mean=7.9, SD=1.3, median=8.0. Ischemic stroke cases were more likely than controls to be current smokers, to have high BMI, and to report a history of atrial fibrillation, angina or revascularization (Table 1). Additionally, cases were more likely to have hypertension, diabetes, and use of lipid-lowering drugs and aspirin. Significant correlations among inflammatory and hemostasis biomarkers were observed, ranging as high as r = 0.51 (p < 0.001) for Factor VII and prothrombin fragment 1+2, and r = 0.47 (p < 0.001) for CRP and IL6 (Table 2). Significant differences (p < 0.05) were present between matched cases and controls in median baseline levels of several of the biomarkers under study (CRP, IL-6, tPA, WBC, neopterin, E-selectin, TNF-alpha, and VCAM-1), whereas case-control differences in D-dimer, homocysteine, and PAI-1 antigen were of borderline statistical significance (p = 0.05 –0.10) (Table 3).
Table 1.
Baseline characteristics among ischemic stroke cases and age- and race-matched controls
| Controls (n=972) | Cases (n=972) | ||||
|---|---|---|---|---|---|
| N | % | N | % | p-value | |
| Age groups, years | |||||
| 50–59 | 95 | 9.8 | 95 | 9.8 | NA |
| 60–69 | 392 | 40.3 | 392 | 40.3 | |
| 70–79 | 485 | 49.9 | 485 | 49.9 | |
| Race/ethnicity | |||||
| American Indian/Alaskan Native | 5 | 0.5 | 5 | 0.5 | NA |
| Asian/Pacific Islander | 21 | 2.2 | 21 | 2.2 | |
| African American | 80 | 8.2 | 80 | 8.2 | |
| Hispanic | 20 | 2.1 | 20 | 2.1 | |
| Other | 13 | 1.3 | 13 | 1.3 | |
| White | 833 | 85.7 | 833 | 85.7 | |
| Smoking | |||||
| Never | 526 | 54.6 | 505 | 52.6 | <0.01 |
| Past | 400 | 41.5 | 377 | 39.2 | |
| Current | 37 | 3.8 | 79 | 8.2 | |
| Alcohol | |||||
| Non-Drinker | 113 | 11.7 | 118 | 12.2 | 0.33 |
| Past Drinker | 179 | 18.5 | 212 | 21.9 | |
| <1 Drink per Month | 119 | 12.3 | 115 | 11.9 | |
| <1 Drink Week | 202 | 20.8 | 201 | 20.7 | |
| 1-<7 a Week | 240 | 24.7 | 199 | 20.5 | |
| 7+ a Week | 117 | 12.1 | 124 | 12.8 | |
| Hormone Use | |||||
| No current hormone use | 603 | 62.0 | 588 | 60.5 | 0.51 |
| Any current hormone use | 369 | 38.0 | 384 | 39.5 | |
| Type of Hormone among Current Users | |||||
| Estrogen Alone | 237 | 64.2 | 271 | 70.6 | 0.60 |
| Estrogen + Progestin | 132 | 35.7 | 113 | 29.4 | |
| BMI | |||||
| <25 | 390 | 40.7 | 335 | 34.8 | <0.01 |
| 25–30 | 346 | 36.1 | 365 | 37.9 | |
| >30 | 222 | 23.2 | 263 | 27.3 | |
| History of Atrial Fibrillation | |||||
| No | 894 | 94.2 | 858 | 90.2 | <0.01 |
| Yes | 55 | 5.8 | 93 | 9.8 | |
| History of Angina | |||||
| No | 906 | 94.5 | 876 | 90.7 | <0.01 |
| Yes | 53 | 5.5 | 90 | 9.3 | |
| History of Revascularization | |||||
| No | 937 | 98.8 | 913 | 96.0 | 0.0001 |
| Yes | 11 | 1.2 | 38 | 4.0 | |
| Diastolic Blood Pressure, mm Hg | |||||
| <90 | 923 | 95.0 | 892 | 92.2 | 0.02 |
| ≥90 | 49 | 5.0 | 75 | 7.8 | |
| Systolic Blood Pressure, mm Hg | |||||
| ≤120 | 329 | 33.9 | 198 | 20.4 | <0.0001 |
| 120– 140 | 384 | 39.5 | 372 | 38.4 | |
| >140 | 259 | 26.7 | 400 | 41.2 | |
| Hypertension* | |||||
| No | 531 | 55.6 | 347 | 36.6 | <0.0001 |
| Yes | 424 | 44.4 | 600 | 63.4 | |
| Use of Hypertensive Medications | |||||
| No | 632 | 65.0 | 511 | 52.6 | <0.0001 |
| Yes | 340 | 35.0 | 461 | 47.4 | |
| Diabetes† | |||||
| No | 889 | 91.7 | 805 | 83.3 | <0.0001 |
| Yes | 81 | 8.4 | 162 | 16.7 | |
| Use of Aspirin | |||||
| No | 732 | 75.3 | 675 | 69.4 | <0.01 |
| Yes | 240 | 24.7 | 297 | 30.6 | |
| High Cholesterol Requiring Pills | |||||
| No | 807 | 84.7 | 768 | 81.0 | 0.05 |
| Yes | 146 | 15.3 | 180 | 19.0 | |
| Controls (n=972) | Cases (n=972) | p-values from paired t-tests | |||
| Continuous Variables | Mean | SD | Mean | SD | |
| BMI | 27.0 | 5.3 | 27.7 | 5.9 | <0.01 |
| Systolic Blood Pressure | 130.1 | 18.0 | 137.2 | 19.4 | <0.001 |
| Diastolic Blood Pressure | 74.1 | 9.5 | 75.5 | 10.1 | <0.01 |
| Low Density Lipoprotein Cholesterol | 139.0 | 36. 7 | 140.8 | 37.4 | 0.31 |
| High Density Lipoprotein Cholesterol | 59.8 | 16.4 | 57.2 | 16.2 | <0.01 |
BMI, body mass index
Subjects with missing values excluded from table.
Cases and controls were matched on age and race/ethnicity.
Hypertension defined as either on medication by self report or SBP>=140 or DBP >=90
Diabetes defined as being on treatment for diabetes by self report or fasting glucose level >126mg/dl
Table 2.
Correlations among inflammatory and hemostasis biomarkers among control study participants
| Prothrombin | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRP | IL-6 | TNF-alpha | Neopterin | E-Selectin | VCAM | Factor VII | Fragment 1+2 | tPA | PAI-1 | D-dimer | Fibrinongen | Homocysteine | |
| WBC | 0.25 | 0.30 | 0.08 | 0.00 | 0.22 | 0.00 | 0.05 | 0.00 | 0.21 | 0.13 | 0.05 | 0.14 | 0.05 |
| <0.0001 | <0.0001 | 0.02 | 0.92 | <0.0001 | 0.96 | 0.14 | 0.99 | <0.0001 | <0.001 | 0.11 | <0.0001 | 0.14 | |
| CRP | 0.47 | 0.14 | 0.11 | 0.15 | −0.01 | 0.15 | 0.04 | 0.15 | 0.06 | 0.18 | 0.27 | 0.01 | |
| <0.0001 | <0.0001 | <0.001 | <0.0001 | 0.83 | <0.0001 | 0.38 | <0.0001 | 0.09 | <0.0001 | <0.0001 | 0.78 | ||
| IL-6 | 0.21 | 0.21 | 0.22 | 0.10 | −0.01 | −0.07 | 0.29 | 0.14 | 0.27 | 0.34 | 0.10 | ||
| <0.0001 | <0.0001 | <0.0001 | <0.01 | 0.87 | 0.07 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.01 | |||
| TNF-alpha | 0.37 | 0.17 | 0.38 | 0.05 | 0.03 | 0.11 | 0.13 | 0.12 | 0.13 | 0.19 | |||
| <0.0001 | <0.0001 | <0.0001 | 0.19 | 0.43 | <0.01 | <0.0001 | <0.001 | <0.001 | <0.0001 | ||||
| Neopterin | 0.09 | 0.41 | −0.02 | −0.02 | 0.14 | 0.05 | 0.21 | 0.11 | 0.30 | ||||
| <0.01 | <0.0001 | 0.48 | 0.70 | <0.0001 | 0.13 | <0.0001 | <0.001 | <0.0001 | |||||
| E-Selectin | 0.08 | 0.07 | 0.01 | 0.30 | 0.18 | 0.16 | 0.05 | 0.10 | |||||
| 0.01 | 0.03 | 0.72 | <0.0001 | <0.0001 | <0.0001 | 0.13 | <0.01 | ||||||
| VCAM | −0.10 | −0.02 | 0.08 | −0.04 | 0.16 | 0.13 | 0.31 | ||||||
| 0.01 | 0.66 | 0.02 | 0.30 | <0.0001 | <0.0001 | <0.0001 | |||||||
| Factor VII | 0.51 | −0.02 | 0.05 | 0.17 | −0.26 | 0.02 | |||||||
| <0.0001 | 0.53 | 0.14 | <0.0001 | <0.0001 | 0.52 | ||||||||
| Prothrombin Fragment 1+2 | −0.17 | −0.04 | 0.17 | −0.17 | −0.02 | ||||||||
| <0.0001 | 0.29 | <0.0001 | <0.0001 | 0.66 | |||||||||
| tPA | 0.32 | 0.02 | 0.17 | 0.16 | |||||||||
| <0.0001 | 0.57 | <0.0001 | <0.0001 | ||||||||||
| PAI-1 | 0.05 | 0.12 | 0.08 | ||||||||||
| 0.13 | <0.001 | 0.02 | |||||||||||
| D-Dimer | 0.17 | 0.18 | |||||||||||
| <0.0001 | <0.0001 | ||||||||||||
| Fibrinogen | 0.07 | ||||||||||||
| 0.03 | |||||||||||||
Data in Table represent: Spearman’s r p-value
CRP, C-reactive protein; IL-6, interleukin-6; tPA, tissue plasminogen activator; WBC, white blood cell count; TNF, tumor necrosis factor; VCAM-1, vascular cell adhesion molecule-1; PAI-1, plasminogen activator inhibitor-1
Note: We observed no significant correlation between CRP and LDL level (Spearman correlation r=−0.03, p = 0.30).
Table 3.
Inflammatory and hemostasis biomarker values among ischemic stroke cases and age- and race- matched controls
| Cases | Controls | Wilcoxon Signed Rank Test | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Median | IQR | Median | Lower 25%ile | Upper 25%ile | IQR | # case-control pairs | p (S) |
| CRP (mg/dL) | 3.6 | 5.7 | 2.6 | 1.1 | 5.2 | 4.1 | 939 | <0.0001 |
| IL-6 (PG/ml) | 2.1 | 1.9 | 1.8 | 1.3 | 2.7 | 1.4 | 949 | <0.0001 |
| tPA (mg/ml) | 9.9 | 5.7 | 8.7 | 6.3 | 11.3 | 5.0 | 776 | <0.0001 |
| D-dimer (ng/mL) | 489.7 | 343.0 | 460.4 | 334.5 | 647.4 | 312.9 | 780 | 0.06 |
| WBC (Kcell/ml) | 6.0 | 2.2 | 5.7 | 4.8 | 6.8 | 2.0 | 940 | <0.0001 |
| Neopterin (ng/mL) | 1.8 | 0.9 | 1.7 | 1.3 | 2.1 | 0.7 | 944 | <0.001 |
| Homocysteine (Umol/L) | 8.5 | 3.7 | 8.2 | 6.6 | 10.2 | 3.6 | 930 | 0.06 |
| E-Selectin (ng/mL) | 31.0 | 18.0 | 29.0 | 21.0 | 38.0 | 17.0 | 957 | <0.0001 |
| Fibrinogen (mg/dL) | 279.0 | 87.0 | 273.0 | 242.0 | 316.0 | 74.0 | 780 | 0.43 |
| TNF-alpha (pg/mL) | 1.4 | 0.8 | 1.4 | 1.0 | 1.8 | 0.8 | 885 | 0.02 |
| VCAM-1 (ng/mL) | 698.0 | 288.0 | 683.5 | 561.0 | 835.0 | 274.0 | 933 | <0.01 |
| Prothrombin fragment 1+2 (nmol/L) | 1.2 | 1.1 | 1.2 | 0.9 | 1.9 | 1.0 | 583 | 0.61 |
| Factor VII (%) | 151.0 | 100.0 | 149.0 | 121.0 | 217.0 | 96.0 | 780 | 0.65 |
| PAI-1 (ng/mL) | 26.6 | 32.1 | 23.6 | 14.6 | 43.2 | 28.6 | 773 | 0.09 |
IQR, interquartile range; CRP, C-reactive protein; IL-6, interleukin-6; tPA, tissue plasminogen activator; WBC, white blood cell count; TNF, tumor necrosis factor; VCAM-1, vascular cell adhesion molecule-1; PAI-1, plasminogen activator inhibitor-1
As an alternative to the Wilcoxon signed rank test, Mann-Whitney U tests were also performed and this provided similar conclusions about significant (p<0.05) p-values, except for D-dimers (<0.01, versus 0.06 in table), VCAM-1 (0.09, versus <0.01 in table), and PAI-1 (0.04, versus 0.09 in table).
Biomarkers and risk of ischemic stroke
We examined the associations of incident ischemic stroke with levels of each individual biomarker. In analyses of linear trends across biomarker quartiles, the associations for CRP (ptrend < 0.001), IL-6 (ptrend < 0.001), tPA (ptrend = 0.02), D-dimer (ptrend = 0.03) and WBC (ptrend = 0.03), met the standard p < 0.05 criteria for statistical significance in models that adjusted for aspirin use, BMI, diabetes, systolic blood pressure, smoking, high cholesterol requiring pills, anti-hypertensive medication use, fasting glucose, LDL and HDL cholesterol (Table 4). Biomarkers for which the trends across quartiles were of borderline significance (p = 0.05 to p = 0.15) were neopterin (ptrend = 0.05) and homocysteine (ptrend = 0.10). Similar results were observed in analyses that compared individuals in the highest versus the lowest quartiles of biomarkers. Specifically, significant (p < 0.05) associations were found for quartile comparisons of CRP (adjusted OR comparing Q4 versus Q1 = 1.78, 95% CI: 1.32–2.39), IL-6 (OR = 1.68, 95% CI: 1.25–2.26), tPA (OR = 1.42, 95% CI: 1.03–1.94), D-dimer (OR = 1.52, 95% CI: 1.12–2.08) and WBC (OR = 1.46, 95%CI: 1.10–1.94), There was no evidence for a relationship between risk of incident ischemic stroke and levels of E-selectin, fibrinogen, TNF-alpha, VCAM-1, prothrombin fragment 1+2, Factor VIIC, or PAI-1 antigen, either as analyses of quartile trends or comparisons of extreme quartiles.
Table 4.
Adjusted analyses of the association between inflammatory and hemostasis biomarker levels with risk of incident ischemic stroke
| Adjusted for known stroke risk factors | Adjusted for known stroke risk factors and other biomarkers | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| QUARTILE CATEGORIES | QUARTILE CATEGORIES (740 cases, 775 controls) | ||||||||||
| 1 | 2 | 3 | 4 | p-trend† | 1 | 2 | 3 | 4 | p-trend† | ||
| C-Reactive Protein cases/controls: 874/892 | OR 95% CI | 1 (reference) | 1.18 (0.88, 1.59) | 1.24 (0.92, 1.67) | 1.78 (1.32, 2.39) | <0.001 | 1 (reference) | 1.19 (0.86, 1.63) | 1.04 (0.75, 1.45) | 1.64 (1.15, 2.32) | 0.01 |
| Interleukin 6 892/912 | OR 95% CI | 1 (reference) | 1.26 (0.94, 1.68) | 1.15 (0.86, 1.55) | 1.68 (1.25, 2.26) | <0.001 | 1 (reference) | 1.07 (0.77, 1.48) | 0.91 (0.64, 1.28) | 1.22 (0.85, 1.75) | 0.43 |
| Tissue Plasminogen Activator 789/820 | OR 95% CI | 1 (reference) | 0.90 (0.66, 1.22) | 1.00 (0.73, 1.36) | 1.42 (1.03, 1.94) | 0.02 | 1 (reference) | 0.93 (0.68, 1.29) | 1.04 (0.75, 1.44) | 1.35 (0.96, 1.89) | 0.06 |
| D-Dimer 791/821 | OR 95% CI | 1 (reference) | 1.5 (1.11, 2.03) | 1.33 (0.97, 1.81) | 1.52 (1.12, 2.08) | 0.03 | 1 (reference) | 1.43 (1.04, 1.97) | 1.20 (0.86, 1.68) | 1.30 (0.92, 1.83) | 0.19 |
| White Blood Cell Count 885/904 | OR 95% CI | 1 (reference) | 1.21 (0.91, 1.60) | 1.03 (0.77, 1.37) | 1.46 (1.10, 1.94) | 0.03 | 1 (reference) | 1.20 (0.88, 1.64) | 0.90 (0.65, 1.24) | 1.20 (0.87, 1.67) | 0.44 |
| Neopterin 878/893 | OR 95% CI | 1 (reference) | 0.79 (0.59, 1.05) | 0.98 (0.74, 1.30) | 1.24 (0.94, 1.63) | 0.05 | 1 (reference) | 0.73 (0.53, 1.00) | 0.92 (0.67, 1.26) | 1.10 (0.79, 1.52) | 0.30 |
| Homocysteine 872/886 | OR 95% CI | 1 (reference) | 1.15 (0.86, 1.52) | 1.23 (0.93, 1.64) | 1.26 (0.95, 1.68) | 0.10 | 1 (reference) | 1.13 (0.83, 1.55) | 1.15 (0.84, 1.59) | 1.11 (0.80, 1.54) | 0.60 |
| E-Selectin 894/910 | OR 95% CI | 1 (reference) | 0.93 (0.70, 1.22) | 1.00 (0.76, 1.31) | 1.15 (0.86, 1.53) | 0.30 | Not included in model | ||||
| Fibrinogen 791/821 | OR 95% CI | 1 (reference) | 0.75 (0.56, 1.01) | 0.89 (0.67, 1.18) | 0.91 (0.67, 1.22) | 0.72 | Not included in model | ||||
| TNF-alpha 851/865 | OR 95% CI | 1 (reference) | 0.98 (0.74, 1.30) | 1.03 (0.78, 1.37) | 1.16 (0.87, 1.54) | 0.27 | Not included in model | ||||
| VCAM-1875/891 | OR 95% CI | 1 (reference) | 0.97 (0.73, 1.28) | 1.00 (0.76, 1.32) | 1.00 (0.75, 1.32) | 0.96 | Not included in model | ||||
| Prothrombin Fragment 1 + 2580/601 | OR 95% CI | 1 (reference) | 0.90 (0.64, 1.27) | 1.18 (0.84, 1.65) | 1.04 (0.74, 1.47) | 0.57 | Not included in model | ||||
| Factor VIIC 791/821 | OR 95% CI | 1 (reference) | 0.77 (0.58, 1.03) | 0.91 (0.68, 1.21) | 0.91 (0.68, 1.21) | 0.75 | Not included in model | ||||
| PAI-1 Antigen 785/820 | OR 95% CI | 1 (reference) | 0.80 (0.59, 1.08) | 1.03 (0.77, 1.38) | 1.00 (0.74, 1.35) | 0.60 | Not included in model | ||||
Adjusted for aspirin use, BMI, diabetes, systolic blood pressure, smoking, high cholesterol requiring pills, anti-hypertensive medication use, fasting glucose, low density lipoprotein and high density lipoprotein.
Test of trend across quartiles of biomarkers were conducted by assigning a numerical value for each quartile (1, 2, 3, 4) and fitting this continuous variable in the model
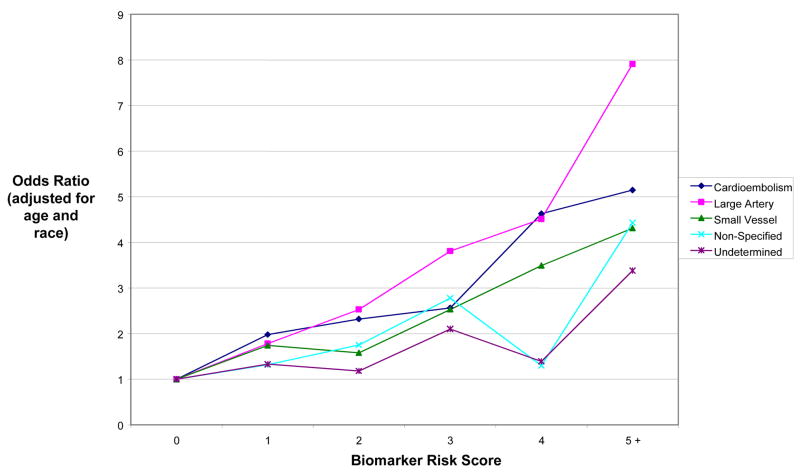
In models that included CRP, IL-6, tPA, D-dimer, WBC neopterin and homocysteine together as predictor variables, CRP retained an independent association with risk of ischemic stroke (adjusted OR comparing Q4 versus Q1 = 1.64, 95% CI: 1.15–2.32, ptrend = 0.01) (Table 4). The only other biomarker that achieved even a borderline level of statistical significance when multiple biomarkers were included in models was tPA (adjusted OR comparing Q4 versus Q1 = 1.35, 95% CI: 0.96–1.89, ptrend = 0.06). In analyses of CRP in relation to subtypes of stroke, the adjusted OR comparing Q4 versus Q1 was 2.27 (95% CI = 1.35, 3.84) for cardioembolic stroke (n=186 cases), 1.35 (95% CI = 0.67, 2.70) for large-artery stroke (n=86 cases), and 1.68 (95% CI = 1.07, 2.63) for small-vessel stroke (n=230 cases).
Biomarker Risk Score and risk of ischemic stroke
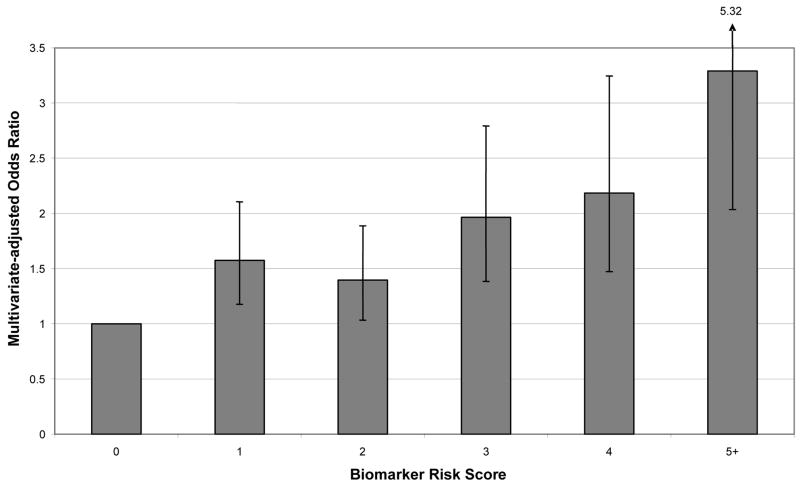
Biomarker Risk Score was derived based on levels of seven biomarkers that met predefined statistical criteria (ptrend <0.15): CRP, IL-6, tPA, D-Dimer, WBC, neopterin, and homocysteine. Individuals were assigned one point for each biomarker measurement that was in the top quartile of the control distribution. In multivariate analyses, Biomarker Risk Score was an independent predictor of ischemic stroke, after adjusting for stroke risk factors (p < 0.001) (Figure 1). Higher Biomarker Risk Score predicted all major etiologic subtypes of stroke (Figure 2). In stratified analyses with tests for interaction, we found no evidence that the ORs describing the association between Biomarker Risk Score and risk of ischemic stroke differed across subgroups of age, estrogen/progestin use, hypertension, diabetes, smoking, race, HDL cholesterol, or LDL cholesterol.
Figure 1. Multivariate-adjusted analyses of Biomarker Risk Score as a predictor of incident ischemic stroke.
Y axis: Multivariate-adjusted odds ratio
X axis: Biomarker Risk Score
Biomarker Risk Score (BRS) defined as number of biomarkers that were above the top 25% of the distribution among controls, from among: CRP, IL-6, tPA, D-Dimer, WBC, neopterin, and homocysteine. See Table 3 for upper quartile cutpoints.
N (%) among controls was 243 (25%) for BRS=0, 263 (27.1%) for BRS=1, 232 (23.9%) for BRS=2, 115 (11.8%) for BRS=3, 79 (8.1%) for BRS=4, and 40 (4.1%) for BRS>=5. N (%) among cases was 141 (14.5%) for BRS=0, 238 (24.5%) for BRS=1, 217 (22.3%) for BRS=2, 160 (16.5%) for BRS=3, 119 (12.2%) for BRS=4, and 97 (10.0%) for BRS>=5.
CRP, C-reactive protein; IL-6, interleukin-6; tPA, tissue plasminogen activator; WBC, white blood cell count; BRS, Biomarker Risk Score
Figure 2. Age- and race-adjusted analyses of Biomarker Risk Score as a predictor of incident ischemic stroke, by stroke subtype.
Y axis: Multivariate-adjusted odds ratio
X axis: Biomarker Risk Score
Biomarker Risk Score was defined as the number of biomarkers that were above the top 25% of the distribution among controls, from among: CRP, IL-6, tPA, D-Dimer, WBC, neopterin, and homocysteine. See Table 3 for upper quartile cutpoints.
CRP, C-reactive protein; IL-6, interleukin-6; tPA, tissue plasminogen activator; WBC, white blood cell count
The c index for prediction of stroke was 0.633 (95% CI: 0.605–0.660) for a model that included standard stroke risk factors (age, race/ethnicity, aspirin use, BMI, diabetes, systolic blood pressure, anti-hypertensive medication use, smoking, lipid-lowering medication use, fasting glucose, LDL cholesterol and HDL cholesterol) but not the Biomarker Risk Score. Addition of the Biomarker Risk Score to the model improved the c index to 0.649 (95% CI: 0.622–0.677), which was a statistically significant increase as compared with the standard stroke risk factor model (p = 0.02). A model including standard stroke risk factors and CRP alone had a c index of 0.640 (95% CI: 0.613–0.668, p = 0.15 as compared with the standard stroke risk factor model). The addition of the Biomarker Risk Score to the model containing CRP and standard stroke risk factors produced a non-significant increase in the c index (p = 0.09).
Biomarker Risk Score and CRP
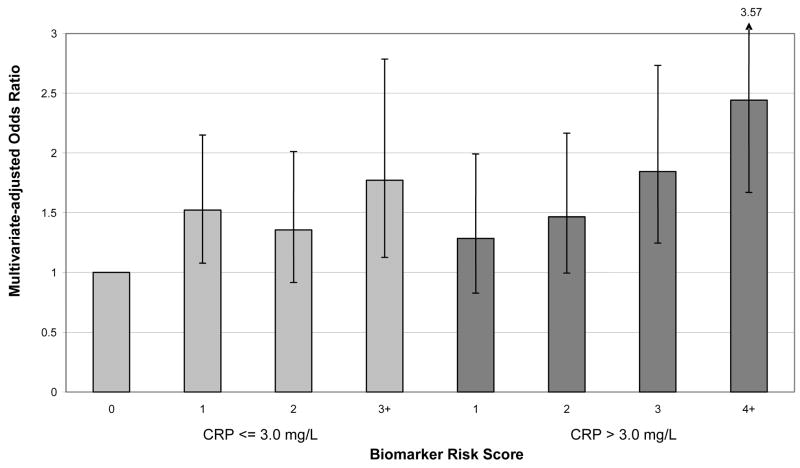
Additional analyses examined the association of the Biomarker Risk Score with risk of stroke among subjects with CRP above and below the “high risk” level of 3.0 mg/L. For these analyses, the reference group was defined as individuals who had CRP <= 3.0 mg/L and who had a Biomarker Risk Score of zero (ie, no elevated biomarkers). A gradient of increasing risk was observed across increasing numbers of elevated biomarkers, particularly among individuals with high CRP (Figure 3). Among individuals with levels of CRP > 3.0 mg/L, almost 40% had zero or one other elevated biomarkers (ie, 15.2% had Biomarker Risk Score = 1 and 23.8% had Biomarker Risk Score = 2). Among these individuals who had high CRP but <= 1 other elevated biomarkers, risk of stroke was similar as compared with those who had CRP below the 3.0 mg/L threshold but who had elevated levels of 1 or more other biomarkers (Figure 3). In analyses of Biomarker Risk Score as continuous variable, the adjusted odds ratio per unit was 1.12 (95% CI: 0.99–1.28) for those with CRP < =3.0 mg/L, and the adjusted odds ratio per unit was 1.26 (95% CI: 1.13–1.40) for those with CRP >3.0 mg/L (p for interaction = 0.53). The addition of the Biomarker Risk Score to the model containing standard stroke risk factors significantly improved the c index both among subjects with CRP < = 3.0 mg/L (p = 0.04) and among subjects with CRP > 3.0 mg/L (p = 0.03). These results stratified on CRP were changed little after residual adjustment for CRP levels.
Figure 3. Multivariate-adjusted analyses of Biomarker Risk Score as a predictor of incident ischemic stroke, in subgroups stratified by low CRP level (< 3.0 mg/l) or high CRP level (>= 3.0 mg/l).
Y axis: Multivariate-adjusted odds ratio
X axis: Biomarker Risk Score
Biomarker Risk Score was defined as the number of biomarkers that were above the top 25% of the distribution among controls. See Table 3 for upper quartile cutpoints for IL-6, tPA, D-Dimer, white blood cell count, neopterin, and homocysteine. In the present analyses within CRP subgroups, in computing the Biomarker Risk Score, one point was assigned for CRP >= 3.0 mg/L, while for the main analyses the CRP upper quartile value of 5.2 mg/L was used to define high levels.
CRP, C-reactive protein; IL-6, interleukin-6; tPA, tissue plasminogen activator; WBC, white blood cell count
Discussion
A multiple-biomarker index (Biomarker Risk Score) derived from levels of seven biomarkers of inflammation and hemostasis (CRP, IL-6, tPA, D-Dimer, WBC, neopterin, and homocysteine) defined a gradient of ischemic stroke risk across this population of 50 to 79 year old postmenopausal women. Discrimination between stroke cases and controls (c index) was significantly improved with addition of the Biomarker Risk Score to standard stroke risk factors including diabetes, hypertension, and smoking.
In the present study, CRP was the only single biomarker that remained associated with stroke after adjustment for standard stroke risk factors and other inflammation and hemostasis-related biomarkers. CRP is a well-established vascular risk factor [18], although the risk associated with elevated CRP may be weaker than previously believed [14]. Moreover, fewer studies of CRP have examined ischemic stroke than have examined coronary disease, and the importance of CRP may be less in older adults than in middle-aged populations. Data from the Women’s Health Study (mean age 53.7 years) suggested that CRP was more strongly associated with risk of ischemic stroke than with risk of coronary events (for stroke, adjusted hazard ratio comparing CRP Tertile 3 [T3] versus Tertile 1 [T1] = 2.76, 95% CI: 1.51–5.05; for coronary disease, adjusted hazard ratio comparing CRP T3 versus T1 = 1.66, 95% CI:1.17– 2.34) [15]. In the present cohort (50 to 79 year old, median age 69 years), results were consistent with this finding but the association was weaker and appeared to be confined to the upper quartile (adjusted OR comparing CRP Q4 versus Q1 = 1.64, 95% CI: 1.15–2.32). Other studies have produced conflicting evidence on the association of CRP with ischemic stroke in older adults. In the Cardiovascular Health Study (CHS) cohort, elevated levels of CRP did not predict risk of stroke among men and women 65 years and older who were free of prior angina, myocardial infarction, or stroke; in CHS, an association between CRP and stroke was only observed among those older adults who had an increased burden of subclinical atherosclerosis as indicated by carotid artery wall thickness [1, 3]. The Health, Aging and Body Composition (Health ABC) Study found that IL-6 and TNF- α, but not CRP, were associated with stroke, heart failure, and coronary disease among 70 to 79 year old adults[12]. In the Rotterdam (>= 55 years old) [16] and Copenhagen (50 to 89 years old) [13] cohorts, elevated CRP had no significant association with risk of stroke after adjustment for standard stroke risk factors. Notable strengths of the present study, shared by some but not all prior investigations, included large sample size, with more than 900 incident strokes and a comparably-sized control population, and neurologist review of medical records to confirm stroke events.
Present clinical guidelines have endorsed measurement of CRP level as an adjunct to standard CVD risk factor screening for guiding CVD prevention efforts [18], and new CVD risk stratification algorithms that have been proposed include CRP in addition to established risk factors [20]. Our study raises the question of whether multiple biomarkers reflecting inflammation or hemostasis might be useful when measured in addition to CRP. For example, a Biomarker Risk Score defined as the total number of inflammation and hemostasis biomarkers that were elevated (ie, in the upper quartile) revealed an approximate two-fold gradient in stroke risk among individuals who had “high risk” CRP levels (>= 3.0 mg/L) [18]. Among women with CRP above 3.0 mg/L, nearly 50% had zero or 1 other elevated biomarkers, and these individuals had a risk of stroke that was similar to those who had CRP below high-risk levels (< 3.0 mg/L) but who had elevated levels of 1 or more other biomarkers. Thus, our data suggest that among individuals with CRP levels in the “high-risk” category, measurement of other inflammatory and hemostasis biomarkers may be clinically useful to provide additional stratification of stroke risk.
In addition to CRP, this study examined other candidate biomarkers that were selected to reflect a variety of relevant etiologic pathways including inflammation, atherosclerosis, platelet activity, coagulation, and fibrinolysis. We confirmed that modest elevations in WBC, an acute-phase reactant, may reflect global inflammation and increased stroke risk, as shown previously in this population and others [21, 22]. IL-6 is a pro-inflammatory cytokine and trigger for liver release of CRP, and we confirmed its previously-reported association with risk of stroke [5, 7, 12]. Two of the other biomarkers that we identified as stroke risk factors reflect fibrinolytic activity, including tPA, the main activator of fibrinolysis, and D-dimer, a marker of fibrin turnover. Both have been previously implicated as vascular risk factors [9, 10]. Also worthy of note are negative findings for several biomarkers in the present study. For example, a recent meta-analysis of over 31 prospective epidemiological studies suggested that elevated levels of fibrinogen, which is involved in inflammation, platelet aggregation, and coagulation cascades, predict the risk of ischemic stroke as well as other vascular events among healthy adults [23]. However, our data showed no significant association between fibrinogen and stroke among postmenopausal women. This appears to confirm prior findings from the Cardiovascular Health Study that fibrinogen does not predict stroke among older women, although it does among older men [24].
Limitations of the present study include a lack of data among men and premenopausal females, limiting the ability to generalize results to these groups. We also lacked comparative data for multiple vascular endpoints in addition to stroke, and had limited statistical power for subgroup analyses by race/ethnicity. It is important to note that this observational study is not able to evaluate whether the identified biomarkers play a causal etiologic role or whether their modification may alter risk of stroke. Validation of these results in another population will be important, although we used cross-validation methods to address the lack of an external validation cohort. It is important to note that biomarkers were only measured at baseline, and may have changed during follow-up due to biological within-individual variation or initiation of medications that affect inflammation, endothelial function, and coagulation status.
Among several inflammatory and hemostasis-related biomarkers that were studied, we identified elevated CRP level as the strongest independent risk factor for stroke among postmenopausal women. A Biomarker Risk Score derived from levels of several biomarkers provided additional useful information for stratifying stroke risk. The findings support the further exploration of multiple-biomarker panels for more accurately stratifying an individual’s risk of stroke, possibly based upon emerging multiplex assay technologies that may reduce technical and cost barriers [25].
Acknowledgments
Funding: The WHI program is funded by the National Heart, Lung and Blood Institute, U.S. Department of Health and Human Services. The HaBPS Study is funded by the National Institute of Neurological Disorders and Stroke, Bethesda, Md., U.S. Department of Health and Human Services. The funders had no role in the design, conduct or manuscript preparation for the HaBPS Study.
WHI Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Elizabeth Nabel, Jacques Rossouw, Shari Ludlam, Linda Pottern, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg, Ruth E. Patterson, Anne McTiernan; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker; (Medical Research Labs, Highland Heights, KY) Evan Stein; (University of California at San Francisco, San Francisco, CA) Steven Cummings.
Clinical Centers: (Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller; (Baylor College of Medicine, Houston, TX) Jennifer Hays; (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn Manson; (Brown University, Providence, RI) Annlouise R. Assaf; (Emory University, Atlanta, GA) Lawrence Phillips; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley Beresford; (George Washington University Medical Center, Washington, DC) Judith Hsia; (Los Angeles Biomedical Research Institute at Harbor- UCLA Medical Center, Torrance, CA) Rowan Chlebowski; (Kaiser Permanente Center for Health Research, Portland, OR) Evelyn Whitlock; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen; (MedStar Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn; (Rush Medical Center, Chicago, IL) Henry Black; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Alabama at Birmingham, Birmingham, AL) Cora E. Lewis; (University of Arizona, Tucson/Phoenix, AZ) Tamsen Bassford; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of California at Davis, Sacramento, CA) John Robbins; (University of California at Irvine, CA) F. Allan Hubbell; (University of California at Los Angeles, Los Angeles, CA) Howard Judd; (University of California at San Diego, LaJolla/Chula Vista, CA) Robert D. Langer; (University of Cincinnati, Cincinnati, OH) Margery Gass; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Hawaii, Honolulu, HI) David Curb; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Massachusetts/Fallon Clinic, Worcester, MA) Judith Ockene; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser; (University of Miami, Miami, FL) Mary Jo O’Sullivan; (University of Minnesota, Minneapolis, MN) Karen Margolis; (University of Nevada, Reno, NV) Robert Brunner; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (University of Tennessee, Memphis, TN) Karen C. Johnson; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski; (University of Wisconsin, Madison, WI) Gloria E. Sarto; (Wake Forest University School of Medicine, Winston-Salem, NC) Denise Bonds; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Susan Hendrix.
Dr. Kaplan had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Disclosure: The authors report no conflicts of interest
Please do not cite or distribute without the permission of the lead author
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jenny NS, Arnold AM, Kuller LH, et al. Serum amyloid P and cardiovascular disease in older men and women: results from the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 2007;27:352–358. doi: 10.1161/01.ATV.0000254150.97741.fe. [DOI] [PubMed] [Google Scholar]
- 2.Cushman M, Arnold AM, Psaty BM, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation. 2005;112:25–31. doi: 10.1161/CIRCULATIONAHA.104.504159. [DOI] [PubMed] [Google Scholar]
- 3.Cao JJ, Thach C, Manolio TA, et al. C-reactive protein, carotid intima-media thickness, and incidence of ischemic stroke in the elderly: the Cardiovascular Health Study. Circulation. 2003;108:166–170. doi: 10.1161/01.CIR.0000079160.07364.6A. [DOI] [PubMed] [Google Scholar]
- 4.Revilla M, Obach V, Cervera A, et al. A -174G/C polymorphism of the interleukin-6 gene in patients with lacunar infarction. Neurosci Lett. 2002;324:29–32. doi: 10.1016/s0304-3940(02)00169-6. [DOI] [PubMed] [Google Scholar]
- 5.Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108:2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 6.Pradhan AD, Manson JE, Rossouw JE, et al. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: prospective analysis from the Women’s Health Initiative observational study. Jama. 2002;288:980–987. doi: 10.1001/jama.288.8.980. [DOI] [PubMed] [Google Scholar]
- 7.Jenny NS, Tracy RP, Ogg MS, et al. In the elderly, interleukin-6 plasma levels and the -174G>C polymorphism are associated with the development of cardiovascular disease. Arterioscler Thromb Vasc Biol. 2002;22:2066–2071. doi: 10.1161/01.atv.0000040224.49362.60. [DOI] [PubMed] [Google Scholar]
- 8.Tanne D, Haim M, Boyko V, et al. Soluble intercellular adhesion molecule-1 and risk of future ischemic stroke: a nested case-control study from the Bezafibrate Infarction Prevention (BIP) study cohort. Stroke. 2002;33:2182–2186. doi: 10.1161/01.str.0000029007.32244.40. [DOI] [PubMed] [Google Scholar]
- 9.Johansson L, Jansson JH, Boman K, et al. Tissue plasminogen activator, plasminogen activator inhibitor-1, and tissue plasminogen activator/plasminogen activator inhibitor-1 complex as risk factors for the development of a first stroke. Stroke. 2000;31:26–32. doi: 10.1161/01.str.31.1.26. [DOI] [PubMed] [Google Scholar]
- 10.Pradhan AD, LaCroix AZ, Langer RD, et al. Tissue plasminogen activator antigen and D-dimer as markers for atherothrombotic risk among healthy postmenopausal women. Circulation. 2004;110:292–300. doi: 10.1161/01.CIR.0000134965.73212.A6. [DOI] [PubMed] [Google Scholar]
- 11.Jenny NS, Arnold AM, Kuller LH, et al. Soluble intracellular adhesion molecule-1 is associated with cardiovascular disease risk and mortality in older adults. J Thromb Haemost. 2006;4:107–113. doi: 10.1111/j.1538-7836.2005.01678.x. [DOI] [PubMed] [Google Scholar]
- 12.Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and cardiovascular disease (The Health, Aging and Body Composition [Health ABC] Study) Am J Cardiol. 2003;92:522–528. doi: 10.1016/s0002-9149(03)00718-5. [DOI] [PubMed] [Google Scholar]
- 13.Kistorp C, Raymond I, Pedersen F, et al. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. Jama. 2005;293:1609–1616. doi: 10.1001/jama.293.13.1609. [DOI] [PubMed] [Google Scholar]
- 14.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 15.Everett BM, Kurth T, Buring JE, et al. The relative strength of C-reactive protein and lipid levels as determinants of ischemic stroke compared with coronary heart disease in women. J Am Coll Cardiol. 2006;48:2235–2242. doi: 10.1016/j.jacc.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bos MJ, Schipper CM, Koudstaal PJ, et al. High serum C-reactive protein level is not an independent predictor for stroke: the Rotterdam Study. Circulation. 2006;114:1591–1598. doi: 10.1161/CIRCULATIONAHA.106.619833. [DOI] [PubMed] [Google Scholar]
- 17.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 18.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 19.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, Buring JE, Rifai N, et al. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. Jama. 2007;297:611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 21.Folsom AR, Rosamond WD, Shahar E, et al. Prospective study of markers of hemostatic function with risk of ischemic stroke. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Circulation. 1999;100:736–742. doi: 10.1161/01.cir.100.7.736. [DOI] [PubMed] [Google Scholar]
- 22.Margolis KL, Manson JE, Greenland P, et al. Leukocyte count as a predictor of cardiovascular events and mortality in postmenopausal women: the Women’s Health Initiative Observational Study. Arch Intern Med. 2005;165:500–508. doi: 10.1001/archinte.165.5.500. [DOI] [PubMed] [Google Scholar]
- 23.Danesh J, Lewington S, Thompson SG, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. Jama. 2005;294:1799–1809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 24.Tracy RP, Arnold AM, Ettinger W, et al. The relationship of fibrinogen and factors VII and VIII to incident cardiovascular disease and death in the elderly: results from the cardiovascular health study. Arterioscler Thromb Vasc Biol. 1999;19:1776–1783. doi: 10.1161/01.atv.19.7.1776. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan RC, Ho GY, Xue X, et al. Within-individual stability of obesity-related biomarkers among women. Cancer Epidemiol Biomarkers Prev. 2007;16:1291–1293. doi: 10.1158/1055-9965.EPI-06-1089. [DOI] [PubMed] [Google Scholar]