Abstract
The establishment of an efficient exchange of information between the cerebral hemispheres is of crucial importance in the developing functionally lateralized brain. The corpus callosum, the major connection between the cerebral hemispheres, grows constantly throughout childhood and adolescence. However, behavioral studies suggest the existence of a critical time period for callosal functional development starting around the age of 6 years. In the present longitudinal study, examining a cohort of 20 children at the age of 6 and 8 years, we assessed the relationship between structural and functional callosal development during this time period. The structural development was quantified by calculating the increase in callosal thickness using a shape-based computational analysis of the mid-sagittal corpus callosum as obtained with magnetic resonance imaging. The functional development was assessed with a speech discrimination task based on the dichotic presentation of consonant–vowel syllables. The statistical analysis revealed that children whose callosal isthmus increased in thickness over the course of 2 years showed a decrease in interhemispheric information transfer. However, children exhibiting a decrease in isthmus thickness revealed an increase in information transfer. These results might indicate a refinement process of the callosal connections to optimize the neuronal communication between the developing cerebral hemispheres.
Keywords: corpus callosum, development, dichotic listening, interhemispheric transfer, longitudinal study
Introduction
Efficient processing in the functionally asymmetric human brain relies on an exchange of information and a coordination of activity between the cerebral hemispheres (Baynes et al. 1998; Gazzaniga 2000), which is mainly provided by fibers running through the corpus callosum (Schmahmann and Pandya 2006). Thus, establishing an effective structural and functional callosal connection between the hemispheres is an important task for the developing brain, and an altered callosal development has been linked to several psychiatric disorders and developmental disabilities, including schizophrenia (e.g., Woodruff et al. 1995; Crow et al. 2007) or dyslexia (e.g., Hynd et al. 1995; von Plessen et al. 2002; Odegard et al. 2009). However, it has also been frequently shown that size and shape of the corpus callosum continue to develop throughout childhood and adolescence (Rauch and Jinkins 1994; Thompson et al. 2000; Lenroot and Giedd 2006) into the third decade of life (Pujol et al. 1993). Moreover, behavioral studies indicate that a critical time period for the functional development of the interhemispheric connections lies between the age of 6 and 12 years (Banich and Brown 2000). In this time period, the interaction between the cerebral hemispheres undergoes significant change and development, at the end of which the interhemispheric interaction resembles its adult form. For example, Chicoine et al. (2000) reported that children aged 6–7 years could not transfer acquired visuomotor skills from one hand (i.e., from the trained hemisphere) to the other hand (i.e., to the untrained hemisphere), while 11- to 12-year-old children were able to transfer acquired skills between the hands and hemispheres. Thus, the results for the 6- to 7-year-old group are comparable with those found in subjects with callosal agenesis, while the 11- to 12-year-old children yielded results similar to performance typically found in adults. A series of other studies using different tasks to measure interhemispheric interaction confirm these observations. More specifically, they show that—toward the end of the first decade of life—bimanual coordination improves (e.g., Jeeves et al. 1988; Steese-Seda et al. 1995; Marion et al. 2003) and the bilateral visual field advantage reaches its adult level (Banich et al. 2000), while the manifestation of mirror movements is reduced (Mayston et al. 1999; Fagard et al. 2001) as well as the interhemispheric transfer time (Brizzolara et al. 1994; Hagelthorn et al. 2000; Fagard et al. 2001). However, although indicating a period of significant changes in the way the cerebral hemispheres interact, all these earlier behavioral studies used a cross-sectional design, and no information about the structural development of the interhemispheric pathways was incorporated into the analyses.
To overcome these shortcomings, we designed the present longitudinal study to examine the relationship of structural and functional development in the critical time period, by studying a cohort of 20 children at the age of 6 and 8 years. While the structural callosal development was assessed based on structural magnetic resonance imaging (MRI), the functional development was assessed applying an dichotic listening paradigm consisting of the simultaneous (one in each ear) presentations of 2 different consonant–vowel (CV) syllables (e.g., /ba/ or /da/) together with the instruction to report the one syllable that was heard best (Hugdahl 2003). It has been frequently shown that a subject's correct report of stimuli presented to the left ear (LE) relies on intact callosal connections (for a review, see Bamiou et al. 2007; Westerhausen and Hugdahl 2008). Consequently, the correct LE report and its development over time were taken to indicate functional callosal development.
Materials and Methods
Subjects
Twenty healthy children (9 boys, 11 girls) were tested at the age of 6 (mean ± standard deviation age at testing: 6.4 ± 0.3 years) and 8 (8.4 ± 0.3 years) and were recruited from preschool day care facilities in Western Norway. The exclusion criteria were impaired sight or hearing, intellectual disability according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria, or diagnoses of any other impairment included in the DSM-IV (various syndromes, neurological impairments). The children had normal IQ as tested with the Wechsler Preschool and Primary Scale of Intelligence at the age of 5 years (i.e., before entering the study). For comparison, we also reanalyzed the data of 17 healthy adult subjects taken from a previous study (for more details, see Westerhausen et al. 2009) and applied the same analysis methods as for the group of children studied here. The adult control group consisted of 17 male subjects (all right-handed, mean age 25.9 ± 6.7 years), only including subjects without any history of psychiatric or neurological disorders. Considering the auditory nature of the applied task, subjects with hearing impairment were excluded from both groups. The study was approved by the Regional Committee for Medical Research Ethics in Western Norway (REK Vest) and conducted according to the principles expressed in the Declaration of Helsinki.
The Dichotic Listening Paradigm
To assess the functional development of the interhemispheric interaction, we applied an auditory speech discrimination task that included the dichotic presentation of CV syllable pairs, that is the simultaneous presentation of 2 syllables, one to the right and one to the LE (Hugdahl 2003). The basic set of 6 CV syllables (/ba/, /da/, /ga/, /pa/, /ta/, /ka/) were used to create 30 pairs of syllables (e.g., /ba/–/da/, /pa/–/ta/) whereby the 2 syllables of each pair were temporally aligned to achieve simultaneous onset of the initial consonants. All syllables were spoken by an adult Norwegian male voice with constant intensity and intonation, and the mean stimulus duration was between 400–500 ms. An experimental session consisted of the presentation of all 30-syllable pairs that were presented with an interstimulus interval of 4000 ms. The subjects were instructed to report the syllable that they heard best on each trial. The number of correctly reported syllables was scored separately for the LE and the right-ear (RE) stimulus. For the children group, the difference in correct LE reports (ΔLE, calculated as LE at 6 years subtracted from LE at 8 years) was taken to indicate functional callosal development (see Bamiou et al. 2007; Westerhausen and Hugdahl 2008). The change in the correct RE reports (ΔRE; RE at 6 years subtracted from RE at 8 years) served as control variable.
The dichotic listening procedure, as applied in the present study, commonly yields high retest reliability (e.g., Hugdahl and Hammar 1997; Gadea et al. 2000) also indicating that practice effects are negligible (even when retested in the same session). Thus, it can be assumed that there are no substantial carry-over or practice effects from the testing at the age 6 to the testing at the age of 8, as performed in the present longitudinal experiment.
MRI Acquisition
Structural imaging at both time points and for both the children and the adult group was performed on the same General Electric Signa 3.0-T scanner equipped with 40 mT/m TwinSpeed gradients. T1-weighted images were acquired with a 3-dimensional Fast spoiled gradient recall (echo time = 14 ms, repetition time = 400 ms, inversion time = 500 ms) sequence. In the group of children, 256 consecutive sagittal slices (1.5-mm thickness) with an in-plane resolution of 1 × 1 mm were acquired at both time points. In the adult control group, the acquired images consisted of 188 consecutive sagittal slices (1.0-mm thickness) with an in-plane resolution 1 × 1 mm.
Measurement of Regional Callosal Thickness
The midsagittal slice of each imaging volume was identified, and the image orientation was corrected for head tilt and alignment using the SPM5 software package (Wellcome Department of Cognitive Neurology, London, UK). This way, an imaginary line connecting the lowest points of the genu and splenium subregions of the corpus callosum was reoriented to be horizontal. In the children group (at both ages) as well as in the adult control group, callosal thickness was determined using a surface-based morphometric approach (for details, see Luders et al. 2006, 2007). In short, the upper and lower boundaries of the corpus callosum were outlined manually using MultiTracer software (http://bishopw.loni.ucla.edu/MultiTracer) by one rater (R.W.), who was blind to age of the subjects and the individual performance in the dichotic listening task. Subsequently, a medial curve (midline) was created by calculating the spatial average from 100 equidistant points representing the callosal upper and lower boundaries. The midline was then taken as a basis to calculate distances between corresponding points along the corpus callosum. Finally, the callosal distance values were used as indicators for “regional callosal thickness” 1) at age 6, 2) at age 8, and 3) for the adult group. In the group of children, the difference between the callosal distance values between age 6 and 8 was used as a measure of “regional callosal growth.”
Statistical Analyses
To statistically evaluate an association of structural and functional development in the children group, callosal growth was related to ΔLE and ΔRE by calculating product–moment correlations at each of the 100 surface points. Likewise, for children (at age 6 and 8) and also for the adult control group, the relationship between structure and function was determined by calculating product–moment correlations between callosal distance values and performance measures, such as LE and RE reports. Additionally, we determine regions of significant callosal growth in the children group by calculating paired t-tests comparing the callosal distance values between age 6 and 8. Since the children group consisted of boys and girls, we also tested for differences in callosal growth between the 2 sexes. For all statistical analyses, a significance level of α < 0.05 was applied, where no adjustments for multiple comparisons were performed in order to retain statistical power (i.e., to keep the type II error probability low) and to allow for the detection also of medium-sized effects (Cohen 1988). A sensitivity analysis using the program G*Power (Version 3.0.10; Faul et al. 2007) revealed that in the present analyses, correlations of |r| > 0.50 (children group, N = 20) and |r| > 0.53 (adult group, N = 17), respectively, could be detected with sufficient statistical power (>0.80).
Results
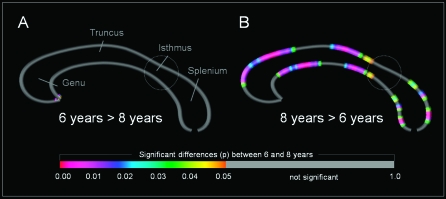
As shown in Figure 1B, we detected a significant increase in callosal thickness between the age of 6 and 8 years in genu, truncus, and splenium of the corpus callosum. Within this time period, there was no significant decrease in any callosal subregion (Fig. 1A). No substantial differences between boys and girls in regional callosal growth were detected, so that all subsequent correlation analyses regarding the association of structural and functional callosal development were performed for the entire children group.
Figure 1.
Differences in callosal thickness between 6- and 8-year-old children. Panel A displays callosal regions that showed a significantly larger thickness at age 6; Panel B displays callosal regions that showed a significantly larger thickness at age 8. The color bar encodes the significance (P). The circle indicates the location of the callosal isthmus.
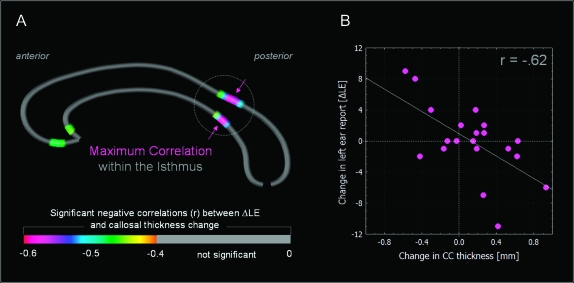
The relationship between structural and functional development of the corpus callosum was examined in 2 sets of analyses. In a first analyses, the regional callosal growth was related to the developmental change in the dichotic listening correct LE and RE report (ΔLE and ΔRE, respectively). Here, callosal growth was found to be significantly correlated with ΔLE, but not with ΔRE (Fig. 2A). The scatter plot of the strongest association (Fig. 2B, r = −0.62), located in the isthmus subregion, illustrates that subjects showing an increase in isthmus thickness between the age of 6 and 8 years also showed a reduction in correct LE report between these time points (as indicated by a negative ΔLE). In contrast, individuals showing a decrease in isthmus thickness also showed an improvement in LE report (i.e., a positive ΔLE).
Figure 2.
Correlations between changes in LE report (ΔLE) and changes in callosal regional thickness between the age of 6 and 8 years. Panel A displays the callosal regions that showed significant negative correlations at P < 0.05. The color bar encodes the correlation coefficient (r). Panel B displays the scatter plot for the highest magnitude correlation, which was located in the isthmus. The circle indicates the location of the callosal isthmus.
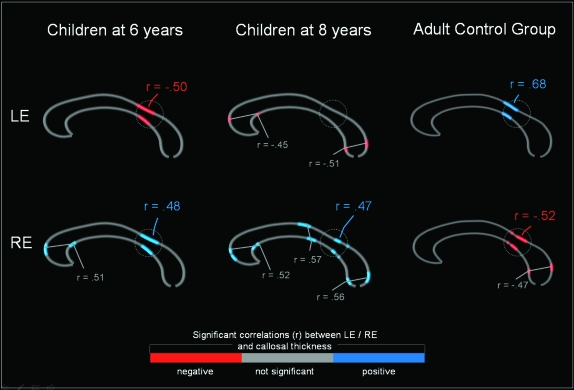
In the second analysis, we were in interested in how the relationship between callosal structure and function develops over time. Here we correlated callosal thickness with correct LE and RE reports separately at the age of 6 and 8 years. As depicted in Figure 3 (left and middle panel), the most prominent developmental changes occurred in the isthmus region. While the LE report was found to be negatively related to the thickness of the isthmus (highest magnitude: r = −0.50) at the age of 6 years, no significant correlation was present at the age of 8 years. In fact, the RE report was found to be positively related to the thickness of the isthmus both at the age of 6 years (maximum r = 0.48) and at the age of 8 years (maximum r = 0.47). As shown in Figure 3 (right panel), in the adult group, the LE report was positively correlated (maximum r = 0.68), while the RE report was negatively correlated (highest magnitude: r = −0.52) with the isthmus thickness.
Figure 3.
Correlations between LE report and RE report and callosal thickness in different age groups. Displayed are the locations of significant negative (red) and positive (blue) correlations between callosal size and LE (upper row) and RE (lower row). The maps are displayed for children at the age of 6 years (left) and 8 years (middle), as well as for an adult control group (right). The maximum correlations (r) are provided for each of the regions in which a significant correlation (P < 0.05) was detected. The circle indicates the callosal isthmus.
Discussion
Mapping the development of the corpus callosum between the age of 6 and 8 years revealed a significant association between structure and function. More specifically, in this period of time, the growth of the isthmus subregion of the corpus callosum was negatively correlated with the development of the LE report (ΔLE). The structural localization of the association is in accordance with the known topographical organization of the corpus callosum since the isthmus and adjacent posterior callosal area contain the fibers interconnecting left and right hemisphere temporal lobe auditory and speech processing areas (e.g., Schmahmann and Pandya 2006; Dougherty et al. 2007; Westerhausen et al. 2009). The functional specificity (i.e., being correlated only with the ΔLE) is in accordance with a body of previous clinical and experimental studies showing that especially the correct LE report in a dichotic listening paradigm can be considered a measure of information transfer via the corpus callosum (Bamiou et al. 2007; Westerhausen and Hugdahl 2008). For example, patients who underwent a complete callosal section are not able to report the LE stimulus in a dichotic listening experiment, while they show no impairment (or even an increase) in the detection of RE stimuli (e.g., Milner et al. 1968; Clarke et al. 1993). Also, less severe alterations of callosal connections (e.g., in multiple sclerosis or after chemotherapy), go along with a significantly reduced LE report (e.g., Benavidez et al. 1999; Gadea et al. 2002; Pollmann et al. 2002; Fujimoto et al. 2006). Thus, both the structural localization and functional specificity of the correlation indicate a developmental process between the age of 6 and 8 years that affects the connectivity between the left and right temporal lobe auditory and speech processing areas. Furthermore, the observation that an increase in isthmus thickness was correlated with a decrease in interhemispheric transfer, while a decrease in thickness was associated with an increase of transfer (see Fig. 2B), suggests a refinement or tuning process of the callosal connections. In an interplay between regressive (reduction in thickness) and progressive (increase in thickness) maturational events, the axons running through the isthmus may be optimized to allow for an efficient neuronal communication between the cerebral hemispheres. An interaction of regressive and progressive processes would also explain the lack of a significant increase in the thickness of the isthmus (see Figure 1). Axonal pruning or alterations in axonal myelination might be the microstructural processes underlying this macrostructural development (Paus et al. 1999; Lenroot and Giedd 2006).
Our second analyses, performed separately for the age of 6 and 8 and the adult control group, revealed a developmental change of the structure–function association over time, which was, again, most obvious in the isthmus region of the corpus callosum. At the age of 6 years, the correct LE report in the dichotic listening paradigm was negatively correlated with isthmus thickness, while no significant correlation was detected at the age of 8 years. However, examining the same association in an adult group, we found a positive correlation of isthmus thickness and correct LE report. Correlating the correct RE report with callosal thickness, we found a positive correlation in the isthmus that was present both at the age of 6 and 8 years, while the adults showed a negative correlation. The change of the structure–function correlation over time might indicate an underlying reorganization or refinement process of the interhemispheric connections in the examined time period. Yet, even at age 8, the correlation pattern was still different from the pattern observed in adults, suggesting that the developmental reorganization process may not be fully completed by age 8. However, any such conclusion is limited by the fact that the present adult control group consisted only of male subjects while the children group consisted of male and female subjects, so that developmental sex differences cannot be excluded. Nevertheless, the present observation agrees well with the results of behavioral studies indicating the critical time period for the callosal development extends, at least, up to the age of 10–12 years (see e.g., Chicoine et al. 2000; Hagelthorn et al. 2000; Fagard et al. 2001).
Furthermore, finding a positive correlation of callosal thickness with the LE report and negative correlation with the RE report in the adult group is in line with previous studies of clinical and healthy samples (for review, see Westerhausen and Hugdahl 2008). For example, an acquired (permanent or transient) loss or degradation of callosal fibers not only results in a reduced LE report but is also associated with increased RE report in the dichotic listening paradigm (e.g., Clarke et al. 1993, Gadea et al. 2002; Fujimoto et al. 2006). The positive association of callosal thickness with the LE report is in accordance with the dichotic listening model proposed by Kimura (1967). According to this, so-called “structural” model, the speech-processing areas located in the left hemisphere have direct access to the stimulus presented to the contralateral right ear. However, since the weaker ipsilateral ascending auditory pathways are inhibited under dichotic stimulation, the stimulus presented to the left ear has to take an indirect route passing through the right hemisphere and the corpus callosum. Thus, an increased callosal thickness might support a better interhemispheric transfer of the LE report, resulting in the positive correlation observed in the adult group. The negative correlation with the RE report can be seen as a by-product of a “dual task”-like situation in which the callosal-transferred LE and “acallosal” RE stimuli compete for the same processing resources in the left hemisphere, with the degree of this competition mediated by the strength of the interhemispheric connections (Westerhausen et al. 2006). However, the children group, both at the age of 6 and 8 years, shows a correlation pattern that substantially differs from the “LE-positive, RE-negative” correlation pattern observed in adults, presumably indicating an ongoing developmental process.
In summary, the results of both analyses indicate a parallel structural and functional development of the interhemispheric connections possibly reflecting a callosal refinement process taking place between the age of 6 and 8 years. The callosal refinement might involve an adjustment of the interhemispheric neural connections in context of an ongoing reorganization of the auditory and speech processing systems. The present developmental effects also temporally coincide with the children's enrolment into school and consequently with the beginning of the formal education to read and write. An important ability acquired during this age range is “phonological awareness,” the ability to recognize and manipulate phonological units of spoken language (Bradley and Bryant 1983; Ziegler and Goswami 2005). Given this temporal association and given the verbal nature of the dichotic listening paradigm, it can be hypothesized that the observed alteration in the isthmus’ structure–function relationship may be related to the development of phonological abilities. This hypothesis is supported by the finding that a child's degree of phonological awareness is directly linked to the strength of the interhemispheric connections between the temporal lobe areas (Dougherty et al. 2007). Furthermore, recent studies have shown that literacy education during childhood has a significant influence on the architecture of the posterior corpus callosum as an adult (Petersson et al. 2007). Thus, the age period investigated in the present study may represent an important developmental period, in which maladjustments with respect to the relationship between callosal connections and hemispheric lateralization may lead to developmental disabilities and impairments, such as seen in dyslexia (e.g., Hynd et al. 1995; von Plessen et al. 2002).
Funding
Research Council of Norway, West-Norway Health Authority, and Haukeland University Hospital Strategic Research Program (to K.H.); Meltzer Foundation, University of Bergen (to T.H.); the National Institutes of Health (NIH) (to E.L., P.M.T., A.W.T.) through the NIH Roadmap for Medical Research, entitled Center for Computational Biology.
Acknowledgments
The authors would like to thank Lisa Austhamn for her assistance in preparing the data. The present research was part of the “speak-up project” (T.H.). Conflict of Interest: None declared.
References
- Bamiou DE, Sisodiya S, Musiek FE, Luxon LM. The role of the interhemispheric pathway in hearing. Brain Res Rev. 2007;56:170–182. doi: 10.1016/j.brainresrev.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Banich MT, Brown WS. A life-span perspective on interaction between the cerebral hemispheres. Dev Neuropsychol. 2000;18:1–10. doi: 10.1207/S15326942DN1801_1. [DOI] [PubMed] [Google Scholar]
- Banich MT, Passarotti AM, Janes D. Interhemispheric interaction during childhood: I. Neurologically intact children. Dev Neuropsychol. 2000;18:33–51. doi: 10.1207/S15326942DN1801_3. [DOI] [PubMed] [Google Scholar]
- Baynes K, Eliassen JC, Lutsep HL, Gazzaniga MS. Modular organization of cognitive systems masked by interhemispheric integration. Science. 1998;280:902–905. doi: 10.1126/science.280.5365.902. [DOI] [PubMed] [Google Scholar]
- Benavidez DA, Fletcher JM, Hannay HJ, Bland ST, Caudle SE, Mendelsohn DB, Yeakley J, Brunder DG, Harward H, Song J, et al. Corpus callosum damage and interhemispheric transfer of information following closed head injury in children 1196. Cortex. 1999;35:315–336. doi: 10.1016/s0010-9452(08)70803-7. [DOI] [PubMed] [Google Scholar]
- Bradley L, Bryant PE. Categorizing sounds and learning to read—a causal connection. Nature. 1983;301:419–421. [Google Scholar]
- Brizzolara D, Ferretti G, Brovedani P, Casalini C, Sbrana B. Is interhemispheric transfer time related to age? A developmental study. Behav Brain Res. 1994;64:179–184. doi: 10.1016/0166-4328(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Chicoine AJ, Proteau L, Lassonde M. Absence of interhemispheric transfer of unilateral visuomotor learning in young children and individuals with agenesis of the corpus callosum. Dev Neuropsychol. 2000;18:73–94. doi: 10.1207/S15326942DN1801_5. [DOI] [PubMed] [Google Scholar]
- Clarke JM, Lufkin RB, Zaidel E. Corpus callosum morphometry and dichotic listening performance: individual differences in functional interhemispheric inhibition? Neuropsychologia. 1993;31:547–557. doi: 10.1016/0028-3932(93)90051-z. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power for the behavioral sciences. Hillsdale (NJ): Lawrence Erlbaum; 1988. [Google Scholar]
- Crow TJ, Paez P, Chance SA. Callosal misconnectivity and the sex difference in psychosis. Int Rev Psychiatry. 2007;19:449–457. doi: 10.1080/09540260701486282. [DOI] [PubMed] [Google Scholar]
- Dougherty RF, Ben-Shachar M, Deutsch GK, Hernandez A, Fox GR, Wandell BA. Temporal-callosal pathway diffusivity predicts phonological skills in children. Proc Natl Acad Sci U S A. 2007;104:8556–8561. doi: 10.1073/pnas.0608961104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagard J, Hardy-Leger I, Kervella C, Marks A. Changes in interhemispheric transfer rate and the development of bimanual coordination during childhood. J Exp Child Psychol. 2001;80:1–22. doi: 10.1006/jecp.2000.2623. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Fujimoto C, Ito K, Iwasaki S, Nakao K, Sugasawa M. Reversible impairment of auditory callosal pathway in 5-fluorouracil-induced leukoencephalopathy: parallel changes in function and imaging. Otol Neurotol. 2006;27:716–719. doi: 10.1097/01.mao.0000194815.15298.8b. [DOI] [PubMed] [Google Scholar]
- Gadea M, Gomez C, Espert R. Test-retest performance for the consonant-vowel dichotic listening test with and without attentional manipulations. J Clin Exp Neuropsychol. 2000;22:793–803. doi: 10.1076/jcen.22.6.793.959. [DOI] [PubMed] [Google Scholar]
- Gadea M, Marti-Bonmati L, Arana E, Espert R, Casanova V, Pascual A. Dichotic listening and corpus callosum magnetic resonance imaging in relapsing-remitting multiple sclerosis with emphasis on sex differences. Neuropsychology. 2002;16:275–281. [PubMed] [Google Scholar]
- Gazzaniga MS. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain. 2000;123(Pt 7):1293–1326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- Hagelthorn KM, Brown WS, Amano S, Asarnow R. Normal development of bilateral field advantage and evoked potential interhemispheric transmission time. Dev Neuropsychol. 2000;18:11–31. doi: 10.1207/S15326942DN1801_2. [DOI] [PubMed] [Google Scholar]
- Hugdahl K. Dichotic listening in the study of auditory laterality. In: Hugdahl K, Davidson RJ, editors. The asymmetrical barin. Cambridge (MA): MIT Press; 2003. pp. 441–475. [Google Scholar]
- Hugdahl K, Hammar Å. Test-retest reliability for the consonant-vowel syllables dichotic listening paradigm. J Clin Exp Neuropsychol. 1997;19:667–675. doi: 10.1080/01688639708403752. [DOI] [PubMed] [Google Scholar]
- Hynd GW, Hall J, Novey ES, Eliopulos D, Black K, Gonzalez JJ, Edmonds JE, Riccio C, Cohen M. Dyslexia and corpus callosum morphology. Arch Neurol. 1995;52:32–38. doi: 10.1001/archneur.1995.00540250036010. [DOI] [PubMed] [Google Scholar]
- Jeeves MA, Silver PH, Milne AB. Role of the corpus callosum in the development of a bimanual motor skill. Dev Neuropsychol. 1988;4:305–323. [Google Scholar]
- Kimura D. Functional asymmetry of the brain in dichotic listening. Cortex. 1967;3:163–178. [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Bilder RM, Thompson PM, Szeszko PR, Hamilton L, Toga AW. Positive correlations between corpus callosum thickness and intelligence. Neuroimage. 2007;37:1457–1464. doi: 10.1016/j.neuroimage.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr KL, Zaidel E, Thompson PM, Jancke L, Toga AW. Parasagittal asymmetries of the corpus callosum. Cereb Cortex. 2006;16:346–354. doi: 10.1093/cercor/bhi112. [DOI] [PubMed] [Google Scholar]
- Marion SD, Kilian SC, Naramor TL, Brown WS. Normal development of bimanual coordination: visuomotor and interhemispheric contributions. Dev Neuropsychol. 2003;23:399–421. doi: 10.1207/S15326942DN2303_6. [DOI] [PubMed] [Google Scholar]
- Mayston MJ, Harrison LM, Stephens JA. A neurophysiological study of mirror movements in adults and children. Ann Neurol. 1999;45:583–594. doi: 10.1002/1531-8249(199905)45:5<583::aid-ana6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Milner B, Taylor L, Sperry RW. Lateralized suppression of dichotically presented digits after commissural section in man. Science. 1968;161:184–186. doi: 10.1126/science.161.3837.184. [DOI] [PubMed] [Google Scholar]
- Odegard TN, Farris EA, Ring J, McColl R, Black J. Brain connectivity in non-reading impaired children and children diagnosed with developmental dyslexia. Neuropsychologia. 2009;47:1972–1977. doi: 10.1016/j.neuropsychologia.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Petersson KM, Silva C, Castro-Caldas A, Ingvar M, Reis A. Literacy: a cultural influence on functional left-right differences in the inferior parietal cortex. Eur J Neurosci. 2007;26:791–799. doi: 10.1111/j.1460-9568.2007.05701.x. [DOI] [PubMed] [Google Scholar]
- Pollmann S, Maertens M, von Cramon DY, Lepsien J, Hugdahl K. Dichotic listening in patients with splenial and nonsplenial callosal lesions. Neuropsychology. 2002;16:56–64. doi: 10.1037//0894-4105.16.1.56. [DOI] [PubMed] [Google Scholar]
- Pujol J, Vendrell P, Junque C, Marti-Vilalta JL, Capdevila A. When does human brain development end? Evidence of corpus callosum growth up to adulthood. Ann Neurol. 1993;34:71–75. doi: 10.1002/ana.410340113. [DOI] [PubMed] [Google Scholar]
- Rauch RA, Jinkins JR. Analysis of cross-sectional area measurements of the corpus callosum adjusted for brain size in male and female subjects from childhood to adulthood. Behav Brain Res. 1994;64:65–78. doi: 10.1016/0166-4328(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber pathways of the brain. New York: Oxford University Press; 2006. [Google Scholar]
- Steese-Seda B, Brown WS, Caetano C. Development of the visuomotor coordination in school-age children: the bimanual coordination test. Dev Neuropsychol. 1995;11:181–199. [Google Scholar]
- Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404:190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- von Plessen K, Lundervold A, Duta N, Heiervang E, Klauschen F, Smievoll AI, Ersland L, Hugdahl K. Less developed corpus callosum in dyslexic subjects–a structural MRI study. Neuropsychologia. 2002;40:1035–1044. doi: 10.1016/s0028-3932(01)00143-9. [DOI] [PubMed] [Google Scholar]
- Westerhausen R, Grüner R, Specht K, Hugdahl K. Functional relevance of interindividual differences in temporal lobe callosal pathways: a DTI tractography study. Cereb Cortex. 2009;19:1322–1329. doi: 10.1093/cercor/bhn173. [DOI] [PubMed] [Google Scholar]
- Westerhausen R, Hugdahl K. The corpus callosum in dichotic listening studies of hemispheric asymmetry: a review of clinical and experimental evidence. Neurosci Biobehav Rev. 2008;32:1044–1054. doi: 10.1016/j.neubiorev.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Westerhausen R, Woerner W, Kreuder F, Schweiger E, Hugdahl K, Wittling W. The role of the corpus callosum in dichotic listening: a combined morphological and diffusion tensor imaging study. Neuropsychology. 2006;20:272–279. doi: 10.1037/0894-4105.20.3.272. [DOI] [PubMed] [Google Scholar]
- Woodruff PW, McManus IC, David AS. Meta-analysis of corpus callosum size in schizophrenia. J Neurol Neurosurg Psychiatry. 1995;58:457–461. doi: 10.1136/jnnp.58.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler JC, Goswami U. Reading acquisition, developmental dyslexia, and skilled reading across languages: a psycholinguistic grain size theory. Psychol Bull. 2005;131:3–29. doi: 10.1037/0033-2909.131.1.3. [DOI] [PubMed] [Google Scholar]