Abstract
Dysfunction of the dorsolateral prefrontal cortex (DLPFC) in schizophrenia is associated with lamina-specific alterations in particular subpopulations of interneurons. In pyramidal cells, postsynaptic γ-aminobutyric acid (GABAA) receptors containing different α subunits are inserted preferentially in distinct subcellular locations targeted by inputs from specific interneuron subpopulations. We used in situ hybridization to quantify the laminar expression of α1, α2, α3, and α5 subunit, and of β1-3 subunit, mRNAs in the DLFPC of schizophrenia, and matched normal comparison subjects. In subjects with schizophrenia, mean GABAA α1 mRNA expression was 17% lower in layers 3 and 4, α2 expression was 14% higher in layer 2, α5 expression was 15% lower in layer 4, and α3 expression did not differ relative to comparison subjects. The mRNA expression of β2, which preferentially assembles with α1 subunits, was also 20% lower in layers 3 and 4, whereas β1 and β3 mRNA levels were not altered in schizophrenia. These expression differences were not attributable to medication effects or other potential confounds. These findings suggest that GABA neurotransmission in the DLPFC is altered at the postsynaptic level in a receptor subunit- and layer-specific manner in subjects with schizophrenia and support the hypothesis that GABA neurotransmission in this illness is predominantly impaired in certain cortical microcircuits.
Keywords: inhibition, in situ hybridization, interneurons, postmortem, prefrontal cortex
Introduction
Fast synaptic inhibition in the cerebral cortex is mediated by the interaction of the neurotransmitter γ-aminobutyric acid (GABA) with heteropentameric postsynaptic GABAA receptors that form ligand-gated chloride channels (Semyanov et al. 2004; Kullmann et al. 2005). The majority of GABAA receptors result from the assembly of a pair of α subunits and a pair of β subunits, in combination with a fifth, either γ or δ, subunit (Mohler 2006). Native GABAA receptors exhibit a preferential assembly for specific αβ pairings. The major combinations that have been identified in the central nervous system are α1β2γ2, α2β3γ2, and α3β3γ2, with α5β3γ2 considered to be highly probable (Mohler 2006; Olsen and Sieghart 2009). Specific α subunits confer distinct kinetic and pharmacological properties to the receptor and are associated with particular sites of subcellular localization. For example, α1-containing receptors show faster kinetics of the GABA-activated Cl− current than those with α2, α3, or α5 subunits (Farrant and Nusser 2005); in pyramidal cells, GABAA receptors containing different α subunits are targeted to specific subcellular locations and contribute to the characteristic nature of the inhibitory response evoked by the class of interneuron innervating that location (Nusser et al. 1996; Nyiri et al. 2001; Klausberger et al. 2002).
Convergent evidence indicates that schizophrenia is associated with alterations in presynaptic markers of specific interneuron subpopulations in the dorsolateral prefrontal cortex (DLPFC) (Lewis et al. 2008b). For example, lower levels of the transcript encoding the 67-kDa isoform of glutamic acid decarboxylase (GAD67), the principal enzyme for GABA synthesis, have been consistently found in the DLPFC of individuals with schizophrenia (Akbarian et al. 1995b; Guidotti et al. 2000; Mirnics et al. 2000; Volk et al. 2000; Straub et al. 2007; Hashimoto et al. 2008a). Furthermore, this deficit is particularly prominent in the subset of GABA cells that express the calcium-binding protein parvalbumin (PV) (Hashimoto et al. 2003) and may also be present in interneurons that contain the neuropeptide somatostatin (SST) (Morris et al. 2008). PV-positive cells in the DLPFC include basket cells, which target synapses enriched in α1-containing GABAA receptors subunits in the soma (and proximal dendrites) of pyramidal neurons and chandelier cells whose terminals contact pyramidal neuron axon initial segments that contain α2 or α3 subunits (Nusser et al. 1996; Loup et al. 1998, 2006; Nyiri et al. 2001). On the contrary, SST-containing Martinotti cells make contacts preferentially onto the apical dendrites of pyramidal neurons and produce inhibitory postsynaptic currents that are mediated by α5-containing GABAA receptors (Serwanski et al. 2006; Ali and Thomson 2008).
Interestingly, alterations in expression of both PV and SST mRNAs in the DLPFC of subjects with schizophrenia appear to be lamina specific. For example, levels of PV mRNA are lower in layers 3 and 4, with no difference in layers 2 and 5 (Hashimoto et al. 2003); the density of PV-positive varicosities (putative basket cell axon terminals) is lower in layers 3 and 4, but not in layer 2 (Lewis et al. 2001; Lewis and Gonzalez-Burgos 2008); and GAD67 mRNA expression is lower in PV-positive neurons in layers 3 and 4 (Hashimoto et al. 2003). On the other hand, SST mRNA expression in the DLPFC is lower principally in layers 2, 3, and 5 in subjects with schizophrenia (Morris et al. 2008), and mRNA expression of the SST2 receptor, which is localized to pyramidal cell apical dendrites, is lower in layer 5 neurons in the same subjects (Morris et al. 2006). Given that GABAA receptors enriched in specific α subunits have a preferential subcellular distribution and that they are particularly targeted by interneurons whose altered expression in the DLPFC in schizophrenia is lamina specific, we used in situ hybridization to analyze the laminar expression of the postsynaptic GABAA receptor subunits α1, α2, α3, and α5 in schizophrenia and matched normal comparison subjects. Due to the preferred assembly of each α subunit with a specific β subunit in cortical GABAA pentamers (Olsen and Sieghart 2009), we also analyzed the expression levels of β1-3 subunits in the same subjects. Our findings support the hypothesis that GABA neurotransmission in the DLPFC is in predominantly in certain cortical microcircuits in schizophrenia.
Material and Methods
Human Subjects
Brain specimens from 46 subjects were obtained after consent from the next-of-kin during autopsies conducted at the Allegheny County Medical Examiner's Office (Pittsburgh, PA). All procedures were approved by the University of Pittsburgh's Committee for the Oversight of Research Involving the Dead and Institutional Review Board for Biomedical Research.
In order to control for experimental variance and to reduce biological variance between groups, each subject with schizophrenia (n = 23) was matched for sex and, as closely as possible, for age and PMI, with one normal comparison subject (for demographic details, see Table 1). Subject groups did not differ in mean age, postmortem interval (PMI), RNA integrity number (RIN), brain pH, or tissue storage time at −80 °C (Table 1). Samples from these same subjects have been used in previous studies of mRNA expression levels for presynaptic markers of GABA neurons by in situ hybridization (Eggan et al. 2008; Morris et al. 2008), and a subset of these subject pairs (n = 14) was also included in a previous quantitative polymerase chain reaction (PCR) study in which GABAA α1 subunit mRNA was found significantly reduced in schizophrenia (Hashimoto et al. 2008a).
Table 1.
Summary of subject characteristics
| Parameter | Comparison | Schizophrenia | t-Test |
| Sex | 17 Male, 6 female | 17 Male, 6 female | |
| Race | 18 White, 5 black | 15 White, 8 black | |
| Age (years) | 48.0 (15.5) | 47.9 (14.1) | t22 = 0.16; P = 0.88 |
| PMI (h) | 18.0 (5.5) | 17.8 (9.3) | t22 = 0.22; P = 0.83 |
| Brain pH | 6.9 (0.2) | 6.8 (0.3) | t22 = 0.62; P = 0.54 |
| RIN | 8.7 (0.4) | 8.4 (0.7) | t22 = 1.84; P = 0.08 |
| Storage time (months at −80 °C) | 113.6 (23.5) | 117.8 (23.5) | t22 = −0.96; P = 0.35 |
Note: Values are mean (±SD).
Tissue Preparation
The right hemisphere of each brain was blocked coronally, immediately frozen, and stored at −80 °C (Volk et al. 2000). Cryostat sections (20 μm) from the anterior–posterior level corresponding to the middle portion of the superior frontal sulcus were cut serially and collected into tubes containing Trizol reagent (Invitrogen) for RNA isolation and RIN determination (Eggan et al. 2008), or mounted on Super frost plus glass slides (VWR International) for Nissl staining or in situ hybridization. The location of DLPFC area 9 was determined by cytoarchitectonic criteria from the Nissl-stained sections (Rajkowska and Goldman-Rakic 1995).
In Situ Hybridization
Histological analysis of gene expression was performed by in situ hybridization performed as described previously (Hashimoto et al. 2003, 2005; Morris et al. 2008) (see Supplementary Methods for additional details).
Quantification of GABAA Receptor Subunit mRNAs
Trans-illuminated autoradiographic film images were captured by a video camera under precisely controlled conditions, digitized, and quantified using a Microcomputer Imaging Device (MCID) system (Imaging Research Inc.). Quantification was performed without knowledge of subject diagnosis by random coding of the sections. Images of Nissl-stained sections were also captured and superimposed onto the autoradiographic images to draw contours of the full thickness of the cortex exclusively in the zones where the cortex was cut perpendicular to the pial surface. Optical density (OD) was measured within the contours and expressed as nCi/g of tissue by reference to radioactive 14C standards (ARC Inc.) exposed on the same autoradiographic film. The mean (standard deviation [SD]) total area of gray matter sampled in each subject was 140 (64) mm2 for normal comparison subjects and 128 (51) mm2 for subjects with schizophrenia.
Levels of mRNA expression in cortical layers 2 and 6 were determined for those subunits for which the total gray matter analysis detected a significant difference between groups, as well as for those subunits that previous reports suggested were altered in a lamina-specific manner in schizophrenia (Volk et al. 2002). Laminar expression was calculated using a series of cortical traverses (1–2 mm in width) extending from the pial surface to the white matter, as previously described (Morris et al. 2008) (see Supplementary Methods for additional details).
Evaluation of mRNA expression at the cellular level was performed for GABAA α1 and β2 transcripts in a subset of 12 subject pairs that showed >15% decrease in mRNA expression. Silver grain accumulation on emulsion-dipped, Nissl-counterstained sections was conducted as previously described (Hashimoto et al. 2003; Beneyto and Meador-Woodruff 2006, 2008; Morris et al. 2008). Briefly, using the MCID imaging software and a Zeiss microscope with a motorized stage, four 1-mm-wide cortical traverses extending from the pial surface to the white matter were placed on each tissue section in locations where area 9 was cut perpendicular to the cortical surface. In each of the cortical traverses, 4 sampling frames (120 × 170 μm) were systemically and randomly placed in deep layer 3 (defines as 35–50% of the distance from the pial surface to the white matter border), corresponding to the laminar distribution of the major change in mRNA expression observed for both GABAA α1 and β2 subunits. The edges of the frames were equidistant from the border of each traverse and the edge of the next sampling frame.
As RNase-A treatment during the in situ hybridization procedure degrades Nissl-stainable substances within the cytoplasm, it is not possible to draw contours of the neuronal soma. Thus, the number of grains/cell was counted in each frame by placing circles over nuclei of cells in a bright-field image. As previously described, grain clusters confined within a 22-μm diameter circle were considered to be interneurons and those within a 30-μm diameter circle were considered to be pyramidal cells (Benes et al. 1986; Rajkowska et al. 1998; Hashimoto et al. 2003; Beneyto and Meador-Woodruff 2006, 2008; Morris et al. 2008). In the corresponding dark-field image, the software determined the number of grains in each circle. Background grain density was measured in each slide by counting the grains in a sampling frame placed over the white matter. The smaller size and intense Nissl staining of glial nuclei distinguished them from the larger, more faintly stained neuronal nuclei. An average of 90 large cells and 60 small cells were sampled per slide (total number, α1: 1552 small cells, 819 from control vs. 732 from schizophrenia subjects, and 2232 large cells, 1120 from control vs. 1112 from schizophrenia subjects; β2: 1320 small, 715 for control vs. 605 for schizophrenia subjects, and 2088 large cells, 996 from control vs. 1092 from schizophrenia subjects). For both subject groups, frequency histograms of the number of grains of all sampled neurons normalized by the background for each slide showed unimodal and normal distribution for both large and small cells in both subject groups.
Antipsychotic-Exposed Monkeys
As described previously, 18 experimentally naive, male macaque monkeys (Macaca fasicularis), 4.5–5.3 years of age, were chronically exposed to twice daily oral doses of haloperidol, olanzapine, or placebo (n = 6 monkeys per group) for 17–27 months (Dorph-Petersen et al. 2005). Trough plasma levels for haloperidol and olanzapine were within the range associated with clinical efficacy in humans.
Animals were euthanized in triads, and serial coronal sections (16 μm) from the right frontal lobe were cut from blocks containing the anterior one-third of the principal sulcus. In situ hybridization and quantification of mRNA levels were performed using methods similar to those described above (see Supplementary Methods for additional details).
All studies were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Statistical Analysis
Two analysis of covariance (ANCOVA) models were used to test group differences for total, laminar, and cellular expression of each mRNA. The first ANCOVA model used diagnostic group as the main effect, pair as a blocking effect, and RIN, brain pH, and storage time as covariates. The pair effect reflects the matching of individual subject pairs for sex, age, and PMI. A second unpaired ANCOVA model was performed to validate the first model using diagnostic group as the main effect and sex, age, PMI, pH, RIN, and storage time as covariates. For the covariates that showed a significant effect on the OD for any of the transcripts analyzed, Person's coefficient analyses were performed and the resulting r values are reported. The results from both models are presented. Because both models produced comparable results for diagnostic group effect, only the unpaired model is reported for analysis of expression differences within layers.
The influences of potential confounding variables on the OD values in subjects with schizophrenia were assessed with ANCOVA models using each variable (sex; schizoaffective disorder; suicide; antidepressants, benzodiazepines or sodium valproate, or antipsychotics at the time of death; diagnosis of substance abuse or dependence at the time of death) as the main effect and sex, age, PMI, brain pH, RIN, and storage time as covariates. A one-way analysis of variance model, with OD as the dependent variable and treatment group as the main effect, was used to compare mRNA expression levels in the DLPFC of antipsychotic-exposed monkeys.
Results
Specificity of Riboprobes and Laminar Expression Patterns
The specificity of the riboprobes was confirmed by the following observations. First, each riboprobe showed a distinctive laminar pattern of expression in the DLPFC (Figs 1 and 2, and Supplementary Fig. 1) that was consistent with previous studies of human prefrontal cortex (Akbarian et al. 1995a; Loup et al. 2000, 2006; Petri et al. 2006). Second, no signal above background was found in sections hybridized with sense riboprobes (data not shown). Third, silver grain clusters in emulsion-dipped sections were located exclusively over neuronal nuclei (Supplementary Fig. 2).
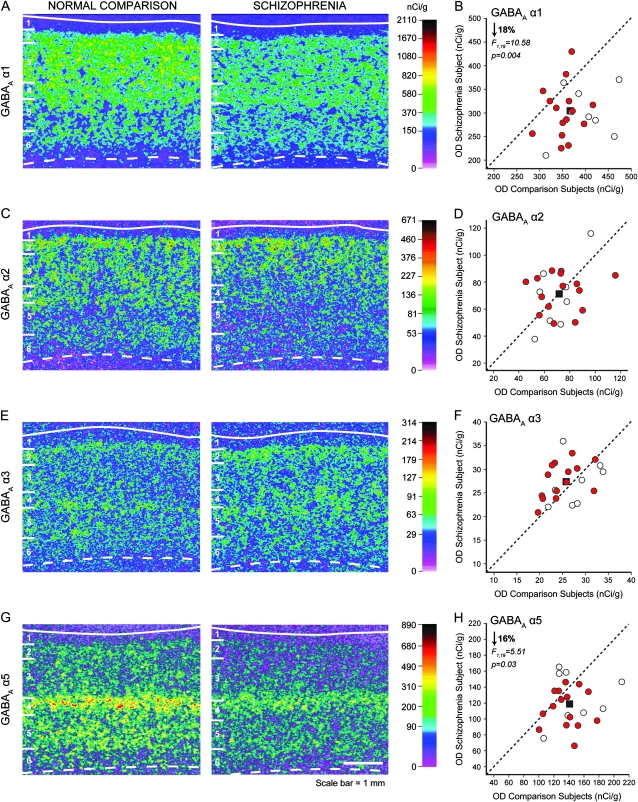
Figure 1.
Representative autoradiograms illustrating expression of GABAA α1 (A), α2 (C), α3 (E), and α5 (G) subunits mRNA in DLPFC area 9 of a comparison subject (left) and a matched subject with schizophrenia (right). The density of hybridization signal is represented in pseudocolor according to the calibration scale (nCi/g) from the 14C standards. Solid and broken lines denote the pial surface and the gray matter–white matter border, respectively. The 6 cortical layers, identified by Nissl staining, are indicated on the left of each panel. Scale bar = 1 mm. Comparison of film autoradiogram OD measures for α1 (B), α2 (D), α3 (F), and α5 (H) in total gray matter of DLPFC in matched pairs of normal comparison subjects and subjects with schizophrenia (red circles) and schizoaffective disorder (open circles). Mean value for the group difference is indicated by the black square. Labels below the dashed unity line indicate pairs for which the subject with schizophrenia or schizoaffective disorder had a lower mean expression level than the matched comparison subject.
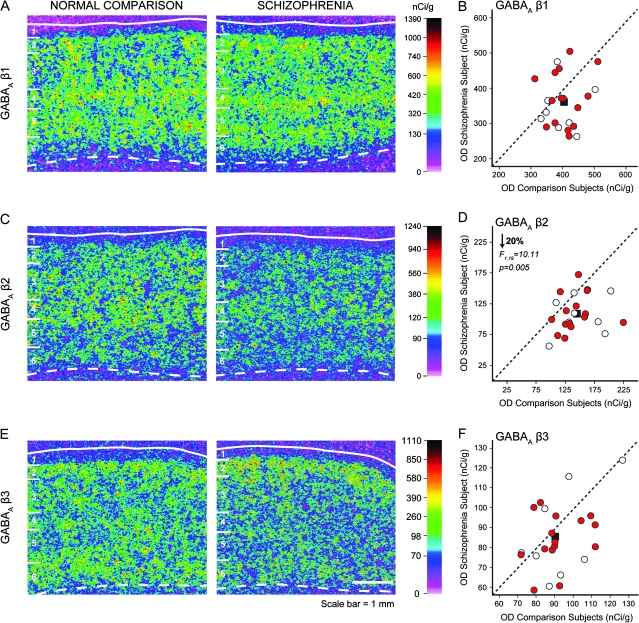
Figure 2.
Representative autoradiograms illustrating expression of GABAA β1 (A), β2 (C), and β3 (E) subunits mRNA in DLPFC area 9 of a comparison subject (left) and a matched subject with schizophrenia (right). For details, refer to Figure 1. Scale bar = 1 mm. Comparison of film autoradiogram OD measures for β1 (B), β2 (D), and β3 (F) in total gray matter of DLPFC in matched pairs of normal comparison subjects and subjects with schizophrenia (red circles) and schizoaffective disorder (open circles). For details, refer to Figure 1.
In normal comparison subjects, α1 mRNA expression was high across layers 3 to superficial 5, intermediate in layers 2 and deep 5, lower in layer 6, and lowest in layer 1 (Figs 1A and 3A–B). GABAA α2 subunit mRNA levels were high in layers 2 and superficial 3, intermediate in layers deep 3, 4, and superficial 5, low in layers deep 5 and 6, and lowest in layer 1 (Figs 1C and 3C–D). Consistent with previous reports in humans (Loup et al. 1998, 2006), but in contrast to rodents (Pirker et al. 2000), the highest expression levels of α3 mRNA were in layers 2 and 5 (Fig. 1E and Supplementary Fig. 3A); however, the overall expression levels were very low compared with the other α subunits (Fig. 1E,F). GABAA α5 subunit mRNA expression was highest in layer 4, moderate in layer 5, low in layers 2, 3, and 6, and lowest in layer 1 (Figs 1G and 3E–F). Although cross-probe comparisons must be made with caution, α1 mRNA levels were relatively higher than those for the other α subunits, consistent with previous reports of the relative abundance of these subunits (Mohler 2006).
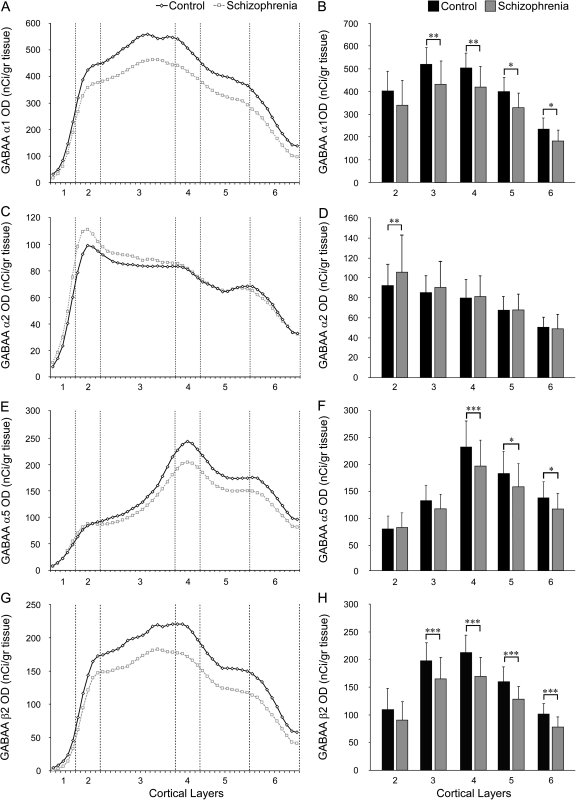
Figure 3.
Laminar expression of the mRNAs for GABAA receptor α1 (A, B), α2(C, D), α5 (E, F), and β2 (G, H) subunits. Left panels: Mean mRNA OD across cortical layers from the pial surface to the white matter border in schizophrenia (gray) and comparison groups (black). Right panels: Mean (SD) film OD for mRNA expression in each cortical layer between comparison and schizophrenia groups. *P< 0.1, **P < 0.05, ***P < 0.01.
GABAA β2 subunit mRNA expression was high in deep layer 3 and layer 4, intermediate in layer 2 and superficial layer 3, and low in layers 5 and 6 (Figs 2C and 3G–H). This laminar distribution, and the relative expression level of the β2 subunit, were very similar to those for the α1 subunit (compare Fig. 3A,G), consistent with the observation that the α1β2γ2 assembly constitutes ∼60% of GABAA receptors in the adult mammalian brain (Mohler 2006). In contrast, expression levels of β1 mRNA were high in all layers except layer 1 (Fig. 2A; Supplementary Fig. 3B), and expression of β3 mRNA was the highest in layer 2, followed by intermediate expression levels in layers 3–6, and low expression in layer 1 (Fig. 3E; Supplementary Fig. 3C).
GABAA α Subunit mRNA Expression in Schizophrenia
The mean (±SD) α1 mRNA expression level, as measured in the full thickness of the gray matter, was significantly decreased by 18% (paired: F1,19 = 10.58, P = 0.004; unpaired: F1,38 = 16.64, P < 0.001) in the schizophrenia subjects (302.6 ± 53.8 nCi/g) relative to the normal comparison group (368.3 ± 46.0 nCi/g) (Fig. 1B). Laminar analysis (Fig. 3A,B) revealed a significantly lower mRNA expression in the schizophrenia group in layer 3 (−17%; F1,38 = 5.07, P = 0.03) and layer 4 (−17%; F1,38 = 4.77, P = 0.035), with trends in the same direction in layer 5 (−17%; F1,38 = 3.87, P = 0.056) and layer 6 (−22%; F1,38 = 3.84, P = 0.057). Group differences in layers 1 and 2 did not reach statistical significance (both F1,38 < 2.20, both P > 0.15).
Total gray matter OD levels for α2 mRNA expression in the schizophrenia subjects did not significantly differ (paired: F1,19 = 2.86, P = 0.12; unpaired: F1,38 = 0.19, P = 0.66) from the matched comparison group (Fig. 1D). However, laminar analysis showed a significant (F1,38 = 6.86, P = 0.013) 14% higher expression of α2 subunit mRNA in layer 2 of the schizophrenia subjects (Fig. 3C,D), consistent with a previous immunocytochemistry study of α2 protein (Volk et al. 2002). Levels of α2 mRNA expression did not differ between diagnostic groups in the remaining cortical layers (all F1,38 < 1.82, all P > 0.19) (Fig. 3C,D).
For GABA α3 mRNA, one comparison subject had an OD value > 2.4 SD from the mean; consequently, this subject and the matched schizophrenia subject were removed for the statistical analyses. The overall expression of α3 mRNA in DLPFC gray matter was not different in the subjects with schizophrenia relative to comparison subjects (paired: F1,18 = 2.74, P = 0.12; unpaired: F1,36 = 1.62, P = 0.21) (Fig. 1F).
Analysis of the OD values for the α5 subunit mRNA revealed a significant (paired: F1,19 = 5.51, P = 0.03; unpaired: F1,38 = 6.98, P = 0.01) 16% lower expression level in the subjects with schizophrenia (118.9 ± 27.4 nCi/g) relative to comparison subjects (140.8 ± 26.3 nCi/g) (Fig. 1H). Laminar analysis (Fig. 3E,F) showed a significant 15% lower expression of α5 subunit mRNA in layer 4 (F1,38 = 5.55, P = 0.024), with trends in the same direction in layer 5 (−14%; F1,38 = 3.4, P = 0.073) and layer 6 (−15%; F1,38 = 3.67, P = 0.063). Expression of α5 mRNA did not differ between diagnostic groups in layers 1–3 (all F1,38 < 2.3, all P > 0.14).
GABAA β Subunit mRNA Expression in Schizophrenia
Total gray matter expression levels of the β1 subunit did not differ between schizophrenia and normal comparison subjects (paired: F1,19 = 1.94, P = 0.18; unpaired: F1,38 = 3.68, P = 0.07) (Fig. 2B). In contrast, total gray matter expression for β2 was significantly 20% lower in schizophrenia relative to comparison subjects (paired: F1,19 = 10.11, P = 0.005; unpaired: F1,38 = 14.21, P = 0.001) (Fig. 2D). Laminar analysis (Fig. 3G,H) revealed that this difference in β2 expression was due to significantly lower expression in layer 3 (−16%; F1,38 = 8.39, P = 0.006), layer 4 (−20%; F1,38 = 15.15, P < 0.001), layer 5 (−20%; F1,38 = 15.99, P < 0.001), and layer 6 (−23%; F1,38 = 11.37, P = 0.002). The overall expression of the β3 subunit in the total gray matter was not significantly different between schizophrenia and comparison subjects (unpaired: F1,38 = 2.38, P = 0.131; paired: F1,19 = 4.35, P = 0.102) (Fig. 2F).
Cellular mRNA Expression Levels of GABAA Subunits α1 and β2
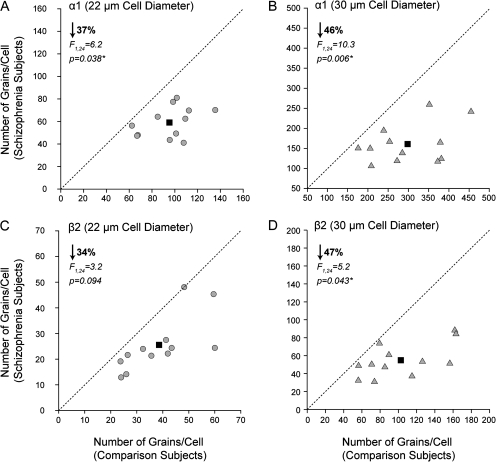
Given that α1 is known to be located at both the synapses from PV-positive basket cells on pyramidal cells as well as in the reciprocal connections between PV-positive basket cells (Nusser et al. 1996; Fritschy et al. 1998a, 1998b; Loup et al. 1998), we decided to analyze if putative pyramidal cells and interneurons exhibited lower α1 subunit expression in layer 3 in schizophrenia. The mean (±SD) number of grains per putative interneuron (i.e., 22-μm diameter sampling circle) was significantly decreased by 37% in subjects with schizophrenia (59.2 ± 13.5) relative to controls (95.3 ± 21.5) (F1,24 = 6.2, P = 0.038) (Fig. 4A). Likewise, the number of grains per putative pyramidal neuron (i.e., 30-μm diameter sampling circle) was significantly (F1,24 = 10.3, P = 0.0061) 46% lower in the subjects with schizophrenia (160.7 ± 48.8) than in the control subjects (298.3 ± 87.4) (Fig. 4B). We also measured the grain density per neuron for the β2 subunit given its preferential assembly with α1 subunits (Mohler 2006). Consistent with the findings from the film analysis, the mean (±SD) number of grains per large neuron was significantly (F1,24 = 5.2, P = 0.043) 47% lower in the subjects with schizophrenia (55.9 ± 18.7) relative to control subjects (102.8 ± 40.4) (Fig. 4D). Finally, the difference in β2 mRNA expression in small neurons, although similar in magnitude to the observed for α1, showed a trend level (F1,24 = 3.2, P = 0.094) 34% reduction in schizophrenia (25.4 ± 10.8) compared with control subjects (38.6 ± 12.9) (Fig. 4C).
Figure 4.
Cellular level analysis of the difference in GABAA subunits α1 and β2 mRNA expression in deep layer 3 of the DLPFC. Comparison of number of grains per cell measures for α1 (A, B) and β2 (C, D) in small (circles) (A, C) and large cells (triangles) (B, D) in matched pairs of normal comparison subjects and subjects with schizophrenia. Mean value for the group difference is indicated by the black square. The dashed unity line indicates no difference in expression levels.
Analysis of Potential Confounding Factors
Pearson's correlation analyses revealed significant associations between RIN and α1 (r = 0.58, P < 0.001), α2 (r = 0.48, P = 0.001), α5 (r = −0.51, P < 0.001), β1 (r = 0.51, P < 0.001), β2 (r = 0.61, P < 0.001) and β3 subunit mRNA expression (r = 0.52, P < 0.001), but not with α3 subunit mRNA expression. Correlation analyses also showed inverse associations between PMI and α1 (r = −0.44, P = 0.002) and β1 subunit mRNA expression (r = −0.41, P = 0.05). Finally, we also observed significant inverse relationships between age and α5 (r = −0.51, P < 0.001) and β3 subunit expression (r = −0.44, P = 0.002). Each of these confounding variables was included as a covariate in the ANCOVAs performed for each GABAA receptor subunit.
The mean OD values for α1, α2, α5, and β2 subunit mRNAs in the subjects with schizophrenia did not differ as function of diagnosis of schizoaffective disorder; suicide; use of antidepressants, benzodiazepines or sodium valproate, or antipsychotics at the time of death; or diagnosis of substance abuse or dependence at the time of death (all F1,16 < 5.1, all P > 0.07; Supplementary Fig. 4). Mean OD values for α1, α5, and β2 in subjects with schizophrenia did not differ by sex (Supplementary Fig. 4A,C–D); but α2 OD levels were greater in male than female subjects with schizophrenia in layer 2 (F1,17 = 4.43, P = 0.05) (Supplementary Fig. 4B). Consistent with these findings, α2 subunit expression in layer 2 was significantly higher in male subjects with schizophrenia than male normal comparison subjects (F1,30 = 9.19; P = 0.005) (Supplementary Fig. 4B). Although no difference in α2 mRNA in layer 2 was observed between female subjects with schizophrenia and female normal comparison subjects (F1,5 = 1.17; P = 0.2), the small sample (n = 6 pairs) of females in our cohort does not provide enough power to draw conclusions regarding the sex specificity of these findings.
To further test for potential effects of antipsychotic medication on the expression of GABAA receptor subunits, we examined the mRNA expression of α1, α2, α5, and β2 subunits in male macaque monkeys with long-term exposure to haloperidol, olanzapine, or placebo. The mean OD values in total gray matter and in each layer did not differ (all F2,15 < 1.1; all P > 0.4) across the subject groups for any of the subunits studied (Supplementary Fig. 5).
Discussion
Our results suggest that GABA neurotransmission in the DLPFC of subjects with schizophrenia is altered at the postsynaptic level in a receptor subunit- and layer-specific manner. These findings do not appear to be attributable to a number of potential confounds, such as antipsychotic treatment. For example, none of the transcripts (α1, α2, α5, or β2) that were altered in schizophrenia showed differences in expression in monkeys chronically exposed to either a typical (haloperidol) or atypical (olanzapine) antipsychotic medication relative to sham-treated animals (Supplementary Fig. 5). Although this approach cannot exclude a potential interaction between illness and medications, we also did not find a difference in the effect of illness in subjects with schizophrenia who were on versus those who were off antipsychotic medications at the time of death. Similarly, the effect of diagnosis did not differ between subjects who were on or off antidepressant medications, benzodiazepines, or valproate at the time of death (Supplementary Fig. 4). Thus, the combination of 1) distinctive laminar pattern of differences between schizophrenia and control subjects for some GABAA receptor subunits, 2) the lack of group differences for other subunits, and 3) the apparent absence of effects for potential confounds suggests that the disease process of schizophrenia alters GABA neurotransmission in specific DLPFC microcircuits.
Potential Relationships between Pre- and Postsynaptic Alterations in Markers of GABA Neurotransmission in Schizophrenia
GABAA Receptors Containing α1 Subunits and PV-Containing Basket Cell Inputs
The inputs from the PV-positive basket cell class of GABA neurons to pyramidal neuron cell bodies are mediated predominately by α1-containing GABAA receptors in the adult cerebral cortex, whereas contacts made by PV-negative basket cell terminals have very low levels of this subunit (Nusser et al. 1996; Fritschy et al. 1998a, 1998b; Loup et al. 1998). Interestingly, the significantly lower expression of α1 mRNA predominantly in layers 3 and 4 of schizophrenia subjects is similar to that previously reported for the following presynaptic markers of PV-positive interneurons in the DLPFC of subjects with schizophrenia: 1) lower PV mRNA expression in layers 3 and 4, with no difference in layers 2 and 5 (Hashimoto et al. 2003); 2) lower density of PV-positive varicosities (putative basket cell axon terminals) in layers 3 and 4, but not in layer 2 (Lewis et al. 2001; Lewis and Gonzalez-Burgos 2008); and 3) lower GAD67 mRNA expression in PV-positive neurons in layers 3 and 4 (Hashimoto et al. 2003). Together, these findings suggest a coordinated pre- and postsynaptic reduction of phasic GABA neurotransmission from PV-positive basket cells to pyramidal cells and/or other interneurons in the middle cortical layers in schizophrenia (Fig. 5). For example, α1-containing GABAA receptors are present postsynaptic to PV-positive basket cells in both pyramidal neurons and other PV-positive basket cells (Klausberger et al. 2002). Our single-cell–level analysis of both α1 and β2 expression by emulsion grain counting showed that both mRNAs are lower in both large cells, putative pyramidal neurons, and in small cells, putative interneurons. However, due to the technical limitations for cell type classification associated with the use of Nissl-stained, RNase-treated samples, confirmation of lower α1 and β2 mRNA in both pyramidal and interneurons awaits dual label in situ hybridization studies with cell type–specific markers.
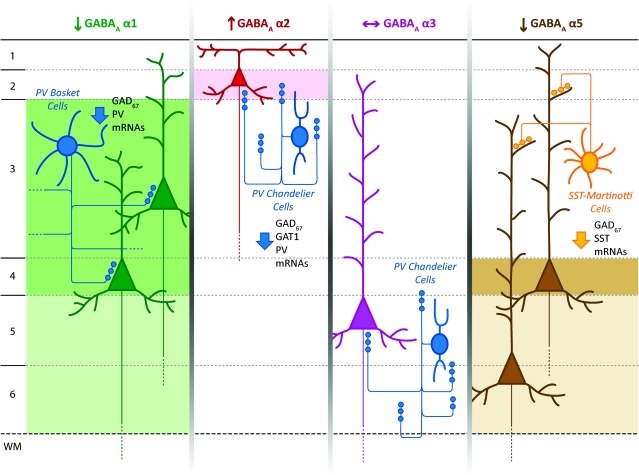
Figure 5.
Schematic summary of hypothesized circuit-specific transcript alterations in pre- and postsynaptic markers of GABA neurotransmission in the DLPFC of subjects with schizophrenia. For each GABAA α subunit, the background shading marks the cortical layers where the indicated change in expression of that subunit was found. The laminar specificity of the decrease in α1 expression matches that of the alterations in GAD67 and PV mRNAs thought to be present in PV-positive basket cells. The increase in α2 expression in layer 2 is consistent with previous findings of pre- and postsynaptic alterations in chandelier cell inputs to the axon initial segment of pyramidal cells in this location. In contrast, the absence of alterations in α3 subunit expression, which is present postsynaptic to chandelier cells in deep layer pyramidal neurons matches the failure to find significant changes in chandelier cell inputs in these layers. The decrease in α5 was observed in deeper layers of DLPFC where the somata of pyramidal neurons whose apical dendrites are known to be innervated by SST+ Martinotti cells, also affected in schizophrenia, are predominantly located.
Consistent with the interpretation of a coordinated pre- and postsynaptic reduction of phasic GABA neurotransmission from PV-positive basket cells in the middle layers of the DLPFC in schizophrenia, mRNA levels for the GABAA receptor β2 (present study) and γ2 (Akbarian et al. 1995a; Huntsman et al. 1998, 2008a) subunits, which typically assemble with α1 subunits in postsynaptic receptors mediating phasic GABA neurotransmission (Farrant and Nusser 2005), are also reduced in the DLPFC in schizophrenia. Indeed, we detected strong positive correlations between the within-subject expression of α1 and β2 subunit mRNAs (r = 0.758, P < 0.001) and between their within-pair percentage differences (r = 0.601, P = 0.002) in the DLPFC. Furthermore, this correlation was also observed at the cellular level, indicating that the reduction in α1β2-containing GABAA receptors likely affects both pyramidal cells and interneurons (large and small cells) in layers 3 and 4 of the DLPFC in schizophrenia. Schizophrenia-associated alterations in expression of α1 and γ2 subunit mRNAs are also positively correlated (Hashimoto et al. 2008a). Given that α1, β2, and γ2 subunits coassemble to form ∼60% of GABAA receptors in the adult cortex (Mohler 2006), a coordinated difference in their expression levels would be expected. The biological relevance of these correlations is further supported by the similar magnitude and identical laminar pattern of the expression alterations in α1 and β2 mRNAs in schizophrenia subjects and by the absence of expression alterations in either β1 or β3 subunits, which do not assemble with α1 subunits, and accordingly have different laminar patterns of expression. Thus, this constellation of findings suggests that the total number of α1β2γ2 GABAA receptors is lower in the middle layers of the DLPFC in subjects with schizophrenia.
Interestingly, α1 subunits can also coassemble with a δ (instead of γ) subunit to form functional receptors (Mertens et al. 1993; Saxena and Macdonald 1994; Bianchi and Macdonald 2003). Cortical GABAA receptors containing δ subunits are extrasynaptic, have a high affinity for GABA, and mediate tonic inhibition, defined as the constant activation of extrasynaptic receptors that, by increasing input conductance, reduces the probability of generating an action potential (Farrant and Nusser 2005). In primate DLPFC, both α1 and δ subunits have similar laminar patterns of expression and undergo similar developmental trajectories, which are distinct from those of GABAA α2 and α4 subunits (Hashimoto et al. 2009; Maldonado-Aviles et al. 2009). In the present study, the laminar pattern of altered α1 mRNA expression matches that exhibited by the δ subunit transcript in the same subjects (Maldonado-Aviles et al. 2009), and the within-subject pair differences in α1 and δ mRNA levels were significantly correlated (r = 0.74, P < 0.0001). Together, these findings suggest that schizophrenia might be associated with a reduced complement of α1βxδ GABAA receptors in the DLPFC. Thus, our findings raise the possibility that both synaptic, phasic inhibition from PV-positive basket cells (via α1β2γ2 receptors) and tonic inhibition (via α1βxδ receptors) are altered in the DLPFC of subjects with schizophrenia (Maldonado-Aviles et al. 2009).
Deficits in both α1 and β2 transcript expression, similar in magnitude and laminar pattern to those observed in the present study, were previously reported in the DLPFC in schizophrenia, although those findings did not achieve statistical significance. Such due to a smaller sample size (Akbarian et al. 1995a). Lower α1 transcript levels in the DLPFC in schizophrenia have also been observed by microarray and quantitative PCR (Hashimoto et al. 2008a, 2008b). However, some studies have reported higher cortical levels of α1 transcript (Impagnatiello et al. 1998; Ohnuma et al. 1999) and protein (Ishikawa et al. 2004) in schizophrenia. These earlier studies utilized subject groups that were not as well matched for age and sex and did not assess RNA integrity markers, such as RIN or brain pH, raising the possibility that the group differences reflect factors other than the disease process of schizophrenia. However, additional studies are required to reconcile the differences in the literature.
GABAA Receptors Containing α2 Subunits and PV-Positive Chandelier Cell Inputs
Our finding of greater expression of GABAA α2 subunit mRNA in DLPFC layer 2 (and superficial layer 3; Fig. 3C,D) from schizophrenia subjects parallels the laminar distribution of alterations in both pre- and postsynaptic markers of inputs from PV-positive chandelier cells to the axon initial segment of pyramidal neurons (Fig. 5). For example, increased α2 subunit protein immunoreactivity (Volk et al. 2002) and decreased ankyrin-G immunoreactivity in pyramidal neuron axon initial segments (Cruz et al. 2009), as well as decreased GABA membrane transporter (GAT-1) immunoreactivity in chandelier neuron axon cartridges (Woo et al. 1998; Pierri et al. 1999), are all preferentially or selectively found in DLPFC layers 2 and superficial 3 of subjects with schizophrenia. Thus, these findings suggest that alterations in chandelier cell-pyramidal cell connectivity differ in laminar location from those found in basket cell-pyramidal cell connectivity. Consistent with this idea that an interaction between GABA cell type and laminar location confers vulnerability in schizophrenia, we did not observe altered mRNA expression of the α3 subunit, which in contrast to the α2 subunit, is enriched in the axon initial segment of pyramidal cells in deeper cortical layers (Nusser et al. 1996; Loup et al. 1998, 2006) where presynaptic alterations chandelier neuron axon cartridges are less prominent (Woo et al. 1998; Pierri et al. 1999). Interestingly, the expression of GAD67 mRNA is not altered in DLPFC layer 6 of subjects with schizophrenia (Akbarian et al. 1995a; Volk et al. 2000), supporting the idea of laminar and circuit specificity of altered GABA neurotransmission in schizophrenia (Fig. 5).
In the hippocampus, α2 subunit–containing GABAA receptors are also present in pyramidal cell bodies postsynaptic to axon terminals from PV-negative basket neurons that express the neuropeptide cholecystokinin (CCK) and that contain the cannabinoid receptor 1 (CB1R) on their axon terminals (Nyiri et al. 2001). Although we cannot exclude an upregulation of α2 subunits postsynaptic to these CCK/CB1R inputs, their upregulation in layers 2 to superficial 3 does not match the high density of CCK/CB1R axon terminals in layers 4 and 6 (Oeth and Lewis 1993; Eggan and Lewis 2007) or the findings that CB1R-positive axons in the DLPFC of subjects with schizophrenia are significantly altered in those layers, but not in layers 2 and superficial 3 (Eggan et al. 2008).
GABAA Receptors Containing α5 Subunits and SST–Martinotti Cell Inputs
Our findings of a 15% reduction in α5 mRNA expression in DLPFC layers 4–6 in schizophrenia are consistent in magnitude and laminar distribution with a previous report showing a trend decrease of ∼22% for α5 mRNA expression (Akbarian et al. 1995a). However, higher expression for α5 subunit transcript expression has also been observed in schizophrenia (Impagnatiello et al. 1998). As for α1 subunit expression, these discrepancies might reflect differences across studies in the extent to which the potential impact of various confounds were controlled, but further studies are warranted.
GABAA receptors containing α5 subunits were previously thought to be almost exclusively extrasynaptic, contributing to the tonic inhibition of pyramidal cells (Caraiscos et al. 2004). However, recent studies conclusively identified the presence of GABAA α5 subunits on pyramidal cell apical dendrites postsynaptic to GABA inputs from SST-containing Martinotti cells (Ali and Thomson 2008). Interestingly, SST mRNA expression is lower in the DLPFC in schizophrenia, principally in layers 2, 3, and 5 (Morris et al. 2008), and mRNA expression of SST2 receptor, which is localized to pyramidal cell apical dendrites, is lower in layer 5 neurons in the same subjects (Morris et al. 2006). Together, these findings suggest a correlated alteration in schizophrenia of GABA and SST inputs to the apical dendrites, present in layers 2 and 3, of pyramidal neurons whose cell bodies are present in the deeper layers of the DLPFC (Fig. 5).
Functional Implications of Lamina-Specific Alterations in GABAA α Subunits
In concert with previous investigations, our findings suggest that cortical GABA neurotransmission in altered in schizophrenia in certain DLPFC microcircuits, defined by the laminar location of coordinated pre- and postsynaptic changes in the inputs from specific subclasses of GABA neurons (Fig. 5). Specifically, the findings suggest altered inhibitory regulation of different domains (cell body, axon initial segment, or dendrites) in separate populations of pyramidal neurons. In contrast, although we cannot exclude alterations in the inhibitory regulation of GABA neurons, neither the results of this study nor of previous studies of the calretinin-containing GABA neurons that provide the majority of such inputs (Woo et al. 1997; Cotter et al. 2002; Hashimoto et al. 2003) provide evidence of such a disturbance. Thus, the composite data suggest a circuit-specific dysregulation of the inhibition, but not of disynaptic disinhibition, of DLPFC pyramidal neurons in schizophrenia.
The synaptic inhibition provided by the affected GABA inputs serves to coordinate the timing of neuronal activity by synchronizing the firing of pyramidal cells at different frequencies. For example, gamma band (30–80 Hz) oscillations appear to require the fast decay of the GABA-mediated Cl− current through α1-containing GABAA receptors; in particular, α1-containing synaptic inputs from PV-positive basket cells to the perisomatic region of pyramidal cells are critical for rhythmic gamma activity (Mann and Paulsen 2005; Bartos et al. 2007), whereas α1-mediated connections between PV-positive basket cells are not (Wulff et al. 2009). Thus, if our findings are indicative of reduced inhibitory transmission in basket cell inputs to pyramidal cells, then one would expect to see a reduction in gamma band oscillations in schizophrenia, which has, in fact, been observed over the DLPFC (Lewis et al. 2005; Cho et al. 2006). Whether this impairment can be attributed specifically to the laminar-specific alteration in PV-positive basket cell-pyramidal cell connectivity remains to be determined, but layer-specific factors in the generation of oscillations have been observed in vitro (Cunningham et al. 2003).
In vivo data (Cardin et al. 2009; Sohal et al. 2009) clearly indicate the dependence of gamma oscillations on PV neurons, but these studies were not able to discriminate between the contributions of basket versus chandelier cells. In studies of hippocampal oscillations in vivo, the firing pattern of chandelier cells strongly suggests a potent inhibitory effect on pyramidal cells, but recent in vitro studies indicate that chandelier neurons can, under certain conditions, powerfully depolarize pyramidal neurons (Szabadics et al. 2006; Khirug et al. 2008). Thus, how pre- and postsynaptic alterations in chandelier cell inputs contribute to the observed deficits in prefrontal gamma oscillations in schizophrenia remains to be determined, but their involvement is supported by the finding that a positive allosteric modulator for α2-containing GABAA receptors increased prefrontal gamma band power during a working memory task in individuals with schizophrenia (Lewis et al. 2008a). Similarly, theta oscillations (4–7 Hz) may depend on GABA inputs from SST-containing Martinotti cells (Fanselow et al. 2008) that are mediated by the slower kinetics of α5-containing GABAA receptors (Ali and Thomson 2008; Zarnowska et al. 2009). Interestingly, gamma oscillations are thought to be embedded in theta frequency oscillations in the context of certain cognitive tasks, such as working memory (Lisman and Idiart 1995), raising the possibility that the working memory disturbances in schizophrenia reflect the behavioral outcome of convergent disturbances in different GABA circuits in the DLPFC.
Although the findings of this study are consistent with earlier data in supporting circuit-specific alterations in GABA neurotransmission in schizophrenia, they do not suggest a simple hypothesis regarding the pathogenetic mechanisms underlying the alterations. For example, prior studies suggested that the upregulation of postsynaptic α2-containing receptors in pyramidal neuron axon initial segments represented a compensatory response to a presynaptic deficiency in GABA release from chandelier neurons due to lower levels of GAD67 (Volk et al. 2002; Lewis et al. 2005). If so, why are α1-containing receptors not upregulated postsynaptic to PV basket cells, given the likelihood that GAD67 mRNA expression is reduced in these neurons as well? One possibility is suggested by findings in mice that GAD67-mediated GABA synthesis is required for the formation of basket cell axon terminals and synapses (Chattopadhyaya et al. 2007). If GAD67 expression is low during postnatal development in individuals who later develop schizophrenia, then fewer basket cell synapses would form on pyramidal cell bodies, reducing the need for postsynaptic GABAA receptors containing α1 subunits. Alternatively, the disease-related differences in the expression of α1 and α2 subunits might reflect disturbances in their opposed patterns of development. For example, in monkey DLPFC, the expression of α2 is high at birth and progressively declines through adolescence, whereas α1 levels are low at birth and progressively increase with age (Hashimoto et al. 2009). Thus, schizophrenia might involve a disruption, or arrest, in the normal developmental trajectories of GABAA α1 and α2 subunit expression. This hypothesis suggests new avenues of investigation for GABA circuit-specific interventions in individuals at high risk for schizophrenia.
Supplementary Material
Supplementary Methods and Figures 1–5 can be found at: http://www.cercor.oxfordjournals.org/
Funding
National Alliance for Research in Schizophrenia and Depression Young Investigator Awards (to M.B. and T.H.); the National Institutes of Health (grant numbers MH043784 and MH084053 to D.A.L.).
Acknowledgments
We thank Mary Brady, BS, for her assistance in editing the graphics, Holly Bazmi, MS, for her assistance in generating templates for the GABRA3 and GABRA5 riboprobes, Jim Kosakowski for developing the Matlab program for laminar analysis, and Katherine C. McTish for her assistance in grain counting. We thank the members of the Clinical Services and Diagnostics Core of the Conte Center for the Neuroscience of Mental Disorders (MH084053) for their assistance in diagnostic assessments. In 2007–2009 D.A.L. served as a consultant in the areas of target identification and validation and new compound development to AstraZeneca, BioLine RX, Bristol-Myers Squibb, Hoffman-Roche, Lilly, Merck, Neurogen, and SK Life Science. Conflict of Interest: David A. Lewis currently receives investigator-initiated research support from the BMS Foundation, Bristol-Myers Squibb, Curridium Ltd, and Pfizer and in 2007-2009 served as a consultant in the areas of target identification and validation and new compound development to AstraZeneca, BioLine RX, Bristol-Myers Squibb, Hoffman-Roche, Lilly, Merck, Neurogen, and SK Life Science.
References
- Akbarian S, Huntsman MM, Kim JJ, Tafazzoli A, Potkin SG, Bunney WE, Jr., Jones EG. GABAA receptor subunit gene expression in human prefrontal cortex: comparison of schizophrenics and controls. Cereb Cortex. 1995a;5:550–560. doi: 10.1093/cercor/5.6.550. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr., Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995b;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Ali AB, Thomson AM. Synaptic alpha 5 subunit-containing GABAA receptors mediate IPSPs elicited by dendrite-preferring cells in rat neocortex. Cereb Cortex. 2008;18:1260–1271. doi: 10.1093/cercor/bhm160. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Benes FM, Davidson J, Bird ED. Quantitative cytoarchitectural studies of the cerebral cortex of schizophrenics. Arch Gen Psychiatry. 1986;43:31–35. doi: 10.1001/archpsyc.1986.01800010033004. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Meador-Woodruff JH. Lamina-specific abnormalities of AMPA receptor trafficking and signaling molecule transcripts in the prefrontal cortex in schizophrenia. Synapse. 2006;60:585–598. doi: 10.1002/syn.20329. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Meador-Woodruff JH. Lamina-specific abnormalities of NMDA receptor-associated postsynaptic protein transcripts in the prefrontal cortex in schizophrenia and bipolar disorder. Neuropsychopharmacology. 2008;33:2175–2186. doi: 10.1038/sj.npp.1301604. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABA(A) receptor channels from low- to high-efficacy gating patterns. J Neurosci. 2003;23:10934–10943. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraiscos VB, Newell JG, You-Ten KE, Elliott EM, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Selective enhancement of tonic GABAergic inhibition in murine hippocampal neurons by low concentrations of the volatile anesthetic isoflurane. J Neurosci. 2004;24:8454–8458. doi: 10.1523/JNEUROSCI.2063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Wu CZ, Knott G, Kuhlman S, Fu Y, Palmiter RD, Huang ZJ. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron. 2007;54:889–903. doi: 10.1016/j.neuron.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter D, Landau S, Beasley C, Stevenson R, Chana G, MacMillan L, Everall I. The density and spatial distribution of GABAergic neurons, labelled using calcium binding proteins, in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia. Biol Psychiatry. 2002;51:377–386. doi: 10.1016/s0006-3223(01)01243-4. [DOI] [PubMed] [Google Scholar]
- Cruz DA, Weaver CL, Lovallo EM, Melchitzky DS, Lewis DA. Selective alterations in postsynaptic markers of chandelier cell inputs to cortical pyramidal neurons in subjects with schizophrenia. Neuropsychopharmacology. 2009;34:2112–2124. doi: 10.1038/npp.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MO, Davies CH, Buhl EH, Kopell N, Whittington MA. Gamma oscillations induced by kainate receptor activation in the entorhinal cortex in vitro. J Neurosci. 2003;23:9761–9769. doi: 10.1523/JNEUROSCI.23-30-09761.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30:1649–1661. doi: 10.1038/sj.npp.1300710. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Hashimoto T, Lewis DA. Reduced cortical cannabinoid 1 receptor messenger RNA and protein expression in schizophrenia. Arch Gen Psychiatry. 2008;65:772–784. doi: 10.1001/archpsyc.65.7.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17:175–191. doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- Fanselow EE, Richardson KA, Connors BW. Selective, state-dependent activation of somatostatin-expressing inhibitory interneurons in mouse neocortex. J Neurophysiol. 2008;100:2640–2652. doi: 10.1152/jn.90691.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Johnson DK, Mohler H, Rudolph U. Independent assembly and subcellular targeting of GABA(A)-receptor subtypes demonstrated in mouse hippocampal and olfactory neurons in vivo. Neurosci Lett. 1998a;249:99–102. doi: 10.1016/s0304-3940(98)00397-8. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Weinmann O, Wenzel A, Benke D. Synapse-specific localization of NMDA and GABA(A) receptor subunits revealed by antigen-retrieval immunohistochemistry. J Comp Neurol. 1998b;390:194–210. [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008a;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008b;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, Sun Z, Sampson AR, Lewis DA. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci. 2005;25:372–383. doi: 10.1523/JNEUROSCI.4035-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Nguyen QL, Rotaru D, Keenan T, Arion D, Beneyto M, Gonzalez-Burgos G, Lewis DA. Protracted developmental trajectories of GABAA receptor alpha1 and alpha2 subunit expression in primate prefrontal cortex. Biol Psychiatry. 2009;65:1015–1023. doi: 10.1016/j.biopsych.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntsman MM, Tran BV, Potkin SG, Bunney WE, Jr., Jones EG. Altered ratios of alternatively spliced long and short gamma2 subunit mRNAs of the gamma-amino butyrate type A receptor in prefrontal cortex of schizophrenics. Proc Natl Acad Sci U S A. 1998;95:15066–15071. doi: 10.1073/pnas.95.25.15066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impagnatiello F, Guidotti AR, Pesold C, Dwivedi Y, Caruncho H, Pisu MG, Uzunov DP, Smalheiser NR, Davis JM, Pandey GN, et al. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci U S A. 1998;95:15718–15723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Mizukami K, Iwakiri M, Hidaka S, Asada T. Immunohistochemical and immunoblot study of GABA(A) alpha1 and beta2/3 subunits in the prefrontal cortex of subjects with schizophrenia and bipolar disorder. Neurosci Res. 2004;50:77–84. doi: 10.1016/j.neures.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Khirug S, Yamada J, Afzalov R, Voipio J, Khiroug L, Kaila K. GABAergic depolarization of the axon initial segment in cortical principal neurons is caused by the Na-K-2Cl cotransporter NKCC1. J Neurosci. 2008;28:4635–4639. doi: 10.1523/JNEUROSCI.0908-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Roberts JD, Somogyi P. Cell type- and input-specific differences in the number and subtypes of synaptic GABA(A) receptors in the hippocampus. J Neurosci. 2002;22:2513–2521. doi: 10.1523/JNEUROSCI.22-07-02513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Ruiz A, Rusakov DM, Scott R, Semyanov A, Walker MC. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why? Prog Biophys Mol Biol. 2005;87:33–46. doi: 10.1016/j.pbiomolbio.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Cho RY, Carter CS, Eklund K, Forster S, Kelly MA, Montrose D. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry. 2008a;165:1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Cruz DA, Melchitzky DS, Pierri JN. Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am J Psychiatry. 2001;158:1411–1422. doi: 10.1176/appi.ajp.158.9.1411. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Morris HM. Cell and receptor type-specific alterations in markers of GABA neurotransmission in the prefrontal cortex of subjects with schizophrenia. Neurotoxicol Res. 2008b;14:237–248. doi: 10.1007/BF03033813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Idiart MA. Storage of 7 ± 2 short-term memories in oscillatory subcycles. Science. 1995;267:1512–1515. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- Loup F, Picard F, Andre VM, Kehrli P, Yonekawa Y, Wieser HG, Fritschy JM. Altered expression of alpha3-containing GABAA receptors in the neocortex of patients with focal epilepsy. Brain. 2006;129:3277–3289. doi: 10.1093/brain/awl287. [DOI] [PubMed] [Google Scholar]
- Loup F, Weinmann O, Yonekawa Y, Aguzzi A, Wieser HG, Fritschy JM. A highly sensitive immunofluorescence procedure for analyzing the subcellular distribution of GABAA receptor subunits in the human brain. J Histochem Cytochem. 1998;46:1129–1139. doi: 10.1177/002215549804601005. [DOI] [PubMed] [Google Scholar]
- Loup F, Wieser HG, Yonekawa Y, Aguzzi A, Fritschy JM. Selective alterations in GABAA receptor subtypes in human temporal lobe epilepsy. J Neurosci. 2000;20:5401–5419. doi: 10.1523/JNEUROSCI.20-14-05401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Aviles JG, Curley AA, Hashimoto T, Morrow AL, Ramsey AJ, O'Donnell P, Volk DW, Lewis DA. Altered markers of tonic inhibition in the dorsolateral prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2009;166:450–459. doi: 10.1176/appi.ajp.2008.08101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EO, Paulsen O. Mechanisms underlying gamma ('40 Hz') network oscillations in the hippocampus–a mini-review. Prog Biophys Mol Biol. 2005;87:67–76. doi: 10.1016/j.pbiomolbio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Mertens S, Benke D, Mohler H. GABAA receptor populations with novel subunit combinations and drug binding profiles identified in brain by alpha 5- and delta-subunit-specific immunopurification. J Biol Chem. 1993;268:5965–5973. [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Mohler H. GABA(A) receptor diversity and pharmacology. Cell Tissue Res. 2006;326:505–516. doi: 10.1007/s00441-006-0284-3. [DOI] [PubMed] [Google Scholar]
- Morris H, Hashimoto T, Lewis DA. Analysis of the mRNA expression of somatostatin receptors in the prefrontal cortex of individuals with schizophrenia. Atlanta (GA): Society for Neuroscience; 2006. [Google Scholar]
- Morris HM, Hashimoto T, Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex. 2008;18:1575–1587. doi: 10.1093/cercor/bhm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Benke D, Fritschy JM, Somogyi P. Differential synaptic localization of two major gamma-aminobutyric acid type A receptor alpha subunits on hippocampal pyramidal cells. Proc Natl Acad Sci U S A. 1996;93:11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyiri G, Freund TF, Somogyi P. Input-dependent synaptic targeting of alpha(2)-subunit-containing GABA(A) receptors in synapses of hippocampal pyramidal cells of the rat. Eur J Neurosci. 2001;13:428–442. doi: 10.1046/j.1460-9568.2001.01407.x. [DOI] [PubMed] [Google Scholar]
- Oeth KM, Lewis DA. Postnatal development of the cholecystokinin innervation of monkey prefrontal cortex. J Comp Neurol. 1993;336:400–418. doi: 10.1002/cne.903360307. [DOI] [PubMed] [Google Scholar]
- Ohnuma T, Augood SJ, Arai H, McKenna PJ, Emson PC. Measurement of GABAergic parameters in the prefrontal cortex in schizophrenia: focus on GABA content, GABA(A) receptor alpha-1 subunit messenger RNA and human GABA transporter-1 (HGAT-1) messenger RNA expression. Neuroscience. 1999;93:441–448. doi: 10.1016/s0306-4522(99)00189-x. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri S, Kollewe K, Grothe C, Hori A, Dengler R, Bufler J, Krampfl K. GABA(A)-receptor mRNA expression in the prefrontal and temporal cortex of ALS patients. J Neurol Sci. 2006;250:124–132. doi: 10.1016/j.jns.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Pierri JN, Chaudry AS, Woo TU, Lewis DA. Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. Am J Psychiatry. 1999;156:1709–1719. doi: 10.1176/ajp.156.11.1709. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: i. Remapping of areas 9 and 46 using quantitative criteria. Cereb Cortex. 1995;5:307–322. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry. 1998;55:215–224. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Assembly of GABAA receptor subunits: role of the delta subunit. J Neurosci. 1994;14:7077–7086. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Serwanski DR, Miralles CP, Christie SB, Mehta AK, Li X, De Blas AL. Synaptic and nonsynaptic localization of GABAA receptors containing the alpha5 subunit in the rat brain. J Comp Neurol. 2006;499:458–470. doi: 10.1002/cne.21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RE, Lipska BK, Egan MF, Goldberg TE, Callicott JH, Mayhew MB, Vakkalanka RK, Kolachana BS, Kleinman JE, Weinberger DR. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Mol Psychiatry. 2007;12:854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311:233–235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- Volk DW, Pierri JN, Fritschy JM, Auh S, Sampson AR, Lewis DA. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 2002;12:1063–1070. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- Woo TU, Miller JL, Lewis DA. Schizophrenia and the parvalbumin-containing class of cortical local circuit neurons. Am J Psychiatry. 1997;154:1013–1015. doi: 10.1176/ajp.154.7.1013. [DOI] [PubMed] [Google Scholar]
- Woo TU, Whitehead RE, Melchitzky DS, Lewis DA. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci U S A. 1998;95:5341–5346. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff P, Ponomarenko AA, Bartos M, Korotkova TM, Fuchs EC, Bahner F, Both M, Tort AB, Kopell NJ, Wisden W, et al. Hippocampal theta rhythm and its coupling with gamma oscillations require fast inhibition onto parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2009;106:3561–3566. doi: 10.1073/pnas.0813176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnowska ED, Keist R, Rudolph U, Pearce RA. GABAA receptor alpha5 subunits contribute to GABAA, low synaptic inhibition in mouse hippocampus. J Neurophysiol. 2009;101:1179–1191. doi: 10.1152/jn.91203.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.