Abstract
Assigning meaning to words, sounds, and objects requires stored conceptual knowledge plus executive mechanisms that shape semantic retrieval according to the task or context. Despite the essential role of control in semantic cognition, its neural basis remains unclear. Neuroimaging and patient research has emphasized the importance of left inferior frontal gyrus (IFG)—however, impaired semantic control can also follow left temporoparietal lesions, suggesting that this function may be underpinned by a large-scale cortical network. We used repetitive transcranial magnetic stimulation in healthy volunteers to disrupt processing within 2 potential sites in this network—IFG and posterior middle temporal cortex. Stimulation of both sites selectively disrupted executively demanding semantic judgments: semantic decisions based on strong automatic associations were unaffected. Performance was also unchanged in nonsemantic tasks—irrespective of their executive demands—and following stimulation of a control site. These results reveal that an extended network of prefrontal and posterior temporal regions underpins semantic control.
Keywords: executive functions, prefrontal cortex, semantic decisions, temporal cortex, transcranial magnetic stimulation
Introduction
Semantic cognition refers to the goal-directed activation of semantic knowledge, encompassing the meanings of words, sounds, actions, symbols, and faces. As such, it brings meaning to our interactions with objects and people around us—and, as a consequence, it plays a vital role in most activities of daily living. Studies of brain-injured patients with debilitating semantic disorders have revealed a great deal about how our store of semantic knowledge is organized in the brain (Martin and Chao 2001; Damasio et al. 2004; Martin 2007; Patterson et al. 2007). However, semantic cognition not only requires us to access our store of semantic facts but also to manipulate this information such that task-relevant aspects of meaning are brought to the fore. This is because we know a vast amount about any given concept—yet only particular aspects of our knowledge will be relevant in a given situation. For example, we know many different things about bananas, including that they are peeled before being eaten and that they can make you slip when dropped on the ground. In order to understand the relationship between “banana” and “slip,” it is necessary to focus on a relatively obscure aspect of meaning (i.e., that bananas have a slimy texture) as opposed to more dominant aspects of meaning that are thought to be retrieved automatically (Thompson-Schill et al. 1997; Wagner et al. 2001; Badre et al. 2005; Jefferies and Lambon Ralph 2006; Badre and Wagner 2007; Jefferies et al. 2007; Corbett, Jefferies, and Lambon Ralph 2009). As a consequence, successful semantic cognition (the application of semantic knowledge in a particular situation) involves 2 interacting but separable components: 1) information within the semantic store itself and 2) executive mechanisms that direct semantic activation so that it is appropriate for the current task or context.
Recent work—encompassing both functional neuroimaging and studies of brain-injured patients—suggests that these 2 basic processes of semantic cognition, semantic representation and control, are underpinned by distinct areas in the human brain. While left temporal regions (especially anterior and mid-ventrolateral aspects) are considered to be critical for the semantic store (Hodges et al. 1992; Vandenberghe et al. 1996; Hickok and Poeppel 2004; Indefrey and Levelt 2004; Rogers et al. 2004; Vigneau et al. 2006; Patterson et al. 2007; Binder et al. 2009; Binney et al. 2010), left inferior frontal gyrus (IFG) is thought to regulate the recovery of semantic information, presumably via top-down signals to temporal cortex (Thompson-Schill et al. 1997; Wagner et al. 2001; Bookheimer 2002; Badre et al. 2005; Ye and Zhou 2009). In functional neuroimaging studies, brain activation increases in left IFG when participants are required to retrieve nondominant aspects of knowledge (such as banana-slip, as opposed to banana-peel) or the subordinate meanings of ambiguous words (e.g., bank-river, as opposed to bank-money) (Bedny et al. 2008; see also Gennari et al. 2007; Zempleni et al. 2007; Whitney et al. 2009). Moreover, this research is consistent with the view that the prefrontal cortex is of fundamental importance in executive control across a wide range of cognitive domains (Duncan and Owen 2000; Owen et al. 2000; Petrides 2005; Duncan 2006, 2010; Badre and D'Esposito 2007; Badre 2008).
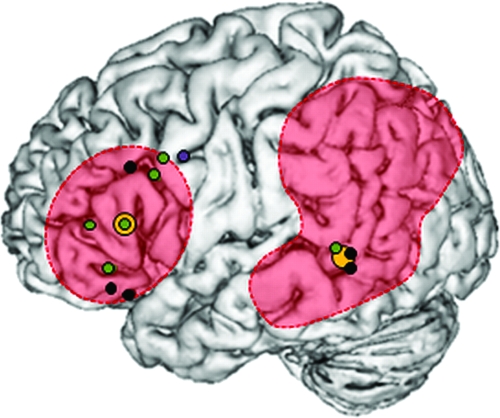
Although functional neuroimaging studies of semantic control have focused almost exclusively on the role of left IFG, the neural underpinnings of this crucial function may be complex, drawing on a large-scale distributed network of interconnected brain regions (see Fig. 1). Often, studies that report left IFG activity during tasks requiring greater semantic control reveal coactivation in parts of the neural network that have been linked to the storage of semantic knowledge instead, especially posterior middle temporal gyrus (pMTG) (e.g., Thompson-Schill et al. 1997; Wagner et al. 2001; Noppeney et al. 2004; Badre et al. 2005; Gold et al. 2006; Zempleni et al. 2007; Kuperberg et al. 2008). Typically, these studies utilize tasks that increase the level of executive semantic control in the context of increased activation of semantic knowledge, for example, when more than one response is appropriate and additional control processes are required to restrain the response set and select the target item as opposed to distracter items. The observed frontotemporal activation pattern is therefore consistent with the proposed neural organization of semantic memory described above—in which modulatory signals from left IFG act upon activation in posterior meaning areas—but equally allows for an alternative interpretation: pMTG may be integrated in a semantic executive system that comprises both frontal and temporal brain structures. The role of pMTG in the semantic network therefore remains highly controversial. In particular, current neuroimaging evidence is unable to decide 1) whether the observed activation in pMTG directly reflects manipulations in semantic control demand as opposed to co-occurring processes in the semantic store and 2) whether there exists a “causal” relationship between pMTG activation and increased executive effort during word retrieval (or whether the pMTG activation is epiphenomenal, reflecting a tendency for activation patterns across connected brain regions to be correlated).
Figure 1.
Semantic control in the brain. Overlap image of TMS stimulation sites (“orange”), lesions in patients with deregulated semantic control after infarction to prefrontal and/or temporoparietal cortex (“red”), and brain activation for high > low executive semantic demands during fMRI (“black,” “green,” “purple”). Activation peaks correspond to studies that were used to generate coordinates of stimulation sites. Note: black = Wagner et al. (2001), green = Badre et al. (2005), and purple = Thompson-Schill et al. (1997).
One way of addressing these critical issues is to selectively disrupt neural processing in either of these 2 components of semantic cognition (IFG, pMTG) and examine performance on comprehension tasks requiring different degrees of semantic control. The advantage of this “interference” design is that it is not obligatory to separate executive from representational demands: if left IFG and/or pMTG play a decisive part in executive aspects of meaning retrieval, brain damage to these regions should produce substantial impairment of executively demanding semantic tasks—irrespective of whether these tasks are accompanied by increased representational demands or not. Interpretations of neuroimaging findings that do not link IFG and/or pMTG to executive semantic processes would thus be no longer valid.
Strong support for the importance of IFG in semantic control is provided by patients with lesions in this area. Such patients show behavioral deficits in situations characterized by strong competition between potential responses, increasing the need for semantic selection (e.g., during sentence completion tasks with low vs. high predictive endings) (Robinson et al. 1998, 2005; Novick et al. 2009; see also Thompson-Schill et al. 1998). Deficits of semantic control can also arise following left temporoparietal lesions (Jefferies and Lambon Ralph 2006) (see Fig. 1). Patients with “semantic aphasia” (SA) following stroke in either left prefrontal or temporoparietal cortex show deregulated semantic cognition across both verbal and nonverbal semantic tasks (Jefferies and Lambon Ralph 2006; Corbett, Jefferies, Ehsan, and Lambon Ralph 2009; Corbett, Jefferies, and Lambon Ralph 2009). These patients are highly sensitive to the executive requirements of semantic tasks—for example, they have difficulty retrieving the subordinate meanings of ambiguous words and struggle to reject highly associated distractor words in synonym judgment (Noonan et al. 2009). They benefit substantially from the provision of cues (e.g., /r/ → “rabbit”), which reduce the requirement for internally generated semantic control (Jefferies et al. 2008). Moreover, they do not show degradation of semantic knowledge per se—they are insensitive to item frequency and their ability to recover information reflects the control demands of tasks as opposed to the identity of the items being tested. This is in sharp contrast to patients with semantic dementia, who exhibit progressive dissolution of semantic knowledge itself (Bozeat et al. 2000; Jefferies and Lambon Ralph 2006; Patterson et al. 2007).
These observations suggest that the deficit in SA results from malfunction in the executive semantic system as opposed to degeneration of information in the semantic store, supporting the view that left IFG and pMTG work in tandem to underpin semantic control. While this hypothesis is consistent with the neuroimaging research reviewed above, SA patients typically have widespread lesions that are not focused on a single brain region (e.g., inferior parietal areas may be compromised along with posterior temporal structures). A higher spatial resolution can be achieved with repetitive transcranial magnetic stimulation (rTMS). Magnetic pulses are applied over a specific brain region in healthy participants, leading to relatively focal, temporary disruption of neural processing. This results in performance deficits in tasks that are underpinned by the targeted brain area (i.e., a “virtual lesion”) (Walsh and Rushworth 1999; Pascual-Leone et al. 2000; Walsh and Cowey 2000). Although most TMS research to date has focused on sensory/motor systems, rTMS can be used to explore the neural basis of language and semantic memory (for a review, see Devlin and Watkins 2007). We recently simulated the pattern observed in semantic dementia by administering rTMS to the lateral anterior temporal lobes (Pobric et al. 2007, 2010; Lambon Ralph et al. 2009). In addition, stimulation of left IFG and temporoparietal cortex interferes with standard semantic tasks, such as picture naming, word–picture verification, and semantic judgments (Flitman et al. 1998; Wassermann et al. 1999; Devlin et al. 2003; Drager et al. 2004; Oliveri et al. 2004). However, rTMS has not been used previously to explore the neural organization of semantic control.
In the present study, we sought evidence for a wider neural network underpinning semantic control by using TMS to produce virtual lesions within 2 sites, left IFG and pMTG, in healthy participants. We explored the effect of TMS on semantic decisions that varied in their requirement for controlled semantic retrieval. Manipulations of semantic control were based on a standard neuroimaging paradigm that reliably evokes left prefrontal and often also posterior temporal activation changes (Wagner et al. 2001; Badre et al. 2005) (see Figs 1 and 2). Participants were required to determine which word was semantically related to a cue in the presence of 2 unrelated distractor items. The target was either strongly or weakly related to the cue word (e.g., strong: salt–pepper, machine, land; weak: salt–grain, radio, adult). Retrieval of the relevant semantic relationship for strong cue–target pairs is thought to occur relatively automatically, facilitated by the normal flow of activation between associated concepts in the semantic network. In contrast, identifying a weak associate requires executive control over semantic retrieval.
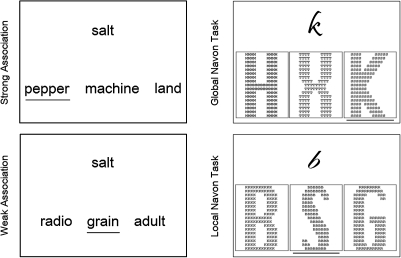
Figure 2.
Stimuli. Example trials for the semantic (“left panel”) and nonsemantic tasks (“right panel”). Participants had to select the target word that was either strongly related to the cue shown above (strong association) or weakly related (weak association). In the Navon tasks, participants had to choose the target compound letter that resembles the cue letter either in its global shape (global Navon) or in its local, smaller elements (local Navon). Note: Target items are underlined, and Navon compound letters are enlarged for illustration purposes.
We made the following predictions: If left IFG and pMTG work together to underpin semantic control, rTMS to both target sites should have parallel effects—namely, greater disruption in the executively demanding weak association condition. In contrast, if pMTG has little or no functional significance in executive aspects of meaning retrieval, rTMS over this site should not interact with the control demands of the semantic task. To examine the specificity of these effects, participants performed easy and difficult versions of a nonsemantic letter-matching task (Navon 1977), for which no interference with rTMS was expected. Furthermore, rTMS was applied over a control site (vertex) to confirm that any effects were specific to the stimulated areas.
Materials and Methods
Participants
Sixteen right-handed native English speakers took part in the TMS experiment (8 females; mean age = 22.25 years, standard deviation [SD] = 3.55). All participants were students from the University of York. Subjects were screened for general TMS and magnetic resonance imaging (MRI) incompatibility (Wassermann 1998) and excluded on grounds of medication or any personal or family history of neurological or psychiatric illness. All participants had normal or corrected-to-normal vision, gave informed consent, and were paid £40 for participation in the study. The study was approved by the local ethics committee.
Task
The current study employed 2 semantic judgment tasks that differed in their level of semantic control demand (cf. Wagner et al. 2001; Badre et al. 2005). In both tasks, a cue word appeared above a row of 3 potential target words, one of which was related to the cue. Participants had to choose the related target by pressing 1 of 3 buttons, corresponding to the position of the response item (left, middle, right), with their right hand. Cue–target relationships were either strong (salt–pepper, machine, land) or weak (salt–grain, radio, adult) (see Fig. 2). Semantic control demands were assumed to be minimal in the strongly associated trials because the correct response could be efficiently identified via automatic spreading activation between associated representations in the semantic network (salt–pepper). Since alternative response options were unrelated to the cue, retrieval of the target could proceed without significant competition from distracter items. In contrast, when cue and targets were weakly related, targets could not be rapidly identified via spreading activation, and the target was less clearly dissociable from the distracter items. Additional executive resources were therefore required to direct the search and recovery of the target item within the semantic network.
Nonsemantic control tasks with the same decision/response demands as the semantic trials were constructed from the Navon letter-matching task (Navon 1977). We produced easy and difficult versions of this task to establish whether rTMS effects over IFG/pMTG remained specific to the semantic domain even when control demands were increased. In both Navon conditions, a cue letter appeared above 3 larger compound letters, which were composed of smaller letters (e.g., a large A made of small Bs; see Fig. 2). In the easy condition, participants had to decide which compound letter matched the cue in “global” shape, irrespective of the letters that appeared as smaller elements inside the compounds. Cognitive control demands were expected to be minimal in these trials because global shape is visually dominant over local features (Navon 1977). Moreover, both distracters were unrelated to the cue, that is, neither global shape nor local letter features matched the cue in this condition (see Fig. 2). In contrast, the more difficult Navon task required participants to match the cue letter to the “local” elements of one of the compounds and, hence, to disregard the dominant, global shape of the stimuli. Cognitive control demands were further increased by presenting a compound letter whose global shape was identical to the cue—this item would have been a strong task-irrelevant competitor (see Fig. 2).
Design and Procedure
A 3 × 2 × 4 fully factorial design was used, with stimulation SITE (IFG, pMTG, vertex), TMS (stimulation vs. no stimulation), and TASK (weak and strong association, local and global Navon) as within-subject factors. Each site was stimulated on a different day, with test sessions separated by at least 1 week. Each session included recordings of task performance immediately after TMS (“post-TMS”) and without any TMS intervention (“baseline”) to identify the influence of TMS on behavior (“TMS effect”). Baseline was either measured before TMS intervention or 30 min after TMS offset, by which time no TMS effects remain (Pobric et al. 2007; Lambon Ralph et al. 2009; Pobric, Jefferies, and Lambon Ralph 2009; Pobric, Lambon Ralph, and Jefferies 2009). For each stimulation site, half of the participants performed the baseline assessment before TMS, and the other half were assessed 30 min post-TMS. The sequence of stimulation site was counterbalanced across subjects. However, due to technical faults with the coregistration equipment, 2 participants received stimulation of the vertex first instead of IFG.
The 6 experimental runs—that is, baseline and post-TMS performance for each stimulation site (vertex, IFG, MTG)—lasted about 6 min each (mean = 5.97 min, SD = 0.46) and included 30 trials per condition. Two miniblocks of 15 consecutive trials were created for each condition and presented in a pseudorandomized order, designed to eliminate effects of testing order effects across participants.
At the beginning of each block, an instruction slide was shown, followed by a fixation cross for 500 ms in the center of the screen. This was replaced by the first experimental trial, which displayed the cue above 3 response options for a maximum of 5 s (Fig. 2). As soon as a response was made, the fixation cross appeared again, followed by the next trial. A computer running E-prime (Psychology software tools) was used to present the stimuli and record the responses.
Stimuli
The 2 semantic conditions (involving strong and weak semantic associations) consisted of 180 cue–target–distractor trials. The trials were arranged into 6 matched sets of 30 trials each, used for each experimental run, and then split into miniblocks of 15 trials, which were equated for word length, frequency, and cue–target association strength. Stimuli were taken from Badre et al. (2005) but restricted to nouns only, and some trials were amended to make them suitable for UK participants. Conditions of high and low semantic control demands were arranged, such that the same cue word was matched with a high or low semantic associate, using several sets of association norms (Postman and Keppel 1970; Moss and Older 1996). Associations included semantic (e.g., categorical) and thematic (e.g., functional, perceptual, and spatiotemporal) cue–target relations. Each cue word was also paired with 2 unrelated distracter items, for which no entry in the association norms was found (e.g., strong: salt–pepper, machine, land; weak: salt–grain, radio, adult). The mean association strength for high and low related cue–target pairs, referring to the proportion of subjects that named the target in response to the cue in free association, differed significantly (high = 0.240, SD = 0.182; low = 0.035, SD = 0.095; P < 0.001). Words in the 2 conditions were similar in frequency (Kucera and Francis 1967; strong: mean = 48.1, SD = 90.4; weak: mean = 54.3, SD = 105.1) and length in letters (strong: mean = 5.2, SD = 1.8; weak: mean = 5.1, SD = 1.4).
For the nonsemantic control condition, 180 trials of the global and local versions of the Navon task were constructed and, again, broken down into sets of 15 trials. Compound letters were based on stimuli taken from Hills and Lewis (2007). These depicted 21 uppercase letters (excluding M, N, Q, V, W) composed of smaller capital letters with a different identity (e.g., an A made out of small Bs). Local elements (width × length: 7 × 7 pixels) were arranged densely in the compound letter (69 × 166 pixels), with no gap in between. There were between 3 and 10 different versions of each of the 21 uppercase letters (made up of different local letters), yielding a total of 125 unique compound letters. The cues in the Navon task were 21 lowercase letters that matched the local elements or global shape of the target compound letter. To increase set size and delay response times, varying script fonts were used (Blackadder, Curlz MT, Bradleyhand, Edwardian Script, and Pristina), yielding 74 individual cue letters. No cue letters were repeated in a single experimental run.
TMS Protocol
A standard offline virtual lesion rTMS protocol was used, which was compatible with established TMS safety guidelines (Wassermann 1998; Rossi et al. 2009). For 10 min, repetitive trains of TMS were delivered at 1 Hz to the target brain area, producing transient disruption of neural processing in underlying tissue and concurrent disruption of behavioral tasks reliant on this brain area (Pascual-Leone et al. 1998; Lambon Ralph et al. 2009; see also Pobric et al. 2007; Pobric, Lambon Ralph, and Jefferies 2009). Since these deficits were assumed to be a consequence of additional noise rather than inactivation of the system (as apparent in lesions), TMS effects were expected to produce delayed reaction times (RTs) rather than a decline in accuracy (Pascual-Leone et al. 2000; Walsh and Cowey 2000; Devlin et al. 2003). Stimulation of a brain area that is irrelevant for a particular cognitive task (e.g., vertex) should not lead to performance deficits; in these cases, TMS often facilitates behavior due to a considerable general alerting effect after brain stimulation (Lambon Ralph et al. 2009; Pobric, Jefferies, and Lambon Ralph 2009).
A 50-mm figure-of-eight coil, attached to a MagStim Rapid2 stimulator (MagStim), was used for the repetitive delivery of magnetic pulses. The center of the coil was aligned to the point that marked the stimulation site on a tight-fitting elastic cap, worn by the participant. The coil was held firmly against the scalp throughout stimulation. Stimulation intensity was determined before each rTMS administration as 120% of active motor threshold (MT). MT was identified as the lowest intensity that produced a visible muscle twitch in the tense right hand when intensity was gradually decreased during single-pulse stimulation of left motor cortex. In addition, intensity threshold was set to a maximum of 65% of stimulator output (mean intensity = 62.56%, SD = 3.46). Coil orientation was manipulated to minimize participants’ discomfort during rTMS (particularly over IFG), as previous research found behavioral effects were insensitive to the orientation of the coil (Niyazov et al. 2005). Also, 6 participants who reported initial discomfort during stimulation of IFG received a slightly lower intensity over this site, ranging from 109% to 116% of individual MT (mean = 113%). Despite these adaptations, IFG stimulation yielded strongest performance deficits, which were comparable in size with the interference effects observed in previous rTMS studies that used the same stimulation protocol and similar semantic tasks (e.g., Pobric et al. 2007; Lambon Ralph et al. 2009). Moreover, differences in sensory experiences across stimulation site (e.g., general discomfort, noise or muscle twitches, which were highest during IFG stimulation) cannot account for the TMS effects since 1) performance was always measured in the absence of any ongoing brain stimulation and 2) nonsemantic control tasks were used to detect any task-independent consequences of TMS.
Localization of Stimulation Sites
Structural T1-weighted MRI scans were used to guide coil positioning using the Ascension Minibird magnetic tracking device (www.ascension-tech.com) and MRIreg software (www.mricro.com/mrireg.html). Five anatomical landmarks (tip and bridge of the nose, left and right tragus, and vertex) were identified for coregistering the participant’s head, stabilized on a chin rest, with the MRI image on the screen. Stimulation sites corresponded to Montreal Neurological Institute (MNI)-coordinates from functional magnetic resonance imaging (fMRI) studies on semantic control that were based on the same stimulus set and/or employed the same tasks (Wagner et al. 2001; Badre et al. 2005). Coordinates were transformed into individual subject space using the transformation matrix from the “Segment” function implemented in SPM5, after the origin of each individual image was aligned to the anterior commissure. Visual inspection ensured that coordinates referred to the target areas by making reference to anatomical landmarks on the images (see Fig. 3).
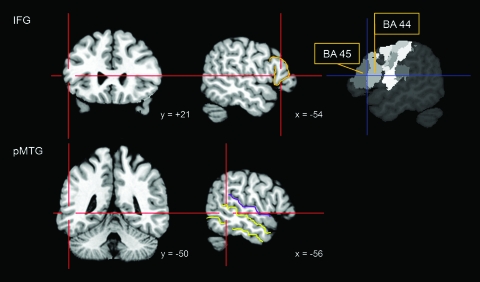
Figure 3.
Stimulation sites. rTMS was delivered to the pars triangularis of left IFG, the left pMTG, and the vertex (not shown). The image on the right depicts probability maps for BA 44 and 45, available from the SPM Anatomy toolbox. Note: Coordinates are in MNI space; orange = pars triangularis, yellow = inferior and superior temporal sulcus, purple = Sylvian fissure.
MNI coordinates for left IFG (−54, 21, 12) were taken from Badre et al. (2005) and corresponded to the left pars triangularis (BA 44/45). This area was interpreted by Badre et al. (2005) as a “convergence zone” for semantic control since it was sensitive to several semantic control manipulations, including weak versus strong cue–target associations in judgments of semantic relatedness.
The location for left pMTG stimulation (−56, −50, 3) lay between the superior and inferior temporal sulcus and was close—in posterior dimension—to an imaginary line that was perpendicular to the most posterior horizontal segment of the Sylvian fissure (cf. Gennari et al. 2007). The site, in BA 21, was identified from the average MNI coordinates of 2 studies (Wagner et al. 2001; Badre et al. 2005), which both reported increased pMTG activity in response to manipulations of controlled semantic retrieval, including judgments of weak versus strong cue–target associations.
The vertex corresponded to the midpoint of the head. This was identified as the intersection of the anterior-to-posterior axis (measured from the bridge of the nose to the inion) and left-to-right axis (measured from the tragus of the left to the right ear).
Data Analysis
The primary performance measure was reaction time (RT), since RT appears to be sensitive to rTMS effects even in the absence of any decline in accuracy (cf. Devlin et al. 2003; Pobric et al. 2007; Lambon Ralph et al. 2009). RT data were screened for errors and outliers (±2 SD) and initially entered into a SITE × TMS × TASK repeated-measures analysis of variance (ANOVA), which yielded a significant 3-way interaction. In a second step, task DOMAIN (i.e., semantic vs. nonsemantic) and level of CONTROL (i.e., low vs. high) was modeled explicitly to further specify the function of each site. Finally, to explore the effects of TMS directly, difference scores were calculated from post-TMS and baseline sessions for each subject in each condition at each individual site. Planned comparisons were then utilized to determine if there were any rTMS-induced effects (2-tailed one sample t-test) and to resolve interactions with stimulation site or task (2-tailed paired t-tests). Error rates were analyzed using the same model.
Results
Reaction Time
The RT results support the view that left IFG and pMTG both play an essential role in semantic control. TMS over both regions disrupted semantic cognition when control demands were high (i.e., for weakly associated cue–target pairs). In contrast, neither area showed effects of TMS when semantic control demands were low (for highly associated cue–target pairs). There were also no significant effects of TMS on the nonsemantic Navon tasks, irrespective of their executive control requirements.
The ANOVA for RT (shown in Table 1) revealed a main effect of TASK (P < 0.001), reflecting the expected effects of executive control manipulations: RTs were longer for the local Navon task than the global version (P < 0.001) and longer for semantic judgments on weak compared with strong cue–target associations (P < 0.001). Semantic decisions were generally slower than those in the Navon task (weak association vs. local Navon: P < 0.001; strong association vs. local Navon: P < 0.05). There were also significant 2-way interactions for SITE by TMS (P = 0.022) and TASK by TMS (P = 0.001) and, most importantly, a 3-way interaction (P = 0.038; see Table 1, Fig. 4). Separate ANOVAs, in which each target region (IFG, pMTG) was compared with the control site (vertex), yielded the same pattern of main effects and interactions. In contrast, no 3- or 2-way interactions with SITE were observed when IFG and pMTG were compared (see Table 1).
Table 1.
F- and P-values of the ANOVA for reaction time (RT)
| SITE | TMS | TASK | SITE × TMS | SITE × TASK | TMS × TASK | SITE × TMS × TASK | |
| df | 2, 30 | 1, 15 | 3, 45 | 2, 30 | 6, 90 | 3, 45 | 6, 90 |
| IFG, pMTG, vertex | 1.59 | 1.99 | 173.62 | 4.33 | <1 | 6.63 | 2.34 |
| P | 0.22 | 0.18 | <0.001 | 0.022 | 0.66a | 0.001 | 0.038 |
| df | 1, 15 | 1, 15 | 3, 45 | 1, 15 | 3, 45 | 3, 45 | 3, 45 |
| IFG, vertex | 2.56 | <1 | 154.16 | 6.33 | <1 | 3.26 | 3.42 |
| P | 0.13 | 0.57 | <0.001 | 0.024 | 0.65a | 0.030 | 0.025 |
| pMTG, vertex | <1 | <1 | 153.40 | 8.10 | <1 | 2.85 | 3.56 |
| P | 0.45 | 0.63 | <0.001a | 0.012 | 0.75a | 0.048 | 0.022 |
| IFG, pMTG | 1.84 | 6.54 | 167.14 | <1 | 1.18 | 9.96 | <1 |
| P | 0.20 | 0.022 | <0.001a | 0.87 | 0.32a | <0.001a | 0.93a |
Note: df, degrees of freedom. The ANOVA includes SITE (IFG, MTG, vertex), TMS (stimulation vs. no stimulation), and TASK (weak and strong association, local and global Navon) as within-subject factors. The first column lists the sites that were entered into the ANOVA.
Sphericity correction (Huynh–Feldt).
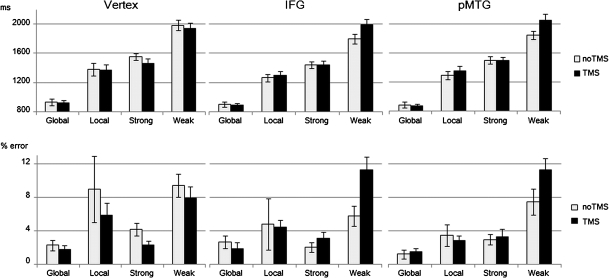
Figure 4.
Behavioral results. Reaction time (RT) and error rate at baseline (no TMS) and directly after stimulation (TMS) to the vertex, left IFG, and pMTG. Note: global = global Navon task, local = local Navon task, strong = strong cue–target association strength, weak = weak cue–target association strength.
In a second step, each site was tested for its sensitivity to DOMAIN (i.e., semantic vs. nonsemantic) and level of CONTROL demand (i.e., low vs. high demand) with and without TMS. This allowed us to determine fine-grained similarities and differences between brain regions in the control network. As expected, a 2 × 2 × 2 ANOVA including the factors DOMAIN, CONTROL, and TMS did not reveal any significant interactions when vertex was stimulated (see Table 2). The ANOVA for IFG showed a significant 3-way interaction (P = 0.005), confirming that this site was specifically engaged by semantic control demands (see Table 2). The pattern for pMTG was somewhat different. There was a 2-way interaction between TMS and CONTROL but the 3-way interaction failed to reach significance (see Table 2). This reflected slower post-TMS performance for both the weak association semantic task and the local Navon task, although the TMS effect was only significant in the semantic domain in planned comparisons (see below). There were no significant differences between IFG and pMTG when these sites were directly compared in a SITE × TMS × DOMAIN × CONTROL ANOVA (for all interactions involving SITE, F < 1.58) but, again, the 3-way interaction was observed (TMS × DOMAIN × CONTROL: F1,15 = 6.35, P = 0.02).
Table 2.
F- and P-values of the ANOVA for reaction time (RT) for each brain site separately (vertex, IFG, pMTG)
| TMS | DOMAIN | CONTROL | TMS × DOMAIN | TMS × CONTROL | DOMAIN × CONTROL | TMS × DOMAIN × CONTROL | |
| df | 1, 15 | 1, 15 | 1, 15 | 1, 15 | 1, 15 | 1, 15 | 1, 15 |
| Vertex | 2.24 | 154.23 | 119.69 | 2.08 | <1 | <1 | <1 |
| P | 0.16 | <0.001 | <0.001 | 0.17 | 0.66 | 0.88 | 0.56 |
| IFG | 5.34 | 136.98 | 289.87 | 3.10 | 9.45 | 1.37 | 10.89 |
| P | 0.04 | <0.001 | <0.001 | 0.10 | 0.008 | 0.26 | 0.005 |
| pMTG | 2.92 | 138.47 | 403.69 | 3.04 | 8.64 | <1 | 2.23 |
| P | 0.11 | <0.001 | <0.001 | 0.102 | 0.010 | 0.94 | 0.16 |
Note: df, degrees of freedom. ANOVAs include TMS (stimulation vs. no stimulation), DOMAIN (semantic vs. nonsemantic), and CONTROL (low vs. high) as within-subject factors.
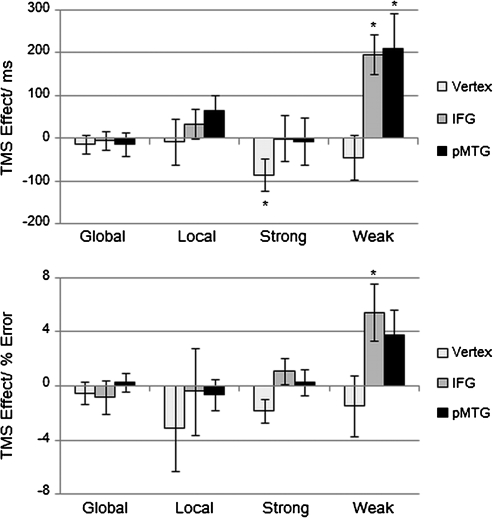
Finally, to determine the effect of rTMS for each condition at each site separately, difference scores were calculated between post-TMS and baseline performance. Three main findings emerged: 1) as predicted, planned comparisons revealed that responses were selectively delayed in the weak association task after rTMS to both IFG (t15 = 4.24, P = 0.001) and pMTG (t15 = 2.51, P = 0.024; see Fig. 5). 2) These TMS effects were significantly larger than the corresponding effect at the vertex for the same condition (vertex vs. IFG: t15 = 3.75, P = 0.002; vertex vs. pMTG: t15 = 3.14, P = 0.007). 3) There were larger TMS effects for the weak than the strongly associated cue–target pairs at both of these semantic sites (IFG: t15 = 3.35, P = 0.004; pMTG: t15 = 2.41, P = 0.029). These findings confirm that the rTMS interference effect was site specific and sensitive to semantic control demands. In contrast, a significant speeding of RT after rTMS was discovered at the vertex for the strongly associated trials (t15 = 2.21, P = 0.043). No further rTMS effects were significant; therefore, IFG and pMTG were largely insensitive to executive control demands beyond the semantic domain.
Figure 5.
TMS effects. Difference in reaction time (top) and error rate (bottom) between TMS and baseline performance (TMS – no TMS) for each stimulation site. Positive values indicate a decline in performance after brain stimulation, while negative values indicate improvement. Note: global = global Navon task, local = local Navon task, strong = strong cue–target association strength, weak = weak cue–target association strength. * P < 0.05.
Error Rate
The ANOVA for error rates largely replicated the RT results, revealing a main effect of TASK (F3,45 = 28.14, P < 0.001), a SITE by TMS interaction (F2,30 = 4.77, P = 0.018), and a TMS by TASK interaction (F3,45 = 3.05, P = 0.048). However, the 3-way interaction failed to reach significance (F6,90 < 1, P = 0.72; Fig. 4). Participants made more errors in the semantic task when cues and targets were weakly associated (P < 0.05). They were also more accurate in the global than local Navon task (P < 0.05). There was no difference in accuracy between the strong association task and the local Navon task (P > 0.2). The difference between the strong association task and the global Navon task approached significance (P = 0.056).
To assess the influence of rTMS on error rates at each site and condition, difference scores were again calculated using post-TMS and baseline performance (Fig. 5). Planned comparisons revealed a TMS effect at IFG for weakly associated trials (t15 = 2.59, P = 0.022), indicating that participants made more errors after rTMS. The effect of rTMS over pMTG approached significance for weakly associated trials (t15 = 2.04, P = 0.059). Following the procedure above, these effects in accuracy were individually compared with vertex stimulation and the high association semantic task at the same site. For decisions about weak semantic associations, the TMS effect was significantly larger for IFG than vertex (t15 = 2.25, P = 0.04), while the difference between pMTG and vertex approached significance (t15 = 1.92, P = 0.074). Differences between the strong and weak association trials approached significance at both sites (IFG: t15 = 1.89, P = 0.077; pMTG: t15 = 1.82, P = 0.088). Again, there was a trend toward improved performance after vertex rTMS for strongly associated trials (t15 = 2.01, P = 0.062).
Discussion
The current investigation used rTMS to identify the extent of the semantic control network in neurologically intact participants. Existing evidence clearly points to a role for left IFG in semantic control but the broader neural network that underlies this function remains unclear, despite its crucial importance in everyday activities. We examined the influence of rTMS over left IFG and pMTG in combination with a standard manipulation of semantic control from the neuroimaging literature: participants made semantic relatedness judgments involving strong or weak associations between cues and targets (Thompson-Schill et al. 1997; Wagner et al. 2001; Badre et al. 2005). The following effects were observed: 1) stimulation of left IFG and pMTG led to equivalent disruption of executively demanding semantic decisions. In contrast, there was no TMS effect for more automatic semantic decisions (based on strong associations). These findings indicate that pMTG, like IFG, plays a crucial role in semantic control but may not be central to semantic representation. 2) rTMS over left IFG and pMTG did not disrupt a nonsemantic Navon task, even when it was designed to place a significant burden on cognitive control. 3) rTMS to a nonsemantic control site (vertex) did not disrupt behavior in any of the tasks—consequently, the effects of rTMS over IFG and pMTG could not be interpreted as nonspecific effects of stimulation.
Converging Evidence for an Extended Semantic Control System
Our novel TMS findings favor an extended executive semantic system that relies on a distributed set of brain areas in left prefrontal and posterior middle temporal cortex. This proposal converges with data from patients with SA, who showed impaired regulation of semantic control after left temporoparietal and/or prefrontal infarction (Jefferies and Lambon Ralph 2006; Jefferies et al. 2007; Corbett, Jefferies, and Lambon Ralph 2009; Noonan et al. 2009). SA patients from these 2 lesion subgroups show highly similar deficits across a wide range of semantic control manipulations—for example, effects of cues and miscues on picture naming, comprehension of dominant and subordinate meanings of ambiguous words, and synonym judgment with strong and weakly associated distracters (Jefferies and Lambon Ralph 2006; Jefferies et al. 2008; Noonan et al. 2009). However, neuropsychological research cannot determine whether the deficits of SA patients with posterior lesions result instead from damage to inferior parietal cortex, which is also typically affected in SA. This question is resolved by the current study, in which TMS was used to induce small virtual lesions in healthy volunteers (i.e., 1–2 cm; Taylor et al. 2008). The finding that stimulation of IFG and pMTG interacted with the control requirements of semantic tasks to an equal degree is reminiscent of the similarity of SA patients with prefrontal and temporoparietal lesions. Following our findings, it is no longer plausible to propose that disconnection of prefrontal control mechanisms from a posterior temporal semantic store causes the deficits of SA patients since disruption of pMTG itself resulted in impaired semantic control functions (after TMS).
The present findings also complement recent neuroimaging studies that report activation of pMTG alongside IFG during situations of high semantic control demands (e.g., Badre et al. 2005; Rodd et al. 2005; Zempleni et al. 2007; Bedny et al. 2008; Whitney et al. 2009). These studies have often observed that IFG-pMTG activation is complemented by increased neural responses in dorso- and ventrolateral prefrontal cortex, anterior cingulate, angular gyrus, and/or superior parietal cortex (e.g., Thompson et al. 1997; Badre et al. 2005; Bedny et al. 2008; Whitney et al., 2010). All brain structures within this network are established components in executive control, either subserving semantic or domain-independent processes, implying that pMTG might serve similar executive purposes during semantic tasks (Owen et al. 2000; Duncan 2006, 2010; Dosenbach et al. 2008; Nagel et al. 2008). Following the present study, there is therefore convergent data from TMS, fMRI, and neuropsychology that pMTG supports the regulation of meaning retrieval, alongside IFG.
The identified semantic control network is by no means exhaustive. Stroke patients with deregulated executive semantic functions suffer from widespread infarction to left prefrontal and/or temporoparietal cortex (Jefferies and Lambon Ralph 2006; Jefferies et al. 2007, 2008; Corbett, Jefferies, and Lambon Ralph 2009). It is therefore possible that additional cortical regions contribute to semantic control alongside IFG and pMTG. Other candidate regions include structures in left parietal cortex, from the angular gyrus to the intraparietal sulcus. These brain areas play an established role in the “multiple-demand” system, which mediates all tasks of high executive requirements irrespective of domain (Owen et al. 2000; Duncan 2006, 2010). The extent to which multiple-demand regions (i.e., parietal lobe) contribute to semantic control is currently being assessed via TMS (Whitney et al., unpublished data).
Functional Heterogeneity in Left Temporal Lobe
Posterior middle temporal areas implicated in semantic control are dissociable from ventrolateral temporal cortex, associated with semantic representation (Sharp et al. 2004; Pobric et al. 2007, 2010; Lambon Ralph et al. 2009; Pobric, Jefferies, and Lambon Ralph 2009; Visser et al. 2009; Binney et al. 2010; Whitney et al., 2010). One line of evidence for this functional dissociation within temporal cortex is provided by comparison of patients with multimodal semantic impairment either in the context of semantic dementia (SD) or after stroke aphasia (which we have referred to as SA: Jefferies and Lambon Ralph 2006). SD patients suffer from a profound degradation of semantic knowledge following progressive bilateral atrophy focused on anterior, inferolateral cortex (Hodges et al. 1992; Bozeat et al. 2000; Mummery et al. 2000). In contrast, although SA patients with left temporoparietal and/or prefrontal lesions show multimodal semantic deficits, their store of semantic knowledge remains relatively intact—for example, they often retrieve irrelevant yet accurate semantic information (e.g., producing the response “nuts” for the item “squirrel” in picture naming) (Jefferies and Lambon Ralph 2006; Jefferies et al. 2008; Soni et al. 2009).
A second line of evidence is provided by neuroimaging. Recently, Whitney and colleagues observed a double dissociation between temporal areas that responded to increased semantic control demands as opposed to representational aspects of word meaning using items with multiple meanings (Whitney et al., 2010). Left mid-inferior temporal gyrus (BA 20) was more strongly engaged when both interpretations of an ambiguous word were relevant to the task (e.g., bank–money and river), suggesting that this region is involved in semantic representation. In contrast, both pMTG and IFG showed their strongest responses when participants were required to retrieve only the less frequent meaning of ambiguous words—that is, when semantic selection and inhibition requirements were maximal.
The present TMS study made no attempt to separate representation and control aspects of semantic cognition but was designed to identify brain structures that make an essential contribution to semantic decisions under conditions of increased control requirements. The conclusions that can be drawn from this study help to resolve some of the controversy regarding the role of pMTG in semantic cognition. Temporarily disruption of pMTG functioning post-TMS directly led to performance deficits during tasks of high semantic control requirements, disproving the alternative view that brain activation in pMTG during fMRI is a by-product rather than a causal consequence of manipulations in executive semantic demands. The next step will be to show a double dissociation between semantic representation and control processes in the temporal lobe within a single study.
Connectivity analyses further support the view that pMTG works in concert with left prefrontal cortex to permit strategic access to semantic representations stored elsewhere in the brain (i.e., anterior and inferior portions of the temporal lobe). pMTG yields strong anatomical and functional links to anterior aspects of IFG (for a review, see Friederici 2009), but it is also well connected with temporal lobe structures implicated in the storage of semantic knowledge (i.e., fusiform gyrus) (Saur et al. 2010). This architecture suggests that pMTG and left IFG (BA 45/47) can act in concert to retrieve semantic knowledge from “meaning areas” in inferior temporal cortex when the automatic recovery of information is hindered. Damage or TMS to this site therefore disrupts access to semantic knowledge with no loss of word meaning per se.
Domain Specificity of Left IFG and pMTG in Executive Control
There is considerable debate about whether executively demanding tasks share a common neural basis (i.e., a multiple-demand system) or whether executive processes can be fractionated into several discrete cognitive and neural components (Stuss and Alexander 2007; Duncan 2010). In light of this controversy, it is interesting that, in the current study, TMS over left IFG and pMTG selectively disrupted tasks involving high semantic control demands—while nonsemantic aspects of executive processing were unaffected. Executive control of semantic cognition might draw on both a unitary multiple-demand system plus domain-specific components adapted to the unique requirements of semantic tasks. In line with this view, neuroimaging studies show that a distributed set of brain regions is activated by multiple executively demanding tasks, both semantic and nonsemantic, including inferior frontal sulcus, dorsolateral prefrontal cortex, supplementary motor areas, adjacent cingulate cortex, and areas in and around the intraparietal sulcus (Duncan and Owen 2000; Wager et al. 2004; Dosenbach et al. 2008; Nagel et al. 2008; Duncan 2010). Left IFG and pMTG coactivate with this multiple-demand system in that they also show a strong response when semantic control requirements are high, but unlike the other regions listed above, they show task-dependant activation (Duncan 2006, 2010; Hon et al. 2006; Nagel et al. 2008; Dumontheil et al. 2010).
Funding
Wellcome Trust grant (078734/Z05/Z to E.J. and M.A.L.R.).
Acknowledgments
We thank David Badre, Peter Hills, and Michael Lewis for supplying their stimulus material. Conflict of Interest: None declared.
References
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn Sci. 2008;12:193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Badre D, D'Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. J Cogn Neurosci. 2007;19:2082–2099. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Bedny M, McGill M, Thompson-Schill SL. Semantic adaptation and competition during word comprehension. Cereb Cortex. 2008;18:2574–2585. doi: 10.1093/cercor/bhn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binney RJ, Embleton KV, Jefferies E, Parker GJ, Lambon Ralph MA. The ventral and inferolateral aspects of the anterior temporal lobe are crucial in semantic memory: evidence from a novel direct comparison of distortion-corrected fMRI, rTMS, and semantic dementia. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq019. Advance Access published February 26, doi: 10.1093/cercor/bhq019. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38:1207–1215. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- Corbett F, Jefferies E, Ehsan S, Lambon Ralph MA. Different impairments of semantic cognition in semantic dementia and semantic aphasia: evidence from the non-verbal domain. Brain. 2009;132:2593–2608. doi: 10.1093/brain/awp146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett F, Jefferies E, Lambon Ralph MA. Exploring multimodal semantic control impairments in semantic aphasia: evidence from naturalistic object use. Neuropsychologia. 2009;47:2721–2731. doi: 10.1016/j.neuropsychologia.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92:179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MF. Semantic processing in the left inferior prefrontal cortex: a combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J Cogn Neurosci. 2003;15:71–84. doi: 10.1162/089892903321107837. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Watkins KE. Stimulating language: insights from TMS. Brain. 2007;130:610–622. doi: 10.1093/brain/awl331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drager B, Breitenstein C, Helmke U, Kamping S, Knecht S. Specific and nonspecific effects of transcranial magnetic stimulation on picture-word verification. Eur J Neurosci. 2004;20:1681–1687. doi: 10.1111/j.1460-9568.2004.03623.x. [DOI] [PubMed] [Google Scholar]
- Dumontheil I, Thompson R, Duncan J. Assembly and use of new task rules in fronto-parietal cortex. J Cogn Neurosci. 2010 doi: 10.1162/jocn.2010.21439. doi: 10.1162/jocn.2010.21439. [DOI] [PubMed] [Google Scholar]
- Duncan J. EPS mid-career award 2004: brain mechanisms of attention. Q J Exp Psychol (Colchester) 2006;59:2–27. doi: 10.1080/17470210500260674. [DOI] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci. 2010;14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Flitman SS, Grafman J, Wassermann EM, Cooper V, O'Grady J, Pascual-Leone A, Hallett M. Linguistic processing during repetitive transcranial magnetic stimulation. Neurology. 1998;50:175–181. doi: 10.1212/wnl.50.1.175. [DOI] [PubMed] [Google Scholar]
- Friederici AD. Pathways to language: fiber tracts in the human brain. Trends Cogn Sci. 2009;13:175–181. doi: 10.1016/j.tics.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Gennari SP, MacDonald MC, Postle BR, Seidenberg MS. Context-dependent interpretation of words: evidence for interactive neural processes. Neuroimage. 2007;35:1278–1286. doi: 10.1016/j.neuroimage.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Balota DA, Jones SJ, Powell DK, Smith CD, Andersen AH. Dissociation of automatic and strategic lexical-semantics: functional magnetic resonance imaging evidence for differing roles of multiple frontotemporal regions. J Neurosci. 2006;26:6523–6532. doi: 10.1523/JNEUROSCI.0808-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hills PJ, Lewis MB. Temporal limitation of Navon effect on face recognition. Percept Mot Skills. 2007;104:501–509. doi: 10.2466/pms.104.2.501-509. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115(Pt 6):1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Hon N, Epstein RA, Owen AM, Duncan J. Frontoparietal activity with minimal decision and control. J Neurosci. 2006;26:9805–9809. doi: 10.1523/JNEUROSCI.3165-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJ. The spatial and temporal signatures of word production components. Cognition. 2004;92:101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Baker SS, Doran M, Lambon Ralph MA. Refractory effects in stroke aphasia: a consequence of poor semantic control. Neuropsychologia. 2007;45:1065–1079. doi: 10.1016/j.neuropsychologia.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Lambon Ralph MA. Semantic impairment in stroke aphasia versus semantic dementia: a case-series comparison. Brain. 2006;129:2132–2147. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Patterson K, Lambon Ralph MA. Deficits of knowledge versus executive control in semantic cognition: insights from cued naming. Neuropsychologia. 2008;46:649–658. doi: 10.1016/j.neuropsychologia.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera H, Francis WN. Computational analysis of present-day English. Providence (RI): Brown University Press; 1967. [Google Scholar]
- Kuperberg GR, Sitnikova T, Lakshmanan BM. Neuroanatomical distinctions within the semantic system during sentence comprehension: evidence from functional magnetic resonance imaging. Neuroimage. 2008;40:367–388. doi: 10.1016/j.neuroimage.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, Pobric G, Jefferies E. Conceptual knowledge is underpinned by the temporal pole bilaterally: convergent evidence from rTMS. Cereb Cortex. 2009;19:832–838. doi: 10.1093/cercor/bhn131. [DOI] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annu Rev Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Martin A, Chao LL. Semantic memory and the brain: structure and processes. Curr Opin Neurobiol. 2001;11:194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- Moss H, Older L. Birbeck word association norms. Hove (UK): Psychology Press; 1996. [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- Nagel IE, Schumacher EH, Goebel R, D'Esposito M. Functional MRI investigation of verbal selection mechanisms in lateral prefrontal cortex. Neuroimage. 2008;43:801–807. doi: 10.1016/j.neuroimage.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navon D. Forest before trees: the precedence of global features in visual perception. Cogn Psychol. 1977;9:353–383. [Google Scholar]
- Niyazov DM, Butler AJ, Kadah YM, Epstein CM, Hu XP. Functional magnetic resonance imaging and transcranial magnetic stimulation: effects of motor imagery, movement and coil orientation. Clin Neurophysiol. 2005;116:1601–1610. doi: 10.1016/j.clinph.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Noonan KA, Jefferies E, Corbett F, Lambon Ralph MA. Elucidating the nature of deregulated semantic cognition in semantic aphasia: evidence for the roles of prefrontal and temporo-parietal cortices. J Cogn Neurosci. 2009;22:1597–1613. doi: 10.1162/jocn.2009.21289. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Phillips J, Price C. The neural areas that control the retrieval and selection of semantics. Neuropsychologia. 2004;42:1269–1280. doi: 10.1016/j.neuropsychologia.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Novick J, Kan IP, Trueswell J, Thompson-Schill SL. A case for conflict across multiple domains: memory and language impairments following damage to ventrolateral prefrontal cortex. Cogn Neuropsychol. 2009;26:527–567. doi: 10.1080/02643290903519367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveri M, Romero L, Papagno C. Left but not right temporal involvement in opaque idiom comprehension: a repetitive transcranial magnetic stimulation study. J Cogn Neurosci. 2004;16:848–855. doi: 10.1162/089892904970717. [DOI] [PubMed] [Google Scholar]
- Owen AM, Schneider WX, Duncan J. Executive control and the frontal lobe: current issues. Exp Brain Res. 2000;133:1–2. [Google Scholar]
- Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15:333–343. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V, Rothwell J. Transcranial magnetic stimulation in cognitive neuroscience—virtual lesion, chronometry, and functional connectivity. Curr Opin Neurobiol. 2000;10:232–237. doi: 10.1016/s0959-4388(00)00081-7. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobric G, Jefferies E, Lambon Ralph MA. Anterior temporal lobes mediate semantic representation: mimicking semantic dementia by using rTMS in normal participants. Proc Natl Acad Sci U S A. 2007;104:20137–20141. doi: 10.1073/pnas.0707383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobric G, Jefferies E, Lambon Ralph MA. Amodal semantic representations depend on both anterior temporal lobes: evidence from repetitive transcranial magnetic stimulation. Neuropsychologia. 2009;48:1336–1342. doi: 10.1016/j.neuropsychologia.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Pobric G, Jefferies E, Lambon Ralph MA. Category-specific versus category-general semantic impairment induced by transcranial magnetic stimulation. Curr Biol. 2010;20:964–968. doi: 10.1016/j.cub.2010.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobric G, Lambon Ralph MA, Jefferies E. The role of the anterior temporal lobes in the comprehension of concrete and abstract words: rTMS evidence. Cortex. 2009;45:1104–1110. doi: 10.1016/j.cortex.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postman L, Keppel G. Norms of word association. New York: Academic Press; 1970. [Google Scholar]
- Robinson G, Blair J, Cipolotti L. Dynamic aphasia: an inability to select between competing verbal responses? Brain. 1998;121(Pt 1):77–89. doi: 10.1093/brain/121.1.77. [DOI] [PubMed] [Google Scholar]
- Robinson G, Shallice T, Cipolotti L. A failure of high level verbal response selection in progressive dynamic aphasia. Cogn Neuropsychol. 2005;22:661–694. doi: 10.1080/02643290442000239. [DOI] [PubMed] [Google Scholar]
- Rodd JM, Davis MH, Johnsrude IS. The neural mechanisms of speech comprehension: fMRI studies of semantic ambiguity. Cereb Cortex. 2005;15:1261–1269. doi: 10.1093/cercor/bhi009. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, Patterson K. Structure and deterioration of semantic memory: a neuropsychological and computational investigation. Psychol Rev. 2004;111:205–235. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D, Schelter B, Schnell S, Kratochvil D, Kupper H, Kellmeyer P, Kummerer D, Kloppel S, Glauche V, Lange R, et al. Combining functional and anatomical connectivity reveals brain networks for auditory language comprehension. Neuroimage. 2010;49:3187–3197. doi: 10.1016/j.neuroimage.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Scott SK, Wise RJ. Retrieving meaning after temporal lobe infarction: the role of the basal language area. Ann Neurol. 2004;56:836–846. doi: 10.1002/ana.20294. [DOI] [PubMed] [Google Scholar]
- Soni M, Lambon Ralph MA, Noonan K, Ehsan S, Hodgson C, Woollams AM. “L” is for tiger: effects of phonological (mis)cueing on picture naming in semantic aphasia. J Neurolinguistics. 2009;22:538–547. [Google Scholar]
- Stuss DT, Alexander MP. Is there a dysexecutive syndrome? Philos Trans R Soc Lond B Biol Sci. 2007;362:901–915. doi: 10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P, Walsh V, Eimer M. Combining TMS and EEG to study cognitive function and cortico–cortico interactions. Behav Brain Res. 2008;191:141–147. doi: 10.1016/j.bbr.2008.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP, Schall JD. Dissociation of visual discrimination from saccade programming in macaque frontal eye field. J Neurophysiol. 1997;77:1046–1050. doi: 10.1152/jn.1997.77.2.1046. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci U S A. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, Swick D, Farah MJ, D'Esposito M, Kan IP, Knight RT. Verb generation in patients with focal frontal lesions: a neuropsychological test of neuroimaging findings. Proc Natl Acad Sci U S A. 1998;95:15855–15860. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak RS. Functional anatomy of a common semantic system for words and pictures. Nature. 1996;383:254–256. doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Visser M, Jefferies E, Lambon Ralph MA. Semantic processing in the anterior temporal lobes: a meta-analysis of the functional neuroimaging literature. J Cogn Neurosci. 2009;22:1083–1094. doi: 10.1162/jocn.2009.21309. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Walsh V, Cowey A. Transcranial magnetic stimulation and cognitive neuroscience. Nat Rev Neurosci. 2000;1:73–79. doi: 10.1038/35036239. [DOI] [PubMed] [Google Scholar]
- Walsh V, Rushworth M. A primer of magnetic stimulation as a tool for neuropsychology. Neuropsychologia. 1999;37:125–135. [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Blaxton TA, Hoffman EA, Berry CD, Oletsky H, Pascual-Leone A, Theodore WH. Repetitive transcranial magnetic stimulation of the dominant hemisphere can disrupt visual naming in temporal lobe epilepsy patients. Neuropsychologia. 1999;37:537–544. doi: 10.1016/s0028-3932(98)00102-x. [DOI] [PubMed] [Google Scholar]
- Whitney C, Grossman M, Kircher TT. The influence of multiple primes on bottom-up and top-down regulation during meaning retrieval: evidence for 2 distinct neural networks. Cereb Cortex. 2009;19:2548–2560. doi: 10.1093/cercor/bhp007. [DOI] [PubMed] [Google Scholar]
- Whitney C, Jefferies E, Kircher T. Heterogeneity of the left temporal lobe in semantic representation and control: priming multiple vs. single meanings of ambiguous words. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq148. Advance Access published August 23, doi: 10.1093/cercor/bhq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Zhou X. Executive control in language processing. Neurosci Biobehav Rev. 2009;33:1168–1177. doi: 10.1016/j.neubiorev.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Zempleni MZ, Renken R, Hoeks JC, Hoogduin JM, Stowe LA. Semantic ambiguity processing in sentence context: evidence from event-related fMRI. Neuroimage. 2007;34:1270–1279. doi: 10.1016/j.neuroimage.2006.09.048. [DOI] [PubMed] [Google Scholar]