Abstract
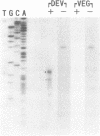
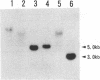
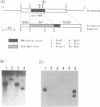
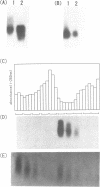
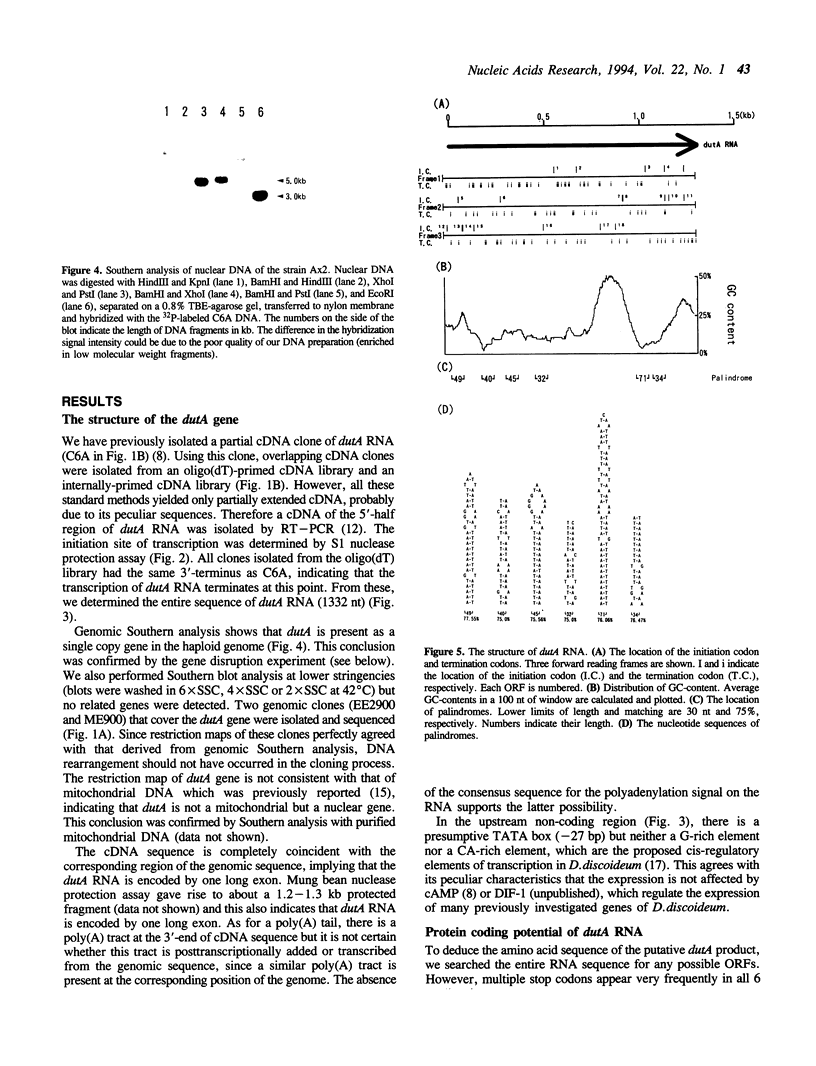
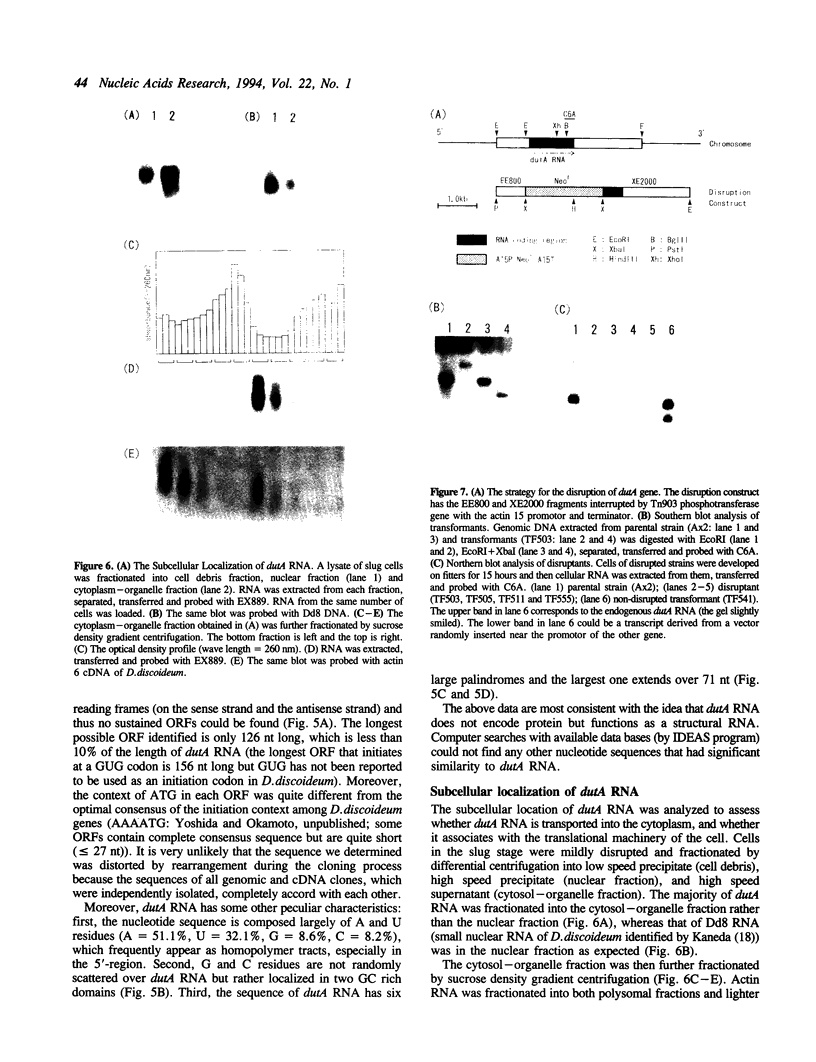
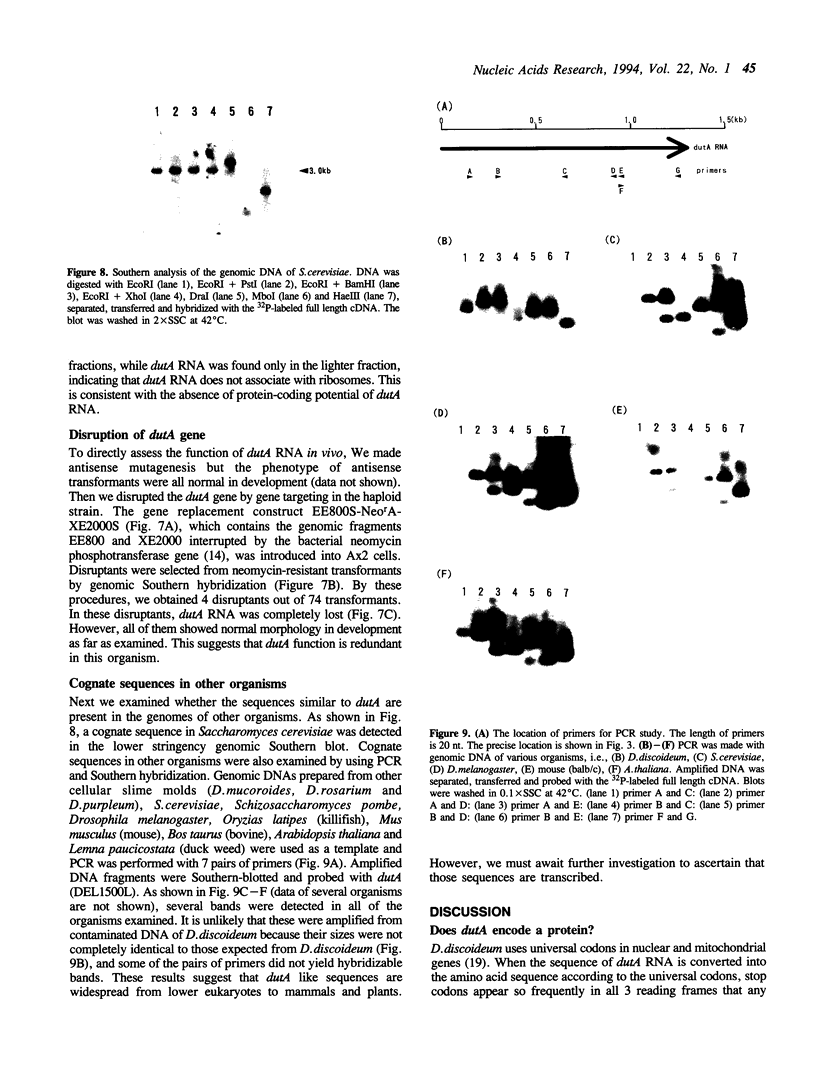
dutA is a gene specifically expressed during the development of Dictyostelium discoideum. Toward understanding its possible role in development, we isolated and characterized the gene and its complete cDNA. We found that dutA is encoded by the nuclear genome as a single copy gene without introns. In addition, the following unique and interesting features of dutA RNA (1322 nt) emerged: (1) it has no sustained ORFs (MAX = 126 nt) (2) it is extremely AU-rich (83%) (3) it contains peculiar sequence motifs (large palindromes, long AU-stretches and GC-clusters) (4) it is localized in the cytoplasm but completely absent from ribosomes. These features suggest that dutA RNA functions without being translated into protein. Disruption of the dutA gene did not cause phenotypic changes, suggesting that the function of dutA is redundant.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartolomei M. S., Zemel S., Tilghman S. M. Parental imprinting of the mouse H19 gene. Nature. 1991 May 9;351(6322):153–155. doi: 10.1038/351153a0. [DOI] [PubMed] [Google Scholar]
- Brannan C. I., Dees E. C., Ingram R. S., Tilghman S. M. The product of the H19 gene may function as an RNA. Mol Cell Biol. 1990 Jan;10(1):28–36. doi: 10.1128/mcb.10.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockdorff N., Ashworth A., Kay G. F., McCabe V. M., Norris D. P., Cooper P. J., Swift S., Rastan S. The product of the mouse Xist gene is a 15 kb inactive X-specific transcript containing no conserved ORF and located in the nucleus. Cell. 1992 Oct 30;71(3):515–526. doi: 10.1016/0092-8674(92)90519-i. [DOI] [PubMed] [Google Scholar]
- Brody E., Abelson J. The "spliceosome": yeast pre-messenger RNA associates with a 40S complex in a splicing-dependent reaction. Science. 1985 May 24;228(4702):963–967. doi: 10.1126/science.3890181. [DOI] [PubMed] [Google Scholar]
- Brown C. J., Hendrich B. D., Rupert J. L., Lafrenière R. G., Xing Y., Lawrence J., Willard H. F. The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell. 1992 Oct 30;71(3):527–542. doi: 10.1016/0092-8674(92)90520-m. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Bass B. L. Biological catalysis by RNA. Annu Rev Biochem. 1986;55:599–629. doi: 10.1146/annurev.bi.55.070186.003123. [DOI] [PubMed] [Google Scholar]
- Cech T. R. Self-splicing of group I introns. Annu Rev Biochem. 1990;59:543–568. doi: 10.1146/annurev.bi.59.070190.002551. [DOI] [PubMed] [Google Scholar]
- Elkins T., Zinn K., McAllister L., Hoffmann F. M., Goodman C. S. Genetic analysis of a Drosophila neural cell adhesion molecule: interaction of fasciclin I and Abelson tyrosine kinase mutations. Cell. 1990 Feb 23;60(4):565–575. doi: 10.1016/0092-8674(90)90660-7. [DOI] [PubMed] [Google Scholar]
- Guerrier-Takada C., Gardiner K., Marsh T., Pace N., Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983 Dec;35(3 Pt 2):849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- Hao Y., Crenshaw T., Moulton T., Newcomb E., Tycko B. Tumour-suppressor activity of H19 RNA. Nature. 1993 Oct 21;365(6448):764–767. doi: 10.1038/365764a0. [DOI] [PubMed] [Google Scholar]
- Kaneda S., Gotoh O., Seno T., Takeishi K. Nucleotide sequence of Dictyostelium small nuclear RNA Dd8 not homologous to any other sequenced small nuclear RNA. J Biol Chem. 1983 Sep 10;258(17):10606–10613. [PubMed] [Google Scholar]
- Myers T. W., Gelfand D. H. Reverse transcription and DNA amplification by a Thermus thermophilus DNA polymerase. Biochemistry. 1991 Aug 6;30(31):7661–7666. doi: 10.1021/bi00245a001. [DOI] [PubMed] [Google Scholar]
- Nellen W., Datta S., Reymond C., Sivertsen A., Mann S., Crowley T., Firtel R. A. Molecular biology in Dictyostelium: tools and applications. Methods Cell Biol. 1987;28:67–100. doi: 10.1016/s0091-679x(08)61637-4. [DOI] [PubMed] [Google Scholar]
- Nellen W., Silan C., Firtel R. A. DNA-mediated transformation in Dictyostelium discoideum: regulated expression of an actin gene fusion. Mol Cell Biol. 1984 Dec;4(12):2890–2898. doi: 10.1128/mcb.4.12.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachnis V., Brannan C. I., Tilghman S. M. The structure and expression of a novel gene activated in early mouse embryogenesis. EMBO J. 1988 Mar;7(3):673–681. doi: 10.1002/j.1460-2075.1988.tb02862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga Y., Yagi T., Ikawa Y., Sakakura T., Aizawa S. Mice develop normally without tenascin. Genes Dev. 1992 Oct;6(10):1821–1831. doi: 10.1101/gad.6.10.1821. [DOI] [PubMed] [Google Scholar]
- Simpson L., Shaw J. RNA editing and the mitochondrial cryptogenes of kinetoplastid protozoa. Cell. 1989 May 5;57(3):355–366. doi: 10.1016/0092-8674(89)90911-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman M. Cultivation and synchronous morphogenesis of Dictyostelium under controlled experimental conditions. Methods Cell Biol. 1987;28:9–29. doi: 10.1016/s0091-679x(08)61635-0. [DOI] [PubMed] [Google Scholar]
- Triglia T., Peterson M. G., Kemp D. J. A procedure for in vitro amplification of DNA segments that lie outside the boundaries of known sequences. Nucleic Acids Res. 1988 Aug 25;16(16):8186–8186. doi: 10.1093/nar/16.16.8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witke W., Nellen W., Noegel A. Homologous recombination in the Dictyostelium alpha-actinin gene leads to an altered mRNA and lack of the protein. EMBO J. 1987 Dec 20;6(13):4143–4148. doi: 10.1002/j.1460-2075.1987.tb02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Yamada Y., Okamoto K. DC6, a novel type of Dictyostelium discoideum gene regulated by secreted factors but not by cAMP. Differentiation. 1991 Apr;46(3):161–166. doi: 10.1111/j.1432-0436.1991.tb00877.x. [DOI] [PubMed] [Google Scholar]