Abstract
Evidence suggests that the right inferior frontal cortex (IFC) plays a specialized role in response inhibition. However, more recent findings indicate a broader role for this region in attentional control. Here, we used functional magnetic resonance imaging to examine the functional role of the right IFC in attention, inhibition, and response control in 2 experiments that employed novel variations of the go/no-go task. Across the 2 experiments, we observed a graded response in the right insula/IFC, whereby increasing response control demands led to an increase in activation. The results are consistent with the hypothesis that this region plays a key role in the integration of bottom-up, sensory information with top-down, response-related information to facilitate flexible, goal-directed behavior.
Keywords: attention, executive function, fMRI, inferior frontal cortex, response inhibition
Introduction
It is widely accepted that the prefrontal cortex (PFC) plays an important role in the top-down control of behavior. However, the precise functions supported by different regions within the PFC remain unclear. Neuroimaging and neuropsychological studies have consistently demonstrated involvement of the right inferior frontal cortex (IFC) in the inhibition of motor responses (Kawashima et al. 1996; Garavan et al. 1999; Konishi et al. 1999; Menon et al. 2001; Rubia et al. 2001; Aron et al. 2003; Kelly et al. 2004; Aron and Poldrack 2006; Li et al. 2006; Hodgson et al. 2007; Leung and Cai 2007), suggesting that this region plays a specialized role in response inhibition.
However, the specificity of this structure–function relationship remains controversial. The right IFC has been shown to be activated across a wide variety of task demands, including attentional reorienting and shifting (Corbetta and Shulman 2002; Hampshire and Owen 2006), oddball and target detection (McCarthy et al. 1997; Downar et al. 2000; Bledowski et al. 2004; Hampshire et al. 2007) and updating attended information (Hon et al. 2006), and is often coactivated with the parietal cortex in neuroimaging studies of executive function. Thus, recruitment of the right IFC during response inhibition does not necessarily imply that this region is a discrete functional module dedicated to response inhibition. An alternative hypothesis suggests that the IFC represents newly attended, task-relevant information as part of a “multiple demand” frontoparietal network recruited across a wide variety of different tasks (Duncan and Owen 2000; Duncan 2006).
Indeed, recent neuroimaging evidence has linked the right IFC with a more general role in attentional control. In one study, Sharp et al. (2010) found that the right IFC/insula did not differentiate between 2 types of trials, both of which required the detection of a novel cue but only one of which required inhibition, suggesting that the detection of the novel cue was sufficient to activate this region. In another study, Duann et al. (2009) examined the functional connectivity of different regions during motor inhibition and found that the pre-SMA but not the right IFC was directly connected to the basal ganglia, supporting differential roles for these regions in inhibitory control, with the right IFC mediating the attentional processing of task-relevant cues and the pre-SMA mediating a direct motor inhibitory function.
Thus, accumulating evidence suggests that attentional processing of task-relevant cues is sufficient to activate the right IFC and may at least partially account for activation in this region during response inhibition tasks. However, other studies have revealed that while the right IFC may be activated by attention, the level of activation in this region is modulated by the particular response requirements of the task. For example, Chikazoe et al. (2009) found that the right IFC showed significantly greater activation during inhibition trials, which required inhibition of a motor response, relative to “continue” trials, which did not require any change to the ongoing response. In another study, Hampshire et al. (2010) compared different versions of a stop-signal task and found that the right IFC was activated regardless of the specific output required—response inhibition, response initiation, or internal counting—but that activation was greater in blocks requiring a motor response.
These latter studies suggest that activation in this region may be driven partly by response requirements. However, no study has yet contrasted different response requirements within a single task, enabling a direct comparison of activation evoked by 2 equally response-relevant cues, unconfounded by factors affecting between-task comparisons such as overall differences in task set, arousal, or motivation
In the present study, therefore, we compared patterns of activation across several different task demands—attentional shifting, motor inhibition, and response initiation—in 2 experiments. In experiment 1, we compared attentional shifting and motor inhibition, and in experiment 2, we compared 2 contrasting aspects of response control: response (motor) inhibition and response initiation.
Experiment 1
In the first experiment, subjects performed a novel go/no-go task in which, on each trial, they saw an overlapping face and house surrounded by a colored border. The color of the border cued subjects to attend to either the face (red) or the house (blue) and changed every few trials, instructing the subject to shift attention from the face to the house or vice versa (shift trial). Subjects were required to make a key-press response to one type of stimulus within each dimension, for example, male faces/2-storey houses and withhold responses from the other type of stimulus, for example, female faces/one-storey houses (inhibition trial).
We reasoned that if the right IFC mediates a purely attentional function, then it should be recruited equally during inhibition and shift trials, both of which required subjects to respond appropriately to infrequent, task-relevant events. Alternatively, if the right IFC is particularly involved in response inhibition, then it should show greater activation during inhibition trials, which required subjects to withhold a prepotent motor response, relative to shift trials.
Method
Participants
The study received ethical approval from the Cambridge Local Research Ethics Committee (ref 08/H0308/65). Participants were 20 healthy, right-handed volunteers (7 females), aged between 18 and 40 years, drawn from the Behavioural and Clinical Neuroscience Institute volunteer panel. None of the participants had any history of psychiatric or neurological disorders. All participants gave informed consent to participate and were reimbursed £20 for their participation.
Stimuli
The task was presented via E-Prime software (Psychological Software Tools) on an IBM personal computer running Windows XP and projected onto a mirror in the magnetic resonance imaging (MRI) scanner. Each stimulus was a square 400 × 400 pixel grayscale picture of an overlapping face and house surrounded by a 5 pixel wide colored border which was either blue or red. Faces and houses were drawn from sets of 20 female faces, 20 male faces, 20 two-storey houses, and 20 one-storey houses. Each item from each of the face sets was paired with a different item from each of the house sets to make a total of 80 novel face–house pairings.
Design
Subjects were scanned in 2 runs, each run consisting of 2 blocks—one in which subjects performed the simple go/no-go version of the task and another in which they performed the complex go/no-go/shift version of the task. Half of the subjects completed the tasks in the order ABAB and the remaining half completed the tasks in the opposite order BABA. The go/no-go rules in both versions were counterbalanced across subjects such that half of the subjects responded to male faces and 2-storey houses and withheld responding to female faces and one-storey houses, while in the remaining half of the subjects, these rules were reversed. The go/no-go rules were also counterbalanced across gender.
Each block of the task consisted of between 158 and 166 trials. The ratio of stop and shift trials to go trials was approximately 1:8, so that in the simple version there were a total of 40 stop and 280 go trials, and in the complex version, there were 40 stop, 40 shift, and 240 go trials. There were 4–12 go trials between consecutive stop trials in both versions and 4–12 go trials between consecutive shift trials in the complex version. In the complex version, the irrelevant stimulus was selected equally often from the 2 categories within that dimension—for example, when attending to faces, the irrelevant stimulus was a 2-storey house on 50% of trials and a one-storey house on the remaining trials. Thus, across all subjects, the mean number of congruent and incongruent stop and shift trials was equal. Specifically, of the 40 stop trials, in 20 the irrelevant stimulus was associated with a stop response (congruent trial), and in 20 trials, the irrelevant stimulus was associated with a go response (incongruent trial). Similarly, in shift trials, the irrelevant stimulus was associated with a go response (congruent trial) in 20 trials, and in the remaining 20 trials, the irrelevant stimulus was associated with a stop response (incongruent trial).
Procedure
Immediately prior to scanning, subjects performed a short practice version of the task, lasting approximately 5 min, in which they were first familiarized with the simple version and subsequently with the complex version.
In the scanner, each block began with an instruction screen that informed subjects which task they would be performing next—complex or simple—and reminded them of the go/no-go and shift rules. The instructions remained on the screen for 10 s. Between each block, which lasted approximately 8.5 min, a screen with the message “take a break” was presented for 20 s.
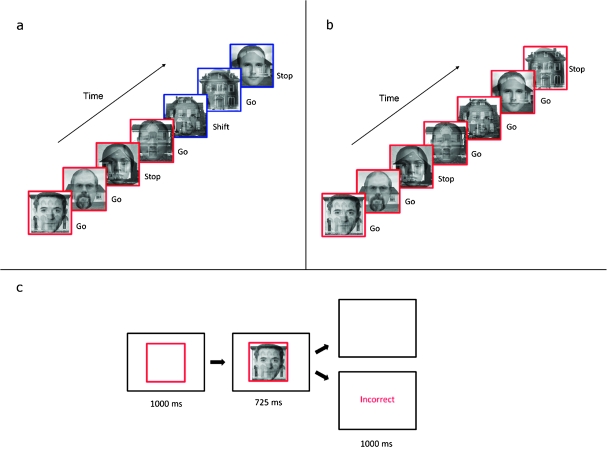
Figure 1 shows a typical sequence of trials from the complex version of the task. On each trial, the cue (a red or blue square border) appeared in the center of the screen for 1000 ms. The cue informed subjects whether to attend to faces (red) or houses (blue). Subsequently, the target picture (an overlapping face and house) appeared inside the border, and both stimuli remained on the screen together for a further 725 ms. On go trials, the subject was required to press a single key (on a button box resting on their stomach) with the index finger of their right hand before the target disappeared. On stop trials, the subject was required to refrain from responding. If the subject responded inappropriately on stop trials or failed to respond within the time limit on go trials, negative feedback (the word “incorrect” in red) was presented for 1000 ms before the next trial began. If the subject responded correctly, a blank screen was presented for 1000 ms before the next trial began. The total trial length was therefore 2725, and trial onset was thus jittered relative to the repetition time (TR), which was 2000 ms.
Figure 1.
Task structure in experiment 1. (a) A sequence of trials from the complex version of the task. In this sequence, the subject was required to respond to male faces and withhold responding to female faces when attending to faces and to respond to 2-storey houses and withhold responding to 1-storey houses when attending to houses. The subject initially attends to faces, as indicated by the red border, and then shifts attention to houses, as indicated by the blue border. (b) A sequence of trials from the simple version of the task. In this sequence, the subject attended to faces and responded to male faces while withholding responses to female faces. (c) The sequence of events in a single trial.
fMRI Data Acquisition and Analysis
Participants were scanned at the Wolfson Brain Imaging Centre (University of Cambridge, UK) on a 3-T Siemens Tim Trio scanner using a head coil. The number of volumes acquired per run varied for each run from 456 to 485 according to the number of trials performed. The first 10 volumes were discarded to avoid T1 equilibrium effects. Each image volume comprised 32 slices of 4-mm thickness, with in-plane resolution of 3 × 3 mm, oriented parallel to the anterior commissure–posterior commissure line. Siemens standard echo-planar imaging (EPI) sequence was used, with TR = 2000 ms, flip angle = 78°, echo time = 30 ms, in a contiguous descending sequence. The field of view was 192 × 192 mm, with matrix 64 × 64, echo spacing .51 ms, and bandwidth 2232 Hz/Px).
All functional magnetic resonance imaging (fMRI) data were preprocessed (transformed) and analyzed using SPM5 software (Wellcome Department of Cognitive Neurology, London). During preprocessing prior to analysis, all images were corrected for slice timing using sinc-interpolation and subject motion corrected using 2nd degree B-spline interpolation. Using the mean realigned image, all images were coregistered to a segmented high-resolution structural scan (voxel size, 1 × 1 × 1 mm) using a normalized mutual information cost function. Images were then normalized, using affine and smoothly nonlinear transformations, to an EPI template in Montreal Neurological Institute (MNI) space. The normalization algorithm determined the optimum 12 parameter affine transformation using a Bayesian framework to maximize the product of the likelihood function and the prior function and then estimated nonlinear deformations, defined by a linear combination of 3D discrete cosine transform basis functions. Finally, all normalized images were spatially smoothed with a 6-mm full-width at half-maximum Gaussian kernel.
The time series were high-pass filtered (128 s), and a canonical haemodynamic response function was modeled to the onsets of the targets.
Statistical Modeling
The following events were modeled at the first level: 1) a random selection of correct go trials in simple blocks matched to the number of correct stop trials in simple blocks, 2) correct stop trials in simple blocks, 3) commission errors in simple blocks, 4) a random selection of correct go trials in complex blocks matched to the number of correct stop trials in complex blocks, 5) a random selection of correct go trials in complex blocks matched to the number of correct shift trials in complex blocks, 6) correct stop trials in complex blocks, 7) shift trials in complex blocks, and 8) commission errors in complex blocks. First level models also included parametric modulators for go trial reaction times (RTs) and shift trial RTs. Combined stop and shift trials (stop trials immediately following a shift cue) were not modeled as there were too few trials to gain a reliable estimate of associated blood oxygen level–dependent (BOLD) signal.
Given that events were rapid, and the trial duration was not variable, go trials may not be particularly separable from the baseline in the General Linear Model (GLM). However, the motivation for including go trials in the first-level models was not to investigate go-related BOLD signal, since the baseline in the GLM is also primarily composed of go trials. Randomly selected subsets of go trials were included in the first-level models to ensure that separate selections of events served as baselines for the 2 contrasts to be entered into the conjunction analysis (stop–go and shift–go). For each participant, the random selections of go trials were determined by listing all go trial numbers in a vector in Matlab 7.0 (www.mathworks.com), generating a paired vector of random numbers, sorting the latter, and then selecting the first n trials, where n is the number of correct stop or shift trials for that subject.
Shift trials where the subject demonstrably did not shift attention were excluded from the model. For example, if the currently relevant dimension was associated with a go response and the previously relevant dimension was associated with a stop response (incongruent go trial), then an omission error indicated a failure to shift attention and the shift trial was excluded. Shift trials were also excluded if the participant made a commission error on an immediately following stop trial—on these trials, it was not possible to ascertain whether the error was due to a failure to shift attention followed by a correct go response to the previously relevant dimension or a successful shift of attention followed by a failure to stop. Stop trials were modeled as commission errors if the subject pressed when the relevant dimension was associated with a stop response. On the basis of these criteria, each first-level model contained an average of 35 shift trials, 28 stop trials in the complex version, and 33 stop trials in the simple version, as well as matched numbers of go trials.
A second model only included shift trials on which participants demonstrably shifted their attention to the newly relevant dimension, as the above method of trial selection did not ensure successful shifts in attention in the modeled shift trials. For instance, in incongruent go trials, a go response may result from a successful shift followed by a correct go response or an unsuccessful shift followed by a commission error. Thus, any activation resulting from the direct contrast of stop–shift trials may result from a difference in the behavioral relevance of stop and shift trials. The second model included only shift trials that were followed shortly thereafter by a successful incongruent stop trial (where the relevant dimension was associated with a stop response and the irrelevant dimension was associated with a go response). In such sequences, if the participant failed to shift attention on the shift trial, they would make a commission error on the subsequent stop trial, and the preceding shift trial was excluded. Only correct incongruent stop trials were entered into the model, so that the total numbers of stop and shift trials were matched. Random selections of go trials, matched to the number of stop and shift trials, were also entered into the model. All incorrect stop trials were modeled as commission errors. The resulting first-level models had a mean of 11 stop trials and 11 shift trials.
Contrasts
In order to examine which regions were activated across both inhibition and shift trials, we performed 2 contrasts at the first level; inhibition trials–go trials and shift trials–go trials. To ensure separate baselines for these 2 contrasts, we randomly selected 2 different sets of go trials for each of these contrasts, with the number of go trials matched to the number of correct inhibition and shift trials for each subject. The contrast images from these contrasts were taken to a random effects conjunction analysis at the second level to test for group level effects.
In order to examine whether any regions showed significantly greater activation for either inhibition or shift trials, we performed 2 further contrasts at the first level; inhibition trials–shift trials and shift trials–inhibition trials. These contrasts were performed directly on the regressors for stop and shift trials. The corresponding contrast images were then taken to 2 separate one-sample t-tests at the second level to test for effects at the group level.
All contrasts were performed at the whole-brain level. Only clusters that were significant at P < 0.05 corrected for false discovery rate (FDR) and contained at least 20 voxels were reported, in order to control for the possibility of making a type I error.
Results of Experiment 1
Behavioral Results
In the complex version, mean percent correct stop trials was 69.0% (standard deviation [SD] 14.6) and mean percent correct go trials was 97.7% (SD 1.5). In the simple version, mean percent correct stop trials was 82.3% (SD 8.6) and mean percent correct go trials was 98.2% (SD 2.0). A paired-samples t-test on the arcsine transformed proportions of correct stop trials revealed that subjects made significantly more commission errors on stop trials in the complex version than in the simple version, t19 = 4.6, P < 0.001, presumably due to the increased task demands in the complex version.
In the complex version, mean RT on go (nonshift) trials was 574 ms (SD 52) when attending to faces and 592 ms (SD 53) when attending to houses. Mean RT on shift trials was 566 ms (SD 55) when shifting from houses to faces and 599 ms (SD 57) when shifting from faces to houses. A paired-samples t-test showed that go RTs were significantly faster in attention-to-face trials than in attention-to-house trials, t19 = −6.1, P < 0.001, indicating increased difficulty of the 2-storey/1-storey discrimination.
In order to examine whether participants shifted attention on trials immediately after shift cues, for each dimension, we compared RTs on go trials with RTs on shift trials (i.e., the trials immediately following a shift cue). Paired-samples t-tests revealed that RTs on shift-to-house trials were significantly slower than RTs on attend-to-face go trials , t19 = 4.1, P < 0.05, while RTs on shift-to-face trials were significantly faster than RTs on attend-to-house go trials, t19 = −3.0, P < 0.05. In view of the overall performance difference between attend-to-face and attend-to-house trials—subjects responded significantly faster when attending to faces than houses—these results indicate that subjects did indeed shift attention on the trial immediately following a shift cue because the difference in performance was immediately apparent on these trials relative to the preceding go trials.
fMRI Results
Regions Commonly Activated during Inhibition and Shift Trials
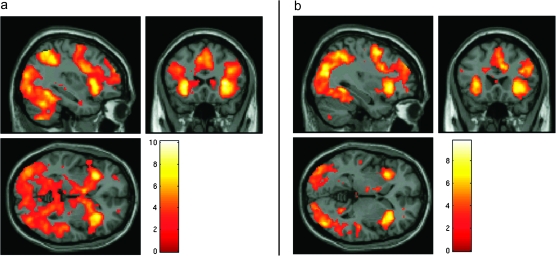
The conjunction analysis revealed an extensive frontoparietal network of regions that was coactivated during stop and shift trials when compared with go trials (Fig. 2). This network consisted of a single large cluster of voxels with a peak in the middle frontal gyrus (28, 0, 50), which extended into the anterior cingulate cortex (ACC) as well as bilaterally into the superior frontal gyrus, inferior frontal gyrus, insula, and inferior parietal lobule. Activation was also observed in the occipital cortex, striatum, midbrain, and cerebellum. In order to reveal the precise location of the right inferior frontal/insula activation, we increased the threshold for rejection of the null hypothesis to P < 0.05 corrected for family-wise error rate. At this threshold, there was an isolated region of activation consisting of 375 voxels with a peak in the right anterior insula (MNI coordinates 32, 22, 6) and encompassing also 2 subclusters in the right inferior frontal gyrus (MNI coordinates 32, 22, −2 and 44, 18, 0).
Figure 2.
fMRI results from experiment 1. (a) Areas commonly activated during stop and shift trials relative to go trials in the complex version of the task from experiment 1, overlaid on the MNI template brain. (b) Areas significantly activated during stop trials relative to go trials in the simple version of the task, overlaid on the MNI template brain. All clusters are significant at P < 0.05 corrected for FDR and contain at least 20 voxels.
Regions Activated in the Direct Contrast of Inhibition and Shift Trials
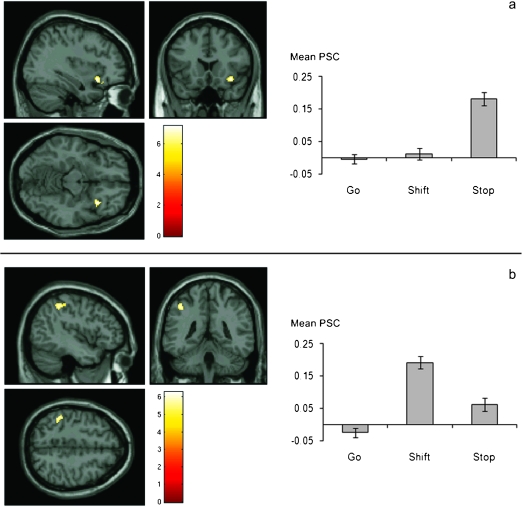
The direct contrast of inhibition and shift trials revealed a single cluster of 62 voxels in the right inferior frontal gyrus which showed significantly greater activation during inhibit trials than shift trials, with a peak voxel at MNI coordinates 34, 18, −12 (BA 47). At the less conservative threshold of P < 0.001 uncorrected for multiple comparisons, this cluster extended dorsally into BA 45. In the opposite contrast, we found 2 clusters of voxels in the left parietal cortex, one in the left inferior parietal lobule in the region of the anterior intraparietal sulcus and the other in the left postcentral gyrus which showed significantly greater activation during shift trials than inhibit trials (inferior parietal lobe: 73 voxels, peak voxel at MNI coordinates −46, −38, 50, BA 40, postcentral gyrus: 25 voxels, peak voxel at MNI coordinates −38, −34, 60) (see Fig. 3). The analysis of the parametric modulators did not reveal any informative results.
Figure 3.
fMRI results from experiment 1. Areas showing significantly greater activation during stop trials relative to shift trials (a) and during shift trials relative to stop trials (b), overlaid on the MNI template brain. All clusters are significant at P < 0.05 corrected for FDR and contain at least 20 voxels. Graphs display mean percent signal change (PSC) across all subjects for go, stop, and shift trials extracted from the right inferior frontal gyrus (top graph) and left inferior parietal lobe (bottom graph). Error bars represent standard error of the mean.
Greater activation in right IFC during stop trials relative to shift trials may be due to subjects failing to shift attention on some attentional shift trials. In order to control for this possibility, we repeated the contrast stop–shift trials using only the subset of trials in which subjects demonstrably shifted attention (shift trials followed by correct incongruent inhibition trials). Using an anatomically defined mask (right inferior frontal gyrus from the talairach daemon in the pickatlas Region of Interest [ROI] toolbox) to restrict the analysis to a region of interest in the right inferior frontal gyrus, there was a cluster of 53 voxels that showed significantly greater activation for stop trials relative to shift trials, with a peak voxel MNI coordinate of 32, 16, −14 (very close to the coordinates of the cluster from the original analysis), which demonstrates that this possibility cannot account for the results.
Comparison of Complex and Simple Blocks
The paired-samples t-test comparing the contrast images from the comparison inhibition trials–go trials in complex and simple blocks showed no difference in activation between the 2 versions. Inhibition-related activation occurred in the same IFC region and with the same magnitude in both the simple and complex versions of the task.
Discussion of Experiment 1
In this experiment, participants performed a novel task involving motor response inhibition and attentional shifting during fMRI. Combining 2 different cognitive processes within a single task enables both a direct contrast of activation associated with each process, unconfounded by factors affecting between-task comparisons such as overall differences in arousal, motivation and task set, as well as an examination of the regions that are coactivated across the 2 different processes when they are engaged under exactly the same task conditions.
We found that the right anterior insula/IFC, together with an extensive network of frontoparietal regions, was coactivated across response inhibition and attentional shift trials when compared with go trials. Additionally, in the direct comparison of inhibition and shift trials, we observed a clear double dissociation—the right IFC showed significantly greater activation during inhibition trials relative to shift trials and the left IPC showed significantly greater activation during shift trials relative to inhibition trials.
The observation that attentional shifting preferentially activates inferior parietal cortex is consistent with previous studies demonstrating a role for this region in mediating attentional flexibility. A meta-analysis of imaging studies of attention shifting identified, among other regions, an area in the left anterior intraparietal sulcus that showed consistent activation during different types of attentional shift (Wager et al. 2004) with a peak very close to the inferior parietal region identified in the present study. Our findings extend those of previous studies by showing that attentional shift-related activation in the left IPC cannot be attributed to an overall increase in executive task demands.
It could be argued that the increase in parietal activation during shift trials is due to the change in the color of the border cue. While it is certainly the case that the color of the border changed on shift trials and not stop trials, and therefore, this perceptual difference could lead to a difference in activation, these kinds of low-level perceptual differences are known to primarily evoke bilateral activation in color-sensitive visual cortical regions, such as V4 (Chawla et al. 1999). Given that the contrast of stop–shift trials resulted in an increase in activation only in the parietal cortex, and only in the left cerebral hemisphere, we think it is unlikely that a simple perceptual difference can account for this finding.
Although studies have consistently demonstrated a significant role for the right IFC in response inhibition, it has remained unclear whether this region constitutes a specialized module dedicated to response inhibition or, alternatively, whether it mediates a more basic, attentional function as part of a multiple-demand frontoparietal network recruited across a variety of different cognitive demands. The present findings suggest that the right IFC and the left IPC are preferentially activated during response inhibition and attentional shifting, respectively. However, it is important to note that this “preferential” activation does not equate to specialization in the absolute sense as the right IFC was in fact active for switching “and” inhibition. Rather, the results suggest that functional differences between frontoparietal network subregions are statistical as opposed to absolute and that these functionally specialized regions exist within an extended, general purpose, frontoparietal executive network.
A noteworthy feature of the present results is that the peak of the inferior frontal region activated in the conjunction analysis was located dorsal and posterior relative to the peak of the region activated in the direct stop-shift contrast. This gives the impression that these regions can be dissociated, with one region mediating a common function across different task demands and the other more specialized for response inhibition. However, after reducing the statistical threshold for the stop-shift contrast to P < 0.001, we found that this cluster spreads more dorsally and overlaps considerably with the cluster from the conjunction analysis. Therefore, we feel it would be inaccurate to conclude that this is a dissociation in the strictest sense. Nevertheless, the findings do suggest the intriguing possibility that process-specific activations may be located toward the edges of a core multiple-demand system.
However, an important feature of no-go trials that could account for an increase in activation in the right IFC, besides response inhibition per se, is the increase in “response control” demands. In the current paradigm, attentional shift cues are task relevant in the sense that they require detection and subsequent initiation of an internal shift in the focus of attention, but they do not require subjects to make any immediate adjustment to their ongoing motor behavior. In contrast, no-go trials do require an immediate adjustment to ongoing behavior—in other words, they require subjects to impose control over their responses. According to this account, when faced with a change in sensory information which instructs the subject to adjust their motor behavior (e.g., a no-go cue), it is not the inhibitory function per se but rather the more general process of initiating response control which drives activation in the IFC. If this is the case, then it may be possible to observe a selective increase in activation in this region in situations when response control demands are high, though where response inhibition demands are minimized.
Another potential confound in experiment 1 that could account for the greater activation in right IFC for stop cues than shift cues is that responding to stop cues relies on processing the identity of an object, that is, the gender of the face, whereas responding to shift cues relies on processing a feature of an object, that is, its color. Thus, increased activation in right IFC during stop trials may reflect a preferential role for this region in identity over feature processing. Accordingly, in experiment 2, we sought to eliminate any differences between task cues.
Experiment 2
In the second experiment, we employed a novel adaptation of the go/no-go task. On each trial of the task, subjects saw a letter presented in the center of the screen (either O, X, or T). On the majority of trials (75%), subjects saw the letter O and simply pressed a key with their index finger before the letter disappeared from the screen (go trials). On 12.5% of trials, subjects saw the letter X and had to withhold their response. On the remaining 12.5% of trials, subjects saw the letter T and had to press an additional key with the middle finger of their right hand at the same time as pressing with their index finger of their right hand (“double trials”).
The crucial comparison of interest in this experiment is between no-go trials and double trials. While response inhibition demands in double trials are minimized—subjects continue to respond with their index finger in these trials, as in preceding simple go trials—response control demands are maximized by the requirement to make a change to their ongoing motor program and coordinate 2 simultaneous button presses. Thus, double trials and no-go trials differentially emphasize response control and response inhibition, respectively.
We hypothesized that if the IFC performs a purely attentional function involving the detection of task-relevant information, then activation in this region should be equal for no-go and double trials, both of which require the detection of equally frequent, equally task-relevant stimuli. On the other hand, if the IFC performs an inhibitory function, then activation in this region should be greater in no-go trials, which require inhibition of the index finger response, relative to double trials, which do not. Finally, if activation in this region is driven by increased response control demands, then we should observe greater activation in double trials relative to no-go trials, due to the increased response control demands in double trials.
Method
Participants
Participants were 17 healthy, right-handed volunteers (8 females), aged between 18 and 40 years, drawn from the Behavioural and Clinical Neuroscience Institute volunteer panel. None of the participants in experiment 2 had also participated in experiment 1. None of the participants had any history of psychiatric or neurological disorders. All participants gave informed consent to participate and were reimbursed £20 for their participation.
Stimuli
The task was presented via E-Prime software (Psychological Software Tools) on an IBM personal computer running Windows XP and projected onto a mirror in the MRI scanner. Letters were presented in black on a gray background.
Design
Subjects were scanned in a single run divided into 4 blocks of trials. Each block of trials consisted of 80 trials—60 go trials, 10 stop trials, and 10 double trials randomly intermixed. There were 320 trials in total—240 go trials, 40 stop trials, and 40 double trials. Thus, the ratio of go:stop trials was 6:1, and the ratio of go:double trials was also 6:1.
Procedure
On each trial, a letter appeared in the center of the screen and remained visible for 725 ms. If the letter was O, subjects were required to make a key press response with the index finger of their right hand (go trial); If the letter was X, subjects were required to withhold responding; and if the letter was T, subjects were required to respond simultaneously with the index and second fingers of their right hand. Subjects were instructed to respond while the letter remained on the screen. If the subject responded incorrectly or failed to respond when they should have done, the word incorrect in red letters appeared immediately after the target letter disappeared. If the subject responded correctly, a blank screen appeared immediately after the letter disappeared. The feedback or blank screen remained visible for 750 ms and was followed by a blank screen for 250 ms before the next target letter appeared. Subjects were given a 30-s break between blocks; after 20 s of the break, the instruction screen appeared for 10 s to remind the subjects which letter corresponded to which trial type.
fMRI Data Acquisition and Analysis
fMRI acquisition and preprocessing were identical to experiment 1.
Statistical Modeling
A canonical haemodynamic response function was modeled to the onset of the targets. The following events were modeled at the first level: 1) a random selection of correct go trials matched to the number of correct stop trials, 2) correct stop trials, 3) commission errors, 4) a random selection of correct go trials matched to the number of correct double trials, 5) correct double trials. The resulting first-level models had a mean of 40 stop trials and 40 shift trials.
Contrasts
In order to examine whether the IFC responded more strongly to stop trials than double trials or vice versa, we computed the contrast stop-double and double-stop for each subject at the first level. These contrasts were performed directly on the regressors for stop and double trials. The contrast images from these contrasts were taken to a random effects conjunction analysis involving an F-test to test whether there were any regions that showed a difference in activation between these 2 conditions and subsequently 2 t-tests to establish directionality of the effects.
Contrasts were performed at the whole-brain level and at the Region of Interest (ROI) level. ROI analyses were carried out using the Marsbar toolbox (Brett et al. 2002). For the ROI analysis, we performed the stop-double and double-stop contrasts on the right inferior frontal cluster activated in the stop-shift contrast from experiment 1. We also performed an additional ROI analysis in which we reran the stop-shift contrast from experiment 1 on a cluster activated in the double-stop contrast from experiment 2. For the purposes of this analysis, because the cluster was large and extended across several regions, we drew a 5-mm radius sphere centered on the peak activated cluster from this contrast.
In the whole-brain analysis, only clusters that were significant at P < 0.05 corrected for FDR and that contained at least 20 voxels were reported, in order to control for the possibility of making a type I error.
Results of Experiment 2
Behavioral Results
One subject’s data were excluded from analysis due to excessive head movement.
Subjects responded correctly on 96% of stop trials and 96% of double trials.
fMRI Results
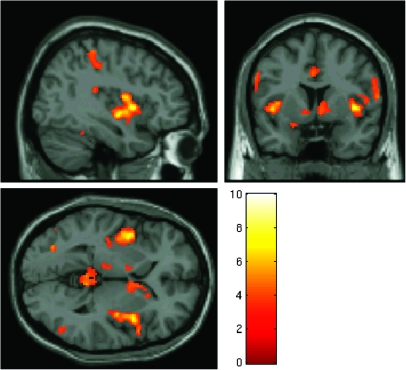
The whole-brain F-test revealed several regions which showed a significant difference in activation between double trials and stop trials, including a prominent cluster of voxels with a peak in the right insula. Whole-brain t-tests revealed that this difference was due to significantly greater activation in double trials relative to stop trials. The results of the double-stop t-test revealed a cluster with a peak in the right insula consisting of 2929 voxels and extended into the right inferior frontal operculum which showed significantly greater activation for double trials than stop trials (MNI coordinates of peak activated voxel = 40, 6, 2—see Fig. 4). Activation was also observed in other regions including bilaterally in the parietal cortex and in the ACC. In contrast, there were no regions that showed significantly greater activation for stop trials than double trials.
Figure 4.
fMRI results from experiment 2. Areas showing significantly greater activation during double response trials relative to stop trials, overlaid on the MNI template brain. All clusters are significant at P < 0.05 corrected for FDR and contain at least 20 voxels.
In order to establish whether the region which showed greater activation for double trials than stop trials in experiment 2 encompassed the region which showed greater activation for stop trials than shift trials in experiment 1, we used the right IFC cluster that was significantly activated in the stop-shift contrast from experiment 1 as an ROI and performed the double-stop contrast from experiment 2 on these voxels. There was an almost significant difference between double and stop trials, t15 = 1.52, P = 0.07.
This result suggests that the right insula/inferior frontal region shows a graded response across the 2 experiments, with greater activation to stop trials than shift trials and greater activation to double trials than to stop trials. However, an alternative possibility is that the insula and IFC constitute separate functional regions, with the IFC more strongly activated by stop trials and the insula more activated by double trials. If this is the case, then the insula may not show a particularly increased response to stop trials in experiment 1. To test this possibility, we drew a 5-mm sphere centered on the peak activated voxel from the double-stop contrast in experiment 2 and reran the stop-shift contrast from experiment 1 on these voxels. This analysis revealed significantly greater activation in this region for stop trials than shift trials, t19 = 2.39, P < 0.05. For comparability, we also reran the contrasts stop-go and shift-go on this ROI. There was significantly greater activation in this ROI for stop trials relative to go trials, t19 = 3.4, P < 0.01 but not for shift trials relative to go trials, t19 = −0.2, P = 0.58.
Discussion of Experiment 2
In a whole-brain analysis of the data from experiment 2, we did not find any regions that showed significantly greater activation for no-go trials than double trials. However, we found that a region with a peak in the right insula that extended into the right frontal operculum region while also encompassing the right IFC region from experiment 1 showed greater activation to double trials than to no-go trials. Furthermore, we found that the insula region that showed significantly greater activation for double trials than stop trials in experiment 2 also showed significantly greater activation for stop trials than shift trials in experiment 1. These results suggest that the right insula and IFC region shows a graded response to the different trial types across the 2 experiments, with the smallest response to attentional shift trials, an intermediate response to no-go trials and the maximal response to double trials.
These results are inconsistent with the hypothesis that activation in the right IFC is preferentially driven by motor response inhibition demands—if this were the case, then activation should be greater for no-go trials than double trials. In fact, these results show that this region is not particularly activated in response inhibition when compared with initiating a less routine motor response.
However, the results are also inconsistent with the hypothesis that activation in this region is preferentially driven by attentional demands—if this were the case, then activation should be equivalent for double trials and no-go trials, both of which require the detection and processing of equally frequent, equally task-relevant stimuli.
The results of experiment 2 are, however, consistent with the hypothesis that activation in this region is sensitive to an increase in the level of response control demands. In double response trials, subjects were required to initiate a change in their motor program. However, crucially, this change did not involve withholding the ongoing index finger response. Instead, subjects were required to add an additional motor response and to coordinate the temporal execution of the 2 responses, a process which we assume would involve considerable response control. This increase in response control was associated with greater activation in the right insula/IFC relative to simple motor response inhibition, indicating that activation in this region is driven at least in part by the specific response requirements of the current task.
General Discussion
Despite the wealth of evidence linking the right IFC with an inhibitory function, the specificity of this relationship has remained controversial. Evidence against such a direct structure–function mapping comes from studies showing that this region is also activated in simple attentional tasks such as target detection (e.g., Hampshire et al. 2007; Sharp et al. 2010), possibly as part of a more extensive network of frontoparietal regions which subserve a multitude of different cognitive processes (Duncan and Owen 2001).
In the present study, we provided several novel findings relating to this debate: First, in experiment 1, we found a double dissociation between motor inhibition and attentional shifting, whereby in the direct contrast of no-go and attentional shift trials, inhibition was associated with a particular increase in activation in the right IFC and attentional shifting was associated with a particular increase in activation in the left inferior parietal cortex. This double dissociation shows that it is possible to demonstrate a degree of functional specialization within separate nodes in the frontoparietal network from the more general-purpose activation of the same regions across multiple task demands.
Second, in experiment 2, we demonstrated, to our knowledge for the first time, that activation in the right insula/IFC can be raised above the level observed during simple motor inhibition trials. Specifically, when subjects were required to perform an additional, rapid motor response within a limited period of time, the direct comparison of these trials with no-go trials revealed significantly greater activation in the right insula/IFC during the additional motor response trials. This finding indicates that activation in this region is modulated by the specific response control demands of the current task.
The results of experiment 2 argue against a purely attentional, target detection-based account of right IFC function, which, given the equal task relevance of no-go and double response trials, would predict equal activation in this region for these 2 trial types. However, an interesting feature of the present results is that the peak of the cluster activated in the double-stop contrast was located in the anterior insula, although the cluster extended into the inferior frontal operculum, and the ROI analysis revealed a marginally significant difference between these trial types in the right IFC region identified in experiment 1. Given this pattern of results, we treat the insula and IFC as an integrated region, consistent with a previous meta-analysis of fMRI data (Duncan and Owen 2000). However, an alternative account would be that there is a graded response across this region, with the IFC showing a more equal response to double and stop trials and the insula showing a greater preference for double trials. If this were the case, it would imply that IFC activation was related to the detection and processing of task-relevant cues, whereas insula activation was related to more effortful processes.
As outlined in the Introduction, several other neuroimaging studies have provided evidence that is consistent with the attentional hypothesis of right IFC function. In the next section, therefore, we discuss how the results of these studies relate to the present findings.
In one study, Hampshire et al. (2010) found that the right IFC showed increased activation to rare targets regardless of the specific response required and even when no overt response was required (although activation was shown to be higher for trials that required a motor response relative to trials that did not). The authors interpreted the results as showing that the right IFC mediates the attentional detection of task-relevant stimuli rather than motor response inhibition per se. However, in that study, the different response demands, that is, inhibition and target detection, were performed in separate blocks and were not contrasted directly. Thus, while the results of that study were suggestive of a response-related modulatory effect in right IFC, the present results provide more direct evidence for this effect.
Two other studies (Chikazoe et al. 2009 and Sharp et al. 2010) attempted to differentiate activation directly related to motor inhibition from activation related to the processing of infrequent stimuli by directly contrasting no-go trials with infrequent go trials. These 2 studies produced somewhat contrasting results with regard to activation in the right IFC: In the Chikazoe et al. (2009) study, the right IFC showed greater activation to no-go trials than to infrequent go trials, whereas in the Sharp et al. (2010) study, there was no difference between the 2 trial types.
The results of the Chikazoe et al. (2009) study are consistent with the response control hypothesis outlined above as they demonstrate that, even when the frequency of the cue is controlled, trials involving an increase in response control (i.e., no-go trials) produce greater activation in right IFC. However, the results of the Sharp et al. (2010) study are inconsistent with this hypothesis as they show no such difference. What, then, is the reason for the discrepancy between these 2 studies?
One possibility is differences in the specific response requirements of the tasks employed in the different studies. In the Chikazoe et al. (2009) study, subjects performed a go/no-go task similar to that used in the present study, whereas in the Sharp et al. (2010) study, subjects performed a stop-signal reaction time (SSRT) task, a more complicated and, arguably, more demanding task, in which subjects make a speeded response to a target (in this case, a left- or right-pointing arrow) but must withhold their response if a rare “stop signal” occurs shortly after target onset. It is possible that infrequent go trials, that is, trials in which a continue signal is presented and subjects make no change to their ongoing response, require a greater degree of response control in the context of the SSRT task than in the go/no-go task due to the trial structure of the SSRT task. This increase in response control requirements may lead to greater activation in the right IFC during infrequent go trials in the SSRT task and, consequently, a smaller difference in activation between no-go and infrequent go trials in this task.
This hypothesis is supported by some additional findings of the Sharp et al. (2010) study. In that study, the contrast infrequent go trials—frequent go trials produced extensive activation in the right IFC, whereas the same contrast in the Chikazoe et al. (2009) study produced activation predominantly in the right inferior frontal junction and not in the IFC. Thus, the right IFC may in fact be sensitive to response control demands but that sensitivity will only be apparent in a contrast of 2 trial types that differ sufficiently in the level of response control required.
Finally, Duann et al. (2009), in an fMRI study of motor inhibition, found that the right IFC showed significant functional connectivity with the pre-SMA as well as with posterior brain regions, including the superior temporal and visual cortices, while the SMA in turn showed significant functional connectivity with the basal ganglia. The authors interpreted these findings as evidence that the IFC plays a role in the attentional processing of the stop signal through the enhancement of visual information processing, while the SMA plays a more direct role in motor inhibition via its connections with the basal ganglia.
While we would agree that a role in the attentional processing of task-relevant visual information is consistent with the strong connections of the right IFC with posterior brain regions (Pandya et al. 1996; Petrides and Pandya 1984), we would also argue that this interpretation rather neglects the response-related functions of this region: First, if the IFC purely enhances visual information, then activation in this region should be equally high for equally task-relevant stimuli. However, as the results of experiment 2 show, this is not the case—activation is greater in trials requiring greater response control. Second, increased connectivity between IFC and pre-SMA was observed during successful stops relative to unsuccessful stops. While this may reflect increased attentional processing of the stop cue during successful trials, a more parsimonious explanation is that it reflects an increase in motor control. Finally, there is mounting evidence that attentional processing of task-relevant information is achieved through increased functional connectivity between higher level frontoparietal regions and lower level sensory-specific cortex (Gazzaley et al. 2007; Browning et al. 2009; Lauritzen et al. 2009). Therefore, it seems more likely that increased connectivity between IFC and pre-SMA during successful motor response inhibition reflects a role for the right IFC in facilitating effective response control.
Any attempt to understand the function of a specific prefrontal cortical region should be grounded within our knowledge of its extensive and wide-ranging connections (Fuster 2001). Therefore, a full account of the function of the right IFC should attempt to take into account not only the wide range of neuroimaging evidence regarding its functional role in cognition but also its position within sensory- and motor-related neural networks.
In the case of the right IFC, its cortical connections may help to resolve the conflicting lines of evidence regarding its functional role in executive control: On the one hand, as demonstrated in experiment 1 and in numerous previous studies, the detection of novel, task-relevant information is sufficient to cause an increase in activation in the right insula/IFC. On the other hand, as demonstrated in experiment 2 and consistent with previous studies of response inhibition, the level of response control required by the current task also appears to be a particularly important driver of activation in this region.
Thus, a complete account of right IFC function may require the integration of these 2 factors—attentional processing and response control. Moreover, such an account would be consistent with the known pattern of connections between the IFC and other cortical regions. The IFC has extensive anatomical connections, not only with posterior brain regions—primarily inferotemporal cortex (Barbas and Pandya 1989; Pandya et al. 1996)—but also with anterior, motor-related brain regions, in particular the ventral premotor cortex (Barbas and Pandya 1987), and is therefore well placed to integrate bottom-up, sensory information with top-down, response-related information.
Therefore, we propose that this region operates at the interface of attention and response control, enhancing attentional processing of sensory stimuli that are relevant to current goals through its connections with posterior, sensory-related brain regions while prioritizing specific actions associated with those task-relevant stimuli through its connections with anterior, motor-related brain regions. This hypothesis may account for a wider range of data than any explanation based solely on a single cognitive function such as attention or inhibition. Furthermore, this account does not consider the IFC as an isolated cortical module but instead attempts to place it within a functional framework that is consistent with its wider role in a more extensive neural network engaged in the production of stimulus-driven, goal-directed behavior.
This proposal is, of course, not entirely novel. Previous authors have made similar claims regarding this region on the basis of neuroimaging results. For example, Hampshire and colleagues have suggested that the IFC lies at the crossover point between bottom-up, stimulus-driven processing and the processing of top-down goal-oriented intentions (Hampshire et al. 2010) and that the relevance of a stimulus to current task goals and actions plays an important role in shaping the response of this region (Hampshire and Owen 2006, 2008). However, until now, direct evidence for this hypothesis, demonstrating that the direct comparison of different response requirements within a single task produces differential activation in this region, has been lacking. Moreover, the present results demonstrate that carrying out an effortful motor response is an important factor driving activation in this region.
In summary, we found that a region encompassing the right insula and IFC, which was commonly activated across 2 different cognitive demands in experiment 1, also showed a graded pattern of activation across the 2 experiments, with the lowest level of activation for attentional shift trials, an intermediate level of activation for no-go trials, and the highest level of activation for double-response trials. These results indicate that it is not motor inhibition or attentional demands per se but rather a combination of attentional and response control demands, which drive activation in this region.
Additionally, in experiment 1, we found a double dissociation between motor response inhibition and attentional shifting, whereby motor inhibition selectively activated the right IFC and attentional shifting selectively activated the left inferior parietal cortex. These latter findings implicate the left inferior parietal cortex and not the IFC in the process of shifting attention between task-relevant stimuli and provide further evidence that different nodes within frontoparietal cortex play specialized functional roles in executive function above and beyond their more general activation as part of a multiple-demand network.
Funding
Program Grant (no. 089589/Z/09/Z) awarded by the Wellcome Trust to T.W.R., B. J. Everitt, A. C. Roberts, and B. J. Sahakian and completed within the University of Cambridge Behavioural and Clinical Neuroscience Institute funded by a joint award from the Medical Research Council and the Wellcome Trust.
Acknowledgments
We would like to thank all participants and the staff at the Wolfson Brain Imaging Centre. Conflict of Interest: None declared.
References
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6(2):115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26(9):2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. J Comp Neurol. 1987;256(2):211–228. doi: 10.1002/cne.902560203. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1989;286(3):353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Bledowski C, Prvulovic D, Goebel R, Zanella FE, Linden DE. Attentional systems in target and distractor processing: a combined ERP and fMRI study. Neuroimage. 2004;22(2):530–540. doi: 10.1016/j.neuroimage.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Presented at the 8th International Conference on Functional Mapping of the Human Brain, June 2–6, 2002, Sendai, Japan. 2002. Region of interest analysis using an SPM toolbox [abstract] Available on CD-ROM in NeuroImage 16(2). Available from: https://cirl.berkeley.edu/mb312/abstracts/Marsbar/marsbar_abs.html. [Google Scholar]
- Browning M, Holmes EA, Murphy SE, Goodwin GM, Harmer CJ. Lateral prefrontal cortex mediates the cognitive modification of attentional bias. Biol Psychiatry. 2009;67(10):919–925. doi: 10.1016/j.biopsych.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla D, Rees G, Friston KJ. The physiological basis of attentional modulation in extrastriate visual areas. Nat Neurosci. 1999;2:671–676. doi: 10.1038/10230. [DOI] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Asari T, Yamashita K, Morimoto H, Hirose S, Miyashita Y, Konishi S. Functional dissociation in right inferior frontal cortex during performance of go/no-go task. Cereb Cortex. 2009;19(1):146–152. doi: 10.1093/cercor/bhn065. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci. 2000;3(3):277–283. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- Duann JR, Ide JS, Luo X, Li CS. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J Neurosci. 2009;29:10171–10179. doi: 10.1523/JNEUROSCI.1300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. EPS Mid-Career Award 2004: brain mechanisms of attention. Q J Exp Psychol. 2006;59(1):2–27. doi: 10.1080/17470210500260674. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23(10):475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Fuster JN. The prefrontal cortex—An update: Time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A. 1999;96(14):8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Rissman J, Cooney J, Rutman A, Seibert T, Clapp W, D'Esposito M. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cereb Cortex. 2007;17(Suppl 1):i125–i135. doi: 10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Duncan J, Owen AM. Selective tuning of the blood oxygenation level-dependent response during simple target detection dissociates human frontoparietal subregions. J Neurosci. 2007;27(23):6219–6223. doi: 10.1523/JNEUROSCI.0851-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Owen AM. Fractionating attentional control using event-related fMRI. Cereb Cortex. 2006;16(12):1679–1689. doi: 10.1093/cercor/bhj116. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Thompson R, Duncan J, Owen AM. The target selective neural response–similarity, ambiguity and learning effects. PLoS. 2008;3(6):e2520. doi: 10.1371/journal.pone.0002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson T, Chamberlain M, Parris B, James M, Gutowski N, Husain M, Kennard C. The role of the ventrolateral frontal cortex in inhibitory oculomotor control. Brain. 2007;130(Pt 6):1525–1537. doi: 10.1093/brain/awm064. [DOI] [PubMed] [Google Scholar]
- Hon N, Epstein RA, Owen AM, Duncan J. Frontoparietal activity with minimal decision and control. J Neurosci. 2006;26(38):9805–9809. doi: 10.1523/JNEUROSCI.3165-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima R, Satoh K, Itoh H, Ono S, Furumoto S, Gotoh R, Koyama M, Yoshioka S, Takahashi T, Takahashi K, et al. Functional anatomy of GO/NO-GO discrimination and response selection—a PET study in man. Brain Res. 1996;728(1):79–89. [PubMed] [Google Scholar]
- Kelly AM, Hester R, Murphy K, Javitt DC, Foxe JJ, Garavan H. Prefrontal-subcortical dissociations underlying inhibitory control revealed by event-related fMRI. Eur J Neurosci. 2004;19(11):3105–3112. doi: 10.1111/j.0953-816X.2004.03429.x. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122(5):981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- Lauritzen TZ, D'Esposito M, Heeger DJ, Silver MA. Top-down flow of visual spatial attention signals from parietal to occipital cortex. J Vis. 2009;9:18–114. doi: 10.1167/9.13.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HC, Cai W. Common and differential ventrolateral prefrontal activity during inhibition of hand and eye movements. J Neurosci. 2007;27(37):9893–9900. doi: 10.1523/JNEUROSCI.2837-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J Neurosci. 2006;26(1):186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Luby M, Gore J, Goldman-Rakic P. Infrequent events transiently activate human prefrontal and parietal cortex as measured by functional MRI. J Neurophysiol. 1997;77(3):1630–1634. doi: 10.1152/jn.1997.77.3.1630. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp. 2001;12(3):131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya DN, Yeterian EH, Fleminger S, Dunnett SB. Comparison of prefrontal architecture and connections. Philos Trans R Soc Lond B Biol Sci. 1996;351:1423–1432. doi: 10.1098/rstb.1996.0127. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Comp Neurol. 1984;228(1):105–116. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Simmons A, Williams SC, Giampietro V, Andrew CM, et al. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13(2):250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, Mehta MA. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc Natl Acad Sci U S A. 2010;107:6106–6111. doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]