Abstract
Variation in the μ-opioid receptor gene has been associated with early social behavior in mice and rhesus macaques. The current study tested whether the functional OPRM1 A118G predicted various indices of social relations in children. The sample included 226 subjects of self-reported European ancestry (44% female; mean age 13.6, SD=2.2) who were part of a larger representative study of children aged 9–17 years in rural North Carolina. Multiple aspects of recent (past 3 months) parent–child relationship were assessed using the Child and Adolescent Psychiatric Assessment. Parent problems were coded based upon a lifetime history of mental health problems, substance abuse, or criminality. Child genotype interacted with parent behavior such that there were no genotype differences for those with low levels of parent problems; however, when a history of parent problems was reported, the G allele carriers had more enjoyment of parent–child interactions (mean ratio (MR)=3.5, 95% CI=1.6, 8.0) and fewer arguments (MR=3.1, 95% CI=1.1, 8.9). These findings suggest a role for the OPRM1 gene in the genetic architecture of social relations in humans. In summary, a variant in the μ-opioid receptor gene (118G) was associated with improved parent–child relations, but only in the context of a significant disruption in parental functioning.
Keywords: genetics, OPRM1, parent–child relations, separation anxiety, childhood
INTRODUCTION
Binding of the endogenous opioids to the μ-opioid receptor (Bodnar, 2008; Kieffer and Evans, 2009; Kreek and LaForge, 2007) activates reward systems by modulating circuitry in the ventral tegmental area (Spanagel et al, 1992) and nucleus accumbens (Simmons and Self, 2009). As such, it is a critical player in reinforcement of both natural and artificial rewards. Among the behavioral systems influenced by the μ-opioid receptor is the infant social attachment system (Nelson and Panksepp, 1998). During periods of interaction with a caregiver, opioids are released, thereby contributing to reinforcement of the attachment bond. A relative reduction in opioid release, which occurs during periods of separation, increases an infant's motivation to seek and maintain proximity to its caregiver (Herman and Panksepp, 1978; Kalin et al, 1995; Knowles et al, 1989). The importance of endogenous opioids for attachment-related behaviors have been validated pharmacologically, as both attachment and separation responses are attenuated with nonsedating doses of the μ-opioid receptor agonist, morphine (Kalin et al, 1988; Panksepp et al, 1978).
Genetic variation that affects μ-opioid receptor function has been demonstrated to influence social behavior in various animal models (Barr et al, 2008; Moles et al, 2004). Mice lacking the OPRM1 receptor gene show prominent deficits in maternal separation-induced ultrasonic vocalizations, preference for maternal cues, and ultrasonic call potentiation after brief maternal exposure (Moles et al, 2004). In rhesus macaques, a nonsynonymous SNP in the OPRM1 gene (rhOPRM1 C77G) that increases reward sensitivity (Barr et al, 2007) predicts increased vocalization during periods of maternal separation and social preference for the caregiver upon reunion (Barr et al, 2008). These data suggest that spontaneous genetic variation at the OPRM1 gene might influence the development of social attachment and other related phenotypes in humans.
In humans, there is a nonsynonymous SNP (OPRM1 A118G) that results in an amino-acid substitution in the N-terminal arm of the receptor. This genetic variant has been shown to predict increased response to ‘reward' in a variety of paradigms. Recent studies also show increased sensitivity to social rejection in G allele carriers (Way et al, 2009). Originally considered a gain-of-function allele (Bond et al, 1998), recent studies examining either in vitro properties or genetic association with substance disorders have produced mixed results (Arias et al, 2006; Kroslak et al, 2007). Given that functional variation appears to influence the development of social attachment in nonhuman species, we wanted to test whether there was an effect of OPRM1 A118G on the quality of human parent–child relations. As studies in other species (Barr et al, 2008; Moles et al, 2004) suggest that genetic variation might produce effects as a function of repeat exposures to separation from a caregiver, we wanted to examine whether there were interactions of genotype with parental inconsistency or unavailability. In this study we examined whether OPRM1 A118G genotype predicted parent–child relations, and whether there were interactive effects of genotype with significant disruption in parental functioning.
MATERIALS AND METHODS
Sample and Procedures
The Caring for Children in the Community study (CCC) is a representative study of psychiatric illness and service use in African-American and White youth in four rural counties in the southeast with high rates of poverty. The two-stage sampling design and methods are described in detail elsewhere (Angold et al, 2002). Briefly, 4500 youths were randomly selected from all 17 117 9–17 year olds in the public school's database. Of these, 3613 were contacted and agreed to complete screens (the externalizing scale of the CBCL). Of these families, 1302 were selected to participate in the interviews, and 920 (70.7%) interviews were completed.
Children were selected with different probabilities from each decile of CBCL scores. Participants were assigned a weight inversely proportional to their probability of selection, so that the results from our analyses reported in this study are representative of the original populations from which the samples were drawn. Parent and child signed informed consent/assent forms approved by the institutional review board of the Duke University Medical Center.
This study sample was limited to subjects of self-reported European ancestry (N=337) because the polymorphism of interest appears to be rare (<1%) in African-derived populations (HapMap). Of the 337 subjects, 71% or 238 consented to provide blood samples using standard blood spot collection procedures. Spots from 12 subjects were too small for extraction. DNA was isolated from 226 dried blood samples using standard extraction procedures.
Measures
Primary outcomes
Both subjects and parents were interviewed with the Child and Adolescent Psychiatric Assessment (CAPA) (Angold and Costello, 2000). All outcomes were assessed over the preceding 3 months. This study did not include a formal assessment of attachment as currently conceptualized. The CAPA does query parents and child about three aspects of the relationship: (1) enjoyment of parent–child activities (scale from 0 to 2); (2) parent–child arguments (count of arguments); and (3) separation anxiety symptoms (count of eight possible symptoms). Assessment of enjoyment of parent–child activities focused on the extent of the time the interviewees enjoyed activities with their parent–child (ie, >75, 25–75, or <25%). When both parent and child report was available, values were averaged for parent–child relation and their arguments. Assessment of separation anxiety was particularly important for discriminating whether the parent–child bond was reinforced by positive affect, anxiety, or both. A separation anxiety symptom was counted as present if it was reported by the primary caregiver, child, or both, as is standard clinical practice. Construct validity of the CAPA, as judged by 10 different criteria including relation to diagnostic rates found using other interviews and relation of CAPA-identified disorders to mental health service use, was good to excellent (Angold and Costello, 1995, 2000).
Specificity analyses
To test whether the genetic effects were specific to parent–child relations, two additional outcomes that were related were included: symptoms of depression and conduct problems. It was hypothesized that OPRM1 would not be associated with either of these outcomes. As with separation anxiety symptoms, depression and conduct symptoms were assessed with the CAPA and counted as present if reported by the primary caregiver, child, or both.
Parent problems
To test whether effects of the OPRM1 G allele on social relations varied by parent behavior, a measure of parental impairment was derived. In animal studies, parental availability is easily manipulated. As this is not possible in human samples, parent problems were chosen that would predispose the parent to be inconsistent, impaired, or unavailable for parent–child relations (see, eg, Jaffee et al, 2006; Lieb et al, 2000; Marcenko et al, 2000). Parents were coded as likely to have been impaired if they reported ever having: (1) significant mental health problems requiring treatment (39.1%, N=93), (2) substance abuse problems requiring treatment (9.5%, N=28), or (3) a criminal conviction (42.9%, N=90). These items were assessed as part of the parental functioning portion of the CAPA interview with the parent (Angold and Costello, 2000). For the mental health and substance-related indicators, parents were asked about specific types of treatment that may have been required (eg, medication, hospitalization). The parental criminality questions include follow-up question about disposition. The κ statistics for 1-year agreement ranged from 0.39 to 0.46 for the three indicators. In all, 59.1% of children (N=138) had a parent reporting at least one of these problems, 25.6% (N=59) reported two or more of these problems, and 5.7% (N=14) reported all three problems.
Genotyping
The 5′ nuclease genotyping assay was used for genotyping. Locus-specific primers and fluorogenic allele-specific probes were obtained from ABI (Assays-on-Demand, identification no. C_8950074). Approximately 10 ng of genomic DNA was amplified by real time-polymerase chain reaction (RT-PCR), using allele-specific probes. The reaction mixture consisted of 5 μl of master mix, 0.25 μl of 20 × assay mix, and 10 ng of genomic DNA diluted in distilled water. Amplification was performed (Gene Amp PCR system 97000; Applied Biosystems) using 384-well plates and the following amplification profile: 50 °C for 2 min and 95 °C for 10 min, followed by 40 cycles of 92 and 60 °C for 1 min. After amplification, end point fluorescence intensity was measured directly in the reaction plates (7900 sequence detector; Applied Biosystems). Four genotyping clusters were identified: A homozygotes, AG heterozygotes, G homozygotes, and no-DNA template controls. Genotype completion and accuracy (based on 10% replicate samples) were both 100%. Results were reported blind to outcome data.
Analytic Strategy
To insure independent analyses, correlations were tested among the three parent–child relations variables. Substantial intercorrelations would suggest nonindependence. The main effect of genotype on each outcome was tested using weighted Poisson regression models. The generalized estimating equations (GEE) option was employed to adjust standard errors of the parameter estimates for the stratified design effects. All models were run in SAS using PROC GENMOD with the GEE option (SAS Institute, 2004). Each model tested genotype as a predictor, adjusting for the covariates of age and sex. For all analyses, the genotype had two levels: A/A and G allele carriers (collapsing heterozygotes and homozygotes). To test for an interaction between genotype and parent behavior, the parent problems variable was entered into the main effect model along with an interaction term between genotype and parent problems. If significant, the LSMEANS statement with the DIFF option was used to compute least-square means estimates for the particular interaction effects and to test differences between such effects.
RESULTS
Of the 226 subjects, 163 had the A/A genotype (weighted percent=66.3), 58 were heterozygous for the G allele (28.7%), and 5 were G homozygotes (5.1%). All results are based upon models in which G/Gs were grouped with heterozygotes (see, eg, Arias et al, 2006). The pattern of results did not differ when analyzed under an additive (G homozygotes in a separate group) or when G homozygotes were excluded. The frequency of the G allele was 15.0%, comparable with the minor allele frequency of 16.7% for the European panel of HapMap (Gibbs et al, 2003). Genotype frequencies did not deviate from Hardy–Weinberg equilibrium (χ2=0.004, p=NS). G allele rates were higher in males than females (10 vs 19%, p=0.03), but genotype was not associated with age (G allele carrier mean=13.1 (SD=2.5) vs A/A mean=13.7 (SD=2.1), p=0.23).
Current parent–child relations were assessed through parent and self-report of the following variables as they were characterized in the previous 3 months: separation anxiety symptoms, enjoyment of parent–child activities, and parent–child arguments (Table 1). The intercorrelations between outcomes were very modest (r range=0.04–0.20).
Table 1. Definitions and Descriptive Information About Outcomes (N=226).
| Outcomes | A/A (N=163) | G allele carriers (N=63) | Overall (N=226) |
|---|---|---|---|
| Primary: parent–child relationship | Mean (SD) | Mean (SD) | Mean (SD) |
| Enjoyment of activities with parent: activities between child and his/her parents are rated according to the degree of pleasure, displeasure, or disinterest associated with them. | 0.30 (1.06) | 0.67 (1.17) | 0.55 (1.16) |
| Parent–child arguments: number of parent–child arguments | 9.07 (26.57) | 5.4 (17.9) | 7.83 (24.49) |
| Separation anxiety symptoms: DSM-IV symptoms of separation anxiety disorder | 0.31 (0.83) | 0.19 (0.77) | 0.27 (0.81) |
| Secondary: prosocial behavior | |||
| Depressive symptoms: non-overlapping DSM-IV symptoms of major depression or dysthymia | 0.65 (1.18) | 0.67 (1.13) | 0.66 (1.16) |
| Conduct symptoms: DSM-IV symptoms of conduct disorder | 1.43 (1.57 | 1.34 (1.53) | 1.40 (1.56) |
OPRM1 genotype influenced all three measures of parent–child relations. After accounting for the effects of sex and age, the OPRM1 118G allele predicted increased enjoyment of parent–child activities (χ2(1)=6.93, p<0.01, means ratio (MR)=2.44 95% CI=1.17, 5.21), lower levels of separation anxiety symptoms (χ2(1)=3.93, p<0.05, MR=0.55 95% CI=0.31, 0.98), and fewer parent–child arguments (χ2(1)=86.34, p<0.01, MR=0.59 95% CI=0.54, 0.67). OPRM1 genotype was not related to other emotional and behavior problems, such as symptoms of depression (χ2(1)=1.29, p=0.26, MR=1.14, 95% CI=0.82, 1.60) or conduct disorder (χ2(1)=0.75, p=0.39, MR=1.16, 95% CI=0.67, 1.99).
There were also interactive effects between parent problems and genotype. There was no significant difference in parental problems by child genotype (A/A: 59.0% (N=99) vs G allele carriers: 60.5% (N=39), χ2(1)=0.01, p=0.92), indicating that genotype was not a proxy for parent problem status. Among subjects whose parent(s) self-reported a history of significant problems, those who were carriers of the G allele displayed a pattern of parent–child relations that differed from that observed in A homozygotes. G allele carriers reported more enjoyment of interactions with parents. However, the effect of genotype was limited to subjects reporting parent problems (Figure 1, genotype by parent problems interaction: χ2(1)=6.6, p=0.01). A similar interaction was observed for parent–child arguments, with the G allele being associated with fewer parent–child arguments, but only as a function of parent problems (Figure 2, genotype by parent problems interaction: χ2(1)=4.7, p=0.03). The G allele did not interact with parent problems to predict the separation anxiety symptoms (Figure 3, genotype by parent problems interaction: χ2(1)=2.0, p=0.16). Follow-up analyses tested the influence of excluding a single indicator (mental health problems, substance problems, or criminality) from the parent problem variable. The pattern of findings did not vary with the omission of any of the specific parent problems.
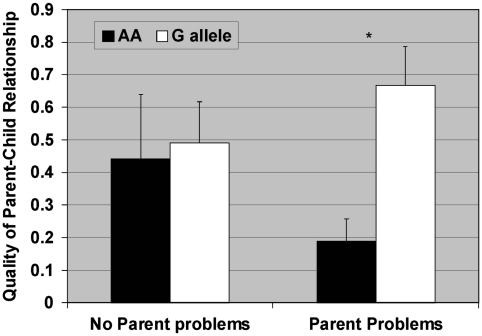
Figure 1.
Results of the Poisson multiple regression analyses estimating the association between parent problems and enjoyment of parent–child interactions as a function of the OPRM1 A118G genotype. The overall main effect for genotype was significant (χ2(1)=4.3, p=0.04, MR=2.0, 95% CI=1.0, 3.8), but not for parent problems (χ2(1)=0.7, p=0.40, MR=1.31, 95% CI=0.7, 2.5). The interaction term (genotype by parent problems) interaction term was also significant (χ2(1)=6.6, p=0.01). For those with no parent problems, genotype status is not significant (χ2(1)=0.04, p=0.84, MR=1.1, 95% CI=0.4, 3.1), whereas for those with parent problems, genotype predicts enjoyment of parent–child interactions (χ2(1)=9.5, p=0.002, MR=3.5, 95% CI=1.6, 8.0).
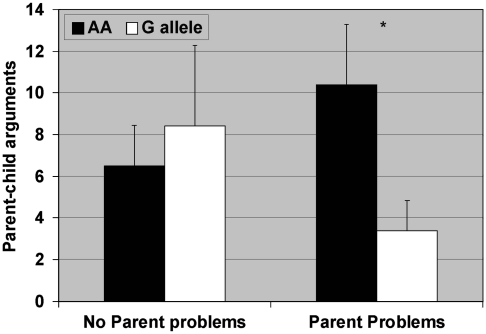
Figure 2.
Results of the Poisson multiple regression analyses estimating the association between parent problems and parent–child arguments as a function of the OPRM1 A118G genotype. Neither of the main effects were significant (genotype: χ2(1)=1.1, p=0.30, MR=1.5, 95% CI=0.7, 3.5; parent problems: χ2(1)=0.4, p=0.54, MR=1.2, 95% CI=0.6, 2.5). The interaction term (genotype by parent problems) interaction term was also significant (χ2(1)=4.7, p=0.03). For those with no parent problems, genotype status is not significant (χ2(1)=0.21, p=0.65, MR=0.8, 95% CI=0.2, 2.4), whereas for those with parent problems, genotype predicts enjoyment of parent–child interactions (χ2(1)=4.5, p=0.03, MR=3.1, 95% CI=1.1, 8.9).
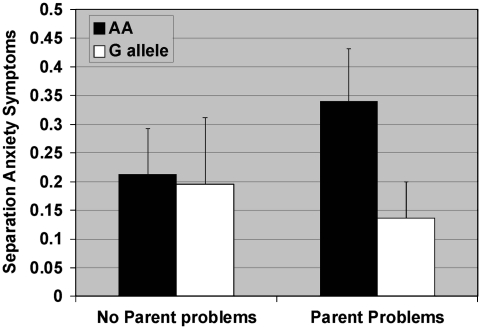
Figure 3.
Results of the Poisson multiple regression analyses estimating the association between parent problems and separation anxiety symptoms as a function of the OPRM1 A118G genotype. Neither of the main effects were significant (genotype: χ2(1)=1.3, p=0.25, MR=1.6, 95% CI=0.7, 4.0; parent problems: χ2(1)=0.0, p=0.90, MR=0.9, 95% CI=0.4, 2.3). The interaction term (genotype by parent problems) interaction term was not statistically significant (χ2(1)=2.0, p=0.16). For those with no parent problems, genotype status is not significant (χ2(1)=0.0, p=0.91, MR=1.1, 95% CI=0.3, 4.3), and for those with parent problems, genotype showed a trend toward predicting enjoyment of parent–child interactions (χ2(1)=2.9, p=0.09, MR=2.5, 95% CI=0.9, 7.4).
DISCUSSION
Understanding the genetic architecture of social relations is critical, given the role of interpersonal functioning in normal development and in the etiologies of almost every major psychiatric disorder (American Psychiatric Association, 1994). In this study, a common OPRM1 variant influenced the quality of parent–child relationships, especially in the context of having a parent with a history of mental health problems, substance problems, or criminality. Each of these problems represents a potentially significant disruption in the parent's functioning and may be associated with a range of parenting problems (see, eg, Jaffee et al, 2006; Lieb et al, 2000; Marcenko et al, 2000). In these cases, OPRM1 G allele carriers were advantaged across two measures of parent–child relations when compared with A/A subjects, but not in the case of separation anxiety symptoms.
The finding that the OPRM1 118G allele was protective in humans converges with results from a knockout study in mice (Moles et al, 2004) and our previous work on the rhOPRM1 C77G SNP in macaques (Barr et al, 2007). This convergence is not trivial given the unavoidable methodological differences among the studies. The mouse study presented an extreme test of the importance of the μ-opioid receptor (ie, genetic deletion), whereas the macaque study focused on a functional SNP. In both studies, it was possible to control the subjects' environments to test attachment behavior under extreme conditions. The current study tested the role of a SNP within a heterogeneous group of children and adolescents and relied upon natural variation in parent behavior rather than experimental control. Indeed, this study did not formally assess attachment as defined in developmental psychological literature, but parent–child relations. This study cannot be considered a ‘replication' of the animal studies. Despite all of these differences, however, each subsequent study has observed the fundamental pattern of variations in caregiver–child interactions as a function of variation in the OPRM1 gene.
Accumulating evidence suggests that oxytocin-, vasopressin-, and opioid-modulated mesocorticolimbic dopaminergic pathway activity accounts for the rewarding aspects of social interactions (Insel, 1997; Nelson and Panksepp, 1998). The endogenous opioids modulate reward pathways via activation of μ-opioid receptors in the ventral tegmental area (Spanagel et al, 1992) as well as the nucleus accumbens (Simmons and Self, 2009). This study supports the hypothesis that at least part of the rewarding/reinforcing aspects of social interactions in humans may be mediated by endogenous opioids. Furthermore, by demonstrating an interaction with parental availability or consistency, these findings reinforce observed similarities between patterns of parent–child interactions and substance withdrawal, wherein lack of positive reinforcement, either through removal of social interaction or the substance, is associated with behavior oriented toward the removed stimulus. This similarity is not unexpected if the positive effects of parent–child interaction are mediated by endogenous opioid binding to the μ-opioid receptor.
There are numerous reports suggesting a functional role for the OPRM1 A118G SNP. However, the exact nature of that role remains unclear and is potentially complex. Originally proposed to be a gain-of-function mutation (Bond et al, 1998), subsequent studies suggest that the in vitro effects of the 118G polymorphism may depend on the cell line and/or outcome measures (Befort et al, 2001; Beyer et al, 2004; Kroslak et al, 2007). Moreover, studies examining OPRM1 A118G genotype as a susceptibility factor for broad substance-related phenotypes are inconsistent (Arias et al, 2006). However, despite the inconsistencies listed above, a variety of studies demonstrate that the G allele behaves in vivo as a gain-of-function allele for opioid-modulated intermediate phenotypes, such as HPA-axis activity (Bart et al, 2006; Wand et al, 2002) pain threshold (Fillingim et al, 2005), and alcohol response (Ray and Hutchison, 2007; Ray, 2005). In fact, robust effects of OPRM1 genotype have been found in functional studies focused on narrowly defined quantitative phenotypes that were most closely related to the proposed function of the genetic variant (see, eg, Wand et al, 2002; Bart et al, 2006; Fillingim et al, 2005; Ray and Hutchison, 2007). This study employed a similar approach. To reduce the signal-to-noise ratio, we also included multiple, independent indices of parent–child interaction phenotypes, tested the specificity of the effect by including secondary outcomes, and stratified based upon a environmental exposure known to affect parenting behavior.
There are a number of scenarios that may lead to false positives in genetic association studies (Sullivan et al, 2001). To guard against type I error, we (1) tested a hypothesis that has been previously supported in two species, (2) limited our focus to a single SNP with the strongest evidence of function, and (3) tested the robustness of the effect by looking at three relatively independent outcomes. Despite these safeguards, all genetic associations must be considered ‘tentative information' until replicated in multiple independent samples (Ioannidis, 2006).
Another potential concern is stratification resulting from population admixture. To minimize this risk, only subjects of self-reported European ancestry were included and the larger epidemiological sample was collected from a rural area with no urban centers. Risk of experimental bias was minimized by blinded genotyping and regenotyping of 10% of all samples. It is important to keep in mind that parental behavior and parent–child interactions were assessed in the same interview. It is not possible, therefore, to clarify temporal sequence. At the same time, all parent–child outcomes were anchored to the 3 months immediately preceding the interview, whereas the parenting behavior variables (ie, mental illness, substance problems, and criminality) were based on whether they had ever occurred. The 1-year κ values for parent problems were relatively low and it is not possible to clarify the timing of the parent problem with respect to the child's birth. Finally, it is important to consider the possibility that OPRM1 A118G may not be the functional variant driving the observed phenotypic differences, but rather a proxy marker for a functional haplotype.
The previous study in macaques speculated that genetic variation that increased reward sensitivity might have conferred a selective advantage at some point in evolutionary history of both rhesus and humans, by increasing attachment in response to caregiver unavailability. This study demonstrates an effect of OPRM1 variation on development of social relations in humans and suggests that the same allele that has been proposed to increase risk for developing substance dependence in adolescence and young adulthood may be protective against poor parent–child relations in childhood. As such, this allele may provide a genetic basis for ‘resilience' to impaired attachment in the face of a significant disruption in the parent's functioning.
Acknowledgments
The work was supported by the National Institute on Alcohol Abuse and Alcoholism Intramural Research Program, the National Institute of Drug Abuse (DA024413 to EJC), and the National Institute for Mental Health (MH080230 to WEC).
The authors declare no conflict of interest.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV) American Psychiatric Press: Washington, DC; 1994. [Google Scholar]
- Angold A, Costello EJ. A test-retest reliability study of child-reported psychiatric symptoms and diagnoses using the Child and Adolescent Psychiatric Assessment (CAPA-C) Psychol Med. 1995;25:755–762. doi: 10.1017/s0033291700034991. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello E. The Child and Adolescent Psychiatric Assessment (CAPA) J Am Acad Child Adolesc Psychiatry. 2000;39:39–48. doi: 10.1097/00004583-200001000-00015. [DOI] [PubMed] [Google Scholar]
- Angold A, Erkanli A, Farmer E, Fairbank J, Burns B, Keeler G, et al. Psychiatric disorder, impairment, and service use in rural African American and white youth. Arch Gen Psychiatry. 2002;59:893–901. doi: 10.1001/archpsyc.59.10.893. [DOI] [PubMed] [Google Scholar]
- Arias A, Feinn R, Kranzler HR. Association of an Asn40Asp (A118G) polymorphism in the [mu]-opioid receptor gene with substance dependence: a meta-analysis. Drug Alcohol Depend. 2006;83:262–268. doi: 10.1016/j.drugalcdep.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Barr C, Schwandt M, Lindell BS, Chen SA, Goldman D, Suomi SJ, et al. Association of a functional polymorphism in the mu-opioid receptor gene (OPRM1C77G) with alcohol response and consumption in male rhesus macaques. Arch Gen Psychiatry. 2007;64:369–376. doi: 10.1001/archpsyc.64.3.369. [DOI] [PubMed] [Google Scholar]
- Barr C, Schwandt M, Lindell BS, Higley JD, Maestripieri D, Goldman D, et al. Variation in the mu-opioid receptor gene (OPRM1) influences attachment behavior in infant primates. Proc Natl Acad Sci USA. 2008;105:5277–5281. doi: 10.1073/pnas.0710225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bart G, LaForge K, Borg L, Lilly C, Ho A, Kreek M. Altered levels of basal cortisol in healthy subjects with a 118G allele in exon 1 of the mu opioid receptor gene. Neuropsychopharmacology. 2006;31:2313–2317. doi: 10.1038/sj.npp.1301128. [DOI] [PubMed] [Google Scholar]
- Befort K, Filliol D, Decaillot F, Gaveriaux-Ruff C, Hoehe M, Kieffer B. A single nucleotide polymorphic mutations in the human mu-opioid receptor severely impairs receptor signaling. J Biol Chem. 2001;276:3130–3137. doi: 10.1074/jbc.M006352200. [DOI] [PubMed] [Google Scholar]
- Beyer A, Koch T, Schroeder H, Schultz S, Hollt V. Effect of the A118G polymorphism on binding affinity, potency, and agonist-medicated edocytosis, desensitization, and resensitization of the human mu-opioid receptor. J Neurochem. 2004;89:553–560. doi: 10.1111/j.1471-4159.2004.02340.x. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ. Endogenous opiates and behavior: 2007. Peptides. 2008;29:2292–2375. doi: 10.1016/j.peptides.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Bond C, LaForge K, Tian M, Melia D, Zhang S, Borg L, et al. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci USA. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, Kaplan L, Staud R, Ness TJ, Glover TL, Campbell CM, et al. The A118G single nucleotide polymorphism of the [mu]-opioid receptor gene (OPRM1) is associated with pressure pain sensitivity in humans. JPain. 2005;6:159–167. doi: 10.1016/j.jpain.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Gibbs RA, Belmont JW, Hardenbol P, Willis TD, Yu F, Yang H, et al. The international HapMap project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- Herman BH, Panksepp J. Effects of morphine and naloxone on separation distress and approach attachment: evidence for opiate mediation of social affect. Pharmacol Biochem Behav. 1978;9:213–220. doi: 10.1016/0091-3057(78)90167-3. [DOI] [PubMed] [Google Scholar]
- Ioannidis J. Grading the credibility of molecular evidence for complex disease. Int J Epidemiol. 2006;35:572–577. doi: 10.1093/ije/dyl003. [DOI] [PubMed] [Google Scholar]
- Insel TR. A neurobiological basis of social attachment. Am J Psychiatry. 1997;154:726–735. doi: 10.1176/ajp.154.6.726. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Belsky J, Harrington H, Caspi A, Moffitt TE. When parents have a history of conduct disorder: how is the caregiving environment affected. J Abnorm Psychol. 2006;115:309–319. doi: 10.1037/0021-843X.115.2.309. [DOI] [PubMed] [Google Scholar]
- Kalin N, Shelton S, Barksdale C. Opiate modulation of separation-induced distress in non-human primates. Brain Res. 1988;440:285–292. doi: 10.1016/0006-8993(88)90997-3. [DOI] [PubMed] [Google Scholar]
- Kalin N, Shelton S, Lynn D. Opiate systems in mother and infant primates coordinate intimate contact during reunion. Psychoneuroendocrinology. 1995;20:735–742. doi: 10.1016/0306-4530(95)00023-2. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Evans CJ. Opioid receptors: from binding sites to visible molecules in vivo. Neuropharmacology. 2009;56 (Suppl 1:205–212. doi: 10.1016/j.neuropharm.2008.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles PA, Conner RL, Panksepp J. Opiate effects on social behavior of juvenile dogs as a function of social deprivation. Pharmacol Biochem Behav. 1989;33:533–537. doi: 10.1016/0091-3057(89)90382-1. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, LaForge KS. Stress responsivity, addiction, and a functional variant of the human Mu-opioid receptor gene. Mol Interv. 2007;7:74–78. doi: 10.1124/mi.7.2.7. [DOI] [PubMed] [Google Scholar]
- Kroslak T, LaForge K, Gianotti R, Ho A, Nielsen D, Kreek M. The single nucleotide polymorphism A118G alters functional properties of the human mu opioid receptor. J Neurochem. 2007;103:77–87. doi: 10.1111/j.1471-4159.2007.04738.x. [DOI] [PubMed] [Google Scholar]
- Lieb R, Wittchen HU, Hofler M, Fuetsch M, Stein MB, Merikangas KR. Parental psychopathology, parenting styles, and the risk of social phobia in offspring: a prospective-longitudinal community study. Arch Gen Psychiatry. 2000;57:859–866. doi: 10.1001/archpsyc.57.9.859. [DOI] [PubMed] [Google Scholar]
- Marcenko MO, Kemp SP, Larson NC. Childhood experiences of abuse, later substance use, and parenting outcomes among low-income mothers. Am J Orthopsychiatry. 2000;70:316–326. doi: 10.1037/h0087853. [DOI] [PubMed] [Google Scholar]
- Moles A, Kieffer B, D'Amato F. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science. 2004;304:1983–1986. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Panksepp J. Brain substrates of infant-mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neurosci Biobehav Rev. 1998;22:437–452. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Herman B, Conner R, Bishop P, Scott J. The biology of social attachments: opiates alleviate separation distress. Biol Psychiatry. 1978;13:607–618. [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study. Arch Gen Psychiatry. 2007;64:1069–1077. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- Ray WA. Observational studies of drugs and mortality. N Engl J Med. 2005;353:2319–2321. doi: 10.1056/NEJMp058267. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc . SAS/STAT® Software: Version 9. SAS Institute: Cary, NC; 2004. [Google Scholar]
- Simmons D, Self D. Role of mu- and delta-opioid receptors in the nucleus accumbens in cocain-seeking behavior. Neuropsychopharmacology. 2009;34:1946–1957. doi: 10.1038/npp.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg T. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci USA. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Eaves LJ, Kendler KS, Neale MC. Genetic case-control association studies in neuropsychiatry. Arch Gen Psychiatry. 2001;58:1015–1024. doi: 10.1001/archpsyc.58.11.1015. [DOI] [PubMed] [Google Scholar]
- Wand GS, McCaul M, Yang X, Reynolds J, Gotjen D, Lee S, et al. The mu-opioid receptor gene polymorphism (A118G) alters HPA axis activation induced by opioid receptor blockade. Neuropsychopharmacology. 2002;26:106–114. doi: 10.1016/S0893-133X(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Way BM, Taylor SE, Eisenberger NI. Variation in the mu-opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proc Natl Acad Sci USA. 2009;106:15079–15084. doi: 10.1073/pnas.0812612106. [DOI] [PMC free article] [PubMed] [Google Scholar]