Abstract
Background
The purpose of this study was to examine factors influencing a woman’s decision to participate in a breast cancer prevention clinical trial. Nine health care organizations in Massachusetts cooperated in the present project.
Methods
We performed a case-control study to compare responses between the study group (STAR trial eligible, but not enrolled) and the control group (STAR trial participants) on 12 factors previously identified as barriers to accrual for clinical trials. Eight hypotheses were tested using multiple logistic regression to estimate the strength of the association for each factor on the dependent variable (study participation).
Results
The study samples were similar to the general population of eligible breast cancer prevention clinical trial subjects in the counties where the participating organizations were located, the state of Massachusetts, and to nationally published STAR-trial data. Results of a mailed questionnaire showed that when adjusting for subject demographics, and in the presence of other questions, four factors (1) clinician expertise and qualifications (p =.012, OR: 4.903; 95% CI: 1.41 to 17.04); (2) personal desire to participate (p =.033, OR: 3.16; 95% CI: 1.10 to 9.06); (3) perceived value of the trial (p =.020, OR: 2.92; 95% CI, 1.18 to 7.21); and, (4) level of trial inconvenience (p =.002, OR: 0.10; 95% CI, 0.02 to 0.44), significantly influenced a woman’s decision to enroll onto a breast cancer prevention clinical trial more than other eligible subjects.
Conclusions
We conclude that addressing these issues in the relationship between patients and clinicians should improve accrual onto breast cancer prevention clinical trials.
Keywords: Breast Cancer, Clinical Trials, Cancer Prevention, Clinical Trial Accrual, Trial Barriers, Accrual Barriers
Background
According to the National Cancer Institute, breast cancer is a critical public health problem. 1 It is estimated by the American Cancer Society that 192,370 newly diagnosed breast cancer cases in women will be reported in the United States and 40,610 will die of the disease in 2009.2 As identified by the Centers for Disease Control and Prevention, breast cancer remains the 7th leading cause of death for women in the U.S.3 In fact, a woman born today in the US has a one in eight chance of being diagnosed with breast cancer at some point in her lifetime.4
Adult accrual onto cancer clinical trials is low. The National Cancer Institute estimates participation rates of 3% to 5% for all eligible adult cancer patients.5 The barriers that influence a patient’s decision to participate or not participate in a clinical research trial are complex and can originate from the patient, the physician, or even the organization where care is being received. From the patient’s perspective, it has been identified that mistrust of the health care system is the most often cited barrier encountered in clinical research.6 Previously, Mansour identified several clinician-oriented factors pertinent to participation rates. Included among these were: clinician’s awareness and access to available clinical trials; clinician’s bias to a particular treatment modality; clinician’s concern of losing patients to follow-up; trial complexities; lack of compensation; and, excessive un-compensated costs. 7 Institutional support has also been cited as an obstacle to patient enrollment by clinicians. This support can be either necessary staff to respond to regulatory requirements, or even the practice credit to clinicians involved in clinical trials. According to Somkin, et al., clinical trial accrual levels are hampered by a lack of infrastructure support to trials, especially additional support staff and research nurses.8
Regardless of the origination of a particular barrier in the accrual onto a cancer clinical trial, the results are detrimental to the health status for the entire patient population. Slow rates of accrual onto clinical trials extend the time required to provide sample sizes of acceptable levels necessary to either test an intervention, or new procedures, to impact the standard of care for patients diagnosed with a particular cancer diagnosis.9 Moreover, it is important to recognize that lower than anticipated participation rates in Phase III clinical trials can result in unintended consequences, since the purposes of Phase III clinical trials is to assess the safety and effectiveness of a new treatment protocol in a large sample (usually 1,000 to 3,000 participants) to timely evaluate overall benefit-risk of new treatment methods against the current standard of care.10
The purpose of this study was to examine factors influencing a woman’s decision to participate or not to participate in a breast cancer prevention clinical trial. Much research has been conducted to identify factors that contribute to accrual barriers onto clinical trials from the enrolled subjects; however, a critical segment of the population has been missing from research projects – that being those patients that were eligible for trial participation, approached for enrollment but subsequently chose not to join the study. Gotay pointed out that research should focus on patient perspectives that accept and decline trial participation, and on specific interventions designed to impact accrual.11 Previously, Friedman articulated that little information had been obtained on the critical impediments to increasing the percentage of eligible patients accrued onto clinical trials.12 Friedman concluded that while an insightful analysis of reasons for non-participation would be methodologically difficult to develop; nonetheless, it is essential to aid in addressing low levels of clinical trial participation.
Using as a basis the Phase III breast cancer prevention clinical trial entitled the Study of Tamoxifen and Raloxifene (STAR)for the Prevention of Breast Cancer in Post-Menopausal Women (NCT00003906) we sought to answer the following three research questions:
Are the screening, eligibility, and accrual rates onto the study at the two selected sites and satellite/affiliated organizations comparable with the community demographics under conside ration?
Are the screening, eligibility, and accrual rates onto the study at the two selected sites and satellite/affiliated organizations comparable with national data reported by the National Surgical Adjuvant Breast and Bowel Project (NSABP) for the Study of Tamoxifen and Raloxifene (STAR) breast cancer prevention clinical trial?
Which of the following factors, if any, differ between women that agreed to join or not to join the clinical trial: Clinician and/or Clinic Staff Relationship; Manner in Which the Trial Information was Presented; Time to Review the Trial Information; Level of Desire to Participate; Influence of Others in Decision to Participate; Time Requirements of the Study; Perceived Value of Participating in the Trial; Randomization; Family Responsibilities; Autonomy; Altruism; and, Practical Considerations?
Methods
Nine health care organizations, in eight cities and towns located in five counties of Massachusetts, agreed to participate in the present project. The cities and towns where the participating organizations are located comprise 15.80% of the total state population, while the selected counties represent 47.97% of the total population of Massachusetts.13,14 All institutions actively participated in the STAR trial (Figure 1). Women who were screened, considered eligible to participate in the STAR trial, and approached for participation at the two identified locations and satellite/affiliate organizations were deemed eligible for participation in this study. A total of 242 women met study eligibility criteria at the participating sites (Table 1). The study population represented 17.8% of STAR trial eligible subjects in Massachusetts.
Figure 1.
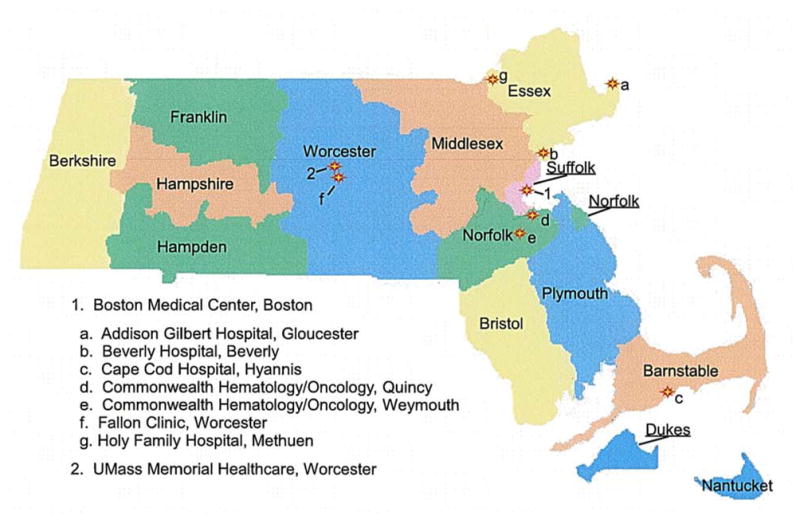
Participating Study Site Locations
Table 1.
Eligible Study Participants by Location in Massachusetts
| Location | Number of Sites | Number of Risk Assessment forms Completed | Number of Women Meeting STAR Study Eligibility Criteria and Approached for Study Enrollment | Number of Women Enrolled onto STAR Study |
|---|---|---|---|---|
| Participating STAR Trial Study Locations | 2 | 519 (25.7%) | 242 (17.8%) | 81 (16.7%) |
| All Other Massachusetts STAR Trial Locations | 11 | 1,497 (74.3%) | 1,118 (82.2%) | 402 (83.2%) |
| Totals | 13 | 2,016 | 1,360 | 483 |
The Institutional Review Board (IRB) at the University of Massachusetts Medical School (UMMS) provided initial review and approval of the study through an expedited review process. The UMMS IRB also agreed to be the IRB of record and to provide study oversight for all other participating sites through the initiation of IRB Authorization Agreements with each cooperating IRB. As part of the review process, the UMMS IRB approved a waiver of informed consent for subjects participating in the study.
The National Surgical Adjuvant Breast and Bowel Project (NSABP) agreed to return Massachusetts level and site specific demographic data back to the primary author to answer the first research question. Publicly available STAR trial results were used to compare the site specific data returned to the author to answer the second research question. Descriptive analysis measures were employed, utilizing a Chi-Square analysis, to compare the observed results with expected results, to answer the first two research questions.
Two instruments were adapted for use in this study to answer the third research question: the “Questionnaire on Understanding and Attitudes to Predict Willingness to Participate in Randomized Oncology Clinical Trials” developed by Ellis, et al.; and, the “Questionnaire to Determine Factors that Influence Individuals to Participate or Not to Participate in a Cancer Clinical Trial” developed by Simon.15,16
The final survey instrument prepared for this research project builds upon both the four factors investigated in the Ellis survey instrument and the eleven constructs addressed by Simon.15,16 Using a 29-question mailed survey instrument, a case-control study was performed to compare responses between the study group (STAR trial eligible, but not enrolled) and the control group (STAR trial participants) on 12 factors built upon previously identified barriers to accrual for clinical trials. The factors included: Clinician and/or Clinic Staff Relationship (2 questions); Manner in Which the Trial Information was Presented (4 questions); Time to Review the Trial Information (2 questions); Level of Desire to Participate (2 questions); Influence of Others in Decision to Participate (3 questions); Time Requirements of the Study (3 questions); Perceived Value of Participating in the Trial (4 questions); Randomization (1 question); Family Responsibilities(3 questions); Autonomy (2 questions); Altruism (1 question); and, Practical Considerations (2 questions). In order to identify the strength of association for those factors that contributed to the decision to participate or not participate in the clinical trial, the questionnaire was formatted using a five-point Likert scale with possible answers of: 1 –strongly disagree; 2 – disagree; 3 – neutral or no opinion; 4 – agree; and, 5 – strongly agree, for respondents to select from.
Eight hypotheses were examined using multiple logistic regression to examine the strength of the association between a factor tested and the dependent variable (study participation) for each hypothesis. The eight hypotheses assessed in this study were:
Participation is related to
The perception of the relationship with clinical staff.
The perception of how the trial was presented for consideration.
The amount of time provided to make the decision.
The perceived value of the trial.
The perceived benefit of the trial.
The level of involvement by others in the decision-making process.
The perceived amount of time or inconvenience involved in participating in the trial.
The amount of outside responsibilities.
For the comparison between non-participants and participants, a standard statistical method for the analysis of case-control studies was applied, treating non-participants as the cases and participants as the controls.17 As such, to answer the third research question four regression models were employed for statistical analysis. These included: (1) Univariate; (2) Uni-Question, adjusting for Questions within Each Hypothesis; (3) Univariate, adjusting for Subject Demographics; and, (4) Multi-question, adjusting for Questions within each Hypothesis and for Subject Demographics. A two-sided p-value based on the Wald statistics and 95% confidence interval was utilized to assess the statistical significance and precision of the odds ratio estimate. All analyses were conducted in SPSS Statistics 17.0.
Results
Observed eligibility results were compared with county specific demographic data available from the U.S. Census Bureau 2005–2007 American Community Survey to answer the first research question. 18 A total of 138 cities and towns were identified as the primary residence for those women that completed risk assessment forms and were considered eligible for STAR trial enrollment at the participating sites for this study. Additionally, it was found that women resided in four states (Connecticut, Maine, Massachusetts, and New Hampshire) that were seen at those organizations participating in the current research project. For those women residing outside of the selected county limits (Barnstable, Essex, Norfolk, Suffolk, and Worcester), but were registered at either Boston Medical Center and its satellites/affiliates, or UMass Memorial Health Care, an assumption was made for the appropriate study site location, and resulting county, based upon actual residence location, proximity, and accessibility to participating study sites.
The results from the analysis of research question one (Table 2) suggested that with the exception of Worcester County, no meaningful differences were detected between the racial/ethnicity demographic characteristics of women screened and considered eligible for participation in the study at the selected Massachusetts sites in Barnstable, Essex, Norfolk, or Suffolk Counties, as compared with related community demographics of the general population within each specific county. As such, it can be inferred that the racial/ethnic demographic composition for those women screened and considered eligible for STAR trial participation at the participating sites are generally comparable to community demographics of the counties in which the sites are located.
Table 2.
Research Question 1 Chi Square Results: Demographic Comparison -Women Ages 35 – 85+ (Racial and Ethnic Comparison)
| STAR Trial Eligible | County Population* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| County | White | African American | Asian/Native American | Hispanic | White | African American | Asian/Native American | Hispanic | χ2 3 d.f. | p-Value |
| Barnstable | 54 | 1 | 2 | 0 | 70,595 | 1,515 | 2,348 | 1,289 | 1.03 | 0.79 |
| Essex | 33 | 1 | 0 | 4 | 168,824 | 5,302 | 9,122 | 28,846 | 2.15 | 0.54 |
| Norfolk | 17 | 0 | 4 | 0 | 164,504 | 9,010 | 17,428 | 4,897 | 3.94 | 0.27 |
| Suffolk | 11 | 10 | 1 | 2 | 91,480 | 34,233 | 19,316 | 30,554 | 7.64 | 0.05 |
| Worcester | 100 | 0 | 2 | 0 | 179,711 | 6,422 | 11,129 | 16,697 | 15.48 | 0.001 |
County population does not include STAR trial eligible subjects.
For demographic composition, statistically significance differences were found for age between the general population and study subjects at the participating locations in all five counties under investigation. The results suggest that risk eligible study participants were older than the general female population in the selected counties. The findings are consistent with STAR trial eligibility criteria requiring post-menopausal women over the age of thirty-five.1
The results from the analysis of research question two (Table 3) produced statistically significant findings in the demographic characteristics between women that completed a risk assessment form at the Massachusetts participating sites as compared with those at nationally participating STAR trial locations. However, as women moved through the critical access points for study participation of eligibility and enrollment, no meaningful differences were detected between the demographic characteristics of women considered eligible for STAR trial participation, or those that actually enrolled onto the study, between the selected Massachusetts sites and nationally participating STAR trial locations, as reported by the NSABP. The findings concerning the comparability of study eligible and enrolled subjects are important because these two populations are components of the mailed questionnaire developed to answer the final research question for this study.
Table 3.
Research Question 2 Results
| Massachusetts Participating Study Locations | National STAR Trial Locations* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study Access Point | White | African American | Asian/Native American | Hispanic | White | African American | Asian/Native American | Hispanic | χ2 3 d.f. | p-Value |
| Risk Assessment | 280 | 187 | 22 | 30 | 145,271 | 21,257 | 9,531 | 7,883 | 311.33 | 0.001 |
| Study Eligibility | 215 | 12 | 9 | 6 | 87,794 | 3,268 | 3,229 | 1,835 | 2.39 | 0.50 |
| Study Enrollment | 77 | 1 | 2 | 1 | 18,369 | 487 | 417 | 393 | 0.81 | 0.85 |
National population excludes Massachusetts participating locations.
As a result of subject withdrawal from the STAR trial, data returned for site specific enrolled women resulted in only 70 STAR trial enrolled women available to participate in the proposed study from the original 81. Ten of the original 81 enrolled women were removed from the roster of study enrolled women for survey mailings to insure compliance with the individual’s request of STAR trial withdrawal and no further contact. One woman was removed at the request of the NSABP STAR trial cluster coordinator at Boston Medical Center due to a medical diagnosis of dementia. However, an additional 11 STAR trial eligible women were added to the roster of women for participation in the proposed research project, resulting in 172 eligible but non-enrolled subjects from the original 161. The additional women were not included in the previous tables returned directly to the author from NSABP for Massachusetts level data due to the women residing out of state; therefore, they were not previously credited to Massachusetts STAR trial participating sites.
A total of 111 of the potential 242 subjects participated in the mailed questionnaire to answer the third research question. Of the respondents, 65 out of 172 women in the study group (STAR trial eligible, but not enrolled) completed the questionnaire for study participation. A total of 46 out of 70 women in the control group (STAR trial participants) completed the questionnaire for study participation. As such, an overall participation rate for the mailed questionnaire equated to 45.9% (111 out of 242).
Initial results of the statistical analysis produced significant findings for each of the eight hypotheses tested, with a total of 14 questions, elements of each hypothesis, being significant. A forward step-wise logistic regression model was then conducted to identify which questions retained the highest level of significance. Results of the forward step-wise logistic regression indicated that four questions (Questions 1, 9, 15, and 19) produced statistically significant results, while adjusting for subject demographics and in the presence of the other questions of the hypotheses (Table 4). The results showed that four factors (1) clinician expertise and qualifications (p =.012, OR: 4.90; 95% CI: 1.41 to 17.04 ); (2) personal desire to participate (p =.033, OR: 3.16; 95% CI: 1.10 to 9.06); (3) perceived value of the trial (p =.020, OR: 2.92; 95% CI, 1.18 to 7.21); and, (4) level of trial inconvenience (p =.002, OR: 0.10; 95% CI, 0.02 to 0.44), significantly influenced a woman’s decision to enroll onto a breast cancer prevention clinical trial more than other eligible subjects.
Table 4.
Research Question 3 Step-Wise Regression Results
| p | O.R. | 95 % C.I. for O.R. | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Q1: The expertise and qualifications of the clinician explaining the trial influenced your decision to participate or not participate in the study. | .012 | 4.903 | 1.411 | 17.043 |
| Q9: The likelihood that this clinical trial would improve your chances for breast cancer prevention influenced your decision to participate or not participate in the study. | .033 | 3.158 | 1.100 | 9.062 |
| Q15: The belief that the clinical trial may involve extra inconvenience, such as further travel, or extra visits to the doctor influenced your decision to participate or not participate in the study. | .002 | .095 | .021 | .435 |
| Q19: Do you believe that the benefits of the clinical trial outweigh any side effects experienced by the drugs on the study? | .020 | 2.921 | 1.184 | 7.209 |
Note: Forward step-wise regression analysis adjusted for subject demographics that included self-reported Age, Marital Status, Race, and Education Level
Conclusions
Results of the first two research questions support the comparability and generalizability of the study findings to the general population for eligible breast cancer prevention clinical trial subjects. These findings were supported at the county, state, and national levels. When comparing the demographic characteristics provided from the NSABP it was found that only the results from one county (Worcester County) were not consistent with the null hypothesis that there were no differences in the demographic characteristics of race and ethnicity for women screened and considered eligible for participation in the study at the selected Massachusetts sites in Worcester County as compared with related community demographics within the county.
Age was found to be significantly different between the general population and study subjects at the participating locations in the five counties under investigation. The results indicate that risk eligible study participants were older than the general population in the selected counties. These findings are consistent with the STAR trial eligibility criteria requiring post-menopausal women over the age of thirty-five. The average age of study eligible subjects at the participating sites was over 60 years of age; whereas, based on Census data, the majority of women in the selected counties were between the ages of 35 to 54. Nationally, only 9% of study enrolled women were between the ages of 35–49, with more than 41% over the age of 60.1
Results of the analysis conducted to measure the comparability between the participants at the selected study sites and nationally published data on the demographic characteristics of women at the three critical access points for STAR trial participation (screening, eligibility, and enrollment) showed similarities between the two groups at the eligibility and enrollment access points. However, statistically significant findings were detected in the demographic characteristics between the women that completed a risk assessment form at the Massachusetts participating sites with those at nationally participating STAR trial locations (Table 3). As women moved through the critical access points for study participation, no differences were detected between the demographic characteristics of women considered eligible for STAR trial participation, or those that actually enrolled onto the study, between the selected Massachusetts sites and nationally participating STAR trial locations, as reported by the NSABP.
Results from the mailed questionnaire support the paramount importance of the notion that the patient-clinician interaction, and subsequent relationship, is central in the accrual onto clinical trials. In fact, Albrecht, et al. concluded that patients were more likely to participate in a trial when their clinician actively nurtured the patient-clinician relationship, to include verbally presenting items normally included in an informed consent document and when their clinicians communicated in a reflective, patient-centered, supportive, and responsive manner.19 Discussion of benefits, side effects, patient concerns and resources to manage concerns regarding trial participation were all associated with increased accrual. The expertise and knowledge of the clinical trial that the clinician is able to effectively convey to the patient is a fundamental element of trust and lays the groundwork of the clinician-patient interaction that can ultimately result in clinical trial accrual. As shown in the analysis of Question One of the survey, a woman is 4.9 times more likely to enroll in a breast cancer prevention clinical trial than other eligible subjects if she feels that the clinician has sufficient expertise and knowledge (Expertise).
A second element of the patient-clinician relationship is the clinician’s ability to objectively foster the patient’s belief that the clinical trial would improve their chances for breast cancer prevention. The direct benefits of the trial to improve cancer prevention, when presented by someone viewed as possessing expertise, knowledge, and the interests of the patient first, should have a greater impact on the patient deciding to participate, or not, in the trial. If a patient believes that the trial will improve their chances of preventing cancer they will be more willing, and have a greater desire, to enroll in the trial. In this instance, as in the previous element, the clinician’s expertise on a particular trial’s ability to improve a patient’s chance for cancer prevention stems from the level of trust the patient has in the clinician to act in their best interest. Ford, et al. identified patient mistrust of the health care system as a most often barrier encountered in clinical research.6 As shown through the results of Question Nine of the survey, a woman is 3.2 times more likely to enroll in a breast cancer prevention clinical trial than other eligible subjects if she feels the trial would improve her chances for breast cancer prevention (Desire).
A third component of the patient-clinician relationship is the clinician’s ability to effectively convey the value of the trial to the patient. A patient must recognize, and believe in, the direct value that the trial can offer on their health status. A clinician’s place in this decision element is essential to answer any questions that may arise in the decision-making process, and offer an impartial risk/benefit perspective. The finding of Kinney, et al. strongly suggested that clinician recommendations regarding trial participation influenced the likelihood that a woman will enroll in a breast cancer prevention clinical trial that includes tamoxifen therapeutic elements.20 These findings are consistent with other work conducted in behavioral research investigating the influence of clinicians in a patient’s decision-making process. Clinical trial enrollment will be easier to achieve once the patient believes in the value of the particular study. In fact, results of Question Nineteen of the survey indicate that a woman is 2.9 times more likely to enroll in a breast cancer prevention clinical trial than other eligible subjects if she believes that the benefits of the trial outweigh any side effects experienced by the drugs on the study (Value).
Previous research has shown that trial inconvenience is a widely stated reason for low accrual onto clinical trials. Inconvenience can be the result of trial related requirements that may include such things as increased office visits, greater travel requirements for the study, requirement to maintain daily logs or specific treatment regiments that must be followed. Perceived inconveniences that disrupt daily lives or family responsibilities are very real to the prospective subject that should be considered at the time of study protocol development. One factor of importance for patient accrual in multi-center studies is the simplicity of the study protocol and forms associated with the study. Clinicians refer to inclusion and exclusion guidelines when considering a patient for a particular study. A major reason cited for patients not being included in a study is that they are ineligible, or that the protocol design itself is not appropriate.21 Overcoming perceived trial inconveniences is difficult because the individual clinician is not in a position to change a clinical trial protocol; however, through their relationship with the patient they can work to reduce this barrier. As shown by the results of Question Fifteen of the survey, a woman is 10.5 times less likely to enroll in a breast cancer prevention trial than other eligible subjects if she feels the clinical trial would be too inconvenient (Inconvenience).
The four factors (expertise, desire, value, and inconvenience), and subsequent interactions, identified in this study that significantly influence a woman’s decision to participate or not participate in a breast cancer clinical trial are presented in Figure 2. It is important to recognize that the three factors of expertise, desire, and value are elements of the clinician-patient relationship; whereas, the inconvenience of a trial is an element that originates with the study itself and is an element of the patient-clinical trial interaction.
Figure 2.
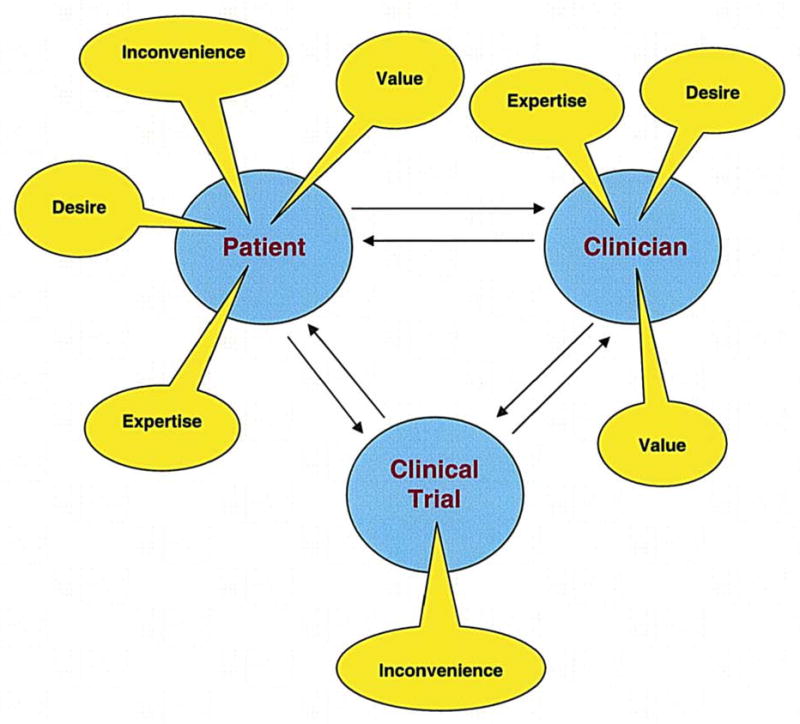
Breast Cancer Prevention Clinical Trial Accrual Barrier Model
We conclude that the results of the study support the importance of the patient-clinician relationship in accrual onto breast cancer prevention clinical trials. Addressing the barriers identified in the relationship between health care providers and patients should help improve accrual onto breast cancer prevention clinical trials. Additionally, a positive patient-clinician relationship can also help in reducing the barrier of trial inconvenience, as identified in this study.
Study Limitations
The emphasis of this study centered on the accrual onto to a specific Phase III breast cancer prevention clinical trial at two participating locations, and cooperating satellite and affiliated organizations, in Massachusetts. Of primary concern is the fact that patients are extremely mobile in seeking care. It is acknowledged that the significant draw of clinical care options that exist for patients to choose from can cloud actual accrual rates reported at the healthcare organizations that are part of this study.
While the study strove for representation of Massachusetts eligible women and comparability with all women eligible to participate in breast cancer prevention clinical trials, sites in other states may have experienced different patterns of accrual. Each of the 516 participating STAR trial sites had significant latitude in the management of trial participation. As such, the patient characteristics of eligible and enrolled patients may have variation depending on the philosophy employed by the site in accruing patients.
The STAR clinical trial began accrual in 1999 and ceased its accrual in 2004. The current research project relied on results for this study based on self-reported reasons for clinical trial participation and non-participation that could produce unintentional errors of omission or misrepresentation by participants due to the time period between initial approach for STAR trial enrollment and the current research study.
The study participants in this research project were all women over the age of 35 and eligible for participation in a breast cancer prevention clinical trial. The results obtained in this study require further research efforts to insure results are generalizable to other patient populations.
Acknowledgments
Sources of Support: None.
The STAR trial was supported by Public Service grants U10-CA-37377, U10-CA-69974, U10-CA-12027, and U10-CA-69651 from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services; AstraZeneca Pharmaceuticals; and, by Eli Lilly & Co.
Footnotes
Financial Disclosures: None
References
- 1. [accessed April 28, 2008];Study of tamoxifen and raloxifene (STAR) trial 2007. Available from URL: http://www.cancer.gov/clinicaltrials/digestpage/STAR/page1.
- 2.American Cancer Society. [accessed October 10, 2009];Cancer facts & figures 2009. Available from URL: http://www.cancer.org/downloads/STT/500809web.pdf.
- 3.Centers for Disease Control & Prevention. [accessed July 27, 2009];Breast cancer statistics: Top 10 causes of death for women in the United States. 2009 Availabe from URL: http://www.cdc.gov/cancer/breast/statistics/
- 4. [accessed September 16, 2008];SEER Stat Fact Sheets: Breast Cancer. NCI, n.d. Available from URL: http://seer.cancer.gov/statfacts/html/breast.html.
- 5.NCI. [accessed January 29, 2008];Cancer clinical trials: The basic workbook. 2002 Available from URL: http://www.cancer.gov/PDF/091e02f3-5bb9-4ba6-a988-9ad35eab6a61/BasicsWorkbook_m.pdf.
- 6.Ford JG, Howerton MW, Bolen S, Gary TL, Lai GY, Tilburt J, et al. Knowledge and access to information on recruitment of underrepresented populations to cancer clinical trials. [accessed November 25, 2007];Evid Rep Technol Assess (Summ) 2005 (122):1–11. doi: 10.1037/e439572005-001. Available from URL: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15989377. [DOI] [PMC free article] [PubMed]
- 7.Mansour EG. Barriers to clinical trials. Part III: Knowledge and attitudes of health care providers. [accessed December 29, 2007];Cancer. 1994 74(9 Suppl):2672–5. doi: 10.1002/1097-0142(19941101)74:9+<2672::aid-cncr2820741815>3.0.co;2-x. Available from URL: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7954284. [DOI] [PubMed]
- 8.Somkin CP, Altschuler A, Ackerson L, Geiger AM, Greene SM, Mouchawar J, et al. Organizational barriers to physician participation in cancer clinical trials. [accessed January 1, 2008];Am J Manag Care. 2005 11(7):413–21. Available from URL: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16044978. [PubMed]
- 9.Swanson GM, Ward AJ. Recruiting minorities into clinical trials: toward a participant-friendly system. [accessed October 10, 2009];J Natl Cancer Inst. 1995 87(23):1747–59. doi: 10.1093/jnci/87.23.1747. Available from URL: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7473831. [DOI] [PubMed]
- 10. [accessed January 20, 2008];Understanding clinical trials. 2007 Available from URL: http://clinicaltrials.gov/ct2/info/understand.
- 11.Gotay CC. Accrual to cancer clinical trials: directions from the research literature. [accessed October 10, 2009];Soc Sci Med. 1991 33(5):569–77. doi: 10.1016/0277-9536(91)90214-w. Available from URL: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1962228. [DOI] [PubMed]
- 12.Friedman MA. Patient accrual to clinical trials. [accessed October 10, 2009];Cancer Treat Rep. 1987 71(6):557–8. Available from URL: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=3581094. [PubMed]
- 13.U.S. Census Bureau. [accessed March 23, 2008 ];Census 2000: Data for the state of Massachusetts. 2003 Available from URL: http://www.census.gov/census2000/states/ma.html.
- 14.U.S. Census Bureau. [accessed January 27, 2008];American Community Survey. 2006 Available from URL: http://factfinder.census.gov/home/saff/main.html?_lang=en.
- 15.Ellis PM, Butow PN, Tattersall MH, Dunn SM, Houssami N. Randomized clinical trials in oncology: understanding and attitudes predict willingness to participate. [accessed May 10, 2008];J Clin Oncol. 2001 19(15):3554–61. doi: 10.1200/JCO.2001.19.15.3554. Available from URL: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11481363. [DOI] [PubMed]
- 16.Simon M. Pilot study of factors that determine whether patients with cancer enter Phase III clinical trials Nursing. MSN Pittsburg: University of Pittsburg; 1995. [Google Scholar]
- 17.Breslow NE, Day NE. Statistical methods in cancer research. Volume I -The analysis of case-control studies. [accessed October 10, 2009];IARC Sci Publ. 1980 (32):5–338. Available from URL: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7216345. [PubMed]
- 18.U.S. Census Bureau. [accessed December 30, 2008];2005–2007 American Community Survey 3-Year Estimates. n.d Available from URL: http://factfinder.census.gov/servlet/DatasetMainPageServlet?_program=ACS&_submenuId=people_10&_lang=en&_ts=
- 19.Albrecht TL, Blanchard C, Ruckdeschel JC, Coovert M, Strongbow R. Strategic physician communication and oncology clinical trials. [accessed January 1, 2008];J Clin Oncol. 1999 17(10):3324–32. doi: 10.1200/JCO.1999.17.10.3324. Available from URL: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10506636. [DOI] [PubMed]
- 20.Kinney AY, Richards C, Vernon SW, Vogel VG. The effect of physician recommendation on enrollment in the Breast Cancer Chemoprevention Trial. [accessed October 21, 2008];Prev Med. 1998 27(5 Pt 1):713–9. doi: 10.1006/pmed.1998.0349. Available from URL: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9808803. [DOI] [PubMed]
- 21.Tournoux C, Katsahian S, Chevret S, Levy V. Factors influencing inclusion of patients with malignancies in clinical trials. [accessed November 29, 2007];Cancer. 2006 106(2):258–70. doi: 10.1002/cncr.21613. Available from URL: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16397866. [DOI] [PubMed]


