Abstract
Speech production is one of the most complex and rapid motor behaviors and involves a precise coordination of over 100 laryngeal, orofacial and respiratory muscles. Yet, we lack a complete understanding of laryngeal motor cortical control during production of speech and other voluntary laryngeal behaviors. In recent years, a number of studies have confirmed the laryngeal motor cortical representation in humans and provided some information about its interactions with other cortical and subcortical regions that are principally involved in vocal motor control of speech production. In this review, we discuss the organization of the peripheral and central laryngeal control based on neuroimaging and electrical stimulation studies in humans and neuroanatomical tracing studies in non-human primates. We hypothesize that the location of the laryngeal motor cortex in the primary motor cortex and its direct connections with the brainstem laryngeal motoneurons in humans, as oppose to its location in the premotor cortex with only indirect connections to the laryngeal motoneurons in non-human primates, may represent one of the major evolutionary developments in humans towards the ability to speak and vocalize voluntarily.
Keywords: motor control, voice, speech, human, non-human primate
Introduction
Voice is essential for human communication. Starting with the first cry at birth, the vocal repertoire develops throughout childhood into unique human speech. The development of the human ability to speak relies on the abilities to listen to speech, comprehend and process the meaning of the heard words, and coordinate laryngeal, respiratory and orofacial muscles to communicate speech sounds. Together with body expressions, we use speech and other vocal gestures to define our needs and thoughts and to project our feelings and emotions.
Research on the mechanism of speech spans the centuries. Commonly, the topic of the brain basis of speech is discussed in relation to speech perception within the auditory cortex and speech production within the inferior frontal gyrus (i.e., Broca's area), while the involvement of the laryngeal motor cortex (LMC) is rarely addressed. Nevertheless, the LMC is imperative for the control of motor coordination of over 100 muscles for voluntary production of voice, swallowing and breathing, all of which represent vital functions for our existence and communication.
In this review, we will first briefly introduce the anatomy and peripheral nervous control of the larynx as an organ for voice production; we will then review the hierarchical organization of voice control from brainstem to the LMC with a special focus on the role of the LMC in the control of voluntary learned voice production and on its interactions with other brain regions associated with speech control. We will present some hypotheses on the LMC evolutionary developments in humans and conclude with a summary and future directions.
Larynx and its peripheral nervous control
The larynx as a structure is phylogenetically much older than its role as a vocal organ. The first vertebrate larynx appeared in the lungfish more than 400 million years ago; however, the first vocalizations appeared much later with the evolution of anurans about 250 million years ago.
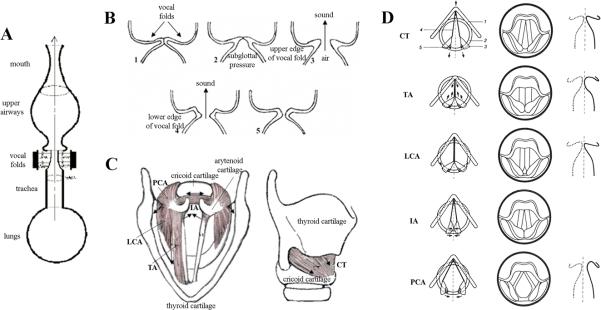
From a phonatory point of view, voice is produced when the expiratory airflow from the lungs sets the closed vocal folds of the larynx into vibration, converting aerodynamic power generated by the thoracic and abdominal muscles (subglottal component) into the basic sound wave (e.g., acoustic power). This sound wave is further filtered and amplified by oral articulators, such as pharynx, tongue, palate, lips, and jaw (supraglottal component), and is emitted from the mouth and nose as sound of voice (Fig. 1A,B).
Figure 1. (A) Schematic view of the vocal and respiratory tracts.
Voice originates in the larynx. First, the expiratory airflow from the lungs reaches the larynx through the trachea, where it sets the closed vocal fold tissue into self-excited oscillatations, due to which the larynx becomes the source of voice sound. Further, pressure from the vocal fold oscillations is resonated through the vocal tract and radiated from the mouth as voice. (B) Schematic sequence of events preceding voice production. (1). The vocal folds close immideatly prior to voice production; (2) subglottal air pressure builds up below the vocal folds during exhalation; (3) lower and upper edge of the vocal folds separate subsequently with the release of air and sound generation; (4) the vocal folds re-approximate, starting from their lower edge, and (5) the vocal folds close completely before the next sound production. (C) Superior and lateral views of the human larynx. Intrinsic laryngeal muscles and cartilages. TA – thyroarytenoid muscle; LCA – lateral cricoarytenoid muscle; PCA – posterior cricoarytenoid muscle; IA – interarytenoid muscle; CT - cricothyroid muscle. The arrows show the directions of the muscle contractions. (D) Schematic presentation of the laryngeal muscle function. The left column shows the location of the cartilages and the edge of the vocal folds when each of the laryngeal muscles is active. The arrows indicate the directions of the force exerted. 1. thyroid cartilage; 2. cricoid cartilage; 3. arytenoid cartilages; 4. vocal ligament; 5. posterior cricoarytenoid ligament. The middle column shows the laryngeal view. The right column shows contours of the frontal section at the middle of the membranous portion of the vocal fold. The dotted line shows a state in which no muscle is activated (reprinted from Hirano, 1981 with permission of Springer Science + Business Media).
Voice onset is required for production of vowels and voiced consonants (e.g., b, d), while voice offset is necessary for production of voiceless consonants (e.g., p, t). During speech production, voice onset is precisely timed, which allows linguistic distinctions between voiced and voiceless consonants, such as /d/ versus /t/. Changes in the subglottal pressure due to changes in lung volume, the elastic properties of the chest wall and the active contraction of the intercostal and abdominal muscles lead to modulations of voice intensity, whereas the resonance characteristics of the supraglottal region (e.g., oral and pharyngeal cavities) influence the spectral properties of the sound.
Vocal fold movements are controlled by intrinsic and extrinsic laryngeal muscles. The intrinsic laryngeal muscles are confined to the larynx and participate in vocal fold closure (thyroarytenoid, TA, lateral cricoarytenoid, LCA, and interarytenoid muscles, IA), opening (posterior cricoarytenoid muscle, PCA), and lengthening (cricothyroid muscle, CT) (Fig. 1C,D). The extrinsic muscles connect the larynx with surrounding structures, such as the hyoid bone, sternum and pharynx, and raise or lower the larynx within the neck relative to the spine to modulate vocal fold length, fundamental frequency, oro-pharyngeal resonance frequencies and formant structure.
All laryngeal muscles, with exception of the IA muscle, receive bilateral motor and sensory innervation from the superior laryngeal (SLN) and recurrent laryngeal (RLN) nerves branching from the vagal nerve. While the internal branch of the SLN and the RLN provides laryngeal sensory innervation, the external motor branch of the SLN innervates the CT muscle; all other intrinsic laryngeal muscles receive their motor innervation from the RLN. The extrinsic muscles are primarily innervated from the ansa cervicalis. The motoneurons of the intrinsic laryngeal muscles are located in the nucleus ambiguus of the brainstem; the motoneurons of extrinsic muscles are situated near the hypoglossal nucleus. Motor and sensory nuclei of the subglottal (respiratory muscles) and supraglottal (oro-facial muscles) components are located in the pons (trigeminal motor nucleus), brainstem (facial nucleus, hypoglossal nucleus, and nucleus ambiguus), and ventral horn of the spinal cord (cervical, thoracic and lumbar regions). Bilateral innervation of almost all laryngeal muscles and, therefore, control of each half of the larynx by both left and right LMC is beneficial in preventing loss of voluntary voice control in patients with unilateral LMC damage, while bilateral LMC lesions render patients unable to speak and sing. On the other hand, damage to the branches of the vagal nerve results in the unilateral paralysis and paresis of the vocal fold (Jurgens, 2002).
Two pathways controlling voice production
Central control of voice production is carried out by two parallel pathways: the limbic vocal control pathway, which is responsible for the control of innate non-verbal and emotional vocalizations, and the laryngeal motor cortical pathway, which regulates the fine motor control of voluntary voice production, such as speech and song, as well as voluntary production of innate vocalizations. These pathways are organized hierarchically, building from the basic levels in the lower brainstem and spinal cord to the most complex levels in the anterior cingulate cortex (ACC) and LMC, respectively (Fig. 2).
Figure 2. Hierarchical organization of central voice control in humans and non-human primates.
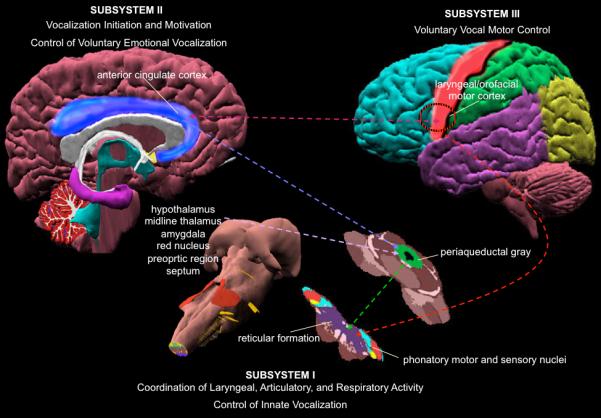
The figure depicts different levels of the voice control system. The lowest level (Subsystem I) is represented by the brainstem and spinal cord sensorimotor phonatory nuclei. This subsystem is responsible for the coordination of laryngeal, articulatory and respiratory control during production of innate vocalizations. The higher level within this system (Subsystem II) is represented by the PAG, ACC and limbic input structures, such as the hypothalamus, midline thalamus, amygdala, red nucleus, preoptic region, septum. This subsystem is responsible for initiation of vocalizations and control of voluntary emotional vocalizations. The highest level is represented by the laryngeal/orofacial motor cortex with its input and output regions (Subsystem III). This subsystem is responsible for voluntary vocal motor control of speech and song production. The dotted lines show simplified connections between different regions within the voice controlling system.
Voice control develops gradually throughout childhood for the control of speech production. As we mentioned earlier, the first vocalization occurs at birth as a shriek of the newborn. This and other types of non-verbal vocalizations in humans, such as an infant's cry and laughter, represent innate vocal reactions whose acoustic structure is genetically preprogrammed (Scheiner et al., 2004). This means that infants do not need to hear the sounds of cry or laughter from others in order to produce them. These basic innate vocalizations are controlled by the sensory and motor nuclei of the lower brainstem (e.g., ambigual, trigeminal, facial, hypoglossal, solitary tract nuclei) and spinal cord (thoracic and lumbar ventral horn), which are responsible for the basic coordination of laryngeal, respiratory and articulatory muscle activity (Fig. 2, subsystem I). The reticular formation of the pons and lower brainstem is another important structure for this type of vocal control. It plays a dual role in establishing connections between different phonatory nuclei and in coordinating basic vocal motor activities. Single-unit recording studies in the squirrel monkey have found that some vocalization-correlated neurons in the reticular formation are active only during specific vocalizations, while other neurons fire during various types of vocalizations or change their discharge rate based on the rhythmic frequency modulations of produced vocalizations (Kirzinger and Jurgens, 1991; Luthe et al., 2000). Furthermore, the reticular formation dorsal to the superior olive contains a vocalization pattern generator, the neuronal activity of which co-occurs with the neuronal firing of the phonatory ambigual, facial and motor trigeminal nuclei (Hage and Jurgens, 2006).
The involvement of the forebrain is not essential for production of innate vocalizations. It has been reported that even anencephalic infants, who lack the entire forebrain but have an intact brainstem, are still able to vocally react to painful stimuli (Monnier and Willi, 1953). However, in older children, innate vocalizations come under voluntary control, sometimes involving mimicking of vocal utterances. For example, cry can be produced in the absence of pain or suppressed in the presence of pain. For this higher level of vocal control, that is, voluntary initiation of innate vocalizations and control of their emotional status, the brainstem phonatory nuclei and the vocal pattern generator require an input from the higher brain regions, such as the periaqueductal gray (PAG) and ACC (Fig. 2, subsystem II). The PAG receives direct projections from the ACC as well as from other cortical and subcortical regions controlling limbic, sensory, motor, cognitive and arousal systems (Dujardin and Jurgens, 2005). The PAG projects to the reticular formation of the lower brainstem, thus representing an neuroanatomical and functional relay station within the ACC-PAG-brainstem pathway. The PAG plays primarily a gating role in triggering a vocal response and modulating its intensity, while the ACC is involved in voluntary control of voice initiation and its emotional intonation. Destruction of the ACC results in a loss of voluntary control of emotional intonations during speaking, while lesions in the PAG lead to mutism. Interestingly, destruction of the ACC does not interfere with the production of vocalizations elicited at the level of the PAG and brainstem reticular formation; however, destruction of the PAG abolishes vocalizations from the ACC but not from the brainstem reticular formation (Jurgens, 2002). Thus, while the ACC and PAG are important in shaping voluntary control of voice initiation and emotional modulation, the reticular formation represents the basic executing level of this pathway.
The highest level within the hierarchy of voice controlling system is represented by the LMC and its input and output structures (Fig. 2, subsystem III). The ability to learn new vocalizations is very limited, perhaps impossible, in non-human primates and other mammals (except whales and dolphins). Their vocalizations are exclusively emotional, with each type of vocalization conveying only one meaning. In humans, in contrast, the development of the ability to produce innumerable learned vocal utterances for speech and song depends on the fine motor control by the LMC. While lesions in this region have almost no effects on monkey vocalizations, they abolish speech production in human patients (Jurgens, 2002). Such patients are occasionally able to initiate phonation, such as grunts, wails and laughs, but do not succeed in voluntary modulations of pitch, intensity and the harmonious quality of their vocalizations. The preservation of non-verbal vocalizations in patients with bilateral damage to the LMC may be due to parallel organization of the LMC and ACC-PAG pathways, one controlling voluntary voice production and the other controlling the initiation of basic vocal reactions.
For proper coordination of learned vocal patterning and voice initiation, the LMC and ACC-PAG pathways converge in at least two regions, as found in neuroanatomical studies in non-human primates. One such region is the ACC itself, which has direct reciprocal connections with the LMC (Simonyan and Jurgens, 2002, 2005a). The other region is the reticular formation of the brainstem, which projects directly to the phonatory motoneurons (Hannig and Jurgens, 2006). Thus, the vocal motor control system seems to be separated into two parallel pathways for learned and innate vocalizations, coordination and interactions of which are indispensible for proper voice control.
Localization of the laryngeal motor cortex
As we stated earlier, the role of the LMC is essential in the control of voluntary laryngeal behaviors, both learned, such as speech and song, and innate, such as production on demand of laughter, coughing, breathing, etc. The ability to control various laryngeal behaviors voluntarily is most prominent in humans, while other species, including non-human primates, have limited ability to produce their vocalizations voluntarily.
First observations of the larynx representation within the motor cortex were made in the 1930s. Shortly after the report by Oscar Foester that bilateral vocal fold movements in humans can be produced with electrical stimulation of the motor strip of one hemisphere (Foester, 1936), Wilder Penfield and colleagues described the representation of vocalization in the inferior portion of the motor cortex above the jaw and below the lip muscles representations (Penfield and Bordley, 1937) (Fig. 3A). Nearly the same somatotopy was observed in the monkey brain with the representation of the larynx region just above the Sylvian fissure (Woolsey et al., 1952) (Fig. 3B). On the other hand, electrical stimulation studies failed to identify the larynx representation in the motor cortex of lower mammals, such as the dog and cat (Milojevic and Hast, 1964).
Figure 3. Laryngeal motor cortical representation.
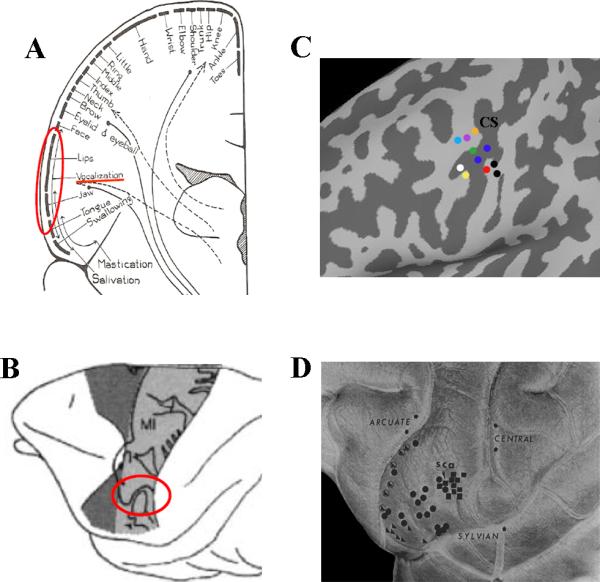
Schematic views of body representation within the motor cortex (A) in humans (“motor homunculus” according to (Penfield and Bordley, 1937)) and (B) in the rhesus monkey (“motor simiculus” according to (Woolsey et al., 1952), reprinted from Fadiga et al., 2000 with permission from Elsevier). (C) The laryngeal motor cortical region in humans as defined in neuroimaging studies. The colored circles represent the reported peaks of activation in the following studies of syllable production: orange - (Bohland and Guenther, 2006); purple - (Olthoff et al., 2008); light blue - (Terumitsu et al., 2006); green - (Loucks et al., 2007); blue - (Brown et al., 2008); red -(Wilson et al., 2004); black - (Simonyan et al., 2009); white - (Riecker et al., 2008), and yellow - (Peeva et al.). CS - central sulcus. (D) The laryngeal motor cortical region in the rhesus monkey. Topographical representation of the laryngeal muscles: cricothyroid - right-angled triangle; thyroarytenoid - circle; combination of the circothyroid and thyroarytenoid - encircled right-angled triangle, and extrinsic laryngeal muscles - square. sca - sulcus subcentralis anterior (subcentral dimple) (reprinted from (Hast et al., 1974) with permission from Elsevier).
Although the implications of the LMC in the control of voice production is significantly different between humans and non-human primates, much of our understanding about the organization of the LMC comes from research in non-human primates, primarily in the rhesus and squirrel monkeys. The LMC in these species is situated between the inferior branch of the arcuate sulcus anteriorly and the subcentral dimple posteriorly (Hast et al., 1974) (Fig. 3D). This region contains small populations of neurons that are selectively active during either conditioned or spontaneous vocalizations (Coude et al., 2009). Among these, the majority of neurons specific for conditioned vocalization discharge before voice onset, while a smaller number of neurons are time-locked with voice onset.
The larynx area is surrounded by the representations of the tongue, lip and masticatory muscles and is considered to be part of the primary motor cortex, although cytoarchitectonically it corresponds to premotor cortex, area 6, in non-human primates (Jurgens, 1974; Simonyan and Jurgens, 2002). Electrical stimulation of this region with simultaneous laryngeal electromyographic (EMG) recordings in the rhesus monkey revealed that the intrinsic and extrinsic laryngeal muscles have separate representations within the LMC, i.e. topographical organization (Hast et al., 1974) (Fig. 3D).
More than half a century later after Foester's and Penfield's seminal observations, studies of the LMC in humans have been recently revived with the advent of non-invasive neuroimaging techniques. Recent investigations in humans have shown that the LMC is located more dorsally from the Sylvian fissure (Khedr and Aref, 2002; Rodel et al., 2004) than originally proposed (Penfield and Bordley, 1937). Similar to non-human primates, the human LMC is organized topographically, although, compared to the monkey's brain, the representation of the laryngeal muscles is reversed, with the CT muscle located more medially than the TA muscle (Rodel et al., 2004). However, in contrast to the monkey, recent studies have substantially revised the location of the LMC in humans. From the earlier works of Penfield and colleagues, it is notable that the motor homunculus identifies a representation of a behavior (vocalization) rather than an organ (larynx) in the inferior portion of the motor cortex (Fig. 3A). As behavior, vocalization requires not only laryngeal muscle activity but also orchestrated activity of the respiratory and orofacial movements. Hence, it is not surprising that, on the map of the motor homunculus, vocalization as behavior, in contrast to the single organ representation, encompasses quite a large region within the motor cortex, overlapping with the lip, jaw and tongue representations, without, however, a specific reference to the larynx. Thus, the location of the human LMC remained largely unknown until recently. Neuroimaging studies using different vocal tasks were finally able to separate and localize the laryngeal region within the primary motor cortex (Brown et al., 2008; Loucks et al., 2007; Rodel et al., 2004; Simonyan et al., 2009; Wilson et al., 2004), predominantly in the area 4p (Fig. 3C).
The differences in the cytoarchitectonic location of the LMC between humans and non-human primates deserve special attention. The representation of the LMC in the primary motor cortex (area 4) in humans, as oppose to its location in the premotor cortex (area 6) in non-human primates, may represent one of the major evolutionary developments in humans towards the ability to speak and vocalize voluntarily. The LMC representation in area 4 of the motor cortex may have enabled the establishment of a unique direct connection between the LMC and laryngeal motoneurons of the brainstem for faster neuronal transmission and direct control over the coordinated activity of complex laryngeal, orofacial and respiratory movements for speech production. In monkeys, this connection is indirect and hence the control of the brainstem laryngeal motoneurons is limited. We hypothesize that3 this neuroanatomical difference may underlie the very limited ability of non-human primates to learn and control their vocalizations voluntarily.
Laryngeal motor cortical networks
Despite the differences in the representation of the larynx area in the motor cortex and in its anatomical and functional distinctions between humans and non-human primates, the latter species still remain a valuable model for studying the neuroanatomical projections of the LMC using invasive tract tracing techniques. Such investigations in non-human primates can be considered complimentary to diffusion tensor tractography (DTT) studies in humans for categorization of directionality of the LMC connections (efferent vs. afferent), which is otherwise impossible to define using only the DTT approach. Hence, in the following, we will review the LMC networks based on studies using both DTT in humans (Simonyan et al., 2009) and neuroanatomical tract tracing in the rhesus and squirrel monkeys (Jurgens, 1976; Simonyan and Jurgens, 2002, 2003, 2005a, b). We will further review the interaction of the LMC with the main cortical and subcortical regions indispensible for voluntary voice production based on neuroimaging studies of voice and speech in humans.
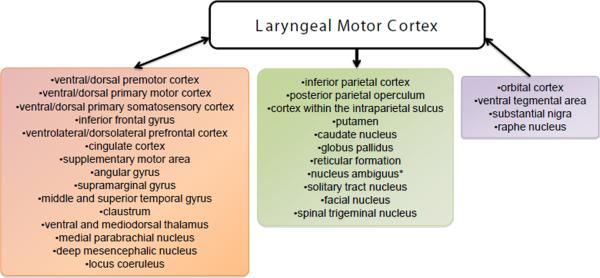
A series of recent investigations have shown that both humans and non-human primates share a common network of extensive cortical and subcortical connections with the LMC (Fig. 4). Nearly all cortical connections of the LMC are reciprocal, that is the LMC both receives and sends information to these regions. The regions reciprocally connected with the LMC are the surrounding ventral and dorsal premotor, primary motor and somatosensory cortices at their orofacial and trunkal representations, inferior frontal gyrus (IFG), ventrolateral and dorsolateral prefrontal cortex, insula, cingulate cortex (CC), supplementary motor area (SMA), angular (AG), supramarginal (SMG), middle (MTG) and superior temporal (STG) gyri, claustrum, ventral and mediodorsal thalamus, medial parabrachial nucleus, deep mesencephalic nucleus and locus coeruleus. The only cortical region projecting to the LMC without receiving an input from it is the orbital cortex, which is assumed to have only a very indirect involvement in vocal control (Price, 1996), as well as subcortically the ventral tegmental area, substantia nigra and raphe nucleus. On the other hand, the cortical regions that receive projections from the LMC but do not send connections back are the inferior parietal cortex, posterior parietal operculum and cortex within the intraparietal sulcus, as well as subcortically the putamen, caudate nucleus, globus pallidus and brainstem nuclei (i.e., reticular formation and spinal trigeminal, solitary tract and facial nuclei).
Figure 4. Cortical and subcortical networks of the laryngeal motor cortex.
Block diagrams illustrate the reciprocal (pink box), outgoing (green box) and incoming (purple box) connections of the laryngeal motor cortex as defined using neuroanatomical tracing studies in non-human primates and diffusion tensor tractography in humans. Asterisk (*) indicates that projection from the laryngeal motor cortex to the nucleus ambiguus exists only in humans but not in non-human primates.
Functionally, the LMC is connected with most of these structures to fulfill its main task - the voluntary control of voice production. Interconnections of the LMC with the surrounding somatosensory cortex and inferior parietal cortex are important for integration of proprioceptive and tactile feedback from the orofacial, respiratory and laryngeal regions during voice production. Neuroimaging studies of overt and covert voice and speech production have consistently reported activation of the primary sensorimotor cortex (e.g., (Bohland and Guenther, 2006; Horwitz et al., 2003; Loucks et al., 2007; Riecker et al., 2000). Activation in this region is organized somatotopically with the orofacial and laryngeal activation occupying the ventral portion of the sensorimotor cortex and the respiratory activation represented as two discrete loci in the dorsal region of the trunk representation and in the ventral region overlapping with the laryngeal and orofacial activation (Brown et al., 2009; Loucks et al., 2007; Simonyan et al., 2007). Interestingly, only voluntary exhalation, which is required for voice production, but not inhalation elicits activation of both dorsal and ventral sensorimotor cortex (Ramsay et al., 1993). The orchestration of simultaneous laryngeal, orofacial and respiratory muscle activity at the cortical sensorimotor level becomes apparent when one of its components is voluntarily modulated or altered. A recent study of functional LMC networks during syllable production showed that when subjects were asked to produce voice with minimal orofacial movements and at the similar breathing depth, that is the modulation of two out of three components of voice production system, the LMC displayed reduced functional connectivity with both orofacial and respiratory sensorimotor cortices to maintain specific level of task production (Simonyan et al., 2009). The influences of the somatosensory cortex on the motor cortex become even more apparent when the auditory feedback, another online monitoring system of correct voice and speech production, is altered and the encoding of auditory input is challenged, for example, in the presence of very loud noise (Guenther, 2006; Peschke et al., 2009). These observations suggest that the ventral sensorimotor region contains not only a simple ensemble of motor and sensory components of the voice controlling system (e.g., laryngeal, orofacial and respiratory), but it also acts as a higher-level centralized region, coordinating exhalatory airflow necessary for setting the vocal folds into vibration and modulating the orofacial articulators for voluntary production of different types of vocal behaviors.
With respect to the inferior parietal cortex, including the SMG and AG, this region represents one of a few higher-order sensorimotor centers for coordination of both speech production and comprehension. The inferior parietal cortex is known to be involved in complex phonological and semantic processing and monitoring of the verbal response to suppress phonemic errors (e.g., (Fiebach et al., 2007; Hocking et al., 2009; Zheng et al., 2010)).
The LMC requires an input from the IFG for motor planning of voice and speech production. The IFG is another brain region where the control of speech production and comprehension converge. Its pars orbitalis is involved in the retrieval of semantic information (de Zubicaray and McMahon, 2009; Tyler et al., 2010) and its pars opercularis is responsible for hierarchical sequencing of linguistic and non-linguistic sequences (Tettamanti et al., 2009; Willems et al., 2009) and articulatory preparation to speech production (Papoutsi et al., 2009; Zheng et al., 2010). Because the IFG is prominent in the processing of information necessary for long-term preparation of learned oro-motor sequences, it is not surprising that the IFG is usually active during production of long sequences of syllables and words (e.g., (Horwitz et al., 2003; Ozdemir et al., 2006; Wise et al., 1999) and only rarely during production of single syllables (Brown et al., 2008; Ghosh et al., 2008; Loucks et al., 2007). Despite the fact that the IFG activation is not always observed in studies of differential complexity of voice and speech production, the strong functional and structural connectivity between the IFG and LMC seems to be responsible for motor preparation and processing of all components of speech production (e.g., laryngeal, orofacial, or respiratory) (Greenlee et al., 2004; Simonyan et al., 2009).
The reciprocal connections of the LMC with the SMA are needed for preparation for vocal motor command execution. Accordingly, it has been shown that the offset of the late vocal-related cortical potentials in the SMA precedes those in the LMC pointing to the involvement of the LMC only after the motor preparatory phase in the SMA (Galgano and Froud, 2008). The SMA is active during various voluntary laryngeal tasks (e.g., (Ghosh et al., 2008; Loucks et al., 2007; Ozdemir et al., 2006; Simonyan et al., 2007). More specifically, it has been shown that activation in the anterior pre-SMA was related to effortful word selection, activation in the posterior pre-SMA was related to the control of syllable sequencing, and activation in the SMA proper was related to overt articulation (Alario et al., 2006). Recently, the SMA has also been identified as a motor component of the speech monitoring network (van de Ven et al., 2009). Furthermore, electrical stimulation of this region has been reported to elicit vocalizations in humans (Penfield and Welch, 1951) but not in monkeys (Jurgens, 2002). Similarly, bilateral lesions in the SMA have no effect on monkey vocalizations. In contrast, in humans, such lesions severely reduce motivation to employ propositional speech, whereas nonpropositional (automatic) speech remains almost intact (so-called transcortical motor aphasia). Functional connections between the LMC and SMA are left-lateralized and are particularly stronger with the pre-SMA during learned voice production compared to innate laryngeal behavior, such as breathing (Simonyan et al., 2009). It, thus, appears that the connectivity between the LMC and the pre-SMA, a region responsible for processing of higher order motor plans for subsequently ordered movement execution (Matsuzaka et al., 1992; Shima and Tanji, 1998; Tanji and Shima, 1996), plays a central role in sequencing and initiation of complex learned vocal movements during speech production.
It is important to note that the LMC has a reciprocal structural connection with the ACC but not with the PAG as identified based on neuroanatomical tract tracing studies in non-human primates. The lack of connections between the LMC and PAG fits well with the separation between the two descending pathways controlling voice production, limbic and motor cortical. Similarly, in the monkey, the PAG blockade has no effect on vocal fold movements elicited from the LMC as oppose to the drastic effects on vocalization elicited by limbic structures (Jurgens, 2002). The LMC, thus, seems to control the voluntary voice production via a pathway by-passing the PAG, although it establishes connections with the ACC for shaping emotional intonations during speech production. As noted earlier, the connection between the LMC and ACC serves as one of the two links at which two pathways controlling innate emotional and learned vocalizations converge.
Another link between the limbic and motor cortical vocal pathways is present at the level of the brainstem reticular formation, specifically in its dorsal and parvocellular reticular nuclei, which are further directly connected with laryngeal motoneurons in the nucleus ambiguus, the articulatory motoneurons in the trigeminal motor, facial and hypoglossal nuclei, and the expiratory motoneurons in the thoracic and upper lumbar spinal cord (Bernard et al., 1990; Thoms and Jurgens, 1987; VanderHorst et al., 2001). Combined electrical stimulation and lesioning studies have shown that bilateral lesions in this region block vocalizations elicited from the PAG and vocal fold movements elicited from the LMC (Jurgens and Ehrenreich, 2007; Shiba et al., 1997). Because both limbic and motor cortical pathways come together in the reticular formation of the brainstem, it has been suggested that this region is involved in vocal motor coordination of both innate and learned voice production (Jurgens and Ehrenreich, 2007). Its functional properties are more important in vocal motor control in non-human primates than in humans due to the lack of direct projections from the LMC to the nucleus ambiguus in the former species and, therefore, their reduced ability to directly modulate activity of brainstem laryngeal motoneurons (Jurgens, 1976; Simonyan and Jurgens, 2003). In humans, in contrast, direct connections do exist between the LMC and the nucleus ambiguus (Iwatsubo et al., 1990; Kuypers, 1958). This direct LMC-ambigual connection together with the LMC representation in the primary motor cortex seem to be very recent acquisitions in hominid evolution and may be regarded crucial as prerequisites for speech control.
In addition to direct LMC-brainstem laryngeal motoneurons connections, the LMC establishes a widespread network of subcortical connections, most of which project further down to the brainstem phonatory motoneurons. The putamen receives the strongest of all telencephalic subcortical projections from the LMC, thus representing the main basal ganglia output structure of the LMC. Putaminal lesions cause dysarthria and dysphonia in humans but have no effect on monkey's vocalizations (Jurgens, 2002), suggesting the involvement of the putamen only in learned voluntary voice and speech production but not in production of innate vocalizations. Motor cortical connections to the putamen are somatotopically organized with the leg area projecting to the rostrodorsal putamen, the arm and trunk occupying its central part, the face area projections to the caudoventral putamen, and the larynx area connecting with the ventral putamen over a large anterio-posterior extent (Kunzle, 1975; Simonyan and Jurgens, 2003). It appears that there is an overlap of projections from the face and larynx areas in the posterior (postcommissural) putamen (i.e., part of the sensorimotor functional striatal loop), whereas the putamen rostral to the anterior commissure (i.e., part of the associative and limbic striatal loops) receives input only from the LMC. Direct connections of the LMC with all striatal functional subdivisions, including the sensorimotor, associative and limbic loops, may represent important factors in the integrative control of different aspects of speech production, ranging from motor control to motivation and cognitive processing of speech.
Another subcortical region that is directly connected with the LMC is the thalamus, specifically its ventral lateral, ventral posterior, medial, and centromedian groups of nuclei. Single-unit recordings in the ventral thalamus have shown voice and speech-related neuronal activity (Farley, 1997; McClean et al., 1990). Furthermore, the ventral thalamus has reciprocal connections with the LMC, IFG and SMA, electrical stimulation of which also produces vocalization in humans (Penfield and Roberts, 1959). Besides the direct role of the ventral thalamus in vocal sensorimotor coordination of learned voice production, it represents an important relay station of the cortico-striato-pallido-thalamo-cortical and cortico-ponto-cerebello-thalamo-cortical loops for integration of sensorimotor information from the basal ganglia and cerebellum. Both pathways, finally, connect the LMC with the laryngeal motoneurons after synapsing in the reticular formation of the brainstem.
In conclusion, the central control of human voice production is organized hierarchically. As the highest level of this control system, the human LMC is indispensible for the control of learned but not innate vocalizations. To fulfill its function, the human LMC has direct connections with the brainstem laryngeal motoneurons, as well as participation in a widespread network of cortical and subcortical connections, which further indirectly connect the LMC with laryngeal motoneurons. Thus, voluntary voice production is controlled by the LMC and is executed through the multiple pathways, both directly and indirectly descending to the brainstem laryngeal motoneurons. This is supported also by the fact that a combined interruption of the direct and indirect LMC-motoneuronal pathways abolishes the ability to produce voice and speech voluntarily, similar to bilateral lesions in the LMC alone (Bauer et al., 1980; Urban et al., 1996).
Future Directions
Although over the past few years we have seen advances in understanding of the LMC's role in human voice and speech production, there are still a number of unanswered questions about its structural and functional organization and interactions with other brain regions in both healthy humans and patients with neurological voice and speech disorders. In particular, further research is needed both in humans and non-human primates for characterization of sub-components of the LMC networks. To date, the LMC projections have been identified using trans-synaptic tracers, such as 3H-leucine, biotin dextran amine, and wheat germ agglutinin conjugated with horseradish peroxidase, in non-human primates and using unconstrained probabilistic DTT in healthy humans. Both techniques provide information about the projecting regions to and from the LMC but lack information about whether the given connection between the LMC and the target region is direct or indirect via other relay stations. To derive a more complete characterization of the LMC structural networks, neuroanatomical studies using tracers that are transported in a time-dependent manner to label synaptically-connected neuronal chains (Kelly and Strick, 2000) should be conducted in non-human primates and further verified in healthy humans using multi-modal neuroimaging techniques.
Future research should also focus on the organization of functional LMC networks during different components of laryngeal behaviors and on interactions between limbic and motor cortical parallel pathways. Similarly, given that speech production, as one of the most rapid human motor actions, has a strong temporal dimension, the temporal characteristics of the LMC activity remain to be explored. The significance of these studies is paramount for future investigations of altered structural and functional links within the central voice control system in patients with neurological voice and speech disorders.
Another unknown aspect is the laryngeal representation in the primary somatosensory cortex and its interactions with the LMC. The earlier studies by Penfield and colleagues lacked the mapping of the larynx representation in the primary somatosensory cortex. Since then, no attempts have been reported to identify the laryngeal somatosensory region, although it is known that lesions in the inferior postcentral gyrus cause dysarthria due to the interruptions of kinesthetic and proprioceptive feedback from the phonatory organs (Luria, 1964).
Finally, it is unknown, to date, how different neurotransmitters (e.g., dopamine, GABA) influence and modulate the human LMC networks during voice and speech production. This information will be crucial in identifying the target brain regions for the development of new neuropharmacological options to modulate the LMC activity in patients with neurological voice problems.
Acknowledgments
This work was supported by the Extramural and Intramural Programs of the National Institute on Deafness and Other Communication Disorders, National Institutes of Health (R00DC009620 to K.S.).
References
- Alario FX, Chainay H, Lehericy S, Cohen L. The role of the supplementary motor area (SMA) in word production. Brain Res. 2006;1076:129–143. doi: 10.1016/j.brainres.2005.11.104. [DOI] [PubMed] [Google Scholar]
- Bauer G, Gerstenbrand F, Hengl W. Involuntary motor phenomena in the locked-in syndrome. J Neurol. 1980;223:191–198. doi: 10.1007/BF00313183. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Villanueva L, Carroue J, Le Bars D. Efferent projections from the subnucleus reticularis dorsalis (SRD): a Phaseolus vulgaris leucoagglutinin study in the rat. Neurosci Lett. 1990;116:257–262. doi: 10.1016/0304-3940(90)90083-l. [DOI] [PubMed] [Google Scholar]
- Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. Neuroimage. 2006;32:821–841. doi: 10.1016/j.neuroimage.2006.04.173. [DOI] [PubMed] [Google Scholar]
- Brown S, Laird AR, Pfordresher PQ, Thelen SM, Turkeltaub P, Liotti M. The somatotopy of speech: phonation and articulation in the human motor cortex. Brain Cogn. 2009;70:31–41. doi: 10.1016/j.bandc.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Ngan E, Liotti M. A larynx area in the human motor cortex. Cereb Cortex. 2008;18:837–845. doi: 10.1093/cercor/bhm131. [DOI] [PubMed] [Google Scholar]
- Coude G, Ferrari P, Roda F, Maranesi M, Rozzi S, Fogassi L. Ventral premotor cortex of the macaque monkey controls conditioned vocalization. Society for Neuroscience; Chicago, IL: 2009. [Google Scholar]
- de Zubicaray GI, McMahon KL. Auditory context effects in picture naming investigated with event-related fMRI. Cogn Affect Behav Neurosci. 2009;9:260–269. doi: 10.3758/CABN.9.3.260. [DOI] [PubMed] [Google Scholar]
- Dujardin E, Jurgens U. Afferents of vocalization-controlling periaqueductal regions in the squirrel monkey. Brain Res. 2005;1034:114–131. doi: 10.1016/j.brainres.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Farley GR. Neural firing in ventrolateral thalamic nucleus during conditioned vocal behavior in cats. Exp Brain Res. 1997;115:493–506. doi: 10.1007/pl00005719. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Friederici AD, Smith EE, Swinney D. Lateral inferotemporal cortex maintains conceptual-semantic representations in verbal working memory. J Cogn Neurosci. 2007;19:2035–2049. doi: 10.1162/jocn.2007.19.12.2035. [DOI] [PubMed] [Google Scholar]
- Foester O. Motorische Felder und Bahnen. Springer; Berlin: 1936. [Google Scholar]
- Galgano J, Froud K. Evidence of the voice-related cortical potential: an electroencephalographic study. Neuroimage. 2008;41:1313–1323. doi: 10.1016/j.neuroimage.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Ghosh SS, Tourville JA, Guenther FH. A neuroimaging study of premotor lateralization and cerebellar involvement in the production of phonemes and syllables. J Speech Lang Hear Res. 2008;51:1183–1202. doi: 10.1044/1092-4388(2008/07-0119). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee JD, Oya H, Kawasaki H, Volkov IO, Kaufman OP, Kovach C, Howard MA, Brugge JF. A functional connection between inferior frontal gyrus and orofacial motor cortex in human. J Neurophysiol. 2004;92:1153–1164. doi: 10.1152/jn.00609.2003. [DOI] [PubMed] [Google Scholar]
- Guenther FH. Cortical interactions underlying the production of speech sounds. J Commun Disord. 2006;39:350–365. doi: 10.1016/j.jcomdis.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Hage SR, Jurgens U. On the role of the pontine brainstem in vocal pattern generation: a telemetric single-unit recording study in the squirrel monkey. J Neurosci. 2006;26:7105–7115. doi: 10.1523/JNEUROSCI.1024-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannig S, Jurgens U. Projections of the ventrolateral pontine vocalization area in the squirrel monkey. Exp Brain Res. 2006;169:92–105. doi: 10.1007/s00221-005-0128-5. [DOI] [PubMed] [Google Scholar]
- Hast MH, Fischer JM, Wetzel AB, Thompson VE. Cortical motor representation of the laryngeal muscles in Macaca mulatta. Brain Res. 1974;73:229–240. doi: 10.1016/0006-8993(74)91046-4. [DOI] [PubMed] [Google Scholar]
- Hocking J, McMahon KL, de Zubicaray GI. Semantic context and visual feature effects in object naming: an fMRI study using arterial spin labeling. J Cogn Neurosci. 2009;21:1571–1583. doi: 10.1162/jocn.2009.21114. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Amunts K, Bhattacharyya R, Patkin D, Jeffries K, Zilles K, Braun AR. Activation of Broca's area during the production of spoken and signed language: a combined cytoarchitectonic mapping and PET analysis. Neuropsychologia. 2003;41:1868–1876. doi: 10.1016/s0028-3932(03)00125-8. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T, Kuzuhara S, Kanemitsu A, Shimada H, Toyokura Y. Corticofugal projections to the motor nuclei of the brainstem and spinal cord in humans. Neurology. 1990;40:309–312. doi: 10.1212/wnl.40.2.309. [DOI] [PubMed] [Google Scholar]
- Jurgens U. On the elicitability of vocalization from the cortical larynx area. Brain Res. 1974;81:564–566. doi: 10.1016/0006-8993(74)90853-1. [DOI] [PubMed] [Google Scholar]
- Jurgens U. Projections from the cortical larynx area in the squirrel monkey. Exp Brain Res. 1976;25:401–411. doi: 10.1007/BF00241730. [DOI] [PubMed] [Google Scholar]
- Jurgens U. Neural pathways underlying vocal control. Neurosci Biobehav Rev. 2002;26:235–258. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Jurgens U, Ehrenreich L. The descending motorcortical pathway to the laryngeal motoneurons in the squirrel monkey. Brain Res. 2007;1148:90–95. doi: 10.1016/j.brainres.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Rabies as a transneuronal tracer of circuits in the central nervous system. J Neurosci Methods. 2000;103:63–71. doi: 10.1016/s0165-0270(00)00296-x. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Aref EE. Electrophysiological study of vocal-fold mobility disorders using a magnetic stimulator. Eur J Neurol. 2002;9:259–267. doi: 10.1046/j.1468-1331.2002.00394.x. [DOI] [PubMed] [Google Scholar]
- Kirzinger A, Jurgens U. Vocalization-correlated single-unit activity in the brain stem of the squirrel monkey. Exp Brain Res. 1991;84:545–560. doi: 10.1007/BF00230967. [DOI] [PubMed] [Google Scholar]
- Kunzle H. Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in Macaca fascicularis. Brain Res. 1975;88:195–209. doi: 10.1016/0006-8993(75)90384-4. [DOI] [PubMed] [Google Scholar]
- Kuypers HG. Corticobular connexions to the pons and lower brain-stem in man: an anatomical study. Brain. 1958;81:364–388. doi: 10.1093/brain/81.3.364. [DOI] [PubMed] [Google Scholar]
- Loucks TM, Poletto CJ, Simonyan K, Reynolds CL, Ludlow CL. Human brain activation during phonation and exhalation: common volitional control for two upper airway functions. Neuroimage. 2007;36:131–143. doi: 10.1016/j.neuroimage.2007.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria AR. Factors and forms of aphasia. In: de Reuck AVS, O'Connor M, editors. Disorders of language. Churchill; London: 1964. pp. 143–167. [Google Scholar]
- Luthe L, Hausler U, Jurgens U. Neuronal activity in the medulla oblongata during vocalization. A single-unit recording study in the squirrel monkey. Behav Brain Res. 2000;116:197–210. doi: 10.1016/s0166-4328(00)00272-2. [DOI] [PubMed] [Google Scholar]
- Matsuzaka Y, Aizawa H, Tanji J. A motor area rostral to the supplementary motor area (presupplementary motor area) in the monkey: neuronal activity during a learned motor task. J Neurophysiol. 1992;68:653–662. doi: 10.1152/jn.1992.68.3.653. [DOI] [PubMed] [Google Scholar]
- McClean MD, Dostrovsky JO, Lee L, Tasker RR. Somatosensory neurons in human thalamus respond to speech-induced orofacial movements. Brain Res. 1990;513:343–347. doi: 10.1016/0006-8993(90)90479-u. [DOI] [PubMed] [Google Scholar]
- Milojevic B, Hast MH. Cortical Motor Centers of the Laryngeal Muscles in the Cat and Dog. Ann Otol Rhinol Laryngol. 1964;73:979–988. doi: 10.1177/000348946407300411. [DOI] [PubMed] [Google Scholar]
- Monnier M, Willi H. [The integrative activity of the nervous system of a mesorhombencephalic anencephalus. II. Anatomical part.]. Monatsschr Psychiatr Neurol. 1953;126:259–273. [PubMed] [Google Scholar]
- Olthoff A, Baudewig J, Kruse E, Dechent P. Cortical sensorimotor control in vocalization: a functional magnetic resonance imaging study. Laryngoscope. 2008;118:2091–2096. doi: 10.1097/MLG.0b013e31817fd40f. [DOI] [PubMed] [Google Scholar]
- Ozdemir E, Norton A, Schlaug G. Shared and distinct neural correlates of singing and speaking. Neuroimage. 2006;33:628–635. doi: 10.1016/j.neuroimage.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Papoutsi M, de Zwart JA, Jansma JM, Pickering MJ, Bednar JA, Horwitz B. From phonemes to articulatory codes: an fMRI study of the role of Broca's area in speech production. Cereb Cortex. 2009;19:2156–2165. doi: 10.1093/cercor/bhn239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeva MG, Guenther FH, Tourville JA, Nieto-Castanon A, Anton JL, Nazarian B, Alario FX. Distinct representations of phonemes, syllables, and supra-syllabic sequences in the speech production network. Neuroimage. 50:626–638. doi: 10.1016/j.neuroimage.2009.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, Bordley E. Somatic motor and sensory representation in the cortex as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- Penfield W, Roberts L. Speech and brain mechanisms. Princeton University Press; Princeton: 1959. [Google Scholar]
- Penfield W, Welch K. The supplementary motor area of the cerebral cortex; a clinical and experimental study. AMA Arch Neurol Psychiatry. 1951;66:289–317. doi: 10.1001/archneurpsyc.1951.02320090038004. [DOI] [PubMed] [Google Scholar]
- Peschke C, Ziegler W, Kappes J, Baumgaertner A. Auditory-motor integration during fast repetition: the neuronal correlates of shadowing. Neuroimage. 2009;47:392–402. doi: 10.1016/j.neuroimage.2009.03.061. [DOI] [PubMed] [Google Scholar]
- Price JL. Vocalization and the orbital and medial prefrontal cortex. In: Davis PJ, Fletcher NH, editors. Vocal Fold Physiology: Controlling Complexity and Chaos. Singular Publishing Group; San Diego: 1996. pp. 171–185. [Google Scholar]
- Ramsay SC, Adams L, Murphy K, Corfield DR, Grootoonk S, Bailey DL, Frackowiak RS, Guz A. Regional cerebral blood flow during volitional expiration in man: a comparison with volitional inspiration. J Physiol. 1993;461:85–101. doi: 10.1113/jphysiol.1993.sp019503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecker A, Ackermann H, Wildgruber D, Dogil G, Grodd W. Opposite hemispheric lateralization effects during speaking and singing at motor cortex, insula and cerebellum. Neuroreport. 2000;11:1997–2000. doi: 10.1097/00001756-200006260-00038. [DOI] [PubMed] [Google Scholar]
- Riecker A, Brendel B, Ziegler W, Erb M, Ackermann H. The influence of syllable onset complexity and syllable frequency on speech motor control. Brain Lang. 2008;107:102–113. doi: 10.1016/j.bandl.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Rodel RM, Olthoff A, Tergau F, Simonyan K, Kraemer D, Markus H, Kruse E. Human cortical motor representation of the larynx as assessed by transcranial magnetic stimulation (TMS). Laryngoscope. 2004;114:918–922. doi: 10.1097/00005537-200405000-00026. [DOI] [PubMed] [Google Scholar]
- Scheiner E, Hammerschmidt K, Jurgens U, Zwirner P. The influence of hearing impairment on preverbal emotional vocalizations of infants. Folia Phoniatr Logop. 2004;56:27–40. doi: 10.1159/000075326. [DOI] [PubMed] [Google Scholar]
- Shiba K, Umezaki T, Zheng Y, Miller AD. The nucleus retroambigualis controls laryngeal muscle activity during vocalization in the cat. Exp Brain Res. 1997;115:513–519. doi: 10.1007/pl00005721. [DOI] [PubMed] [Google Scholar]
- Shima K, Tanji J. Both supplementary and presupplementary motor areas are crucial for the temporal organization of multiple movements. J Neurophysiol. 1998;80:3247–3260. doi: 10.1152/jn.1998.80.6.3247. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Jurgens U. Cortico-cortical projections of the motorcortical larynx area in the rhesus monkey. Brain Res. 2002;949:23–31. doi: 10.1016/s0006-8993(02)02960-8. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Jurgens U. Efferent subcortical projections of the laryngeal motorcortex in the rhesus monkey. Brain Res. 2003;974:43–59. doi: 10.1016/s0006-8993(03)02548-4. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Jurgens U. Afferent cortical connections of the motor cortical larynx area in the rhesus monkey. Neuroscience. 2005a;130:133–149. doi: 10.1016/j.neuroscience.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Jurgens U. Afferent subcortical connections into the motor cortical larynx area in the rhesus monkey. Neuroscience. 2005b;130:119–131. doi: 10.1016/j.neuroscience.2004.06.071. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Ostuni J, Ludlow CL, Horwitz B. Functional but not structural networks of the human laryngeal motor cortex show left hemispheric lateralization during syllable but not breathing production. J Neurosci. 2009;29:14912–14923. doi: 10.1523/JNEUROSCI.4897-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Saad ZS, Loucks TM, Poletto CJ, Ludlow CL. Functional neuroanatomy of human voluntary cough and sniff production. Neuroimage. 2007;37:401–409. doi: 10.1016/j.neuroimage.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji J, Shima K. Supplementary motor cortex in organization of movement. Eur Neurol 36 Suppl. 1996;1:13–19. doi: 10.1159/000118878. [DOI] [PubMed] [Google Scholar]
- Terumitsu M, Fujii Y, Suzuki K, Kwee IL, Nakada T. Human primary motor cortex shows hemispheric specialization for speech. Neuroreport. 2006;17:1091–1095. doi: 10.1097/01.wnr.0000224778.97399.c4. [DOI] [PubMed] [Google Scholar]
- Tettamanti M, Rotondi I, Perani D, Scotti G, Fazio F, Cappa SF, Moro A. Syntax without language: neurobiological evidence for cross-domain syntactic computations. Cortex. 2009;45:825–838. doi: 10.1016/j.cortex.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Thoms G, Jurgens U. Common input of the cranial motor nuclei involved in phonation in squirrel monkey. Exp Neurol. 1987;95:85–99. doi: 10.1016/0014-4886(87)90009-4. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Shafto MA, Randall B, Wright P, Marslen-Wilson WD, Stamatakis EA. Preserving syntactic processing across the adult life span: the modulation of the frontotemporal language system in the context of age-related atrophy. Cereb Cortex. 2010;20:352–364. doi: 10.1093/cercor/bhp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban PP, Hopf HC, Connemann B, Hundemer HP, Koehler J. The course of cortico-hypoglossal projections in the human brainstem. Functional testing using transcranial magnetic stimulation. Brain. 1996;119(Pt 3):1031–1038. doi: 10.1093/brain/119.3.1031. [DOI] [PubMed] [Google Scholar]
- van de Ven V, Esposito F, Christoffels IK. Neural network of speech monitoring overlaps with overt speech production and comprehension networks: a sequential spatial and temporal ICA study. Neuroimage. 2009;47:1982–1991. doi: 10.1016/j.neuroimage.2009.05.057. [DOI] [PubMed] [Google Scholar]
- VanderHorst VG, Terasawa E, Ralston HJ., 3rd Monosynaptic projections from the nucleus retroambiguus region to laryngeal motoneurons in the rhesus monkey. Neuroscience. 2001;107:117–125. doi: 10.1016/s0306-4522(01)00343-8. [DOI] [PubMed] [Google Scholar]
- Willems RM, Ozyurek A, Hagoort P. Differential roles for left inferior frontal and superior temporal cortex in multimodal integration of action and language. Neuroimage. 2009;47:1992–2004. doi: 10.1016/j.neuroimage.2009.05.066. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Saygin AP, Sereno MI, Iacoboni M. Listening to speech activates motor areas involved in speech production. Nat Neurosci. 2004;7:701–702. doi: 10.1038/nn1263. [DOI] [PubMed] [Google Scholar]
- Wise RJ, Greene J, Buchel C, Scott SK. Brain regions involved in articulation. Lancet. 1999;353:1057–1061. doi: 10.1016/s0140-6736(98)07491-1. [DOI] [PubMed] [Google Scholar]
- Woolsey CN, Settlage PH, Meyer DR, Sencer W, Pinto Hamuy T, Travis AM. Patterns of localization in precentral and “supplementary” motor areas and their relation to the concept of a premotor area. Res Publ Assoc Res Nerv Ment Dis. 1952;30:238–264. [PubMed] [Google Scholar]
- Zheng ZZ, Munhall KG, Johnsrude IS. Functional overlap between regions involved in speech perception and in monitoring one's own voice during speech production. J Cogn Neurosci. 2010;22:1770–1781. doi: 10.1162/jocn.2009.21324. [DOI] [PMC free article] [PubMed] [Google Scholar]