INTRODUCTION
Ureaplasma spp. are members of the Mollicutes class that colonize human mucosal surfaces of the respiratory and urogenital tracts 1. Ureaplasma has 14 known serovars and is separated into two new species; U. parvum (serovars 1, 3, 6, and 14) and U. urealyticum (serovars 2, 4, 5, and 7–13) 2. The characteristics of all serovars include lack of cell walls, limited biosynthetic abilities, small genome size, hydrolysis of urea to generate ATP, and mucosal association in the human host 1. Ureaplasma spp. can be found as commensals in the genital tract of 40–80% of sexually mature asymptomatic women and, hence, they have been considered of low virulence 1, 3. However, the Ureaplasma spp. are associated with multiple pregnancy complications including chorioamnionitis, stillbirth, preterm delivery, neonatal morbidity, and perinatal death 1, 3. They are the most common perinatally acquired pathogens in preterm infants 3, 4. Vertical transmission from mothers to their infants occurs either in utero or during delivery.
Ureaplasma respiratory tract colonization has been associated with respiratory distress syndrome 5, persistent pulmonary hypertension 6, neonatal pneumonia 7, and the development of bronchopulmonary dysplasia (BPD) 1, 8, 9. Two meta-analyses confirmed a significant association between Ureaplasma respiratory colonization and the development of BPD whether defined as oxygen-dependence at 28 d or at 36 weeks post-menstrual age (PMA) 8, 9. It has been suggested that infants with a pattern of persistent respiratory colonization/infection are more likely to develop BPD than infants with transient colonization 10. Due to recent improvements in perinatal care, BPD has become a disease limited to the most immature infants, but it remains the major respiratory morbidity of prematurity 11, 12 occurring in 30% of infants ≤ 30 weeks gestation11. Currently, no treatment modality has been shown to prevent or treat Ureaplasma-mediated neonatal lung injury.
There is conflicting evidence concerning whether there are differences in virulence among the Ureaplasma serovars. It has been suggested that some Ureaplasma serovars have greater association with adverse pregnancy outcomes than others 13–16. However, there has been no study to date that has investigated the relationship of specific Ureaplasma serovars and the development of BPD. The aim of this study was to determine the distribution of Ureaplasma serovars in neonatal respiratory secretions by real-time PCR using serovar-specific primers/probes and to assess whether there are predominant serovars associated with BPD in a prospective cohort of preterm infants less than 33 weeks gestation.
METHODS
Study design
The University of Maryland School of Medicine Institutional Review Board approved the study protocol and parental consent was obtained. Eligible subjects were premature infants without congenital anomalies with gestational age <33 weeks admitted to the neonatal intensive care unit <72 h age at the University of Maryland Medical Center from May 2007 to March 2010. During the first sampling period (1–3 d), both a tracheal aspirate and nasopharyngeal wash were obtained from intubated infants and a nasopharyngeal wash only was obtained from non-intubated infants for Ureaplasma culture. At subsequent sampling periods (7–10 d and 28–30 d), a single sample for culture was obtained from each infant as a tracheal aspirate from infants who remained intubated or a nasopharyngeal wash from infants who were not intubated. Sterile, preservative-free-saline (0.5 ml) was instilled into the endotracheal tube or nostril and then immediately aspirated back into a suction trap and the process was repeated. The suction catheter was flushed with 2 ml 10B broth 17, and the samples were transported on ice until processed.
Sample collection and PCR
Nasopharyngeal (NP) and/or endotracheal (ET) specimens were vortexed and 0.2 mL of each specimen was added to 1.8 mL of 10B broth prepared as described by Shepard 17. Serial 10-fold dilutions were made to 10−4. Tubes were incubated at 37°C. If color change occurred, 0.2 ml of inoculum was plated onto A8 agar (Northeast Laboratory, Waterville, ME) and incubated at 37°C in 5% CO2/air. Tube cultures and plates were examined daily for 1 week for color change and typical colonies of Ureaplasma as described previously. A positive culture was defined as a positive broth (color change) confirmed by typical colony morphology on A8 agar. All remaining original samples and positive isolates were aliquoted and stored at − 80°C for later DNA extraction and amplification.
DNA was extracted from tracheal and nasopharyngeal aspirate samples and positive isolates using QiAmp DNA Blood Mini kits (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. The DNA was first analyzed by multiplex real-time PCR to differentiate the two Ureaplasma species simultaneously as previously described using the Roche LightCycler 2.0 18. Serovars of each species were then determined by monoplex real-time PCR assays 18. Each primer/probe set was confirmed to amplify the designated serovar but none of the other 13 serovars when the optimized PCR conditions previously described were used 18. All primers were synthesized by Invitrogen (Carlsbad, CA) and probes were manufactured by Roche Diagnostics (Indianapolis, IN).
Clinical outcomes
BPD severity was defined according to NIH criteria 12. Infants receiving supplemental oxygen at 28 d were assessed at 36 wk PMA or discharge whichever came first, and were classified as mild BPD if breathing room air; moderate BPD, if receiving supplemental oxygen <30%; or severe BPD, if receiving positive pressure support and/or supplemental oxygen ≥ 30% at the assessment time point. Other clinical variables that were recorded included (1) duration of ventilator and oxygen support, (2) duration of hospitalization, (3) incidence of clinical chorioamnionitis 19, (4) occurrence of preterm labor (POL) or preterm premature rupture of membrane (PPROM), and (5) demographic variables such as birth weight, gestational age, sex, and race.
Statistical analysis
Based on BPD rates reported for infants colonized with U. parvum (25%) and U. urealyticum (50%) by Abele-Horn et al. 14, and an expected 40% respiratory colonization rate, we estimated that 360 infants <33 wks gestation would need to be enrolled to detect a 25% difference in rates of moderate-severe BPD between the species with alpha=0.05 and 80% power. This report contains an interim analysis of the first 136 infants enrolled in this prospective study.
Continuous variables were analyzed by the two-tailed Student t-test or ANOVA if normally distributed or the Mann-Whitney U test or Wilcoxon rank sum test if not normally distributed. The Z-test for 2 proportions was used to determine if differences between two groups were significant. The Chi 2 or Fisher exact test was used to compare categorical variables. Univariate odds ratios and 95% confidence intervals were calculated for association of Ureaplasma variables (species, respiratory sample source, and colonization pattern) with BPD outcome. A P-value of < 0.05 was considered significant. All statistical analyses were performed using Stata 7.0 (Stata Corp., College Station, TX, USA).
RESULTS
Subject and Ureaplasma characteristics
Of 324 infants <33 wks gestation who were eligible for the study, 20 were missed due to lack of parental contact, 168 declined consent and parental consent was obtained for the remaining136 infants. Four hundred twenty aspirates (114 ET (27%) and 306 NP (73%) were analyzed by culture and species-specific PCR (Table 1). There were only 96 subjects available at 28–30 d for sampling due to death (N=2) or discharge/transfer (N=38) prior to this timepoint. Five samples were positive by PCR only. Paired ET/NP aspirates were obtained from 52 subjects (38%). There was significant agreement between ET and NP paired sample culture/PCR results (88.7%, kappa 0.722, p<0.001).
Table 1.
Ureaplasma status by respiratory source at each sampling period*
| Sampling Period | Source | Ureaplasma positive N (%) |
|---|---|---|
| Day 1–3 | ET (N=52) | 15 (29) |
| NP (N=136) | 30 (22) | |
| Total (N=188) | 45 (24) | |
| Day 7–10 | ET (N=38) | 10 (26) |
| NP (N=98) | 28 (29) | |
| Total (N=136) | 38 (28) | |
| Day 28–30 | ET (N=24) | 5 (21) |
| NP (N=72) | 14 (19) | |
| Total (N=96) | 19 (20) |
Both ET and NP sample obtained d 1–3 if subject intubated and NP only if not intubated. For other sampling periods, ET samples only from infants who remained intubated and NP samples only from non-intubated infants. Number of subjects sampled per sampling period: day 1–3, 136; day 7–10, 136; day 28–30, 96 due to death (N=2) or discharge/transfer (N=38) prior to 28 d. A sample was classified as Ureaplasma positive if 10B broth had color change and confirmed morphology on A8 agar and/or positive PCR.
Fifty-one infants (37.5%) had one or more positive Ureaplasma NP and/or ET aspirate cultures during the first month of life. Respiratory colonization was inversely related to gestational age (OR = 0.821, CI: 0.720–0.935). Sixty-five percent of infants <26 wk gestation compared with 31% infants ≥ 26 wk were culture/PCR positive (see figure, (Supplemental Digital Content 1, http://links.lww.com/INF/A677) which demonstrates the percentage of Ureaplasma culture/PCR positive infants at each gestational age). Thirty-seven culture/PCR positive infants (77%) who had ≥ 2 positive aspirates during this period were classified as persistent colonization, while 11 (23%) who had a single culture positive in the first week of life were classified as transient colonization. Two infants who were Ureaplasma-positive at the 28 d only and one infant who was culture-positive on day 7, but not day 1, and expired on day 16, were not assigned to a colonization pattern group.
PCR assays demonstrated that U. parvum was the predominant species (N=32, 63%), compared with U. urealyticum (N=17, 33%) regardless of sample source or colonization pattern (see figure, (Supplemental Digital Content 2, http://links.lww.com/INF/A678) which demonstrates the distribution of Ureaplasma species at each gestational age). Cultures from 2 infants contained mixtures of both species (4%). There were no significant differences in clinical variables between infants colonized with U. parvum compared with those colonized with U. urealyticum (table, (Supplemental Digital Content 3, http://links.lww.com/INF/A679) which shows the comparison of clinical variables in subjects with U. parvum and U. urealyticum respiratory colonization).
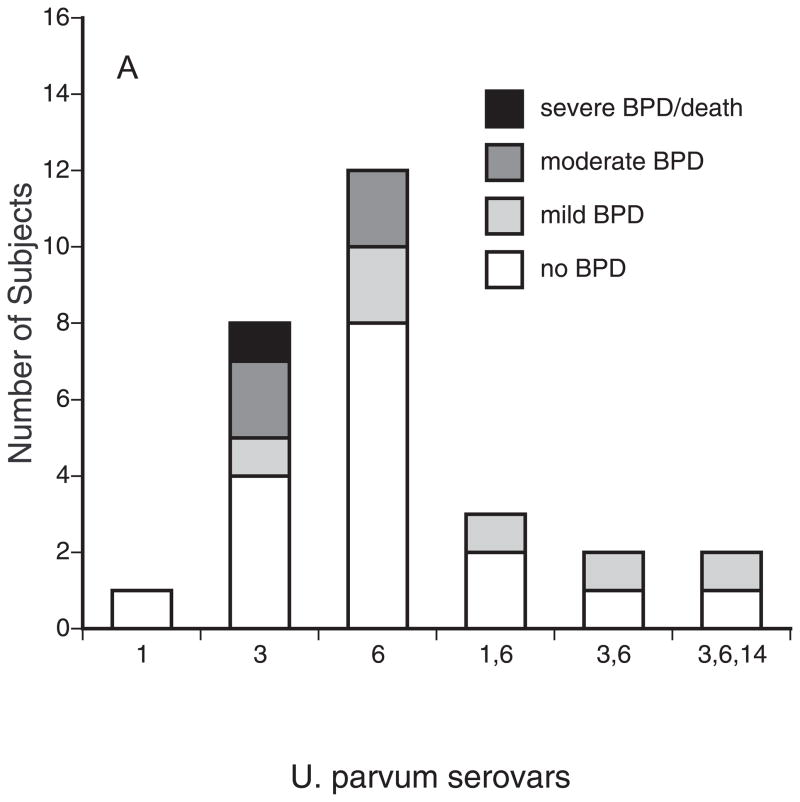
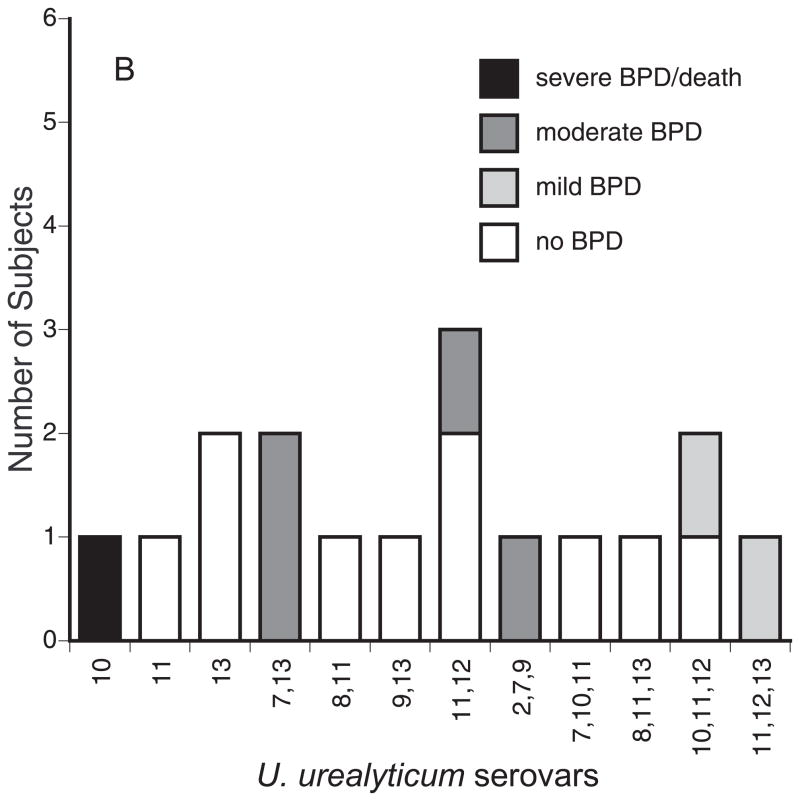
Serovar genotyping was performed on at least one bacterial isolate from 45 positive subjects. As shown in Figure 1A, serovars 3 and 6, alone and in combination, accounted for 96% (27/28) of U. parvum isolates. Serovar 14 was detected only in an aspirate from one infant as a mixture with serovars 3 and 6. The U. urealyticum isolates were commonly a mixture of 2 or more serovars (Figure 1B). Serovar 11 was the most common U. urealyticum serovar alone or in combination with other serovars (10/18, 56%). Serovars 4 and 5 were not detected in any sample.
Figure 1.
BPD severity among infants colonized with U. parvum (A) and U. urealyticum serovars. Serovars were determined by monoplex real-time PCR. BPD severity was defined using NIH criteria 12.
Relationship of Ureaplasma species and serovars to BPD
We analyzed the association of Ureaplasma respiratory tract colonization variables with pulmonary outcomes. We did not observe evidence of a difference in BPD rates between the Ureaplasma species (Table 2). However, with the current sample size, we are not able to rule out the possibility that true differences exist within the confidence interval range (Table 2). Previously, a pattern of persistent, but not transient respiratory tract colonization was associated with BPD 10. However, in the present study, persistent colonization during the first month of life alone did not increase the risk for moderate-severe BPD (Table 2). In contrast, the source of the respiratory sample was an important factor. Infants who had been mechanically ventilated for any duration and had a positive tracheal aspirate with or without a paired positive NP sample had a 7.9-fold increased risk (OR = 7.86, CI: 1.31–47) to develop moderate-severe BPD than mechanically ventilated infants with a positive NP sample alone. Moreover, we compared the BPD severity of infants colonized with the different serovars. There were no statistical differences among the U. parvum and U. urealyticum serovars in BPD severity (Fig. 1), but the sample size was inadequate to detect a difference at the serovar level. The moderate to severe BPD rate for infants colonized with serovar mixtures was similar to the rate for infants colonized with single serovars (Table 2).
Table 2.
Ureaplasma respiratory tract colonization variables associated with BPD outcome.
| Variable | None or mild BPD | Moderate to Severe BPD* | Odds Ratio | P value |
|---|---|---|---|---|
| Species | ||||
| Ureaplasma parvum (N=31) | 23 (74) | 8 (26) | 1.19 (0.39–3.52) | 0.760 |
| U. urealyticum (N=17) | 13 (76) | 4 (24) | ||
| Serovar mixture | ||||
| Single serovar (N=23) | 19 (83) | 4 (17) | 1.19 (0.26–5.52) | 0.827 |
| Multiple serovar (N=20) | 16 (80) | 4 (20) | ||
| Aspirate source | ||||
| NP (N=33) | 30 (91) | 3 (9) | 7.86† (1.31–47.04) | 0.024 |
| ET (N=17) | 7 (41) | 10 (59) | ||
| Persistence‡ | ||||
| Transient (N=11) | 10 (91) | 1 (9) | 3.67 (0.41–32.49) | 0.243 |
| Persistent (N=37) | 26 (70) | 11 (30) | ||
Moderate to severe BPD was defined as receipt of supplemental oxygen and/or positive pressure support at 36 wk PMA or discharge which ever came first.
Regression analysis restricted to subjects who were mechanically ventilated for any duration during the first month of life.
Respiratory tract colonization was considered persistent if Ureaplasma spp. were ≥ 2 positive aspirates during the first month of life.
DISCUSSION
The ability to differentiate the Ureaplasma serovars in clinical samples has long been a challenge for investigators. Previous antibody-based phenotyping methods 20–25 and serologic testing 26 were time-consuming and results were difficult to reproduce and interpret due to multiple cross-reactions and poor ability to discriminate among multiple serovars in clinical samples. More recently, gel-based traditional 2, 27, 28 and real-time PCR 29–32 methods targeting the urease gene, the mba gene, or 16s rRNA gene have been developed to discriminate between the two Ureaplasma species. Real-time PCR assays yield quantitative results and are more rapid, specific, sensitive, and less subject to contamination than culture and traditional PCR methods. Previous attempts to distinguish all 14 serovars by genotyping have been only partially successful, particularly for discriminating among the 10 U. urealyticum serovars. Availability of the genomic sequences for all 14 Ureaplasma serovars enabled primers/probes to be designed that are specific for each serovar without any cross-reactions 18. In this paper, we report for the first time, the frequency of the 14 serovars in respiratory secretions in a prospective cohort of preterm infants at risk for BPD.
Our study confirms previous observations 5, 33 that U. parvum is the predominant species colonizing the respiratory tract of preterm infants. In a study of a previous cohort from the University of Maryland, U. parvum was the more common species detected in samples from multiple compartments (blood, CSF, and tracheal aspirates) 4. In contrast, Heggie et al. 34 reported a similar distribution of the two species by species-specific PCR of culture-positive broths from endotracheal aspirates. Differences in study results may be due to differences in study populations or in selection bias of the DNA source. By performing PCR only on culture-positive broths, Heggie et al. 34 may have missed PCR-positives of culture-negative aspirates and broth medium may have supported differential growth of one or the other of the two species.
It has been debated whether one species is more pathogenic than the other in development of BPD in preterm infants. In agreement with Heggie et al. 34, we did not observe evidence of a difference in the incidence of moderate to severe BPD between infants less than 33 weeks gestation colonized with U. parvum than infants colonized with U. urealyticum. Katz et al. 33 also found no difference in BPD rates between infants colonized with either species, but a higher rate of BPD in infants positive for both species. A limitation of that study was that the study samples were endotracheal aspirates submitted for culture for clinical indications rather than samples collected prospectively using a defined protocol. Abele-Horne et al. 14 reported that the BPD rate was 2-fold higher for U. urealyticum-colonized infants than the rate for U. parvum-colonized infants. However, the infants colonized with U. urealyticum were significantly less mature and had lower birth weight than U. parvum-colonized infants. This may explain, in part, the species difference in BPD rates in their study 14.
Using antibody-based methods, previous investigators sought to ascertain differential pathogenicity of ureaplasmas for adverse pregnancy and neonatal outcomes at the serovar level. Serovar 4 was 4-fold more common in cervical samples from women with recurrent abortions compared with controls 13. Quinn et al. 35 reported that infants of mothers with histories of pregnancy losses had increased mean antibody titers for serovars 6 and 8, while the mothers had elevated mean titers to serovar 4 and 8. Serologic responses were assessed in preterm infants with respiratory diseases 26. Compared with infants without lung disease, infants with lung disease had elevated titers to serovars 4, 7, and 8. For serovars 4 and 8, the mean titers were higher in non-survivors than in survivors, while titers to serovar 5 were elevated in survivors. In the current study using genotyping, the most common serovars alone and in combinations with other serovars were U. parvum serovars 3 and 6 and U. urealyticum serovar 11. Interestingly, serovars 4 and 5 were not detected in any sample. There was no difference in BPD severity among the serovars. This supports the contention that Ureaplasma virulence is species and serovar-independent with regards to neonatal lung disease, but this will need to be confirmed in a larger sample.
In summary, using serovar-specific real-time PCR tests, we have provided a description of the distribution of the Ureaplasma serovars among respiratory secretions from preterm infants. Many isolates, predominantly U. urealyticum, contained multiple serovars. It is unknown whether these isolates represent mixtures of multiple serovars or are hybrids of a single organism expressing markers of multiple serovars as the result of horizontal gene transfer as is now known to occur in Ureaplasma spp. (Xiao, L., Paralanov, V., Glass, J.I., Duffy, L.B., Cassell, G.H. Waites, K.B. Extensive horizontal gene transfer in human ureaplasmas questions the utility of serotyping for diagnostic purposes. Abstract, 18th Congress of the International Organization for Mycoplasmology, Chianciano Terme, Italy, July, 2010). There were no differences in BPD rates between infants colonized with multiple serovars compared with infants colonized with a single serovar. These findings suggest that serotyping of ureaplasmas for diagnostic purposes may be of limited value. A search for specific virulence genes that may be common across multiple serovars may identify targets to prevent or ameliorate the adverse effects of Ureaplasma infection in the immature lung.
Supplementary Material
Percent of Ureaplasma culture/PCR positive infants at each gestational age. An infant was considered Ureaplasma-positive if one or more endotracheal or nasopharyngeal sample was culture-confirmed or PCR-positive. The colonization rate was higher for infants <26 weeks than for infants ≥ 26 weeks gestation.
The distribution of Ureaplasma species at each gestational age. An infant was considered Ureaplasma-positive if one or more endotracheal or nasopharyngeal sample was culture-confirmed or PCR-positive. Two infants <26 weeks gestation were colonized with both species.
Comparison of clinical variables in subjects with U. parvum and U. urealyticum respiratory colonization
Acknowledgments
NIH grant HL087166
This work was funded by NIH grant HL087166 and 5RO1A1072577. The authors gratefully acknowledge the technical support of Mary Spence, R.N., and Elise Janofsky, R.N.
References
- 1.Waites KB, Katz B, Schelonka RL. Mycoplasmas and ureaplasmas as neonatal pathogens. Clin Microbiol Rev. 2005;18:757–789. doi: 10.1128/CMR.18.4.757-789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kong F, Ma Z, James G, Gordon S, Gilbert GL. Species identification and subtyping of Ureaplasma parvum and Ureaplasma urealyticum using PCR-based assays. J Clin Microbiol. 2000;38:1175–1179. doi: 10.1128/jcm.38.3.1175-1179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viscardi RM. Ureaplasma species: role in diseases of prematurity. Clin Perinatol. 2010;37:393–409. doi: 10.1016/j.clp.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viscardi RM, Hashmi N, Gross GW, Sun CC, Rodriguez A, Fairchild KD. Incidence of invasive Ureaplasma in VLBW infants: relationship to severe intraventricular hemorrhage. J Perinatol. 2008;28:759–765. doi: 10.1038/jp.2008.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abele-Horn M, Peters J, Genzel-Boroviczeny O, Wolff C, Zimmermann A, Gottschling W. Vaginal Ureaplasma urealyticum colonization: influence on pregnancy outcome and neonatal morbidity. Infection. 1997;25:286–291. doi: 10.1007/BF01720398. [DOI] [PubMed] [Google Scholar]
- 6.Waites KB, Crouse DT, Philips JB, Canupp KC, Cassell GH. Ureaplasmal pneumonia and sepsis associated with persistent pulmonary hypertension of the newborn. Pediatrics. 1989;83:79–85. [PubMed] [Google Scholar]
- 7.Panero A, Pacifico L, Roggini M, Chiesa C. Ureaplasma urealyticum as a cause of pneumonia in preterm infants: analysis of the white cell response. Arch Dis Child. 1995;73:F37–F40. doi: 10.1136/fn.73.1.f37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang EL, Ohlsson A, Kellner JD. Association of Ureaplasma urealyticum colonization with chronic lung disease of prematurity: Results of a metaanalysis. J Pediatr. 1995;127:640–644. doi: 10.1016/s0022-3476(95)70130-3. [DOI] [PubMed] [Google Scholar]
- 9.Schelonka RL, Katz B, Waites KB, Benjamin DK., Jr Critical appraisal of the role of Ureaplasma in the development of bronchopulmonary dysplasia with metaanalytic techniques. Pediatr Infect Dis J. 2005;24:1033–1039. doi: 10.1097/01.inf.0000190632.31565.83. [DOI] [PubMed] [Google Scholar]
- 10.Castro-Alcaraz S, Greenberg EM, Bateman DA, Regan JA. Patterns of colonization with Ureaplasma urealyticum during neonatal intensive care unit hospitalizations of very low birth weight infants and the development of chronic lung disease. Pediatrics. 2002;110:E45–45. doi: 10.1542/peds.110.4.e45. [DOI] [PubMed] [Google Scholar]
- 11.Viscardi RM, Hasday JD. Role of Ureaplasma species in neonatal chronic lung disease: epidemiologic and experimental evidence. Pediatr Res. 2009;65(5 Pt 2):84R–90R. doi: 10.1203/PDR.0b013e31819dc2f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 13.Naessens A, Foulon W, Breynaert J, Lauwers S. Serotypes of Ureaplasma urealyticum isolated from normal pregnant women and patients with pregnancy complications. J Clin Microbiol. 1988;26:319–322. doi: 10.1128/jcm.26.2.319-322.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abele-Horn M, Wolff C, Dressel P, Pfaff F, Zimmermann A. Association of Ureaplasma urealyticum biovars with clinical outcome for neonates, obstetric patients, and gynecological patients with pelvic inflammatory disease. J Clin Microbiol. 1997;35:1199–1202. doi: 10.1128/jcm.35.5.1199-1202.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grattard F, Soleihac B, De Barbeyrac B, Bebear C, Seffert P, Pozzetto B. Epidemiologic and molecular investigations of genital mycoplasmas from women and neonates at delivery. Pediatr Infect Dis J. 1995;14:853–858. doi: 10.1097/00006454-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Hannaford K, Todd DA, Jeffrey H, John E, Byth K, Gilbert GL. Role of Ureaplasma urealyticum in lung disease of prematurity. Arch Dis Child Fetal Neonatal Ed. 1999;81:F162–F167. doi: 10.1136/fn.81.3.f162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shepard MC. Culture Media for Ureaplasmas. In: Razin S, Tully JG, editors. Methods in Mycoplasmology. Vol. 1. New York: Academic Press; 1983. pp. 137–146. [Google Scholar]
- 18.Xiao L, Glass JI, Paralanov V, et al. Detection and characterization of human ureaplasma species and serovars by real-time PCR. J Clin Microbiol. 2010;48:2715–2723. doi: 10.1128/JCM.01877-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viscardi RM, Muhumuza CK, Rodriguez A, et al. Inflammatory markers in intrauterine and fetal blood and cerebrospinal fluid compartments are associated with adverse pulmonary and neurologic outcomes in preterm infants. Pediatr Res. 2004;55:1009–1017. doi: 10.1203/01.pdr.0000127015.60185.8a. [DOI] [PubMed] [Google Scholar]
- 20.Echahidi F, Muyldermans G, Lauwers S, Naessens A. Development of monoclonal antibodies against Ureaplasma urealyticum serotypes and their use for serotyping clinical isolates. Clin Diagn Lab Immunol. 2000;7(4):563–567. doi: 10.1128/cdli.7.4.563-567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Echahidi F, Muyldermans G, Lauwers S, Naessens A. Development of an enzyme-linked immunosorbent assay for serotyping Ureaplasma urealyticum strains using monoclonal antibodies. Clin Diagn Lab Immunol. 2001;8:52–57. doi: 10.1128/CDLI.8.1.52-57.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stemke GW, Robertson JA. Modified colony indirect epifluorescence test for serotyping Ureaplasma urealyticum and an adaptation to detect common antigenic specificity. J Clin Microbiol. 1981;14:582–584. doi: 10.1128/jcm.14.5.582-584.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turunen H, Leinikki P, Jansson E. Serological characterisation of Ureaplasma urealyticum strains by enzyme-linked immunosorbent assay (ELISA) J Clin Pathol. 1982;35:439–443. doi: 10.1136/jcp.35.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson HL, Blalock DK, Cassell GH. Variable antigens of Ureaplasma urealyticum containing both serovar-specific and serovar-cross-reactive epitopes. Infect Immun. 1990;58:3679–3688. doi: 10.1128/iai.58.11.3679-3688.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng X, Watson HL, Waites KB, Cassell GH. Serotype diversity and antigen variation among invasive isolates of Ureaplasma urealyticum from neonates. Infect Immun. 1992;60:3472–3474. doi: 10.1128/iai.60.8.3472-3474.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quinn PA, Li HCS, Th’ng C, Dunn M, Butany J. Serological response to Ureaplasma urealyticum in the neonate. Clin Infect Dis. 1993;17 (Suppl 1):S136– S143. doi: 10.1093/clinids/17.supplement_1.s136. [DOI] [PubMed] [Google Scholar]
- 27.Robertson JA, Vekris A, Bebear C, Stemke GW. Polymerase chain reaction using 16S rRNA gene sequences distinguishes the two biovars of Ureaplasma urealyticum. J Clin Microbiol. 1993;31:824–830. doi: 10.1128/jcm.31.4.824-830.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanchard A, Hentschel J, Duffy L, Baldus K, Cassell GH. Detection of Ureaplasma urealyticum by polymerase chain reaction in the urogenital tract of adults, in amnioitic fluid, and in the respiratory tract of newborns. Clin Infect Dis. 1993;17(Suppl 1):S148–S153. doi: 10.1093/clinids/17.supplement_1.s148. [DOI] [PubMed] [Google Scholar]
- 29.Yi J, Yoon BH, Kim EC. Detection and biovar discrimination of Ureaplasma urealyticum by real-time PCR. Mol Cell Probes. 2005;19:255–260. doi: 10.1016/j.mcp.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida T, Ishiko H, Yasuda M, et al. Polymerase chain reaction-based subtyping of Ureaplasma parvum and Ureaplasma urealyticum in first-pass urine samples from men with or without urethritis. Sex Transm Dis. 2005;32:454–457. doi: 10.1097/01.olq.0000158932.78183.95. [DOI] [PubMed] [Google Scholar]
- 31.Cao X, Jiang Z, Wang Y, Gong R, Zhang C. Two multiplex real-time TaqMan polymerase chain reaction systems for simultaneous detecting and serotyping of Ureaplasma parvum. Diagn Microbiol Infect Dis. 2007;59:109–111. doi: 10.1016/j.diagmicrobio.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Cao X, Wang Y, Hu X, Qing H, Wang H. Real-time TaqMan polymerase chain reaction assays for quantitative detection and differentiation of Ureaplasma urealyticum and Ureaplasma parvum. Diagn Microbiol Infect Dis. 2007;57:373–378. doi: 10.1016/j.diagmicrobio.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Katz B, Patel P, Duffy L, Schelonka RL, Dimmitt RA, Waites KB. Characterization of ureaplasmas isolated from preterm infants with and without bronchopulmonary dysplasia. J Clin Microbiol. 2005;43:4852–4854. doi: 10.1128/JCM.43.9.4852-4854.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heggie AD, Bar-Shain D, Boxerbaum B, Fanaroff AA, O’Riordan MA, Robertson JA. Identification and quantification of ureaplasmas colonizing the respiratory tract and assessment of their role in the development of chronic lung disease in preterm infants. Pediatr Infect Dis J. 2001;20:854–859. doi: 10.1097/00006454-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Quinn PA, Shewchuk AB, Shuber J, et al. Serologic evidence of Ureaplasma urealyticum infection in women with spontaneous pregnancy loss. Am J Obstet Gynecol. 1983;145:245–250. doi: 10.1016/0002-9378(83)90500-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Percent of Ureaplasma culture/PCR positive infants at each gestational age. An infant was considered Ureaplasma-positive if one or more endotracheal or nasopharyngeal sample was culture-confirmed or PCR-positive. The colonization rate was higher for infants <26 weeks than for infants ≥ 26 weeks gestation.
The distribution of Ureaplasma species at each gestational age. An infant was considered Ureaplasma-positive if one or more endotracheal or nasopharyngeal sample was culture-confirmed or PCR-positive. Two infants <26 weeks gestation were colonized with both species.
Comparison of clinical variables in subjects with U. parvum and U. urealyticum respiratory colonization