Abstract
Cardiac myocytes, although large enough to make up most of the heart volume, are only a minority of cells within the heart with fibroblasts and blood vessel components (endothelial and smooth muscle cells) making up the remainder of the heart. In recent years, there has been increasing interest in the non-myocyte population within the heart. This is due, in part, to our increasing understanding of the biology of the non-myocyte cell types and additionally it is due to our awakening realization that these cells are not static but rather, that they are dynamic in nature indicating that they play a more active role in cardiac function than previously imagined. Studies now show that fibroblasts are involved in formation of the extracellular matrix and they control of the size of the extracellular matrix. Additionally they participate in the repair process by differentiating into myofibroblasts which are cells involved in the inflammatory response to injury. Myofibroblasts migrate to the sites of injury where they produce cytokines thus enhancing the inflammatory response. This review will discuss both structural and functional differences between the two cell types and examine the different roles of these two different cell types in the heart.
Fibroblasts
The normal heart is comprised of four major cell types; myocytes, endothelial cells, smooth muscle cells (within vessels) and the fibroblasts. The proportion of each cell types varies with species (1) but overall myocytes make up less than half of the cellular population of the heart. The fibroblasts make up a large portion of the cellular mass ranging from 40 to over 60% of the total cell population (1). These cells are important for the structural support that they give but also for their interaction with the cardiac myocytes.
Fibroblasts are found in every tissue of the body. They are of mesenchymal origin and depending on their location display multiple morphologies. During cardiac development fibroblasts develop from mulitpotent progenitor cells or mesenchymal stem cells (2). In normal heart, they are arranged in sheets and strands and appear as flattened elongated cells which lie along the cardiac myocytes within the endomysial collagen network. This localization pattern led early anatomists to suggest that these cells form the “glue” which holds the heart together under the duress of contraction. We now know that this is a very simplistic view of the role of the fibroblast. These cells have been shown to form a network of cells connected by long filapodia (1). They respond to both mechanical and chemical stimuli and through paracrine factors have been shown to interact with cardiac myocytes. In addition to holding the tissue together the fibroblast plays an active role in producing and maintaining the extracellular matrix, signaling to myocytes through mechanical stress and providing response elements for the immune system which are activated during myocardial injury. Unlike the cardiomyocytes, whose cell number is established and stable several weeks after birth, the number of fibroblasts continues to expand and the cells themselves grow and differentiate with increasing heart size (3). In the non-disease state, once the heart has fully grown and developed, fibroblast turnover is minimal. However, following cardiac insult new fibroblasts can arise from bone-marrow derived cells known as fibrocytes. Fibrocytes express characteristics of fibroblasts as well as leukocytes and hematopoitic progenitor cells by both playing a role in wound healing and also functioning in the immune response (1,4).
Fibroblasts in the heart show a morphology that is somewhat different than fibroblasts in other tissues. Rather than the normal flattened stellate shape, the cardiac fibroblast is an elongated cell with a highly elaborated endoplasmic reticulum indicative of high cellular activity (2) (Figure 1A). Their branched cytoplasm surrounds an elliptical shaped nucleus which can contain either one or two nucleoli (3). They have highly convoluted rough endoplasmic reticulum, large Golgi apparatus and like all fibroblasts cardiac fibroblasts do not have a basement membrane. Fibroblasts are directly coupled to one another via gap junctions, primarily made of Connexin43 (Cx43) and Connexin45 (Cx45) (5). Thus, along with the myocyte syncytium, the heart contains a separate syncytium of fibroblasts. While fibroblasts do not possess a definitive cell marker, a fact that makes understanding and studying these unique cells much more challenging, they do contain proteins which other cardiac cells do not have. Two such proteins used in studying fibroblasts are vimentin, present in the fibroblast's intermediate filaments, and the collagen receptor Discoidin Domain Receptor 2 (DDR2). DDR2 is the more specific of the two and although it has also been found in leukocytes and in tumors, it is not present in cardiac myocytes or cardiac endothelial cells (3). The use of these markers with a secondary cellular marker may aid in the identification of fibroblasts.
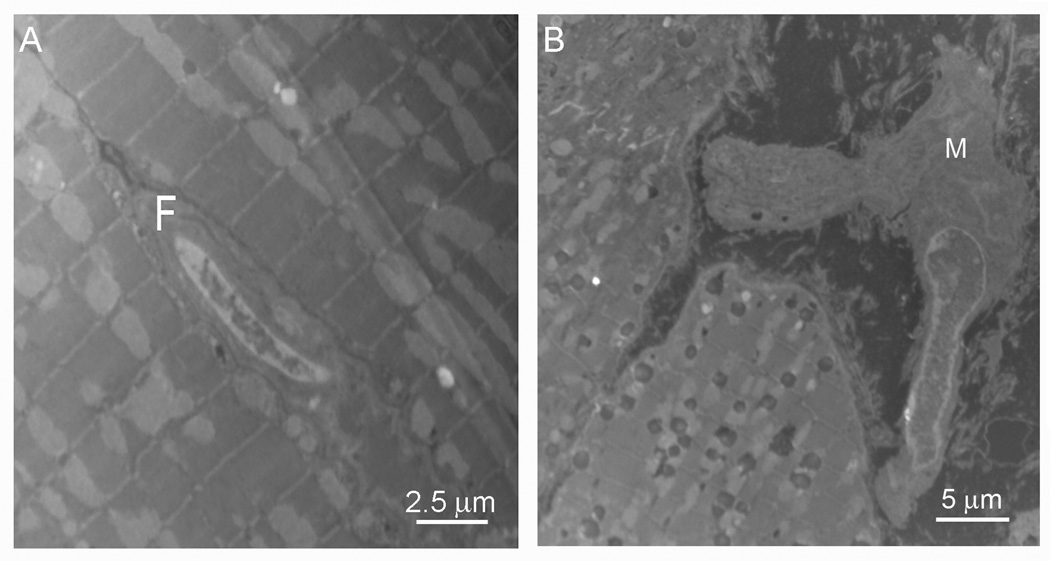
Figure 1.
Electron micrograph of a cardiac fibroblast (F, Panel A) and a cardiac myofibroblast (M, Panel B). Note that the fibroblast has a smooth, regular surface with a large nucleus (A, micro bar=2.5 µm). The myofibroblast is much larger (micron bar=5.0 µm) and has an undulating membrane and multiple processes (B).
These cells have distinct electrophysiological properties and are easily distinguished from neighboring myocytes. Fibroblasts cultured from neonatal rat hearts have a resting membrane potential around −30 mV (6) while acutely isolated adult fibroblasts have a resting membrane potential that is slightly more negative, −37 mM (7). These cells have multiple ion channels including several potassium channels (KCa1.1, Kv1.5 and 1.6, Kv4.2 and 4.3, Kir2.1, and Kir2.3), sodium channels (Na(V)1.2, 1.3, 1.5 and 1.7) and the Clnc3 channel (8). Despite the presence of these channels, fibroblasts are not electrically excitable cells and on their own cannot maintain action potential propagation although they will pass electrical signals if externally stimulated.
All regions of the myocardium have fibroblasts but the level of these cells varies from region to region. For example, the sino-atrial node is surrounded by areas of fibroblasts that are devoid of myocytes, electrically insulating this region. Coupling via gap junctions, although reported to occur between myocytes and fibroblasts of the SA node (9,10), does not allow for depolarization of the atrial myocytes outside the node. In the working myocardium of the atria and ventricles fibroblasts are in lower density than in the SA node region. Along with their structural support, fibroblast produce the extracellular matrix components, primarily collagens type I and III. This is a continuous process with new collagen being deposited as older collagen breaks down (4,11). In normal heart there is a balance between synthesis and degradation such that collagen levels remain stable. This fine balance is achieved by an orchestrated production of cytokines, growth factors and matrix metalloproteinases (MMPs). Over time the balance slowly tips towards the side of synthesis, setting the stage for age related fibrosis in the heart (4). This balance is also altered by cardiac pathology during which activation of fibroblasts occurs causing, among other things, expansion of the collagen network, fibrosis and extracellular matrix degradation. It has been found that the level of MMPs, in particular, increases dramatically during the disease state which leads to excessive extracellular matrix degradation and subsequent impaired healing at the site of injury (1, 2). Cardiac pathology also affects the physical mechanics of fibroblasts with increased stress and loading leading to impaired fibroblast function, increased fibrosis and changes in extracellular matrix metabolism. The release and regulation of cytokines by fibroblasts also changes in response to altered mechanical stretch with increases in TGF-α, TGF-β and ET-1 being observed (2). This stimulation leads to changes in gene expression which causes differentiation of fibroblasts to myofibroblasts which actively participate in the inflammatory response to injury.
Myofibroblasts
Myofibroblasts are large cells with ruffled membranes and highly active endoplasmic reticulum (Figure 1B). Myofibroblasts are not part of normal cardiac tissue and appear only following cardiac injury. They are distinguishable from fibroblasts at the level of the EM by their high level of exocytotic vesicles and their stress fibers (11). At the level of the light microscope they can be distinguished by the presence of smooth muscle actin staining. Myofibroblasts possess bundles of microfilaments which terminate at the cell surface in a specialized adhesion complex, termed the fibronexus or mature local adhesion. This complex bridges the myofibroblast's internal microfilaments with extracellular fibronectin domains thus functioning as a contractile mechanism that enables these cells to generate force to the surrounding extracellular matrix. This contractile force is maintained over time and reinforced by the deposition of collagen (13). Myofibroblasts migrate to and are highly responsive to chemokines released at the site of injury. In the case of ischemia or heart failure, the injury is wide spread, thus the myofibroblasts can be found in large areas of the heart. Myofibroblasts produce and secrete a number of cytokines themselves (See Table) which help to maintain the inflammatory response to injury.
Table 1.
Various Elements Secreted by Myofibroblasts
| Chemokines | IL-1α and β, IL-6, IL-10, TNF-α |
| Cytokines | ENA-78, GRO-1α, IL-8. MCP-1, MIP-1α, MIP-2, RANTES |
| Growth Factors | CSF-1, bFGF, GM-CSF, HGF, IGF-I, IGF-II, KGF, NGF, PDGF-AA, PDGF-BB, SCF, TGF-β |
| Inflammatory Mediators | CO, H2O2, HETEs, NO, O−, PAF, PGE2, Phospholipase A2 activating protein, Prostacyclin |
bFGF= Basic fibroblast growth factor, CSF= Colony-stimulating factor, ENA= Epithelial neutrophil-activating peptide, GM-CSF= Granulocyte/macrophage colony-stimulating factor, GRO= Melanoma growth-stimulatory activity, HETEs= Hydroxyeicosatetraenoic acids, HGF= Hepatocyte growth factor, IGF= Insulin like growth factor, IL=Interleukin KGF=Keratinocyte growth factor, MCP= Monocyte chemoattractant protein, MIP=Macrophage protein, NGF= Nerve growth factor, PAF= Platelet activating factor PDGF= Platelet-derived growth factor, RANTES= Regulated upon activation, normal T cell expressed and secreted, SCF= Stem cell factor, TNFα = Tumor necrosis factor alpha, TGFβ=Transforming growth factor beta, VEGF= Vascular endothelial growth factor
Studies have shown that levels of smooth muscle actin and thus myofibroblast expression peak anywhere from 5 to 14 days following cardiac insult then decrease 21 to 28 days post injury as the scar matures (14). Others have reported a biphasic pattern of expression, showing an increase of αSMA seven days post-MI, a decrease at day fourteen and then a significant increase 20 weeks after myocardial injury (15). It is therefore unclear exactly how long myofibroblasts persist in the injured heart, or even whether the cells seen in later stages of cardiac disease are the same myofibroblasts as were seen in early stages.
Like fibroblasts, myofibroblasts are not excitable cells and are not directly involved in conduction in the post MI heart. Instead, they are likely to produce barriers to conduction as they intercalate themselves between myocytes thus increasing the distance between adjacent myocyte membranes while decreasing myocyte to myocyte coupling via gap junctions. Much has been made of the possibility of myofibroblasts coupling directly to myocytes via gap junctions. These cells contain the connexin proteins which form gap junctions including Connexin43 and Connexin45 both of which are also found in the atrial and ventricular myocytes (16). It would seem that this coupling should be possible and indeed when neonatal myocytes are cultured with myofibroblasts they clearly do couple. Additionally work from Peter Kohl’s group has shown immunofluorescence imaging of a Cx43 protein sitting between a fibroblast and a myocyte in the sinus node of a normal rabbit heart (9,10) suggestive of coupling in this region of the heart. Further studies, however, examining both normal and diseased myocardium have yet to find strong evidence for this coupling in vivo implying that if this coupling does occur it does so at levels so low as to be unlikely to affect cardiac physiology.
Fibroblast to Myofibroblast Transdifferentiation
Upon injury to the myocardium fibroblasts are stimulated both mechanically by altered activation patterns and chemically by inflammatory mediators to undergo differentiation into the myofibroblast phenotype. The steps in this process are not well understood and, in particular, the molecular mechanisms by which this differentiation occurs in vivo are unknown. Fibroblasts of the normal heart express particular markers as noted above but once they begin to differentiate into myofibroblasts their protein profiles change dramatically. In vitro studies have shown that in early stages of differentiation fibroblasts show an increase in focal adhesion proteins (17) indicating a proto-myofibroblast phenotype which emerges with mechanical stress on the cells (18). Further stimulation causes further differentiation to the myofibroblast phenotype characterized by expression of smooth muscle actin (16). Examination of this phenomenon in cultured fibroblasts has shown that fibroblasts retain fibroblast markers only in early passage cultures but with increased cell passages they begin to transdifferentiate into myofibroblasts (17). The first myofibroblast markers (paxillin, vincullin and tensin) appear as early as the second passage (17). This indicates that studies done on fibroblasts in culture are likely to be representative of myofibroblasts unless the studies were done on cells prior to the third passage in culture.
Differentiation of fibroblasts to myofibroblasts in culture by exogenous addition of inflammatory mediators found in diseased heart such as TGF-β and IL-1β led to the hypothesis that the inflammatory response in the heart may be a primary trigger for this phenotype switch. Following cardiac injury inflammatory cells migrate into the tissue via the vascular system. Upon arrival they begin to secrete large amounts of both TGF-β and IL-1β. Receptors for both of these cytokines are present on fibroblast membranes. Receptor stimulation activates signaling pathways which in turn increases transcription of the gene encoding smooth muscle actin. Studies have shown other key marker proteins including vimentin and the embryonic isoform of smooth muscle myosin heavy chain (Smemb) are also upregulated following differentiation and transformation from fibroblast to myofibroblast. Increases in the focal adhesion proteins paxillin, tensin and ED-A fibronectin have also been observed (13, 14) and a rise in collagen synthesis in myofibroblasts as compared to fibroblasts is a defining characteristic between cell types. Based on their dispersed placement in the diseased heart it has been hypothesized that myofibroblasts are not static, rather they migrate through the tissue to sites of injury, likely in response to chemokines released at the site of injury (4, 16). The timing of this is not well described but it is known to occur within 48 hours of injury (17). What is clear is that over time, the transformation produces migratory myofibroblasts which infiltrate the injured regions of the heart and lay down collagenous septa between myocytes.
In diseased hearts both TGF-β and IL-1β stimulate the transformation of fibroblasts to myofibroblasts. TGF-β plays an essential role in extra-cellular matrix remodeling, cell mobility and modulation of immune function. Its action is also crucial to cell differentiation and proliferation and this does not exclude fibroblast to myofibroblast transformation. TGF-β has also been shown to increase collagen deposits and the level of smooth muscle actin in a dose dependent fashion with more abundant bundles of smooth muscle actin being observed in more concentrated areas of TGF-β (17). Inhibiting or blocking TGF-β expression has been shown to dramatically decrease alpha smooth muscle actin expression, with some studies reporting up to a 60% reduction. Associated with this decrease is a concurrent decrease in collagen production and subsequent fibrosis (18).
Whereas TGF-β increases myofibroblast proliferation, IL-1β inhibits differentiation mainly by affecting the levels and expression of SMA and other contractile proteins. Several studies have demonstrated that the decrease in SMA expression observed in response to IL-1β occurs via the induction of nitric oxide production. More recently it has been shown that the activation of nitric oxide synthase by IL-1β leads to apoptosis in select myofibroblasts and that apoptosis levels go up in the presence of increasing amounts of IL-1β (19.20). Also, like TGF-β, IL-1β impacts SMA protein levels and mRNA expression in a dose dependent manner. The other major impact of IL-1β is its effect on Cx43, the major ventricular gap junction protein in the heart. IL-1β is known to downregulate and close Cx43 channels (21). The loss of Cx43 leads to impaired cell to cell coupling and slowed cellular communication. Thus, following cardiac injury the high levels of circulating IL-1β produced and maintained by fibroblasts and then myofibroblasts may significantly impact electrical propagation in the heart.
Conclusions
Regardless of the stimuli leading to differentiation of fibroblasts to myofibroblasts, the end result is a loss of the carefully regulated levels of the extracellular matrix. Excess collagen deposition with a loss of collagen degradation by MMPs leads to fibrosis which has a long term impact on the function of the heart. Separation of myocytes by collagenous septa insulates myocytes and decreases myocyte-myocyte interactions leading to an overall loss of normal function. It is clear that what happens with fibroblasts and myofibroblasts in the cell culture dish is not exactly what happens in the in vivo heart therefore we need to develop new techniques to study these cells in vivo and understanding the biology of these cells is key to determining whether or not they are potential therapeutic targets.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Souders CA, Bowers SLK, Baudino TA. Cardiac Fibroblast: The Renaissance Cell. Circulation Research. 2009;105:1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009;123(2):255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Camelliti P, Borg TK, Kohl P. Structural and functional characterization of cardiac fibroblasts. Cardiovascular Research. 2004;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 4.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaudesius G, Miragoli M, Thomas SP, Rohr S. Coupling of Cardiac Electrical Activity Over Extended Distances by Fibroblasts of Cardiac Origin. Circulation Research. 2003;93(5):381–383. doi: 10.1161/01.RES.0000089258.40661.0C. Jun;288(6):H2931-9. [DOI] [PubMed] [Google Scholar]
- 6.Chilton L, Ohya S, Freed D, George E, Drobic V, Shibukawa Y, Maccannell KA, Imaizumi Y, Clark RB, Dixon IM, Giles WR. K+ currents regulate the resting membrane potential, proliferation, and contractile responses in ventricular fibroblasts and myofibroblasts. Am J Physiol Heart Circ Physiol. 2005 doi: 10.1152/ajpheart.01220.2004. [DOI] [PubMed] [Google Scholar]
- 7.Kamkin A, Kiseleva I, Isenberg G. Activation and inactivation of a non-selective cation conductance by local mechanical deformation of acutely isolated cardiac fibroblasts Cardiovascular. Res. 2003;57(3):793–803. doi: 10.1016/s0008-6363(02)00775-7. [DOI] [PubMed] [Google Scholar]
- 8.Li GR, Sun HY, Chen JB, Zhou Y, Tse HF, Lau CP. Characterization of multiple ion channels in cultured human cardiac fibroblasts. PLoS One. 2009 Oct 6;4(10):e7307. doi: 10.1371/journal.pone.0007307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohl P, Camelliti P. Cardiac myocyte-nonmyocyte electronic coupling: Implications for ventricular arrythmogenesis. Heart Rhythm. 2007;4(2):233–235. doi: 10.1016/j.hrthm.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Kohl P, Camelliti P, Burton FL, Smith GL. Electrical coupling of fibroblasts and myocytes: relevance for cardiac propagation. Journal of Electrocardiology. 2005;38:45–50. doi: 10.1016/j.jelectrocard.2005.06.096. [DOI] [PubMed] [Google Scholar]
- 11.Petrov VV, Fagard RH, Lijnen PJ. Stimulation of collagen production by transforming growth factor-beta1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension. 2001;39:258–263. doi: 10.1161/hy0202.103268. [DOI] [PubMed] [Google Scholar]
- 12.Eyden B. The myofibroblast: phenotypic characterization as prerequisite to understanding its functions in translational medicine. J. Cell. Mol. Med. 2008;1:22–37. doi: 10.1111/j.1582-4934.2007.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 14.Frangogiannis NG, Michael LH, Entman ML. Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb) Cardiovascular Research. 2000;48:89–100. doi: 10.1016/s0008-6363(00)00158-9. [DOI] [PubMed] [Google Scholar]
- 15.Bryant JE, Shamhart PE, Luther DJ, Olson ER, Koshy JC, Costic DJ, Mohile MV, Dockry M, Doane KJ, Meszaros JG. Cardiac myofibroblast differentiation is attenuated by α3 integrin blockade: Potential role in post-MI remodeling. Journal of Molecular and Cellular Cardiology. 2008;46:186–192. doi: 10.1016/j.yjmcc.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 16.Rohr S. Myofibroblasts in diseased hearts: new players in cardiac arrhythmias? Heart Rhythm. 2009;6(6):848–856. doi: 10.1016/j.hrthm.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 17.Santiago JJ, Dangerfield AL, Rattan SG, Bathe KL, Cunnington RH, Raizman JE, Bedosky KM, Freed DH, Kardami E, Dixon IMC. Cardiac fibroblast to myofibroblast differentiation in vivo and in vitro: Expression of focal adhesion components in neonatal and adult rat ventricular myofibroblasts. Developmental dynamics. 2010;239:1573–1584. doi: 10.1002/dvdy.22280. [DOI] [PubMed] [Google Scholar]
- 18.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodeling. Nature Reviews. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Weber KT. Infarct Scar: a dynamic tissue. Cardiovascular Research. 2000;46:250–256. doi: 10.1016/s0008-6363(00)00032-8. [DOI] [PubMed] [Google Scholar]
- 20.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-β1 induces α-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. Journal of Cell Biology. 1993;122(1):103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shephard P, Martin G, Smola-Hess S, Brunner G, Krieg T, Smola H. Myofibroblast differentiation is induced in keratinocyte-fibroblast co-cultures and is antagonistically regulated by endogenous transforming growth factor-beta and interleukin-1. Am J Pathol. 2004;164(6):2055–2066. doi: 10.1016/s0002-9440(10)63764-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang HY, Phan SH. Inhibition of myofibroblast apoptosis by transforming growth factor beta(1) Am J Respir Cell Mol Biol. 1999;21(6):658–665. doi: 10.1165/ajrcmb.21.6.3720. [DOI] [PubMed] [Google Scholar]
- 23.Zhang HY, Gharaee-Kermani M, Phan SH. Regulation of lung fibroblast alpha-smooth muscle actin expression, contractile phenotype, and apoptosis by IL-1beta. J Immunol. 1997;158(3):1392–1399. [PubMed] [Google Scholar]
- 24.Duffy HS, John G, Lee SH, Brosnan C, Spray DC. Reciprocal regulation of junctional proteins by Interleukin-1β (IL-1β) J. Neurosci. 2000;20:1–6. doi: 10.1523/JNEUROSCI.20-23-j0004.2000. RC114. [DOI] [PMC free article] [PubMed] [Google Scholar]