Abstract
OBJECTIVES
The embryonic stem cell (ESC) self-renewal gene Nanog has been shown to be expressed in several tumor types and to regulate tumor development. The aim of this study was to perform a detailed analysis of Nanog expression in human endometrial adenocarcinoma (EAC).
METHODS
Immunohistochemical analysis and reverse transcriptase-polymerase chain reaction (RT-PCR) were used to characterize Nanog, Sox2, and Oct4 expression in tissue arrays containing EAC, benign endometrium samples, and tumorosphere cells. Tumorosphere formation of EAC-derived cells in stem cell culture medium was also analyzed. Nanog expression was then analyzed in secondary tumors initiated by injection of tumorospheres or tumorosphere-derived differentiated cells into fifteen female nude mice. Apoptosis and cell proliferation were detected in fluorescence activated cell sorter (FACS) and 3-( 4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT) experiments, respectively.
RESULTS
The Nanog protein was expressed in a majority of EAC samples (45/55, 81.8%), but not in benign endometrium samples (0/26, 0.0%). Oct4 and Sox2 were also commonly expressed in EAC samples (42/55, 76.4%; 39/55, 70.9%, respectively). Subsets of cancer cells from all EAC samples (15/15, 100%) exhibited the capacity to form Nanog-positive tumorospheres. The tumorospheres also expressed Nanog, Oct4, and Sox2 mRNA and showed a higher proliferation potential than differentiated cells. All 15 mice that were injected with tumorosphere cells formed tumors, while only 3/15 mice injected with differentiated cells derived from tumorospheres developed tumors. All secondary xenograft tumors still expressed Nanog protein as well as Nanog, Oct4, and Sox2 mRNA, and had higher proliferation and lower apostosis than did differentiated cells.
CONCLUSION
Overexpression of Nanog in EACs suggests that Nanog may represent a potential therapeutic target for EAC. Additionally, Nanog may be useful as a biomarker in an immunohistochemical panel to differentiate between EAC and benign endometrial tissues. Expression of Nanog in tumorospheres may be indicative of the presence of a population of endometrial cancer stem cells (ECSCs), and its expression in xenograft tumors suggests that Nanog may also be associated with tumor metastasis.
Keywords: endometrial adenocarcinoma, Nanog, cancer stem cell, tumorosphere, endometrium
Introduction
Endometrial cancer is the most common gynecological malignancy affecting women in the Western world and the second most common affecting women in China [1, 2]. Approximately 95 of every 100 endometrial cancers are adenocarcinomas [1]. As carcinogenic mutations can be acquired over many years, it is likely that only adult stem/progenitor cells have a lifespan sufficiently long to accumulate the genetic damage necessary to give rise to cancer. These cancer stem cells (CSCs) have, therefore, been hypothesized to be responsible for carcinoma initiation [3].
A rare population of epithelial stem/progenitor cells was identified recently in the human endometrium [4]. Therefore, it is possible that these cells or their progeny may serve as a source of putative endometrial adenocarcinoma (EAC) stem cells involved in the initiation and maintenance of EAC. Emerging evidence suggests that human endometrial carcinomas do, in fact, contain endometrial cancer stem cells (ECSCs) [5]. In 1998, characterization of a uterine carcinosarcoma-derived cell line revealed the presence of colony-initiating cells that proliferated for more than 50 serial passages and that were believed to be the stem cells responsible for the propagation of the cell line [6]. More recently, cells freshly isolated from endometrial adenocarcinoma tissues were investigated for CSC-associated properties. These studies revealed a sub-population of freshly isolated cancer cells that had the capacity to initiate clones that underwent self-renewing cell divisions and initiated tumors in vivo, providing further evidence for the existence of ECSCs [4].
Nanog, Sox2, and Oct3/4 are transcription factors that form a core regulatory network that determines ESC self-renewal and differentiation [7]. These ESC-associated proteins may also contribute to tumorigenesis [8]. In support of this hypothesis, clinical studies have shown that elevated expression of Nanog is associated with retinoblastoma [9], prostate cancer [10], embryonal carcinoma [11], metastatic germ cell tumors [12], and ovarian cancer [13]. Additionally, Hubbard et al. detected Nanog expression in secondary clones derived from endometrial carcinoma cells [14]. Nanog overexpression has also been shown to promote proliferation and transformation of NIH3T3 cells [15]. Together, these findings suggest that abnormal expression of Nanog in stem cells and tumor tissues plays a critical role in transformation, tumorigenicity, and metastasis. However, a subset of these studies did not directly analyze expression of the Nanog protein. Nanog has multiple associated pseudogenes whose products can mimic true Nanog mRNA expression, resulting in the production of false-positive real-time polymerase chain reaction (RT-PCR) results. To date, comprehensive and systematic analyses of Nanog protein expression in human EAC specimens are still lacking. Herein, we have investigated the expression of the Nanog protein in EAC.
Materials and Methods
Tissue array samples
EAC and benign endometrium (BE) array slides containing formalin-fixed paraffin-embedded tissues were purchased from Shaanxi Chaoyin Biological Company (Xi’an, China). Each slide contained 81 tissue specimens obtained prior to any treatment from 81 Chinese female patients (mean age 42 ± 12 years, range 35–76 years) who underwent a hysterectomy. The primary indications included uterine fibroids (n = 11), endometriosis (n = 9), endometrial hyperplasia (n = 6), and EAC (Stage IB, n = 21; Stage IC, n = 19; Stage IIA, n = 9; Stage IIb, n = 6; total, 55). BE tissues included 6 post-menopause samples, 17 proliferative endometrium samples, and 9 secretory endometrium samples. All clinical and pathological diagnoses were made according to the standards of the Federation of Gynecology and Obstetrics (FIGO).
Tumorosphere culture
This study was approved by the Medical Ethics Committee of Yunyang Medical University. All patients provided informed written consent. Fifteen EAC samples (Stage IB, n = 8; Stage IC, n = 5; Stage IIa, n = 2) were obtained by surgical resection (Table 1). The samples were immediately washed in phosphate-buffered saline (PBS) containing 500 U/L penicillin G (Gibco, Carlsbad, CA, USA) and 500 mg/L streptomycin (Gibco) to remove blood cells. The samples were then cut into small pieces, followed by digestion overnight in DMEM/F12 supplemented with 0.5 mg/mL collagenase IV (Gibco). Unsorted cells were diluted in serum-free medium (SFM), which was comprised of DMEM-F12 containing 10 ng/mL fibroblast growth factor, 20 ng/mL epidermal growth factor, 5 kg/mL insulin, 2.75 mg/mL transferrin, 2.75 ng/mL selenium (insulin-transferrin-selenium solution), 1 × 105 U/L penicillin, and100 mg/L streptomycin (all reagents from Gibco). The cells were plated at a density of 5 × 105 cells per 100-mm plate and were cultured at 37 °C in a humidified atmosphere containing 5% CO2. Tumorospheres were dissociated every 7–10 d by incubation in a non-enzymatic cell dissociation solution (Sigma, St. Louis, MO, USA) for 2 min at 37 °C and were passaged at a density of 1 × 103 cells per 100-mm plate. Tumorosphere cells were induced to differentiate in stem cell medium by the addition of 10% fetal calf serum (FCS).
Table 1.
Patient characteristics.
| Sample No. | Age (y) | Diagnosis |
|---|---|---|
| 1 | 34 | EAC, Stage IB |
| 2 | 41 | EAC, Stage IB |
| 3 | 49 | EAC, Stage IB |
| 4 | 47 | EAC, Stage IB |
| 5 | 56 | EAC, Stage IB |
| 6 | 54 | EAC, Stage IB |
| 7 | 57 | EAC, Stage IB |
| 8 | 70 | EAC, Stage IB |
| 9 | 61 | EAC, Stage IC |
| 10 | 46 | EAC, Stage IC |
| 11 | 67 | EAC, Stage IC |
| 12 | 34 | EAC, Stage IC |
| 13 | 51 | EAC, Stage IC |
| 14 | 66 | EAC, Stage IIA |
| 15 | 63 | EAC, Stage IIA |
Immunohistochemistry
Tissue samples were fixed in 10% phosphate-buffered formalin and embedded in paraffin. Formalin-fixed, paraffin-embedded sections were cut at a thickness of 4 μm. Tissue microarray sections were dewaxed in xylene and rehydrated in alcohol. Antigen retrieval was performed by heating samples to 100 °C for 10 min in 0.01 M sodium citrate buffer (pH 6.0). After three 5-min rinses in PBS, the sections were immersed in 3% H2O2 for 30 min to suppress endogenous peroxidase activity. After rinsing in PBS, the sections were incubated with normal mouse serum at 37 °C for 15 min to block non-specific antibody binding, followed by incubation with mouse monoclonal anti-human Nanog antibody (1:100 in PBS, Santa Cruz Biotechnology, USA) for 2 h at room temperature. After three 5-min washes in PBS, the sections were then incubated with biotinylated secondary antibody (Santa Cruz Biotechnology, USA) and subsequently rinsed in PBS. After incubation in a solution containing streptavidin-HRP (Kangwei Century Biotechnology, China) and rinsing in PBS, bound antibodies were visualized by reaction with 3,3′-diaminobenzidine (DAB), and the tissues were counterstained with hematoxylin. Immunohistochemistry for Sox2 and Oct4 was performed as described above using primary antibodies diluted 1:100 from Santa Cruz Biotechnology, USA.
Tumorosphere cells and tumorosphere-derived differentiated cells in co-cultures were seeded on poly-D-lysine/laminin-coated coverslips (BD Biosciences, USA). The tumor cells were then fixed in 4% paraformaldehyde in PBS for 6 min at room temperature, followed by two brief rinses with PBS. Fixed cells were permeabilized by incubation in 0.02% Triton X-100 in PBS for 6 min. The cells were then washed briefly three times with PBS and stained using the antibodies and technique described above for tissue sections. Nanog immunoreactivity was quantified by determining the percentage of Nanog positive cells in 300 cells under light microscopy.
Semiquantitative reverse transcription-PCR of Nanog, Oct4, and Sox2
Total RNA samples from tumorospheres and their differentiated cells or xenograft tumors were purified using TRIzol reagent (Invitrogen, USA) and reverse-transcribed with a reverse transcription kit (Invitrogen, USA). We used 2 μL of each reaction for PCR with 1 μL each of sense and antisense primers (Table 2), 1 μL of β-actin primer, 2.5 μL of MgCl2 (25 mmol/L), 1 μL of deoxynucleotide triphosphates, 2.5 μL of 10× PCR buffer, 5 units of Taq DNA polymerase, and 14 μL of double-distilled water in a 25-μL reaction volume. The cycling variables were: one 4-min cycle at 94 °C, 20 cycles at 94 °C for 50 s, 59 °C for 1 min, 72 °C for 50 s; final extension proceeded at 72 °C for 10 min. The PCR products (1 μL) were electrophoresed on 1.5% agarose gels and the gray scale ratios of the PCR products were calculated.
Table 2.
PCR primers and products
| Symbol. | Primers | Products (bp) |
|---|---|---|
| Nanog | Sence: AGCCTCCAGCAGATGCAA Antisence: CCTGGTGGTAGGAAGAGTA |
190 |
| Oct4 | Sence: AGCTGGAGAAGGAGAAGC Antisence: AAAGCGGCAGATGGTCGT |
194 |
| Sox2 | Sence: CAATAGCATGGCGAGCGG Antisence: GTCGTAGCGGTGCATGGG |
196 |
| β-actin | Sence: CCAGAGCAAGAGAGGCATCC Antisence: CCGTGGTGGTGAAGCTGTAG |
437 |
Tumorosphere and xenograft tumor proliferation assays
Tumorosphere cells or cells from digested xenograft tumors were plated in 96-well microwell plates in 0.1-ml volumes of SFM supplemented with growth factors, at a density of 1000 cells/well. Cell proliferation assays were performed with 24-h postplating using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide-based Colorimetric Assay Cell Proliferation kit 1 (Roche, USA). Viable cells were quantified by reading UV absorption spectrums at 575 nm on a Versamax microplate reader.
Implantation of tumorosphere and tumorosphere-derived differentiated cells into nude mice
Following dissociation of tumorospheres and tumorosphere-derived differentiated cells from 15 EAC patients in a non-enzymatic cell dissociation solution, cells were washed in serum-free Hank’s Balanced Salt Solution (HBSS). The cells were then suspended in a 1:1 (v/v) mixture of serum-free DMEM/F12 and Matrigel, followed by subcutaneous (s.c.) injection of 1 × 105 cells into the right (differentiated cells) or left (tumorosphere cells) mid-abdominal area using a 23-gauge needle. Animals were subjected to necropsy 28 d after cell implantation, and tumor growth was assessed by measuring their volume with a straight ruler. The tumor volume was calculated using the following formula: V = 1/2 × (L × W2). Visible tumors were excised for histological examination. Harvested tissues were fixed in 10% buffered formalin, embedded in paraffin, cut into 4-μm sections, and labeled with Nanog-specific antibodies as described above.
Quantitative cancer cell apoptosis assay
Xenograft tumors were trypsinized as mentioned as above, washed once in ice-cold PBS, and at least 400 cells were incubated with annexin-V–fluoroescein/PI (Boehringer Mannheim, USA) in calcium-containing HEPES buffer; the cell quantities were then immediately analyzed with a FACScan machine (BD, USA).
Statistical analysis
To identify significant changes in transcription factor expression between the EAC and BE samples, we applied an independent-sample t-test comparing the two groups. The frequency of transcription factor expression in EAC and BE samples was compared by a χ2. P-values less than 0.05 were considered to be statistically significant in all cases. SPSS software for PC (version 13.0 for Windows, SPSS, Inc., Chicago, IL, USA) was used for statistical analyses.
RESULTS
Nanog, Oct4, and Sox2 protein expression in EAC and BE samples
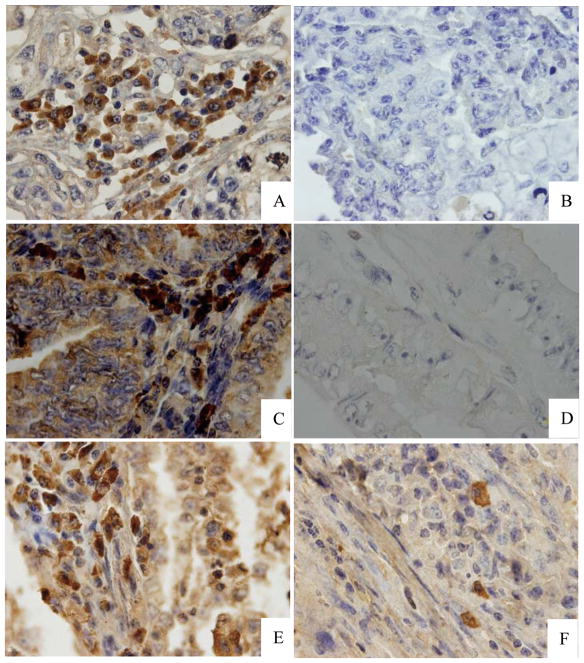
Most (45/55) of the EAC specimens were immunopositive for Nanog protein. Nanog-specific staining was visible in the cytoplasm and nucleus of cancer cells (Fig. 1A–C). However, Nanog expression was not detected in benign samples (P < 0.001 vs. EACs, Fig. 1D). Oct4 and Sox2 were also found to be expressed in EAC specimens (42/55, 76.4%; 39/55, 70.9%, respectively, Fig 1E, F). In particular, samples from six post-menopausal patients with endometrial hyperplasia did not exhibit Nanog expression, indicating that Nanog is not expressed in benign, post-menopausal endometrial tissues.
Figure 1. Immunohistochemical analysis of Nanog, Oct4, and Sox2 in EAC and BE samples.
(A) Positive control showing a cervical carcinoma sample labeled with anti-human Nanog antibodies. (B) An EAC tissue section labeled with an irrelevant antibody shown as a negative control. (C) Nanog expression in a representative EAC sample. (D) Lack of Nanog expression in a representative BE sample. (E) Oct4 expression in a representative EAC sample. (F) Sox2 expression in a representative EAC sample. (Magnification, 1000×)
Tumorosphere formation by cancer cells isolated from EACs
When single-cell suspensions of EAC cells were prepared by enzymatic dissociation and cultured in stem cell medium at cloning densities for 15 d, a few cells within each of the 15 EAC samples examined formed individual colonies (100%). Large colonies exhibited a high proliferative potential, possibly due to the presence of EAC stem/progenitor cells (Fig. 2A). The smaller colonies were thought to have been initiated by more mature cells, which we propose may be transit amplifying cells (Fig. 2A).
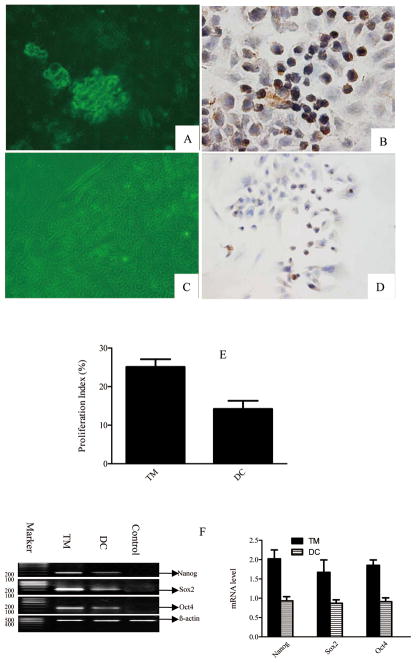
Figure 2. Analysis of tumorosphere formation from EAC cells and assessment of Nanog expression in tumorosphere and tumorosphere-derived differentiated cells.
(A) Tumorosphere formation by cells isolated from EAC samples. (B) Nanog expression in a representative tumorosphere. (C) Differentiated cells derived from a tumorosphere. (D) Nanog expression in representative differentiated cells derived from tumorospheres. (Magnification, 400×) (E) Comparison of the proliferative potential between tumorosphere cells and tumorosphere-derived differentiated cells. (F) RT-PCR results showing Nanog, Oct4, and Sox2 mRNAs in tumorosphere cells and tumorosphere-derived differentiated cells.
Immunohistochemical staining of enzymatically-dissociated tumorosphere cells revealed that Nanog expression was primarily detected in the nucleus, unlike in EAC tissues where Nanog was also detected in the cytoplasm. Few differentiated cells exhibited Nanog expression; the percentage of positive cells was lower among differentiated cells than among tumorosphere cells. Moreover, all of the tumor sphere cell populations assayed demonstrated greater proliferative capacity than that observed for differentiated cells (Fig. 2E). Furthermore, we observed greater Nanog, Sox2 and Oct4 mRNA levels in tumorosphere cells than in differentiated cell (Fig. 2F, P = 0.014)..
Analysis of the tumorigenicity of cells from EAC-derived tumorospheres
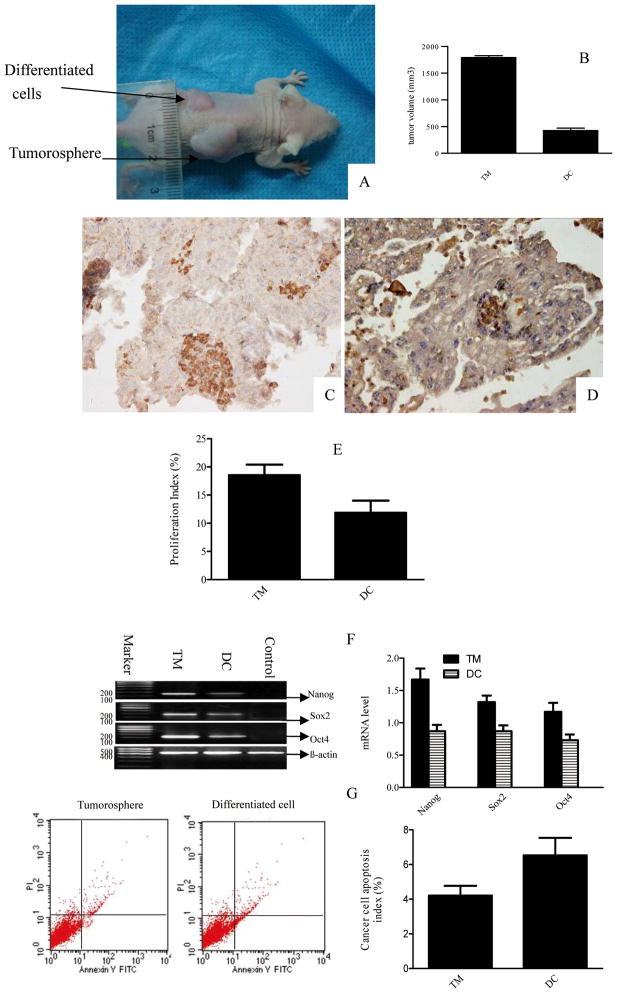
To test whether the EAC-derived tumorospheres exhibit highly tumorigenic properties, tumorospheres were dissociated, and tumorosphere cells or control tumorosphere-derived differentiated cells were injected into female nude mice. Analysis of the resulting tumors revealed that injection of cells from 15 tumorospheres derived from 15 different patients caused tumor formation in mice. In contrast, only 3 of the 15 injections of differentiated cells derived from the tumorospheres resulted in the formation of tumors, and those tumors were relatively small (Fig. 3A). Tumorospheres containing Nanog-positive cells were highly tumorigenic in comparison to tumorosphere-derived differentiated cells (Fig. 3B). The pattern of Nanog expression present in secondary tumors (Fig. 3C, D) was similar to that observed in patient tumors. Realtive to differentiated cells, secondary tumor cells showed higher proliferation rates, higher mRNA levels, and lower apostosis rates (Fig. 3E–G, both of P < 0.001).
Figure 3. Tumor formation in nude mice injected with tumorosphere cells and tumorosphere-derived differentiated cells.
(A) A representative mouse showing the formation of a large tumor at the site of the tumorosphere cell injection and a smaller tumor at the site of the differentiated cell injection. (B) Comparison of the volumes of tumors derived from tumorosphere cells and differentiated cells. (C) Nanog expression in xenograft tumors drived from tumorosphere cell. (D) Nanog expression in xenograft tumors derived from differentiated cancer cells. (Magnification, 400×) (E) Nanog, Oct4, and Sox2 mRNA were detected in xenograft tumors. (F) Comparison of the proliferative potential of xenograft tumors that developed from tumorosphere cells versus those that developed from tumorosphere-derived differentiated cells. (G) Comparison of apoptosis in xenograft tumors that developed from tumorosphere cells versus those that developed from tumorosphere-derived differentiated cells.
Discussion
In this study, we characterized the expression of Nanog in EAC biopsy samples, and tumor-derived cell cultures. Nanog immunohistochemistry revealed Nanog immunoreactivity in the majority of EAC tumors analyzed. Using a novel cell isolation and culture approach, we successfully cultured primary cultures derived from clinical adenocarcinoma samples. In these cultures, we found that a sub-population of cancer cells formed tumorospheres that expressed Nanog in a portion of their constituent cells. Nanog was expressed in more cells in tumorospheres than in EAC tissue samples. Tumorosphere cells and xenograft tumors showed high levels of cell proliferation. And fewer apoptitic cells were observed in tumors derived from tumoroshphere cells than in tumors derived from differentiated cells. Expression of the Nanog protein in EACs suggests that Nanog may play an important role in EAC tumorigenesis and progression and may represent a potential target for EAC-targeted therapies.
Self-renewal, an undifferentiated state, and the capability to differentiate into heterogeneous mature cell types are the hallmarks of stem/progenitor cells [16]. Nanog has been suggested to be one of four major factors that control reprogramming of adult cells into germ-line-competent induced pluripotent stem cells [17]. Nanog expression has been observed in human breast cancer stem-like cells, suggesting that its expression may be involved in self-renewal and tumorigenesis via activation of downstream target genes [18]. Several molecular and cell surface markers of ECSCs have recently been identified, including CD133, Musashi-1, and NAC1 [5]. However, the identification of CSCs in tumors have been limited by the lack of accessibility of cells within solid tumors, the absence of functional assays suitable for detecting and quantifying normal stem cells present in many organs, and the lack of identification of cell surface markers required to isolate CSCs. The expression of Nanog in EAC suggests a potential role for this protein in the tumorigenesis of EACs and mandates further investigation. Nanog could represent a novel molecular marker for indicating the presence of CSC-like cells in tumors [9–12, 19]. Clarifying whether Nanog is a marker of CSCs or tumor-initiating cells in EAC will require further investigation. Even so, given Nanog’s known roles in tumorigenesis and in maintenance of cancer stem cell properties, Nanog-expressing cells may define a specific subset of cells that could be targeted for novel, effective cancer treatments.
Acknowledgments
We wish to thank the Pathology Department of Renmin Hospital for assistance with histological staining. We are also grateful to Yun Zhao for his assistance in the writing of this manuscript. This work was supported by the Shiyan Biologic Research Foundation (No. 045D).
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Xue F, Broaddus RR, et al. Clinicopathological features in endometrial carcinoma associated with Lynch syndrome in China. Int J Gynecol Cancer. 2009;19:651–6. doi: 10.1111/IGC.0b013e3181a12fb9. [DOI] [PubMed] [Google Scholar]
- 3.Ebben JD, Treisman DM, Zorniak M, et al. The cancer stem cell paradigm: a new understanding of tumor development and treatment. Expert Opin Ther Targets. 2010;14:621–32. doi: 10.1517/14712598.2010.485186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gargett CE, Schwab KE, Zillwood RM, et al. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80:1136–45. doi: 10.1095/biolreprod.108.075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hubbard S, Gargett C. A cancer stem cell origin for human endometrial carcinoma? Reproduction. 2010;140:23–32. doi: 10.1530/REP-09-0411. [DOI] [PubMed] [Google Scholar]
- 6.Gorai I, Yanagibashi T, Taki A, et al. Uterine carcinosarcoma is derived from a single stem cell: an in vitro study. Int J Cancer. 1998;72:821–7. doi: 10.1002/(sici)1097-0215(19970904)72:5<821::aid-ijc19>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 7.Okumura-Nakanishi S, Saito M, Niwa H, et al. Oct-3/4 and Sox2 regulate Oct-3/4 gene in embryonic stem cells. J Biol Chem. 2005;280:5307–17. doi: 10.1074/jbc.M410015200. [DOI] [PubMed] [Google Scholar]
- 8.Boer B, Kopp J, Mallanna S, et al. Elevating the levels of Sox2 in embryonal carcinoma cells and embryonic stem cells inhibits the expression of Sox2:Oct-3/4 target genes. Nucleic Acids Res. 2007;35:1773–86. doi: 10.1093/nar/gkm059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seigel G, Hackam A, Ganguly A, et al. Human embryonic and neuronal stem cell markers in retinoblastoma. Mol Vis. 2007;13:823–32. [PMC free article] [PubMed] [Google Scholar]
- 10.Bae KM, Su Z, Frye C, et al. Expression of pluripotent stem cell reprogramming factors by prostate tumor initiating cells. J Urol. 2010;183:2045–53. doi: 10.1016/j.juro.2009.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boer B, Cox J, Claassen D, et al. Regulation of the Nanog gene by both positive and negative cis-regulatory elements in embryonal carcinoma cells and embryonic stem cells. Mol Reprod Dev. 2009;76:173–82. doi: 10.1002/mrd.20943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santagata S, Ligon KL, Hornick JL. Embryonic stem cell transcription factor signatures in the diagnosis of primary and metastatic germ cell tumors. Am J Surg Pathol. 2007;31:836–45. doi: 10.1097/PAS.0b013e31802e708a. [DOI] [PubMed] [Google Scholar]
- 13.Bapat S, Mali A, Koppikar C, et al. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65:3025–9. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- 14.Hubbard SA, Friel AM, Kumar B, et al. Evidence for cancer stem cells in human endometrial carcinoma. Cancer Res. 2009;69:8241–8. doi: 10.1158/0008-5472.CAN-08-4808. [DOI] [PubMed] [Google Scholar]
- 15.Piestun D, Kochupurakkal BS, Jacob-Hirsch J, et al. Nanog transforms NIH3T3 cells and targets cell-type restricted genes. Biochem Biophys Res Commun. 2006;343:279–85. doi: 10.1016/j.bbrc.2006.02.152. [DOI] [PubMed] [Google Scholar]
- 16.Morrison S, Shah N, Anderson D. Regulatory Mechanisms Review in Stem Cell Biology. Cell. 1997;88:287–298. doi: 10.1016/s0092-8674(00)81867-x. [DOI] [PubMed] [Google Scholar]
- 17.Alberio R, Campbell K, Johnson A. Reprogramming somatic cells into stem cells. Reproduction. 2006;132:709–20. doi: 10.1530/rep.1.01077. [DOI] [PubMed] [Google Scholar]
- 18.Ezeh U, Turek P, Reijo R, et al. Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma. Genesis. 2005;104:2255–65. doi: 10.1002/cncr.21432. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Wang X, Chen B, et al. Expression of Nanog gene promotes NIH3T3 cell proliferation. Biochem Biophys Res Commun. 2005;338:1098–102. doi: 10.1016/j.bbrc.2005.10.071. [DOI] [PubMed] [Google Scholar]