Abstract
Purpose
The function of the medial collateral ligament (MCL) during gait has not been investigated. Our objective was to measure the kinematics of the medial collateral ligament during the stance phase of gait on a treadmill using a combined dual fluoroscopic imaging system (DFIS) and MRI technique.
Methods
Three-dimensional models of the knee were constructed using magnetic resonance images of 7 healthy human knees. The contours of insertion areas of the superficial MCL (sMCL) and deep MCL (dMCL) on the femur and tibia were constructed using the coronal plane MR images of each knee. Both the sMCL and the dMCL were separated into 3 portions: the anterior, mid, and posterior bundles. The relative elongation of the bundles was calculated using the bundle length at heel strike (or 0% of the stance phase) as a reference.
Results
The lengths of the anterior bundles were positively correlated with the knee flexion angle. The mid-bundles of the sMCL and dMCL were found to function similarly in trend with the anterior bundles during the stance phase of the gait and their lengths had weak correlations with the knee flexion angles. The elongations of the posterior bundles of sMCL and dMCL were peaked at mid-stance and terminal extension/pre-swing stance phase. The lengths of the posterior bundles were negatively correlated with the knee flexion during the stance phase.
Conclusion
The data of this study demonstrated that the anterior and posterior bundles of the sMCL and dMCL have a reciprocal function during the stance phase of gait. This data provide insight into the function of the MCL and a normal reference for the study of physiology and pathology of the MCL. The data may be useful in designing reconstruction techniques to better reproduce the native biomechanical behavior of the MCL.
Level of evidence
IV.
Keywords: Knee kinematics, Gait, Stance phase, Medial collateral ligament (MCL)
Introduction
The medial collateral ligament (MCL) has two components: the longitudinal fibers of the superficial MCL (sMCL) and the deep MCL (dMCL) [8, 16]. Knowledge of the MCL function is important for prevention and treatment of the MCL injury as well as soft tissue balance in total knee arthroplasty (TKA). The biomechanical function of the MCL was well documented in in vitro cadaveric and clinical studies [4, 5, 12–14, 18, 25, 28, 29, 34]. Most biomechanical assessments of the function of the MCL have been performed by cutting selected ligament bundles [11, 20, 33].
However, the MCL function during in vivo dynamic knee motion has not been well studied. Understanding the role of the MCL under physiological loading conditions is important for improving the techniques of MCL repair and reconstruction surgeries. A few in vivo studies measured the length changes of the human MCL during quasi-static knee flexions [21, 30]. For example, Park et al. reported that the length changes of the MCL depend on the knee flexion angles during the single leg lunge [21] and Van de Velde et al. found that the ACL deficiency can affect the length changes of the sMCL and dMCL from 0 to 90° during in vivo weight bearing flexion [30].
Walking is the most commonly performed daily activity of humans. However, to our knowledge, there is no quantitative data reported on the biomechanical function of the MCL during walking. Recently, a combined dual fluoroscopic imaging system (DFIS) and MR imaging technique was used to determine the human knee kinematics during gait [17]. The purpose of the present study was to investigate the length change patterns of the MCL during the stance phase of the treadmill gait. Based on the current knowledge of the MCL biomechanics, we hypothesize that different portions of the MCL, namely the anterior and posterior bundles of the sMCL and dMCL, will demonstrate reciprocal elongation patterns during the stance phase of gait.
Materials and methods
Seven subjects (aged 32–49 years, two women and five men), with average body mass index (BMI) of 23.5 kg/m2, without previous knee injury, surgery, and systemic disease were recruited and consented for the study according to the requirements of the Institutional Review Board. Each participant had one knee scanned (two right and five left) at full extension using a 3-Tesla scanner (MAGNETOM Trio®, Siemens, Erlangen, Germany) and a double-echo water excitation sequence. This way, parallel, sagittal MR images were taken that were then imported into a modeling software (Rhinoceros®, Robert McNeel & Associates, Seattle, WA), placed in parallel, sagittal planes separated by 1 mm, and the contours of the femur and tibia were manually digitized to construct the 3-dimensional (3D) computer models of the knee.
Next, the dual fluoroscopic imaging system (DFIS) previously validated for treadmill gait analysis was used to determine knee kinematics during the stance phase of gait [17, 19]. The subject practiced the gait on the treadmill for 1 min at a treadmill speed of 1.5 miles per hour (MPH), i.e. 0.67 m/s. Two thin pressure sensors (Force Sensor Resistor (FSR), Interlink Electronics Specifications, Camarillo, CA) were fixed to the bottom of each shoe, to determine the moment between heel strike and toe-off of the stance phase during gait. The knee was imaged during three consecutive strides using 30 pulsed snap shots per second (8 ms/pulse). The stance phase was divided into four portions: Loading response (LR): 0–20%; Mid-stance (MS) phase: 20–60%; Terminal extension (TE): 60–85%; Pre-swing phase (PS): 85–100%.
Finally, the fluoroscopic images were imported into the modeling software [17]. The 3D MRI-based bone models of the knee were imported into the software and manipulated in 6° of freedom until the projections of each model matched the outline of the knee on each fluoroscopic image, reproducing the position of the knee during gait. This process was repeated at each 10% progress of the stance phase from heel strike to toe-off.
sMCL and dMCL kinematics
Using MR images of each knee, the attachment sites of the sMCL and dMCL were digitized on the sagittal MR images [21] (Fig. 1). The insertion areas of each ligament were evenly divided into 3 equal portions to create 3 equal fiber bundles: the anterior bundle, the mid-bundle, and the posterior bundle. The length of each bundle was measured as the distances between the centroids of the femoral and tibial insertions of the bundle from the series of matched knee models. Because the sMCL ligament wraps around the femoral condyles and the tibial plateau, the line connecting the bundle centroids on the femur and tibia was projected on the bony surfaces to create a curved line (Fig. 1). The length of this projected curve was measured as the ligament bundle length [21]. To examine the relative length change of the ligament, the relative elongation of a bundle was calculated using its length at heel strike as a reference.
Fig. 1.
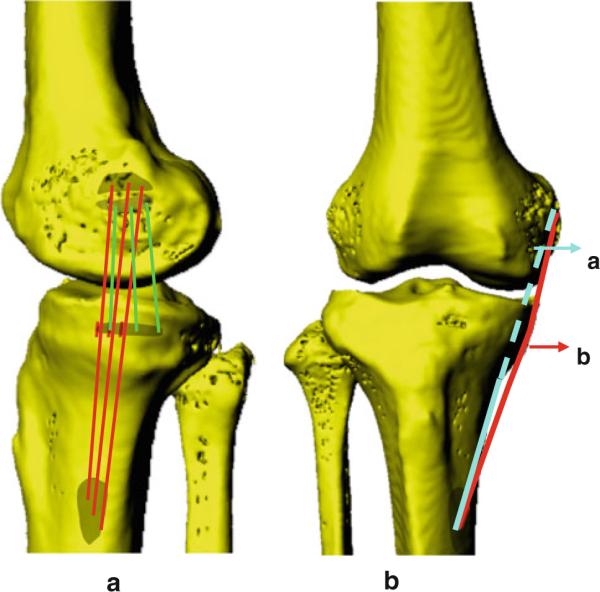
A typical 3D model of the knee created from sagittal plane MRI. a lateral view of a knee model shows the attachment of the sMCL and dMCL; b AP view of a knee model. a, the direct line between the centroid of attachment of sMCL; b, the direct line is projected on the bony surfaces to create a curved ligament
A repeated measure ANOVA and the Student-Newman-Kuels test were used to detect statistically significant differences between bundle lengths and elongations at the 0, 20, 60 and 100% stance. Differences were considered statistically significant when P <.05. The relationship between the relative elongations of the MCL bundles and the flexion-extension were evaluated by regression analysis.
Results
Superficial medial collateral ligament
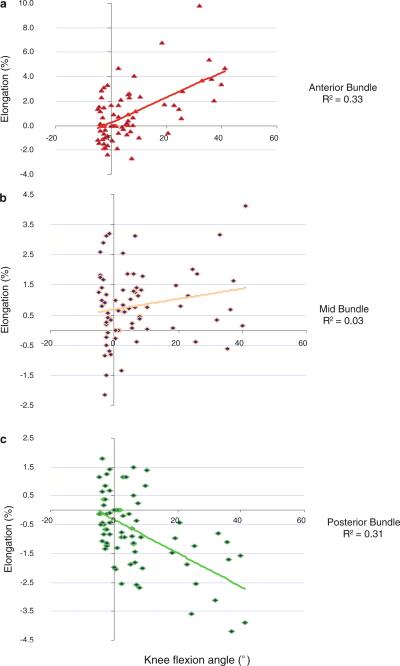
From heel strike to 20% stance, the average length of anterior bundle increased slightly from 86.6 ± 9.8 mm (mean ± SD) to 87.4 ± 9.6 mm (Table 1) with a relative elongation of 1% (Fig. 2a). Thereafter, it decreased slightly at 60% of the stance phase and increased again to a second peak at toe-off when it was measured 90.5 ± 9.2 mm, representing the largest increase of 4.6% relative to its length at heel strike (P < 0.05). The elongation of the anterior bundle of sMCL was correlated positively with the knee flexion angle (R2 = 0.33, P < 0.01) (Fig. 3a).
Table 1.
Length changes of the anterior, mid, and posterior bundles of both the sMCL and dMCL during stance phase of gait (Value ± SD)
| 0% | 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | 90% | 100% | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anterior sMCL | 86.6 ± 9.8 | 87.1 ± 10.1 | 87.4 ± 9.6 | 87.0 ± 9.9 | 86.8 ± 10.2 | 86.6 ± 10.2 | 86.6 ± 9.8 | 86.8 ± 10.8 | 87.5 ± 10.3 | 88.4 ± 10.2 | 90.6 ± 9.2 |
| Mid-sMCL | 94.3 ± 9.7 | 95.0 ± 10.2 | 95.3 ± 10.2 | 95.3 ± 9.2 | 95.1 ± 10.3 | 95.0 ± 10.7 | 95.0 ± 10.7 | 94.9 ± 10.6 | 95.0 ± 10.3 | 95.2 ± 10.5 | 95.6 ± 11.1 |
| Posterior sMCL | 103.4 ± 11.1 | 102.9 ± 10.4 | 102.4 ± 9.7 | 102.4 ± 9.8 | 102.9 ± 10.2 | 103.4 ± 10.4 | 103.5 ± 10.8 | 103.0 ± 10.6 | 102.2 ± 10.4 | 101.5 ± 10.1 | 100.9 ± 10.1 |
| Anterior dMCL | 41.5 ± 3.9 | 42.2 ± 3.7 | 42.5 ± 3.6 | 41.9 ± 2.7 | 41.4 ± 3.3 | 41.2 ± 3.6 | 41.1 ± 3.8 | 41.3 ± 4.0 | 41.8 ± 4.2 | 43.0 ± 4.1 | 45.1 ± 4.3 |
| Mid-dMCL | 41.9 ± 4.2 | 42.5 ± 3.9 | 42.8 ± 3.8 | 42.3 ± 3.8 | 41.8 ± 3.5 | 41.7 ± 3.5 | 41.7 ± 3.6 | 41.8 ± 3.2 | 41.9 ± 2.8 | 42.6 ± 2.9 | 43.5 ± 2.9 |
| Posterior dMCL | 42.8 ± 3.0 | 42.5 ± 2.9 | 42.2 ± 3.5 | 42.2 ± 3.3 | 42.5 ± 3.0 | 42.8 ± 3.2 | 43.0 ± 3.4 | 42.7 ± 3.1 | 42.2 ± 2.9 | 41.3 ± 3.0 | 40.3 ± 3.1 |
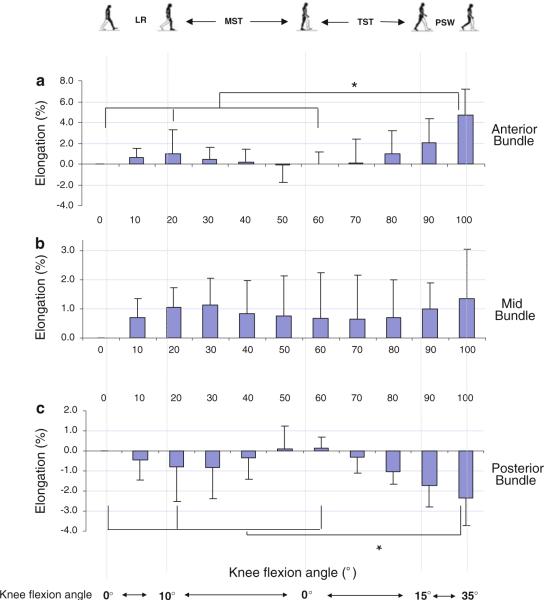
Fig. 2.
The elongation of the anterior (a), mid (b), and posterior (c) portions of the sMCL during the stance phase of gait. (LR loading response, MST mid-stance, TST terminal stance, PSW pre-swing) An asterisk indicates a statistically significant difference (P<0.05)
Fig. 3.
Correlation between the relative elongations of the sMCL portions and the knee flexion angle during the stance phase of gait. a elongation of the anterior portion is positively correlated with the knee flexion angle, b mid-portion, c elongation of the posterior portion is negatively correlated with the knee flexion angle
There was neither a statistically significant change in length of the middle bundle of the sMCL at all studied time-points of the stance phase (Fig. 2b), nor a significant correlation between the elongation of the mid-bundle and the knee flexion angle (R2 = 0.03) (Fig. 3b).
On average, the posterior bundle remained relatively unchanged from 103.4 ± 11.1 mm to 102.4 ± 9.7 mm at heel strike to 20% of the gait cycle (Table 1) with a decrease of −0.8% in relative elongation (Fig. 2c). The length at pre-swing phase was significantly decreased (P < 0.05) with a decrease of 2.4% relative to its length at full extension. The elongation of the posterior bundle of sMCL was negatively correlated with the flexion (R2 = 0.31, P < 0.001) (Fig. 3c).
Deep medial collateral ligament
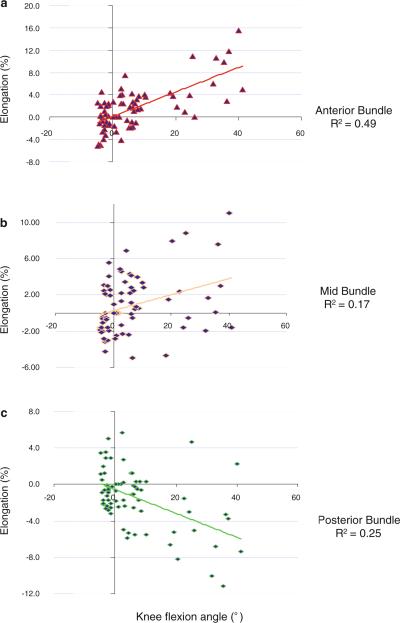
From heel strike to 20% stance, the length of anterior bundle increased slightly from 41.5 ± 3.9 mm to 42.5 ± 3.6 mm (Table 1) with an increase of 2.5% in relative elongation (Fig. 4a). Thereafter, it decreased slightly to 41.1 ± 3.8 mm at 60% of the stance phase and increased to a second peak at toe-off when it was measured 45.1 ± 4.3 mm, which was significantly different from the length measured at all other intervals of the stance phase (P < 0.05), representing an increase of 8.8% relative elongation compared to its length at heel strike of the knee. The elongation of the anterior dMCL was positively correlated with the knee flexion angle (R2 = 0.49, P < 0.01) (Fig. 5a).
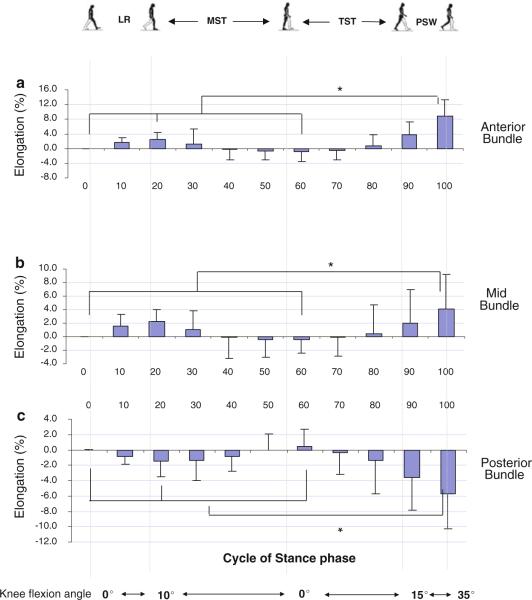
Fig. 4.
The elongation of the anterior (a), mid (b), and posterior (c) portions of the dMCL during the stance phase of gait. An asterisk indicates a statistically significant difference (P<0.05)
Fig. 5.
Correlation between the relative elongations of the dMCL portions and the knee flexion angle during the stance phase of gait. a elongation of the anterior portion is positively correlated with the knee flexion angle, b mid-portion, c elongation of the posterior portion is negatively correlated with the knee flexion angle
The middle portion of the sMCL slightly increased from 41.9 ± 4.2 mm to 42.8 ± 3.8 mm from heel strike to 20% stance (Table 1), representing a relative elongation of 2.3% (Fig. 4b). Thereafter, it deceased to 41.7 ± 3.6 mm at 60% of the stance phase and increased to 43.5 ± 2.9 mm, at toe-off, representing a 4.1% increase in length (P < 0.05). The elongation of the mid-portion of the dMCL was significantly correlated with the knee flexion during the stance phase of gait (R2 = 0.17, P < 0.01) (Fig. 5b).
The posterior portion decreased slightly in length (1.4%) from 42.8 ± 3.0 mm to 42.2 ± 3.5 mm (Table 1; Fig. 4c). Thereafter, it increased to 43.0 ± 3.4 mm at 60% of stance and decreased to a second peak at toe-off when it was measured 40.3 ± 3.1 mm, representing a 5.8% reduction in length compared to that at heel strike (P < 0.05). The elongation of the posterior portion of the dMCL was negatively correlated with the knee flexion angle (R2 = 0.25, P < 0.01) (Fig. 5c).
Discussion
The most important finding of this study was that the anterior and posterior portions of the sMCL and dMCL were demonstrated to have a reciprocal function during the stance phase of gait. Overall, the anterior portions had increased lengths with flexion while the posterior portions had increased lengths with extension. The lengths of the mid-portions were relatively constant during the entire stance phase.
A few studies investigated the biomechanical function of the MCL during in vivo dynamic activities of the knee [21, 30]. Park et al. measured the length patterns of the sMCL and dMCL portions and found that the anterior and posterior portions of the sMCL and dMCL function in a reciprocal fashion and their lengths depend on the knee flexion angles [21]. In a computer simulation of human gait, Shelburne et al. [27] found that the superficial MCL forces decreased with knee flexion during the stance phase of the simulated gait. These data were consistent with the length change patterns of the MCL observed in this study. Early cadaveric studies have shown that during passive knee flexion, the anterior border of the sMCL had increased length with flexion at low flexion angles and the posterior border showed decreased length with flexion [2, 33]. This in vitro data are consistent with our data that were obtained during in vivo gait at low flexion angles.
The data of this study may have implications on further research of MCL injuries. Various surgical reconstructions of the MCL have been reported in literature [1, 3, 18, 32, 35]. Single portion reconstruction of the MCL has been reported in various studies [1, 18, 32, 35]. Several studies have also proposed double portion reconstruction techniques [3, 7]. Due to the reciprocal function of the MCL portions, a single portion reconstruction may not fully reproduce the MCL function during walking or flexion/extension of the knee. Double portion reconstruction that is aimed to reproduce the anterior and posterior portions of the MCL may better restore the complicated function of the MCL. Recently, Borden et al. [3] reported a double portion reconstruction technique that was attempted to restore the anterior and posterior portion function of sMCL in patient with isolated MCL or combined ACL-MCL injuries in both the acute and the chronic settings. The anterior portion was suggested to be secured at 30° and the posterior portion at 60°. However, according to the data of in vivo MCL functions obtained in this study and previous reports [21, 30], the posterior portion may need to be fixed at a lower flexion angle since it elongates more at low flexion angles; and the anterior portion fixed at a higher flexion angle since it elongates more as flexion angle increases, similar to those adopted in double portion ACL reconstruction [9, 26]. A biomechanical test should be carried out in future to examine the effect of flexion angles of graft portion fixations in a double portion MCL reconstruction.
The dMCL has been studied to a much smaller extent when compared to the sMCL [10, 15, 22, 24, 25, 29]. The dMCL is also connected to the medial meniscus. Injuries to the dMACL were not reported as often as the sMCL [15]. The dMCL is usually resected during total knee arthroplasty surgeries [6, 23, 31]. Currently, the in vivo biomechanical function of the dMCL is still unclear. This data indicated that the dMCL has a complicated function during walking. Future studies are necessary to investigate the effect of individual portions of the dMCL on knee joint kinematics.
Several limitations of this study should be noted. The sample size was relatively small. A complete gait cycle consists of the stance and swing periods. Due to the size limitation of the fluoroscopy, we only studied the stance phase of gait, not including the swing phase. Furthermore, only one cycle was studied for each subject at a relatively slow walking speed (0.67 m/s) in order to minimize their radiation exposure. We studied the length change of the MCL. The strain or forces of the MCL were not studied. Nonetheless, we believed that the findings of this paper should provide new insight into the study of the MCL during walking. Further research is required to fully understand the functional role of the MCL during the dynamic activities such as the running and stair climbing.
Conclusion
This study investigated the in vivo kinematics of the sMCL and dMCL during the stance phase of gait using a non-invasive imaging technique. The data of this study demonstrated that the anterior and posterior bundles of the sMCL and dMCL have a reciprocal function during the stance phase of gait. These data may have useful implications to propose effective surgical reconstructive techniques of MCL injury that can restore normal knee kinematics and as well as for the surgical release of the MCL during TKA.
References
- 1.Adachi N, Ochi M, Deie M, Izuta Y, Kazusa H. New hamstring fixation technique for medial collateral ligament or posterolateral corner reconstruction using the mosaicplasty system. Arthroscopy. 2006;22:e571–e573. doi: 10.1016/j.arthro.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Bartel DL, Marshall JL, Schieck RA, Wang JB. Surgical repositioning of the medial collateral ligament. An anatomical and mechanical analysis. J Bone Joint Surg Am. 1977;59:107–116. [PubMed] [Google Scholar]
- 3.Borden PS, Kantaras AT, Caborn DN. Medial collateral ligament reconstruction with allograft using a double-bundle technique. Arthroscopy. 2002;18:E19. doi: 10.1053/jars.2002.32235. [DOI] [PubMed] [Google Scholar]
- 4.Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee. A biomechanical study. J Bone Joint Surg Am. 1980;62:259–270. [PubMed] [Google Scholar]
- 5.Ellsasser JC, Reynolds FC, Omohundro JR. The non-operative treatment of collateral ligament injuries of the knee in professional football players. An analysis of seventy-four injuries treated non-operatively and twenty-four injuries treated surgically. J Bone Joint Surg Am. 1974;56:1185–1190. [PubMed] [Google Scholar]
- 6.Engh GA. The difficult knee: severe varus and valgus. Clin Orthop Relat Res. 2003;416:58–63. doi: 10.1097/01.blo.0000092987.12414.fc. [DOI] [PubMed] [Google Scholar]
- 7.Feeley BT, Muller MS, Allen AA, Granchi CC, Pearle AD. Biomechanical comparison of medial collateral ligament reconstructions using computer-assisted navigation. Am J Sports Med. 2009;37:1123–1130. doi: 10.1177/0363546508331134. [DOI] [PubMed] [Google Scholar]
- 8.Fetto JF, Marshall JL. Medial collateral ligament injuries of the knee: a rationale for treatment. Clin Orthop Relat Res. 1978;132:206–218. [PubMed] [Google Scholar]
- 9.Fu FH, Shen W, Starman JS, Okeke N, Irrgang JJ. Primary anatomic double-bundle anterior cruciate ligament reconstruction: a preliminary 2-year prospective study. Am J Sports Med. 2008;36:1263–1274. doi: 10.1177/0363546508314428. [DOI] [PubMed] [Google Scholar]
- 10.Griffith CJ, LaPrade RF, Johansen S, Armitage B, Wijdicks C, Engebretsen L. Medial knee injury: part 1, static function of the individual components of the main medial knee structures. Am J Sports Med. 2009;37:1762–1770. doi: 10.1177/0363546509333852. [DOI] [PubMed] [Google Scholar]
- 11.Grood ES, Noyes FR, Butler DL, Suntay WJ. Ligamentous and capsular restraints preventing straight medial and lateral laxity in intact human cadaver knees. J Bone Joint Surg Am. 1981;63:1257–1269. [PubMed] [Google Scholar]
- 12.Halinen J, Lindahl J, Hirvensalo E, Santavirta S. Operative and nonoperative treatments of medial collateral ligament rupture with early anterior cruciate ligament reconstruction: a prospective randomized study. Am J Sports Med. 2006;34:1134–1140. doi: 10.1177/0363546505284889. [DOI] [PubMed] [Google Scholar]
- 13.Holden DL, Eggert AW, Butler JE. The nonoperative treatment of grade i and ii medial collateral ligament injuries to the knee. Am J Sports Med. 1983;11:340–344. doi: 10.1177/036354658301100511. [DOI] [PubMed] [Google Scholar]
- 14.Indelicato PA. Non-operative treatment of complete tears of the medial collateral ligament of the knee. J Bone Joint Surg Am. 1983;65:323–329. [PubMed] [Google Scholar]
- 15.Jones L, Bismil Q, Alyas F, Connell D, Bell J. Persistent symptoms following non operative management in low grade mcl injury of the knee—the role of the deep mcl. Knee. 2009;16:64–68. doi: 10.1016/j.knee.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Last RJ. Some anatomical details of the knee joint. J Bone Joint Surg Br. 1948;30:683–688. [PubMed] [Google Scholar]
- 17.Li G, Kozanek M, Hosseini A, Liu F, Van de Velde SK, Rubash HE. New fluoroscopic imaging technique for investigation of 6dof knee kinematics during treadmill gait. J Orthop Surg Res. 2009;4:6. doi: 10.1186/1749-799X-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lind M, Jakobsen BW, Lund B, Hansen MS, Abdallah O, Christiansen SE. Anatomical reconstruction of the medial collateral ligament and posteromedial corner of the knee in patients with chronic medial collateral ligament instability. Am J Sports Med. 2009;37:1116–1122. doi: 10.1177/0363546509332498. [DOI] [PubMed] [Google Scholar]
- 19.Liu F, Kozanek M, Hosseini A, Van de Velde SK, Gill TJ, Rubash HE, Li G. In vivo tibiofemoral cartilage deformation during the stance phase of gait. J Biomech. 2010;43:658–665. doi: 10.1016/j.jbiomech.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monahan JJ, Grigg P, Pappas AM, Leclair WJ, Marks T, Fowler DP, Sullivan TJ. In vivo strain patterns in the four major canine knee ligaments. J Orthop Res. 1984;2:408–418. doi: 10.1002/jor.1100020414. [DOI] [PubMed] [Google Scholar]
- 21.Park SE, DeFrate LE, Suggs JF, Gill TJ, Rubash HE, Li G. The change in length of the medial and lateral collateral ligaments during in vivo knee flexion. Knee. 2005;12:377–382. doi: 10.1016/j.knee.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Price CT, Allen WC. Ligament repair in the knee with preservation of the meniscus. J Bone Joint Surg Am. 1978;60:61–65. [PubMed] [Google Scholar]
- 23.Ritter MA, Faris GW, Faris PM, Davis KE. Total knee arthroplasty in patients with angular varus or valgus deformities of >or = 20°. J Arthroplasty. 2004;19:862–866. doi: 10.1016/j.arth.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Robinson JR, Bull AM, Amis AA. Structural properties of the medial collateral ligament complex of the human knee. J Biomech. 2005;38:1067–1074. doi: 10.1016/j.jbiomech.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 25.Robinson JR, Bull AM, Thomas RR, Amis AA. The role of the medial collateral ligament and posteromedial capsule in controlling knee laxity. Am J Sports Med. 2006;34:1815–1823. doi: 10.1177/0363546506289433. [DOI] [PubMed] [Google Scholar]
- 26.Seon JK, Song EK, Bae BH, Park SJ, Yoon TR, Cho SG, Lee JJ, Kim MS. Kinematic study following double-bundle, anterior cruciate ligament reconstruction. Int Orthop. 2007;31:623–628. doi: 10.1007/s00264-006-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shelburne KB, Torry MR, Pandy MG. Muscle, ligament, and joint-contact forces at the knee during walking. Med Sci Sports Exerc. 2005;37:1948–1956. doi: 10.1249/01.mss.0000180404.86078.ff. [DOI] [PubMed] [Google Scholar]
- 28.Shoemaker SC, Markolf KL. Effects of joint load on the stiffness and laxity of ligament-deficient knees. An in vitro study of the anterior cruciate and medial collateral ligaments. J Bone Joint Surg Am. 1985;67:136–146. [PubMed] [Google Scholar]
- 29.Sims WF, Jacobson KE. The posteromedial corner of the knee: medial-sided injury patterns revisited. Am J Sports Med. 2004;32:337–345. doi: 10.1177/0363546503261738. [DOI] [PubMed] [Google Scholar]
- 30.Van de Velde SK, DeFrate LE, Gill TJ, Moses JM, Papannagari R, Li G. The effect of anterior cruciate ligament deficiency on the in vivo elongation of the medial and lateral collateral ligaments. Am J Sports Med. 2007;35:294–300. doi: 10.1177/0363546506294079. [DOI] [PubMed] [Google Scholar]
- 31.Verdonk PC, Pernin J, Pinaroli A, Ait Si Selmi T, Neyret P. Soft tissue balancing in varus total knee arthroplasty: an algorithmic approach. Knee Surg Sports Traumatol Arthrosc. 2009;17:660–666. doi: 10.1007/s00167-009-0755-7. [DOI] [PubMed] [Google Scholar]
- 32.Wahl CJ, Nicandri G. Single-achilles allograft posterior cruciate ligament and medial collateral ligament reconstruction: a technique to avoid osseous tunnel intersection, improve construct stiffness, and save on allograft utilization. Arthroscopy. 2008;24:486–489. doi: 10.1016/j.arthro.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Warren LA, Marshall JL, Girgis F. The prime static stabilizer of the medical side of the knee. J Bone Joint Surg Am. 1974;56:665–674. [PubMed] [Google Scholar]
- 34.Wijdicks CA, Griffith CJ, LaPrade RF, Spiridonov SI, Johansen S, Armitage BM, Engebretsen L. Medial knee injury: Part 2, load sharing between the posterior oblique ligament and superficial medial collateral ligament. Am J Sports Med. 2009;37:1771–1776. doi: 10.1177/0363546509335191. [DOI] [PubMed] [Google Scholar]
- 35.Yoshiya S, Kuroda R, Mizuno K, Yamamoto T, Kurosaka M. Medial collateral ligament reconstruction using autogenous hamstring tendons: Technique and results in initial cases. Am J Sports Med. 2005;33:1380–1385. doi: 10.1177/0363546504273487. [DOI] [PubMed] [Google Scholar]