Abstract
The GTP binding proteins Rhes and AGS1/Dexras1 define a subfamily of the Ras superfamily and have been shown to affect signaling by G protein-coupled receptors. We tested the effects of both proteins at an early stage of signaling by dopamine receptors—activation of adenylyl cyclase. Rhes decreased dopamine D1 receptor agonist-stimulated cAMP accumulation in a pertussis toxin-sensitive manner. It had no effect on cAMP accumulation in the absence of agonist. AGS1/Dexras1, on the other hand, decreased cAMP accumulation in both vehicle-treated and agonist-treated cells, resulting in a higher percent stimulation by agonist, or a higher signal-to-noise ratio. The effects of AGS1/Dexras1 on cAMP accumulation were not blocked by pertussis toxin, suggesting that it may produce these effects through interaction with a Gαi monomer. Both Rhes and AGS1/Dexras1 associated with GTP-bound Gαi in pull-down assays. However, Rhes had no effect on the ability of activated D2 receptor to inhibit cAMP. Neither Rhes nor AGS1/Dexras1 interacted with the D1 receptor in pull-down assays. These findings show that in addition to its well-known effects on signaling through Gi-coupled receptors, AGS1/Dexras1 can affect signaling through a Gs/olf-coupled receptor. Furthermore, they suggest that Rhes exerts some of its effects by interacting with Gαi.
Keywords: RASD1, RASD2, cAMP, Ras, G protein
INTRODUCTION
Signaling by G protein-coupled receptors (GPCR) is modulated by interactions with several different types of proteins. For example, regulators of G protein signaling (RGS) are a large family of proteins that can attenuate GPCR signaling by enhancing the GTPase activity of the G protein (Ross and Wilkie, 2000). More recently, a structurally diverse family of activators of G protein signaling (AGS) has been identified based on a functional screen (Cismowski et al., 2000; Blumer et al., 2005). Rhes, the Ras Homolog Enriched in Striatum, forms a novel subfamily of the Ras superfamily along with a member of the AGS family termed AGS1/Dexras1. In addition to the Ras core domains, they contain an extended carboxyl terminus, thus making them intermediate in size between Ras-like small GTPases and α subunits of heterotrimeric G proteins (Falk et al., 1999). AGS1/Dexras1, whose expression is regulated by dexamethasone (Kemppainen and Behrand, 1998), has been shown to have a complicated role in ligand-mediated versus basal signaling through Gi/o, whereas Rhes has been shown to affect signaling through Gs/olf- and Gi/o-coupled receptors by an unknown mechanism.
Rhes is a 266-amino acid protein that shares 62% identity with AGS1/Dexras1. It was originally identified by subtractive hybridization based on its enrichment in striatum (Falk et al., 1999; Usui et al., 1994). Although it is preferentially expressed in striatum of rodents, rhes mRNA also displays light to moderate expression in hippocampus, cerebellum, olfactory bulb, and thalamic nuclei, particularly during early postnatal development (Falk et al., 1999; Vargiu et al., 2004; Harrison and LaHoste, 2006; Harrison et al., 2008). However, the significance of the striatal enrichment was recently demonstrated in that Rhes promotes striatal-specific cell death in Huntington’s Disease (Subramaniam et al., 2009). Rhes expression in striatum is modulated by thyroid hormone during development (Falk et al., 1999; Vargiu et al., 2001) and by dopamine innervation in adult rats (Harrison and LaHoste, 2006; Harrison et al., 2008). Behavioral studies with rhes mutant mice have also highlighted the importance of Rhes expression in striatum and indicate that it normally inhibits certain dopamine-mediated behaviors. Both locomotor activity and stereotypy are increased in rhes−/−mice relative to rhes+/+ mice after administration of dopaminergic drugs. Furthermore, rhes−/− mice display more D2 receptor antagonist-induced catalepsy than rhes+/+ mice (Errico et al., 2008; Quintero et al., 2008).
Early evidence indicated that Rhes and AGS1/Dexras1 differ in the heterotrimeric G proteins that they modulate, with AGS1/Dexras1 preferentially affecting Gi/o and Rhes affecting Gs/olf. For example, AGS1/Dexras1 can act as a guanine nucleotide exchange factor (GEF) for monomeric Gαi in vitro (Cismowski et al., 2000) and can inhibit ligand-mediated signaling through βγ subunits liberated by Gi/o-coupled receptors (Graham et al., 2002; Takesono et al., 2002; Nguyen and Watts, 2005). Initial investigations into the mechanisms of Rhes action demonstrated an effect at Gs/olf-coupled receptors. Thus, although Rhes did not affect reporter gene activation by the Gi/o-coupled M2 muscarinic receptor, it inhibited reporter gene activation by the Gs-coupled thyroid stimulating hormone receptor (Vargiu et al. 2004). However, a recent study by Thapliyal et al. (2008) has provided evidence that Rhes, like AGS1/Dexras1, can affect Gi/o. Both AGS1/Dexras1 and Rhes inhibited N-type calcium channels but attenuated the ability of ligands for Gi/o-coupled receptors to inhibit these channels, an effect mediated by βγ subunits liberated from pertussis toxin (PTX)-sensitive G proteins (Thapliyal et al., 2008).
There is thus much evidence that Rhes affects signaling by GPCRs, but the exact locus and mechanism(s) of these effects are unknown. We have hypothesized that the ability of dopamine D1 receptors to activate adenylyl cyclase (AC) is inhibited by Rhes. Here we demonstrate that Rhes can physically interact with Gαi and can interfere with AC activation by the Gs/olf-coupled dopamine D1 receptor in a PTX-sensitive manner. AGS1/Dexras1 was also shown to affect D1 receptor-mediated activation of AC, but by an apparently different mechanism.
Materials and Methods
Materials
AGS1-Dexras1/pcDNA3.1 containing the full coding sequence of human AGS1 was obtained from the Missouri S&T cDNA Resource Center (www.cDNA.org). All other expression constructs were prepared in our laboratory using rat brain cDNA (Clontech; Mountain View, CA) and the pcDNA3.1 or pET expression plasmid (Invitrogen; Carlsbad, CA). Immunoassay kits for cAMP were purchased from either Millipore (Billerica, MA) or Applied Biosystems (Carlsbad, CA). SKF 83822, quinpirole, and pertussis toxin were from Tocris (Ellisville, MO). All other reagents were from Sigma (St. Louis, MO) unless otherwise noted.
Animals
C57/BL6 mice were used to obtain striatal tissue for pull-down assays. Mice were housed 4–5 to a cage under a 12 hour light/dark cycle with water and food provided ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of the Louisiana State University Health Sciences Center and were in accord with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Every effort was made to minimize the suffering of the animals and to reduce the number of animals used.
CHO Cell Culture and cAMP Assays
CHO-K1 cells were seeded in 24-well plates at a density of 1.75–2.00 × 105 cells/well and grown in DMEM/10% FBS. Dopamine D1 or D2 (long) receptors in pcDNA3.1 (0.8 μg) were transiently co-transfected with either Rhes/pcDNA3.1, AGS1-Dexras1/pcDNA3.1, or empty vector (0.8 μg) by using Lipofectamine2000 (Invitrogen). Assays for cAMP accumulation were performed 24 hours later, at a time of maximum expression of Rhes protein (Harrison et al., 2008). For stimulation assays, cells were switched to serum-free medium, and 1 mM IBMX was added. After a 5-minute incubation at 37°C, drug, forskolin, or vehicle was added, and cells were incubated at 37°C for 1 hour. Accumulation of cAMP was measured with a chemiluminescent immunoassay kit from either Millipore or Applied Biosystems according to the manufacturer’s instructions, using an Appliskan luminometer. For some assays, cells were pre-incubated for ~16 hours with pertussis toxin (PTX, 100 ng/ml). For inhibition assays, cells were switched to PBS, and pre-incubated for 5 min at 37°C with 0.5 mM IBMX and drug or vehicle. Forskolin (25 μM) was then added, and cells were incubated at 37°C for exactly 10 minutes followed by assay for cAMP as above.
Pull-Down Assays
Pull-down assays were performed with the ProFound Pull-Down PolyHis Protein:Protein Interaction Kit (Pierce/Thermo; Rockford, IL). “Bait” was either a His-tagged Rhes protein or a His-tagged AGS1/Dexras1 protein prepared as follows. The full-length coding sequence of Rhes (30 kDa form) was amplified from rat brain cDNA by PCR and directionally inserted into pET (Invitrogen) in frame with a poly-His tag at the N-terminus. Sequence was verified by dideoxynucleotide sequencing. AGS1/Dexras1 was PCR-amplified from AGS1/pcDNA3.1 with primers allowing for directional cloning into pET in frame with the His tag at the N-terminus. Rhes or AGS1/Dexras1 protein was expressed in BL21 Star cells, and His-tagged proteins from lysate were immobilized onto a cobalt resin. “Prey” was lysate from striatal punches taken from C57/BL6 mice (age 2–4 months). Bait and prey were incubated in the presence of either 30 μM GDP or 25 mM MgCl2/30 μM GTPγS (Bernard et al., 2001) for 1 hour at 4°C. After washing of the cobalt resin, samples were eluted and analyzed by Western Blotting.
Western Blotting
For tests of protein expression in CHO cells, cells were seeded and transiently transfected as described above. Cells were lysed in buffer containing 50 mM Tris, pH 8, 150 mM NaCl, 5 mM MgCl2, 1% Triton-X 100, 0.5% sodium deoxycholate, 0.1% SDS, and protease inhibitor cocktail (Sigma); protein concentration was determined with a Bio-Rad Detergent Compatible Protein Assay kit. For Western blots, proteins (20 μg cell lysate or 20 μl of eluate from Pull-Down assays) were separated by SDS-PAGE using 12.5% Criterion polyacrylamide gels (Bio-Rad; Hercules, CA) and transferred to PVDF membranes. After blocking with 5% Carnation® milk in TBS-T (20 mM Tris, 150 mM NaCl, 0.1% Tween-20), membranes were incubated at 4°C overnight in TBS-T/5% BSA in primary antibody [Rhes, 1:500 or 1:1000 (custom-made by Bethyl Laboratories; Montgomery, TX); Dexras1/2, 1:500 (Santa Cruz; sc-16404); Gαi-3, 1:1000 (Santa Cruz; sc-262); Gαs/olf, 1:1000 (Santa Cruz; sc-383); or D1, 1:500 (Santa Cruz; sc-14001)]. Membranes were washed and incubated for 1 hour in HRP-labeled goat anti-rabbit or rabbit anti-goat antibody. Bands were visualized by using the SuperSignal chemiluminescence detection kit (Pierce/Thermo Fisher).
Data Analysis
For cAMP assays, percent stimulation was calculated as: [(stimulated response-baseline response)/baseline response] X 100. Concentration-response curves were fitted by nonlinear regression with GraphPad Prism, and EC50 and Emax values, or IC50 and Imax values, were determined for individual assays. These values were then analyzed by one-factor ANOVA, with post-hoc Newman-Keuls or Bonferroni tests where appropriate, or by Student’s t-test. Results from PTX experiments were analyzed by either two-factor ANOVA with Bonferroni post-hoc tests or by one-factor ANOVA with Newman-Keuls post-hoc tests.
Results
A transient expression system in CHO cells was used to measure the effects of Rhes and AGS1/Dexras1 on cAMP accumulation induced by dopamine D1 receptor activation. As shown in Figure 1, CHO cells do not endogenously express either Rhes or AGS1/Dexras1, but at 24h post-transfection, the proteins are expressed in the appropriately transfected cells. A custom-made antibody to Rhes (Bethyl Laboratories) detected a protein of ~30 kDa in Rhes/pcDNA3.1-transfected cells (Figure 1, top panel). A commercially-available antibody to a region of the C-terminus of Rhes that cross-reacts with AGS1/Dexras1 (Dexras1/2, Santa Cruz) detected each protein in the appropriately transfected cells (Figure 1, middle panel). Furthermore, CHO cells did not endogenously express the D1 receptor, but after transient transfection, the D1 receptor was detected in a high molecular weight, likely oligomeric, form (Figure 1, bottom panel). Although the Western blots are not quantitative, there were no gross differences in D1 expression levels when it was expressed alone or co-expressed with AGS1/Dexras1 or Rhes.
Figure 1.
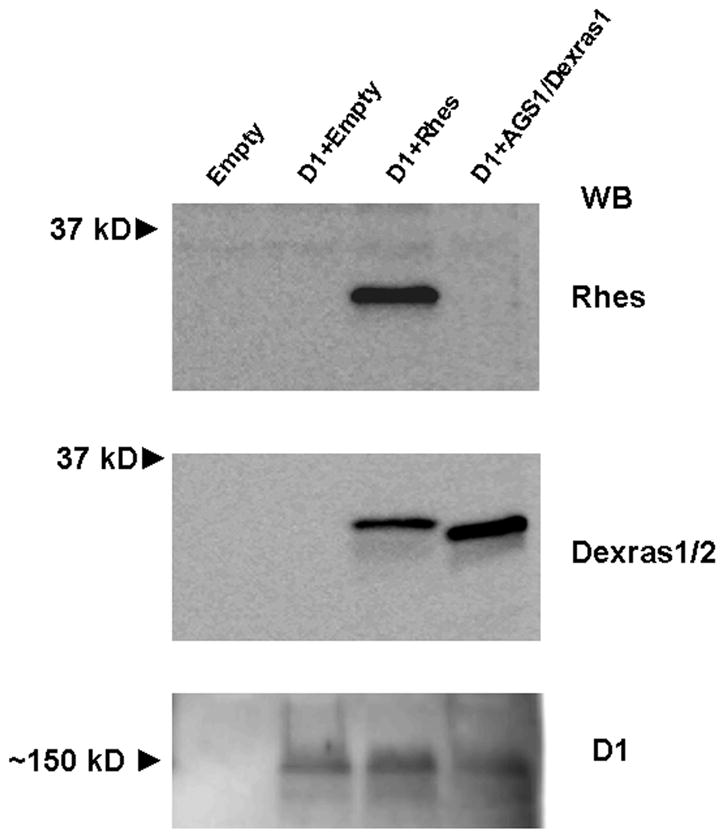
Expression of Rhes, AGS1/Dexras1, and D1 receptors in CHO cells. Shown are Western blots of cell lysates (20 μg total protein) of CHO cells assayed 24 hours after transfection with either empty vector, or a combination of D1 and either Rhes or AGS1/Dexras1. Empty vector-transfected cells did not express any of the proteins. A ~30 kD protein was detected by the anti-Rhes antibody in Rhes-transfected cells, whereas the antibody to Dexras1/2 detected a ~30 kD protein in cells transfected with either Rhes or AGS1/Dexras1. D1 receptor was detected in all D1-transfected cells, in a large, likely oligomeric, form. Blots shown are representative of 4 assays. WB = Western blot.
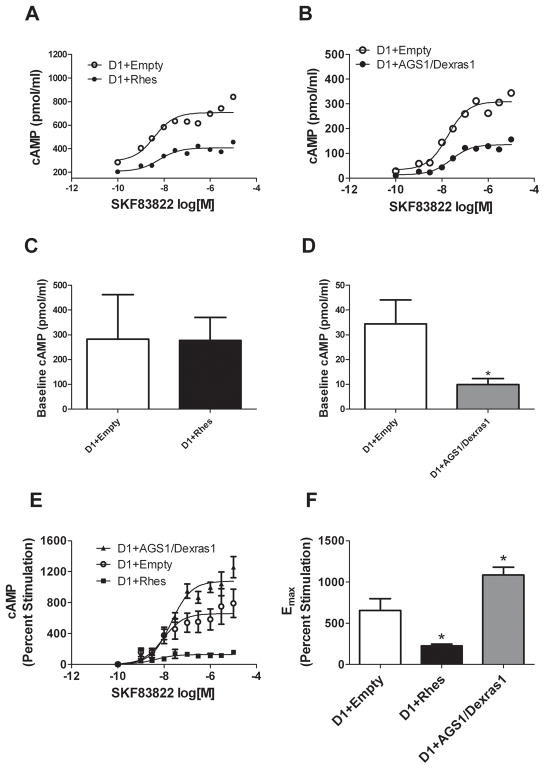
The ability of Rhes and AGS1/Dexras1 to affect D1 dopamine receptor signaling was measured at the level of cAMP accumulation. In CHO cells co-transfected with D1 receptors and empty vector, the D1 agonist SKF 83822, which stimulates AC but not phospholipase C (Undie et al., 1994), concentration-dependently increased cAMP production over a range from 1 nM to 10 μM. However, when either Rhes or AGS1/Dexras1 was co-transfected with D1, the amount of cAMP induced by D1 receptor activation was reduced (Figure 2a and b). Although there was some assay-to-assay variability in absolute numbers, the pattern of responses among all assays was consistent. Rhes had no effect on basal cAMP levels (p = 0.9659 by paired t-test; mean ± SEM: 283 ± 179 for D1 + Empty, 278 ± 94 for D1 + Rhes), whereas AGS1/Dexras1 significantly decreased basal cAMP (p = 0.0217 by paired t-test; mean ± SEM: 34.3 ± 9.8 for D1 + Empty, 10.0 ± 2.4 for D1 + AGS1/Dexras1; Figure 2c and d). In Figure 2e, values are presented as percent stimulation of cAMP above baseline. Whereas Rhes attenuated cAMP stimulation by SKF 83822, co-transfected AGS1/Dexras1 increased the percent stimulation of cAMP by SKF 83822. Two-factor ANOVA (treatment × concentration) indicated significant main effects of treatment [F = 47.2, p<0.0001] and concentration [F = 13.4, p<0.0001], and a significant interaction [F = 2.6, p = 0.001] (Figure 2e). Emax values for percent stimulation were (mean ± SEM): 655 ± 142 for D1+Empty, 225 ± 22 for D1+Rhes, and 1086 ± 95 for D1+AGS1/Dexras1. One-factor ANOVA of Emax values indicated a significant overall effect [F = 7.85, P = 0.0038], with both the D1+Rhes and the D1+AGS1/Dexras1 conditions differing from D1+Empty (p<0.05, Figure 2f). EC50 values determined from individual assays were (mean ± SEM): 10.4 ± 2.4 nM for D1+Empty, 8.0 ± 2.8 nM for D1+Rhes, and 22.7 ± 6.6 for D1+AGS1/Dexras1. One-Factor ANOVA indicated a significant overall effect [F = 3.72, p = 0.0458], but post-hoc tests did not indicate any significant differences between individual treatment groups (data not shown).
Figure 2.
Effects of Rhes and AGS1/Dexras1 on D1 receptor-induced cAMP accumulation in CHO cells. Both Rhes (A) and AGS1/Dexras1 (B) decreased SKF83822-induced cAMP accumulation when co-expressed with D1 receptors. Data are presented as mean pmol/ml cAMP (n = 5 for each of A and B). (C) Rhes did not affect basal cAMP levels (p = 0.966 by Student’s t-test, n = 5), whereas AGS1/Dexras1 (D) significantly decreased basal cAMP (*p<0.05 by Student’s t-test, n = 7). (E) Concentration-response curves for data expressed as percent stimulation of cAMP in CHO cells transfected with dopamine D1 receptors and either empty vector, AGS1/Dexras1, or Rhes. Data are mean ± SEM for percent stimulation over vehicle-treated cells. (F) Emax values (mean ± SEM) calculated from individual assays and analyzed by one-factor ANOVA and post-hoc Newman-Keuls tests. *p<0.05 versus D1+Empty. For E and F, n = 10 (D1+Empty), n = 5 (D1+ AGS1/Dexras1 and D1+Rhes).
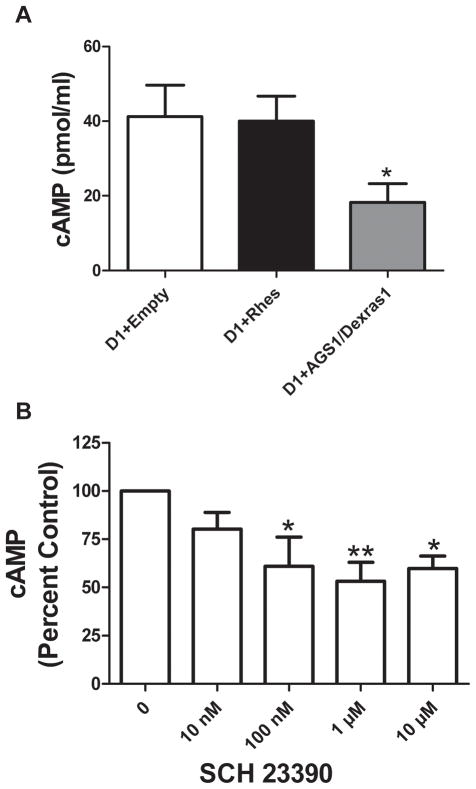
As the absolute values for cAMP differed in assays with Rhes and AGS1/Dexras1, we confirmed their differential effects on baseline cAMP values in side-by-side comparisons in vehicle (<0.2% ethanol)-treated cells and using a cAMP ELISA kit from a single manufacturer. As shown in Figure 3a, Rhes again did not affect the baseline cAMP values, whereas AGS1/Dexras1 consistently and significantly decreased basal cAMP (F = 6.995, p = 0.0175 by One-Factor ANOVA). Thus, the absolute amount of measurable baseline cAMP did not affect Rhes or AGS1/Dexras1. This basal cAMP accumulation could be in part accounted for by constitutive activation of D1 receptors in that the D1 receptor antagonist SCH 23390 dose-dependently attenuated cAMP accumulation in D1-transfected cells (Figure 3b; F = 6.787, p <0.01 by repeated measures One-Factor ANOVA).
Figure 3.
Baseline cAMP in CHO cells. (A) When assayed simultaneously, Rhes did not affect baseline cAMP values, whereas AGS1/Dexras1 did (*p<0.05 by One-Factor ANOVA with post-hoc Bonferroni tests, n = 5). (B) The D1 receptor antagonist SCH23390 attenuated baseline cAMP accumulation in a concentration-responsive fashion. Data were analyzed by One-Factor ANOVA followed by Bonferroni post-tests. *p<0.05, **p<0.01, n = 4.
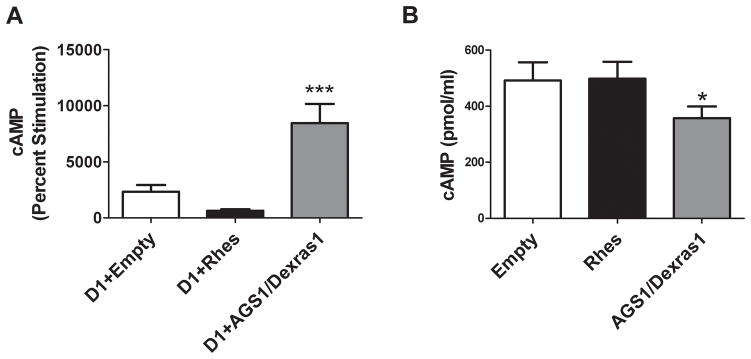
The effect of forskolin (10 μM) to stimulate adenylyl cyclase was tested in cells expressing AGS1/Dexras1 or Rhes. As shown in Figure 4a, cells transfected with AGS1/Dexras1 showed a significantly higher percent stimulation than cells transfected with Rhes or empty vector, whereas Rhes did not affect forskolin-stimulated cAMP accumulation relative to empty vector [F = 13.5, p = 0.0002]. Figure 4b shows absolute values of cAMP (pmol/ml) after forskolin stimulation in cells not expressing the D1 receptor in order to eliminate any effects of D1 constitutive activity on cAMP levels. Rhes had no effect on cAMP accumulation by forskolin, whereas AGS1/Dexras1 significantly reduced cAMP (F = 7.631, p = 0.0097 by One Factor ANOVA). This finding suggests that AGS1/Dexras1 may act by a more general mechanism, whereas Rhes specifically affects activated receptor-stimulated cAMP accumulation.
Figure 4.
Effects of Rhes and AGS1/Dexras1 on forskolin (10 μM)-stimulated cAMP accumulationin CHO cells. (A) Data (mean ± SEM) are presented as percent stimulation above vehicle-treated cells and were analyzed by One-Factor ANOVA. ***p<0.001 versus D1+Empty by Newman-Keuls post-hoc tests. n = 10, D1+Empty; n = 5, D1+ AGS1/Dexras1 and D1+Rhes. (B) Data (mean ± SEM) are presented as pmol/ml and were analyzed by One-Factor ANOVA. *p<0.05by Bonferroni post-tests, n = 6.
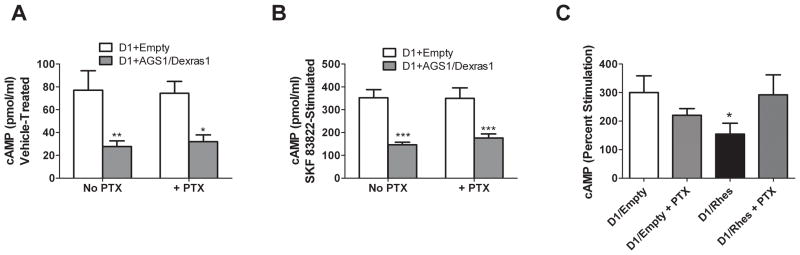
AGS1/Dexras1 is well known for its ability to act as a GEF for Gαi/o (Cismowski et al., 2000). We therefore sought to determine if this property could account for its effects on the Gs/olf-coupled D1 receptor—that is, an increase in the inhibitory tone on AC could indirectly affect cAMP accumulation by D1 receptors. Thus, CHO cells were treated with pertussis toxin in order to assess the role of Gi/o in the effects of AGS1/Dexras1. As AGS1/Dexras1 affects both basal and stimulated cAMP accumulation, both parameters were tested. Pertussis toxin (100 ng/ml) did not affect the ability of AGS1/Dexras1 to decease cAMP levels in vehicle-treated (Figure 5a) or SKF 83822 (10 μM)-treated cells (Figure 5b). In vehicle-treated cells, two-factor ANOVA indicated a significant main effect for AGS1/Dexras1 [F = 20.44, p<0.0001], but not for PTX [F = 0.006, p = 0.94]. Similarly, in SKF 83822-treated cells, there was a significant main effect for AGS1/Dexras1 [F = 21.05, p = 0.0003], but not for PTX [F = 1.009, p = 0.33].
Figure 5.
Effects of pertussis toxin on the abilities of Rhes and AGS1/Dexras1 to alter cAMP accumulation. CHO cells expressing D1 receptors with or without Rhes or AGS1/Dexras1 were treated for ~16 hours with PTX (100 ng/ml) and assayed for cAMP accumulation in the presence of vehicle or 10 μM SKF 83822. cAMP accumulation (pmol/ml, mean ± SEM) in vehicle (0.2% ethanol)-treated cells (A) and in SKF 83822 (10 μM)-treated cells (B) is significantly decreased by AGS1/Dexras1 in a non-PTX reversible manner. Data were analyzed by two-factor ANOVA followed by Bonferroni post-tests. *p<0.05, **p<0.01, ***p<0.001 versus D1+Empty in the same toxin condition. n = 9. (C) Pertussis toxin blocks the ability of Rhes to attenuate D1 receptor-mediated cAMP accumulation. Data were analyzed by One-Factor ANOVA, followed by Newman-Keuls Post-hoc tests. * p<0.05 versus D1/Empty and versus D1/Rhes + PTX. n = 4.
Although AGS1/Dexras1 is known to affect signaling through Gi/o but not Gs (Cismowski et al., 2000), evidence suggests that Rhes may interact with both of these pathways (Vargiu et al., 2004; Thapliyal et al. 2008). We therefore tested the effect of PTX on Rhes action. As Rhes does not affect basal cAMP accumulation (Figures 2 and 3), data are shown as percent stimulation. As shown in Figure 5C, PTX completely blocked the ability of Rhes to attenuate D1-mediated cAMP accumulation [F = 8.302, p<0.05]. This result suggests that the effect of Rhes on D1 receptor-mediated cAMP accumulation is mediated through an interaction of Rhes and Gi/o.
The PTX results with Rhes suggest that this protein may interact with Gαi. Such an interaction may affect the ability of Gi-coupled receptors to inhibit cAMP. Therefore, we tested the effect of Rhes on the ability of the dopamine D2/D3 agonist quinpirole to inhibit cAMP accumulation in D2-transfected CHO cells. As shown in Figure 6, quinpirole induced a significant inhibition of forskolin-stimulated cAMP accumulation regardless of the presence of Rhes. Two-factor ANOVA indicated a highly significant effect of concentration [F = 16.09, p<0.0001], but no significant effect of treatment [F = 0.434, p = 0.5113] or a concentration x treatment interaction [F = 0.5115, p = 0.8608]. IC50 values were (mean ± SEM) 7.6 ± 1.9 nM for D2+empty and 12.3 ± 5.7 nM for D2+Rhes; p = 0.9941). Imax values were (mean ± SEM) 54.6 ± 2.4 for D2+empty and 57.3 ± 6.1 for D2+Rhes; p = 0.6507. Thus, like previous reports for AGS1/Dexras1 (Nguyen and Watts, 2005), Rhes did not affect the ability of the Gi/o-coupled dopamine D2 receptor to inhibit cAMP accumulation.
Figure 6.
Rhes does not affect inhibition of forskolin (25 μM)-stimulated cAMP accumulation by dopamine D2 receptors. Concentration-response curves for cells treated with vehicle or the D2/D3 receptor agonist quinpirole over a range of 1 nM to 10 μM. Data are mean ± SEM for percent maximum (vehicle-treated). n = 7.
In order to investigate potential interaction of Rhes with Gi/o and other G proteins, we performed pull-down assays with His-tagged Rhes as “bait” and mouse striatal lysate as “prey”. Assays were performed in the presence of 30 μM GDP or 25 mM MgCl2/30 μM GTPγS. Rhes was found to interact with Gαi, but only in the GTP-bound state (Figure 7a). It is likely that this GTPγS binding was to Gα subunits, rather than to Rhes, as preloading Rhes with GTPγS and then carrying out the binding reaction in the presence of GDP did not pull down this Gα subunit (data not shown). Rhes showed no appreciable interaction with Gαs/olf. AGS1/Dexras1 showed a similar ability to interact with GTP-bound Gαi, but not with Gαs/olf (Figure 7b). As there was some background binding of Gαi to the cobalt resin, even after extensive washing, we tested whether the “pull-down” of this protein was indeed due to the bait. As shown in Figure 7c, under identical incubation conditions, considerably more Gαi was pulled down by Rhes-transformed bacterial lysate than by lysate of bacteria transformed with empty vector, thus suggesting an interaction of Rhes and Gαi. Neither Rhes nor AGS1/Dexras1 showed interaction with the D1 receptor (Figure 7d).
Figure 7.

Rhes and AGS1/Dexras1 interact with Gαi, but not Gαs or D1 receptor. (A) Western blot of eluates from pull-down assays with His-tagged Rhes and mouse striatal lysates. Lane 1: Only His-tagged Rhes applied to resin; Lane 2: only striatal lysates applied to resin; Lane 3: Both His-tagged Rhes and striatal lysates applied to resin together with GDP; Lane 4: Both His-tagged Rhes and striatal lysates applied to resin together with GTP. (B) AGS1/Dexras1 showed a similar pattern as Rhes in pull-down assays as described in (A). (C) Lysates from bacteria transformed with His-Rhes-expressing vector are compared with lysates from bacteria transformed with empty vector for the ability to pull down Gαi. Although there was some “pull-down” of Gαi/GTP in the empty vector group, much more protein was pulled down when His-Rhes was present. (D) Neither Rhes (top) nor AGS1/Dexras1 (bottom) pulled down the D1 receptor from striatal lysates. Lane 1: striatal lysate input showing D1 receptor in likely monomeric and dimeric forms; Lane 2: His-tagged bait protein; Lane 3: striatal lysate alone applied to resin; Lane 4: His-tagged bait and striatal lysate applied to the resin.
WB = Western blot. In all cases, blots are representative of 3–4 experiments.
Discussion
The present results indicate that the related proteins Rhes and AGS1/Dexras1 can affect signaling through AC by dopamine D1 receptors. Although both proteins decreased the amount of agonist-stimulated cAMP, only AGS1/Dexras1 decreased cAMP in non agonist-stimulated cells. This property of AGS1/Dexras1 contributed to a higher percent stimulation and thus an increased signal-to-noise ratio. In addition, our data suggest that like AGS1/Dexras1, Rhes can interact with Gαi subunits.
Rhes is highly enriched in striatum, where it is known to be involved in the pathology of Huntington’s Disease (Subramaniam et al., 2009), but thus far little is known about its physiological functions. The ability of Rhes to attenuate D1 receptor-mediated cAMP accumulation is in agreement with the work by Vargiu et al. (2004) showing that Rhes attenuated reporter gene activation by the Gs-coupled thyroid stimulating hormone receptor and by a constitutively active β2-adrenergic receptor. Our findings extend these results to show that the effect of Rhes can be detected as proximal in the signaling pathway as activation of AC. The lack of effect of Rhes on forskolin-induced cAMP accumulation is also in agreement with previous results showing that Rhes does not affect reporter gene activation by forskolin or a constitutively active Gαs mutant (Vargiu et al., 2004). Together these findings suggest that Rhes affects the ability of receptors to activate Gs/olf, analogous to the ability of AGS1/Dexras1 to attenuate receptor-mediated activation of Gi/o (e.g. Graham et al., 2002). However, a decrease in AC activity could also be accounted for by an increase in Gαi/o activation, as is apparently the case for AGS1/Dexras1, and Rhes now appears to have complex effects on signaling that involve multiple G proteins. Indeed, a recent study demonstrated Rhes interaction with Gi/o signaling (Thapliyal et al., 2008), and we found that PTX blocked the effect of Rhes on AC and that Rhes is able to interact with Gαi under certain conditions. The full extent of Rhes protein-protein interactions is currently unknown. It interacts with Gβ1, Gβ2, and Gβ3 through its cationic region in the C-terminus (Hill et al., 2009), and it remains possible that it interacts with Gαs and other α-subunits and proteins.
Our findings suggest important similarities and differences between Rhes and AGS1/Dexras1 that may speak to their different physiologies. Their roles in GPCR signaling are quite complex, with effects on (1) both Gα and Gβγ, (2) both Gi and Gs, and (3) differential effects depending on whether or not an activated receptor is involved. Effects on Gα signaling include AGS1/Dexras1 GEF activity at Gαi (Cismowski et al., 2000) and inhibition of AC stimulation by constitutively active Gαs (Graham et al., 2004), as well as Rhes inhibition of AC (present results) and decrease of phosphorylation by protein kinase A (Errico et al., 2008). As for Gβγ-mediated effects, AGS1/Dexras1 promotes basal activation of ERK1/2, but inhibits agonist-stimulated activation (Graham et al., 2002), whereas Rhes has been shown to have no effect on basal ERK1/2 activation (Vargiu et al., 2004), but to affect Gβγ-mediated calcium channel activation (Thapliyal et al., 2008). Rhes effects on AC activation, measured as cAMP accumulation or as activation of downstream factors, appear to require the presence of an activated receptor, evidenced by the lack of effect on forskolin responses. AGS1/Dexras1, on the other hand, has been previously shown to inhibit AC activation by a constitutively active Gαs mutant and to inhibit forskolin-stimulated CREB transactivation (Graham et al., 2004), and here we have shown that it inhibits basal and forskolin-stimulated (as well as D1 receptor-stimulated) AC activity. There is evidence for constitutive activity of D1 receptors in some systems (Cho et al., 1996; Jin et al., 1998). However, in our system only part of the basal activity is likely to be D1-mediated, as the D1 antagonist SCH23390 only partially blocked the basal cAMP.
There are two implications of our overall findings. First, the locus of Rhes action may be at the level of receptor-G protein coupling, as proposed by Vargiu et al. (2004), whereas AGS1/Dexras1 may act further downstream as well. Second, Rhes and AGS1/Dexras1 may act on different forms of heterotrimeric G protein. Graham et al. (2004) have proposed that AGS1/Dexras1 preferentially interacts with monomeric Gαi/o, in agreement with the findings of Cismowski et al. (2000) showing GEF activity of AGS1/Dexras1 on monomeric Gαi. Our findings with PTX and AGS1/Dexras1 were surprising but also point to an effect of AGS1/Dexras1 on a Gαi monomer. The concentration and incubation conditions (100 ng/ml PTX for 16 h) are standard and were effective at blocking the effect of Rhes on D1-mediated cAMP accumulation. Furthermore, the experiments were performed a total of nine times with different lots of PTX and showed strikingly consistent results. As the PTX-insensitive Gαz is not present in CHO cells (Ozawa et al., 1999), it is likely that AGS1/Dexras1 is acting through its well-known interaction with Gαi/o. This finding of non-PTX-reversible effects is similar to effects with NG-GPA (Ribas et al., 2002) and is in agreement with a model of interaction with a monomer, as PTX is only able to ADP-ribosylate the heterotrimer (Tsai et al., 1984). PTX does block the effects of AGS1/Dexras1 on βγ-mediated ERK1/2 activation (Graham et al., 2002). Thus, it may be that AGS1/Dexras1 can interact with both a heterotrimer and an α-monomer, with effects on AC being mediated by its continued association with the α-subunit. One interpretation of the present results is that Rhes has a novel interaction with heterotrimeric G proteins. Indeed, we have shown that Rhes can interact with Gαi, and Hill et al. (2009) have shown that it interacts with Gβ subunits. Thus, Rhes may interact with a heterotrimer, which would preferentially affect receptor-mediated activation of these proteins (Fung, 1983; Jockers et al., 1994). However, a mechanism for the involvement of Gαi in D1 receptor signaling is currently unknown. Furthermore, neither Rhes (present results) nor AGS1/Dexras1 (Nguyen and Watts, 2005) is involved in the ability of D2 receptor-activated Gαi to inhibit AC.
The present results agree with in vivo results showing a role for Rhes in dopamine receptor-mediated signaling and behavior. Rhes−/− mice generally show an increase in stimulatory tone on AC pathways, such as increased dopamine receptor-mediated locomotor activity and stereotypy, and increased phosphorylation of GluR1 (Errico et al., 2008; Quintero et al., 2008). Here we have shown that this effect begins at least at the level of activation of AC. Although AGS1/Dexras1 is not enriched in striatum, it is localized in other brain areas expressing dopamine receptors, such as cortex and hippocampus (Fang et al., 2000), where the proteins can interact. Indeed amphetamine induces AGS1/Dexras1 expression in the prefrontal cortex via a mechanism involving dopamine D2 receptors (Schwendt and McGinty, 2010). However, the effects of both Rhes and AGS1/Dexras1 are not specific to dopamine receptors, and we have shown that neither protein interacts with D1 receptors in pull-down assays. Rhes and AGS1/Dexras1 may affect many receptor types with which they are co-localized to play a fundamental role in modulating intracellular signaling.
Acknowledgments
Supported by the National Institutes of Health (P20RR016816) and the Louisiana Board of Regents [LEQSF(2009-11)-RD-A-11)].
We thank Franklin Lee and John Norbert for expert technical assistance.
References
- Bernard ML, Peterson YK, Chung P, Jourdan J, Lanier SM. Selective interaction of AGS3 with G-proteins and the influence of AGS3 on the activation state of G-proteins. J Biol Chem. 2001;276:1585–1593. doi: 10.1074/jbc.M005291200. [DOI] [PubMed] [Google Scholar]
- Blumer JB, Cismowski MJ, Sato M, Lanier SM. AGS proteins: receptor-independent activators of G-protein signaling. Trends Pharmacol Sci. 2005;26:470–476. doi: 10.1016/j.tips.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Cho W, Taylor LP, Akil H. Mutagenesis of residues adjacent to transmembrane prolines alters D1 dopamine receptor binding and signal transduction. Mol Pharmacol. 1996;50:1338–1345. [PubMed] [Google Scholar]
- Cismowski MJ, Ma C, Ribas C, Xie X, Spruyt M, Lizano JS, Lanier SM, Duzic E. Activation of heterotrimeric G-protein signaling by a Ras-related protein: Implications for signal integration. J Biol Chem. 2000;275:23421–23424. doi: 10.1074/jbc.C000322200. [DOI] [PubMed] [Google Scholar]
- Errico F, Santini E, Migliarini S, Borgkvist A, Centonze D, Nasti V, Carta M, De Chiara V, Prosperetti C, Spano D, Herve D, Pasqualetti M, Di Lauro R, Fisone G, Usiello A. The GTP-binding protein Rhes modulates dopamine signaling in striatal medium spiny neurons. Mol Cell Neurosci. 2008;37:335–345. doi: 10.1016/j.mcn.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Falk JD, Vargiu P, Foye PE, Usui H, Perez J, Danielson PE, Lerner DL, Bernal J, Sutcliffe JG. Rhes: A striatal-specific Ras homolog related to Dexras1. J Neurosci Res. 1999;57:782–788. [PubMed] [Google Scholar]
- Fang M, Jaffrey SR, Sawa A, Ye K, Luo X, Snyder SH. Dexras1: A G protein specifically coupled to neuronal nitric oxide synthase via CAPON. Neuron. 2000;28:183–193. doi: 10.1016/s0896-6273(00)00095-7. [DOI] [PubMed] [Google Scholar]
- Fung BK-K. Characterization of transducin from bovine retinal rod outer segments. I. Separation and reconstitution of subunits. J Biol Chem. 1983;258:10495–10502. [PubMed] [Google Scholar]
- Graham TE, Prossnitz ER, Dorin RI. Dexras1/AGS1 inhibits signal transduction from the Gi-coupled formyl peptide receptor to Erk-1/2 MAP kinases. J Biol Chem. 2002;277:10876–10882. doi: 10.1074/jbc.M110397200. [DOI] [PubMed] [Google Scholar]
- Graham TE, Qiao Z, Dorin RI. Dexras1 inhibits adenylyl cyclase. Biochem Biophys Res Comm. 2004;316:307–312. doi: 10.1016/j.bbrc.2004.02.049. [DOI] [PubMed] [Google Scholar]
- Harrison LM, LaHoste GJ. Rhes, the Ras homolog enriched in striatum, is reduced under conditions of dopamine supersensitivity. Neuroscience. 2006;137:483–492. doi: 10.1016/j.neuroscience.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Harrison LM, LaHoste GJ, Ruskin DN. Ontogeny and dopaminergic regulation in brain of Ras homolog enriched in striatum (Rhes) Brain Res. 2008;1245:16–25. doi: 10.1016/j.brainres.2008.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C, Goddard A, Ladds G, Davey J. The cationic region of Rhes mediates its interactions with specific Gβ subunits. Cell Physiol Biochem. 2009;23:01–08. doi: 10.1159/000204075. [DOI] [PubMed] [Google Scholar]
- Jin H, Nip S, O’Dowd BF, George SR. D1 dopamine receptor activity is not altered by a mutation in the first intracellular loop. Biochem Biophys Acta. 1998;1402:165–170. doi: 10.1016/s0167-4889(97)00159-6. [DOI] [PubMed] [Google Scholar]
- Jockers R, Linder ME, Hohenegger M, Nanoff C, Bertin B, Strosberg AD, Marullo S, Freissmuth M. Species differences in the G protein selectivity of the human and bovine A1-adenosine receptor. J Biol Chem. 1994;269:32077–32084. [PubMed] [Google Scholar]
- Kemppainen RJ, Behrand EN. Dexamethasone rapidly induces a novel Ras superfamily member-related gene in AtT-20 cells. J Biol Chem. 1998;273:3129–3131. doi: 10.1074/jbc.273.6.3129. [DOI] [PubMed] [Google Scholar]
- Nguyen CH, Watts VJ. Dexras1 blocks receptor-mediated heterologous sensitization of adenylyl cyclase 1. Biochem Biophys Res Comm. 2005;332:913–920. doi: 10.1016/j.bbrc.2005.05.041. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Nakagawa T, Minami M, Satoh M. Supersensitization of the adenylyl cyclase system in Chinese hamster ovary cells co-expressing cloned opioid receptors and Gz, a PTX-insensitive G protein. Neurosci Lett. 1999;267:117–120. doi: 10.1016/s0304-3940(99)00347-x. [DOI] [PubMed] [Google Scholar]
- Quintero GC, Spano D, LaHoste GJ, Harrison LM. The Ras homolog Rhes affects dopamine D1 and D2 receptor-mediated behavior in mice. Neuroreport. 2008;19:1563–1566. doi: 10.1097/WNR.0b013e3283118434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas C, Takesono A, Sato M, Hildebrandt JD, Lanier SM. Pertussis toxin-insensitive activation of the heterotrimeric G-proteins Gi/Go by the NG108-15 G-protein activator. J Biol Chem. 2002;277:50223–50225. doi: 10.1074/jbc.C200567200. [DOI] [PubMed] [Google Scholar]
- Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: Regulators of G protein signaling (RGS) and RGS-like proteins. Ann Rev Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- Schwendt M, McGinty JF. Amphetamine up-regulates activator of G-protein signaling 1 mRNA and protein levels in rat frontal cortex: The role of dopamine and glucocorticoid receptors. Neuroscience. 2010;168:96–107. doi: 10.1016/j.neuroscience.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S, Sixt KM, Barrow R, Snyder SH. Rhes, a striatal specific protein, mediates mutant-Huntingtin cytotoxicity. Science. 2009;324:1327–1330. doi: 10.1126/science.1172871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesono A, Nowak MW, Cismowski M, Duzic E, Lanier SM. Activator of G-protein signaling 1 blocks GIRK channel activation by a G-protein-coupled receptor: Apparent disruption of receptor signaling complexes. J Biol Chem. 2002;277:13827–13830. doi: 10.1074/jbc.M201064200. [DOI] [PubMed] [Google Scholar]
- Thapliyal A, Banister R, Hanks C, Adams BA. The monomeric G proteins AGS1 and Rhes selectively influence Gαi-dependent signaling to modulate N-type (Cav2.2) calcium channels. Am J Physiol Cell Physiol. 2008;295:C1417–C1426. doi: 10.1152/ajpcell.00341.2008. [DOI] [PubMed] [Google Scholar]
- Tsai SC, Adamik R, Kanaho Y, Hewlett EL, Moss J. Effects of guanyl nucleotides and rhodopsin on ADP-ribosylation of the inhibitory GTP-binding component of adenylate cyclase by pertussis toxin. J Biol Chem. 1984;259:15320–15323. [PubMed] [Google Scholar]
- Undie AS, Weinstock J, Sarau HM, Friedman E. Evidence for a distinct D1-like dopamine receptor that couples to activation of phosphoinositide metabolism in brain. J Neurochem. 1994;62:2045–2048. doi: 10.1046/j.1471-4159.1994.62052045.x. [DOI] [PubMed] [Google Scholar]
- Usui H, Falk JD, Dopazo A, de Lecea L, Erlander MG, Sutcliffe JG. Isolation of clones of rat striatum-specific mRNAs by directional tag PCR subtraction. J Neurosci. 1994;14:4915–4926. doi: 10.1523/JNEUROSCI.14-08-04915.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargiu P, Morte B, Manzano J, Perez J, de Abajo R, Sutcliffe JG, Bernal J. Thyroid hormone regulation of rhes, a novel Ras homolog gene expressed in striatum. Brain Res Mol Brain Res. 2001;94:1–8. doi: 10.1016/s0169-328x(01)00140-1. [DOI] [PubMed] [Google Scholar]
- Vargiu P, de Abajo R, Garcia-Ranea JA, Valencia A, Santisteban P, Crespo P, Bernal J. The small GTP-binding protein, Rhes, regulates signal transduction from G protein-coupled receptors. Oncogene. 2004;23:559–568. doi: 10.1038/sj.onc.1207161. [DOI] [PubMed] [Google Scholar]