Abstract
Previous studies have shown changes in cyclic AMP response element-binding protein (CREB) signaling pathway in CA1 and CA3 regions of the rostral hippocampus with 10 µg estrogen treatment for 14 days. It appears that estrogen action on CREB phosphorylation in brain structures may depend on other estrogen doses and lengths of treatment. We, therefore, examined effects of moderate regimens (2.5 µg estradiol benzoate [EB] for 4 or 14 days) on mean numbers of neuron-specific neuronal protein (NeuN)-positive cells and phosphorylated CREB (pCREB)-positive cells and subregion volume defined by NeuN and pCREB immunolabeling and compared those results to the high regimen (10 µg EB for 14 days) in CA1, CA2 and CA3 regions and dorsal (DDG) and ventral (VDG) dentate gyrus and hilus of the hippocampus of ovariectomized rats by stereology. For whole hippocampus, all regimens increased mean neuronal (NeuN) numbers and pCREB-positive cell and volume compared to sesame oil (SO) in CA1, CA2 and CA3 regions, DDG and VDG dentate gyrus and hilus. In rostral hippocampus, however, some hippocampal subregions were not responsive to the high regimen and the moderate regimens appear more effective in increasing mean number of NeuN-positive neurons and pCREB-positive cells and subregion volume. Heterogeneity in responsiveness to estrogen was mainly seen within rostral, but not whole, hippocampal subregions. Our results indicate that responsiveness of cells expressing NeuN and pCREB to different EB regimens may vary depending on the specific region of the hippocampus.
Keywords: Estrogen, Hippocampus, NeuN, Phosphorylated CREB, Stereology
Introduction
Estrogen effects in the hippocampus involve virtually all of its subregions, including CA1, CA3, and dentate gyrus (DG). Estrogen increases dendritic spine and synaptic density on CA1 pyramidal cells (Woolley, 1998); decreases neuronal loss and rescues the downregulation of brain-derived neurotrophic factor (BDNF) mRNA levels in the CA3 region and cognitive impairments induced during chronic restraint stress in the CA3 region (Takuma et al., 2007); affects neurogenesis in the DG (Tanapat et al., 2005; Barha et al., 2009); and increases cell proliferation while decreasing cell survival in the DG (Barker and Galea, 2008). Estrogens may exert some of their actions in the hippocampus via effects on second messenger pathways, such as those that result in the phosphorylation of the gene transcription factor, cyclic AMP response element binding protein (CREB). We demonstrated estrogen effects on levels of phosphorylated CREB (pCREB), and components of the CREB signaling cascade, including, Ca2+/calmodulin-dependent protein kinase IV (CaMK IV), the phosphatase calcineurin, and BDNF in regions of the rostral hippocampus of ovariectomized rats using a high dose of estradiol benzoate (EB) (10 µg EB for 14 days) (Carlstrom et al., 2001; Zhou et al., 2001, 2004, 2005).
However, studies are emerging that underscore the importance of different estrogen regimens in eliciting various neurochemical, morphological or behavioral changes (cf. Galea et al., 2008). For example, Walf and Frye (2009) showed effects of moderate, proestrus-like estrogen levels in ameliorating anxiety- and depressive-like behaviors in female rodents. Shors and Leuner (2003) reported optimal performance levels of the classically conditioned eyeblink response with moderate levels of estrogen, but poor performance with either very low or very high levels of hormone. Recently, we demonstrated dose- and time-dependent estrogen-induced increases in mean number of pCREB-positive cells and neuron-specific neuronal protein (NeuN)-labeled neurons in the medial, but not central, amygdala (Fan et al., 2008a). However, it is less clear if estrogen produces a similar response in various structures of hippocampus.
In the present study, we, therefore, examined labeled neurons and pCREB-immunolabeled cells and mean volume in subregions of the hippocampus using stereology. We selected the regimens of 2.5 µg estradiol benzoate (EB) for 4 days and 2.5 µg EB for 14 days based the observation that disparate responses to EB treatment are obtained when the hormone treatment is extended for more than 4 days compared to doses which are in the physiological range (cf. Becker, 1999). We included the 10 µg for 14 days treatment, to compare with our previously published data (e. g., Cohen and Pfaff, 1981; Carlstrom et al., 2001; Zhou et al., 2001, 2004, 2005; Fan et al., 2008a, b), and reports of others (Gu et al., 1996) to optimize the detection of hormonal action on cellular responses and, as a positive control, to relate our findings to published data on estrogen effects on the CREB signaling cascade, including pCREB (Gu et al., 1996; Zhou et al., 1996; Ábrahám et al., 2003; Szegő et al., 2006; cf. Rønnekleiv et al., 2007). NeuN was selected as a marker because of its suitability for immunocytochemical visualization of neurons and analysis of neuronal number by stereology following ovarian steroid treatment (Hoffman et al., 2003, 2005; Fan et al., 2008a). Our aforementioned studies on the effects of estrogen on pCREB and other second messengers in the hippocampus were performed in rostral hippocampus only (Carlstrom et al., 2001; Zhou et al., 2001, 2004, 2005). Given the heterogeneity of estrogen receptor (ER) distribution in the hippocampus (Shughrue et al., 1997; Weiland et al., 1997; Milner et al., 2005), we compared the effects of different regimens of EB on NeuN and pCREB immunolabeling in whole and rostral hippocampus.
Materials and Methods
Animals and Estrogen Treatments
Animals
In the present study, we used brain sections from rats previously used to determine the effects of moderate and high doses and different time courses of EB treatment on NeuN and pCREB immunolabeling in the medial and central amygdala (Fan et al., 2008a). Here, we extend our analysis of the effects of EB on NeuN and pCREB immunolabeling to the hippocampus. Ovariectomized Sprague Dawley rats (200–250 g; approximately 8 weeks old at 250 g) were obtained from Harlan (Madison, WI). The duration between ovariectomy and perfusion is approximately 45 days, when the animals are about 14 weeks old. Animals were adapted to a reverse 12/12-hr light/dark cycle (lights on at 22.0 hours) for 19 days, and then females were tested for completeness of ovariectomy (during rats’ dark period, at approximately 11:00AM) using the lordosis test (Kow and Pfaff, 1975) for six days; none of the rats exhibited the lordosis response. Rat chow (neither phytoestrogen- or soy isoflavone-free) and water were available ad libitum. Immediately following the adaptation period, animals were randomly divided into four groups. Approximately forty-eight hours after lordosis testing (and approximately 28 days following ovariectomy), they were injected subcutaneously with one of the following regimens: vehicle sesame oil (SO) for 10 days followed by 2.5 µg estradiol benzoate (EB) for 4 days (2.5µg/4d) (N=5); 2.5 µg EB for 14 days (2.5µg/14d) (N=5), or, for comparison, 10 µg EB for 14 days (10µg/14d) (N=4); SO was the control (N=5) (Fan et al., 2008a). Because the rodent estrus cycle is characterized by increases in estradiol plasma levels for less than 24 hours, the timing with which these injections were given did not replicate the physiological condition. Also, due to the rapid clearance of estradiol and since plasma estradiol levels were measured a day after the last injection, the observed levels provide a relative, but not precise, indication of circulating estradiol levels during the experiment. All animal procedures were approved by the University of Illinois at Chicago Animal Care Committee and guidelines for the humane and proper treatment of animals were followed.
Radioimmunoassay Procedure for Plasma Estradiol Measurements
Measurements were taken from plasma obtained in the early afternoon of the next day after the last EB injection (see below for details). Blood was collected in BD Vacutainer 10 ml sterile plastic vials with sodium heparin during perfusion by cutting the left ventricle. Blood was allowed to sit at room temperature for between 30 and 60 min, then spun down for 30 min at 2,000 rpm in the cold. Plasma was siphoned off the top and placed into 3 small Eppendorf tubes (1 ml each), then frozen at –80°C. Radioimmunoassays (RIAs) for plasma estradiol levels were performed at the Endocrinology Laboratory of the Cornell New York State Animal Health Diagnostic Laboratory, College of Veterinary Medicine (Ithaca, N.Y., USA). Details regarding the assay are as follows.
RIAs for estradiol were performed using a Coat-A-Count Kit (Diagnostic Products Corp., Los Angeles, Calif., USA) with modifications (Reimers et al., 1991). Three to five ml of plasma were concentrated to 335 µl. Of that amount, 200 µl were used for duplicates for the estrogen assay. The remainder was used for calculating the percent recovery for each individual sample. Specifically, plasma estradiol levels were quantified following extraction of samples with ethyl ether and the extract was reconstituted in phosphate-buffered saline (0.01 M) solution containing 0.5% bovine serum albumin, pH 7.2. Only the polypropylene tubes coated with a rabbit antibody to estradiol and the iodinated estradiol, as tracer, were used from the kit; standard solutions accompanying the kit were not used, but were prepared by the Endocrinology Laboratory of the Cornell New York State Animal Health Diagnostic Laboratory. Immunological specificity was determined by serially diluting 3 pools of commercial rat serum and plasma spiked with estradiol and tested for parallelism. Serial dilutions of rat specimens inhibited the binding of radiolabeled antigen to the antibody in a way parallel to that seen by the inhibition curve generated by the standard solutions. Tritiated [3H] estradiol was used for the quantification of extraction recoveries for each sample; the volume extracted did not affect the accuracy of the assay (Reimers et al., 1991). This procedure results in a minimum detectable dose of 7.1 pg/ml, an intra-assay coefficient of variance of less than 15% and an interassay coefficient of variance of 10% or lower (Lamb, unpublished observations) (Fan et al., 2008a).
Measurements of Uterine Weight
The uteri were removed immediately after perfusions, free of fat and connective tissues. Since some residual fluid and perfusate remained in the uterine tissue, the uteri were subsequently dried for one hour prior to weighing (Fan et al., 2008a).
Immunoperoxidase Staining for NeuN and pCREB Proteins
On the next day after the last injection, animals were anesthetized with Nembutal (50mg/kg, ip), and perfused intracardially with normal saline, followed by ice cold 4% paraformaldehyde in 0.1M phosphate buffer (PB) (250–300ml). To measure plasma estradiol levels, blood was collected during perfusion by cutting the left ventricle (cf. Fan et al., 2008a). Brains were post-fixed in 4% paraformaldehyde in PB overnight, and a notch was cut along the left cortex to maintain laterality throughout the analysis. The olfactory bulb and cerebellum were removed from the brain. Brains were placed into 30% sucrose in phosphate buffer saline (PBS) for cryoprotection and stored in the −80°C. Forty micrometer frozen, serial, coronal sections were cut on a sliding knife microtome (Spencer Lens, Buffalo, USA) through the brain and were stored in cryoprotectant at −20°C. Every sixth section was collected with a random start from the first series. The separation between every sixth serial section used in the analysis was 240 µm. Sections were labeled with either mouse anti-NeuN antibody (Chemicon, Temecula, CA) or rabbit anti-pCREB antibody (Upstate Biotechnology, Waltham, MA) according to standard procedures in our laboratory as detailed in Fan et al. (2008a).
Characterization of the Antibodies
Mouse anti-NeuN monoclonal antibody (Chemicon, Temecula, CA), raised against purified cell nuclei from mouse brain, recognizes the transcription factor neuron-specific protein in the nucleus and cytoplasm of mature neurons. It was shown to recognize 2–3 bands in the 46–48 kDa range and, possibly, another band at 66kDa (Mullen et al., 1992). Lorenz et al. (2005) further confirmed the specificity of this antibody by comparing the consistency of the morphology and temporal appearance of immunoreactivity in their studies with others demonstrating expression of the protein. This antibody was also characterized by Lyck et al. (2006), who demonstrated its applicability for stereological studies. Specifically, the labeling is consistent with the morphology and distribution of neurons, as evaluated by morphological criteria. Antibodies to pCREB (Upstate Biotechnology, Waltham, MA) have been well characterized in our laboratory by Western blots, gel shift assays and immunocytochemistry (Carlstrom et al., 2001; Pandey et al., 2005).
Neuroanatomy: Delineation of Hippocampus and its Subdivisions
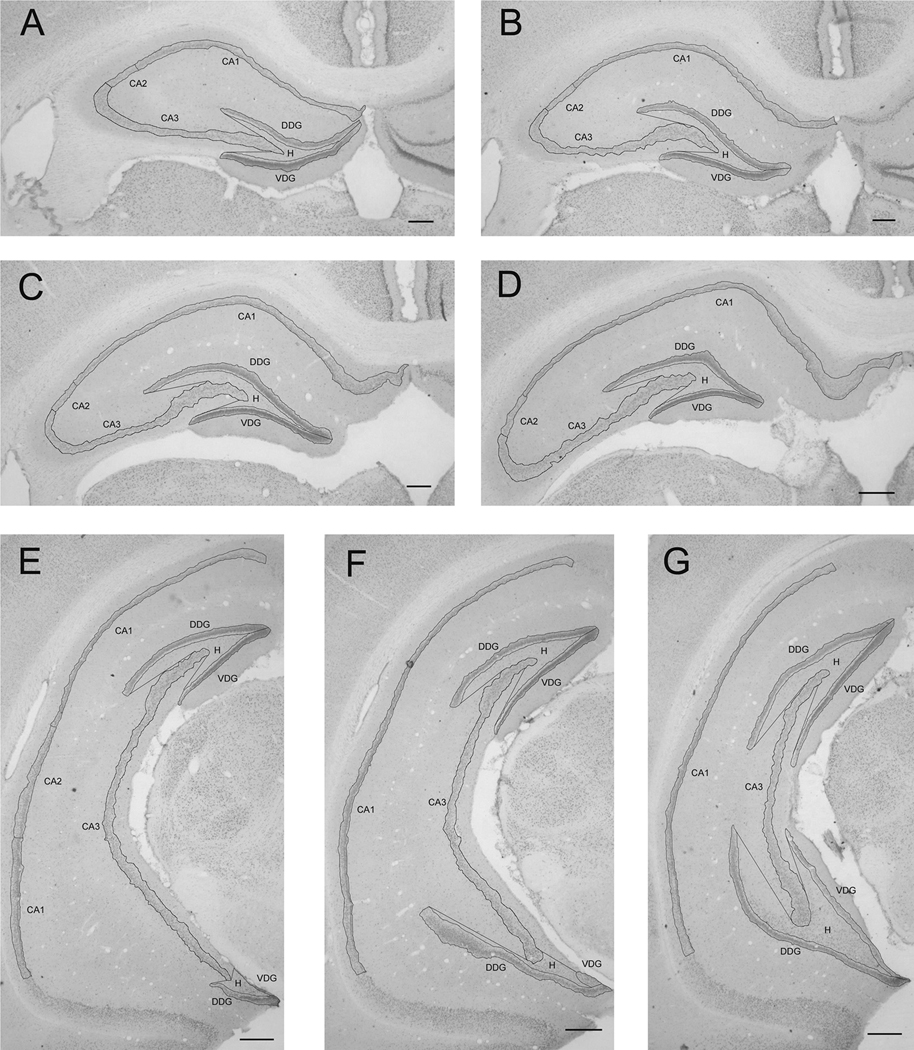
Following mounting of the brain sections, CA1, CA2 and CA3 regions, dorsal and ventral blades of the dentate gyrus (DG), and hilus of the DG were identified on the appropriate sections using the Paxinos and Watson rat atlas (1998) as a guide. Reports of Lister et al. (2006) were used to define hippocampal areas and those of Barker and Galea (2008) and Nakagawa et al. (2002) were used to define the subgranular zone of the dentate gyrus. For the CA1, CA2 and CA3 regions, only the pyramidal cell layers were analyzed; for the dorsal and ventral blades of DG, only the granule cell layer and subgranular zone were analyzed. The lateral border of the hilus of the DG was found by drawing straight lines from the most lateral extents of the dorsal and ventral blades of the DG to the most medial aspect of the pyramidal layer of CA3 (Figure 1). Using a Nikon ECLIPSE 80i microscope, the most rostral section of the each of the areas was identified using the Bregma levels of the atlas and, from there, successive sections were identified in the same manner, until the most caudal section was ascertained. Bregma levels: CA1: −2.20 mm to −6.80 mm; CA2: −2.20 mm to −6.00 mm; CA3: −1.80 mm to −6.00 mm; dorsal blade of DG: −2.20 mm to −6.60 mm; ventral blade of DG: −1.80 mm to −6.60 mm; hilus of DG: −2.20 mm to −6.60 mm. In addition to analyzing each entire region, a separate analysis was performed on only the rostral portion of each area (from the most rostral extent of each area to only Bregma −4.00 mm).
Figure 1.
A series of seven pCREB-labeled sections (A–G, anterior to posterior, separated by 480 µm) showing traced boundaries of hippocampal regions studied (bar = 250 µm). The pyramidal layer of the CA1 to CA3 portions of the hippocampus consists of relatively large, closely packed, densely chromophilic neurons situated between the cell-sparse molecular and polymorphic layers. The CA1 region gives way on one end to the more loosely packed cells of the subiculum. On the other end, the CA2 region can be recognized because its cells are slightly larger and less densely packed than those of the CA1 region. Similarly, the CA3 cells are larger and less densely packed than those in the CA2 region. Here, we included in the dorsal and ventral blades of the dentate gyrus, the densely packed cells of the granular layer and the more loosely packed cells of the subgranular layer. The hilus of the dentate gyrus consists of the entire polymorphic cellular layer located between the dorsal and ventral blades of the dentate gyrus, but excludes the densely packed CA3 cells that extend into the hilus. The lateral border of the hilus of the dentate gyrus was found by drawing straight lines from the most lateral extent of the dorsal and ventral blades of the dentate gyrus to the most medial aspect of the pyramidal layer of CA3. CA1: pyramidal layer of CA1 region of hippocampus; CA2: pyramidal layer of CA2 region of hippocampus; CA3: pyramidal layer of CA3 region of hippocampus; DDG: granular and subgranular layers of dorsal blade of dentate gyrus; VDG: granular and subgranular layers of ventral blade of dentate gyrus; H: hilus of dentate gyrus.
We defined the brain regions in two ways in two separate analyses, one with NeuN immunolabeling and, the other, with pCREB immunolabeling. Previously, using these sections, we compared the neuroanatomical boundaries using NeuN- and pCREB-immunolabeled sections counterstained with cresyl violet in the medial amygdala (see Fig. 2, Fan et al., 2008a). The cellular boundaries of these amygdalar regions were consistent irrespective of the antibody (to NeuN or pCREB) or cell stain (i. e., cresyl violet) used. This indicates that the boundaries of the brain regions analyzed were not different for NeuN or pCREB immunolabeling or cresyl violet staining.
Stereology: Estimations of Cell Numbers and Volume Using Unbiased Stereology
All analyses were conducted blindly with no knowledge as to the treatment status of the animals. A series of sections (every sixth section throughout the rostrocaudal extent of the brain except olfactory bulb and cerebellum) with a random start to ensure an equal probability of being chosen for sampling (Morris et al., 2005; Schumann and Amaral, 2005), was labeled with antibodies to NeuN or pCREB, and mounted on slides; no sections were missing. Boundaries of the hippocampal subregions are shown in Figure 1. Stereological analyses of mean number of NeuN- and pCREB-immunolabeled cells or nuclei in subregions of the hippocampus and volume of these regions were performed using the Optical Fractionator probe in stereological software Stereo Investigator ® (MicroBrightField, Colchester, VT). Our stereology system consists of a Nikon ECLIPSE 80i microscope, coupled to a MicroFire™ S99808 camera (Optronics) and a Ludl X-Y-Z motorized stage controlled by a Dell™OptiPlex™ GX280 computer. We used Adobe Photoshop 6.0 to store and process photographs.
Cell and nuclear number analyses
The numbers of NeuN-immunolabeled neurons and pCREB-immunolabeled cells in left and right sides of each hippocampal region were estimated by an optical fractionator method. For each animal, the thickness of every section included in the analysis was measured at a minimum of three points within the area of interest (average is 16.8 µm thickness and there is 58% shrinkage due to tissue processing [Lyck et al., 2006]) and then the optical dissector height was determined according to the minimum thickness in each series of the sections, leaving the total upper and lower guard zones (the distance between section surface and the optic dissector to prevent errors of estimation resulting from of artifacts of cutting) more than 3 µm. Using a computer mouse, the borders of each area were traced in successive sections throughout the rostral-caudal axis under a low magnification objective (X2), and the cell counting was conducted at high magnification with a 100X (1.40 NA) oil objective within the borders or contours by using an optical dissector probe, i.e., inside or touching the drawn contour. All cells or nuclei that exhibited a brown color, irrespective of the density of the label, were counted (García-Segura et al., 1998). A cell was counted if the cell body (for NeuN staining) or top of the nucleus (for pCREB staining) came into focus within the counting frame and did not touch either solid line of the counting frame (see Figure 2). Tissue shrinkage is not an issue with this method because it is independent of volume measurements (Morris, et al., 2005). The series of sections were analyzed according to a systematic random sampling scheme (in this study, 40 × 40 µm counting frames were used and evenly distributed in the contours with the x-y intervals of 200× 200 µm). The estimated total number of cell bodies or nuclei for each animal was calculated by the optical fractionator program according to the following factors: total number of cell bodies or nuclei counted at optical disectors, section interval, disector height/mean section thickness, and area of counting frame/area of grid. The coefficients of error (CE) are the variation in sampling within each animal and represent the estimated precision of the population size calculated by optical fractionator (Gundersen et al., 1999). CE values less than 0.10 were considered acceptable.
Figure 2.
A typical NeuN counting frame. The following stereological rules were followed: (1) the top of any NeuN-labeled cell that came into focus was counted; (2) any NeuN-labeled cell that fell entirely inside the square indicated by solid (left and bottom of counting frame) and dotted (right and top of counting frame) lines was counted; (3) any NeuN-labeled cell touching dotted lines was counted; (4) any NeuN-labeled cells touching solid lines was not counted; (5) if a NeuN-labeled cell that touched both solid and dotted lines, it was not counted; and (6) cell had to either be within or touching the line (white line at right top of this image) showing the contour limit of the region of interest. These counting rules ensured an unbiased counting, which means no cell would be counted more than once. The same rules were used to count pCREB-labeled nuclei. This micrograph was taken using a 100X (1.40 NA) oil objective mounted on a Nikon ECLIPSE 80i microscope. In this example, those NeuN-labeled cells with a “+” marker were counted, but those with a “−” mark were not counted. Those with a “X” mark were not counted as the tops of the cell bodies were not in focus. The white line in the right top of this image shows the limit of CA3 as identified at lower magnification. Bar = 25 µm
Volume analyses
The camera captured the image of the areas of interest at 70X (2X objective to trace, 70X final magnification on screen) and 140X (4X objective to modify trace to ensure the exact borders) magnification from the microscope. The areas of interest were traced in successive sections throughout the rostral-caudal axis. The estimated total volume for each hippopcampal area in the left and right hemispheres was calculated by planimetric data according to the traced area, block section thickness (40 µm when cut) and section interval (every sixth section) by StereoInvestigator software (Young et al., 2004) (MicroBrightField, Colchester, VT, USA). The mean volumes of each of the hippocampal areas for each of the four hormonal conditions were consistent for both NeuN and pCREB immunolabeling.
Statistical analysis
No tissue was damaged, so that all tissue from all animals was included in the analyses. Because of the low numbers of cells in the region, the CE values for the rostral hilus were not less than 0.10 and, therefore, these data are not reported. We merged the data from the right and left hemispheres to be consistent with our previous studies on the effects of estrogen on components of the CREB signaling cascade and BDNF in the hippocampus (Carlstrom et al., 2001; Zhou et al., 2005).
Both counting of NeuN-immunolabeled neurons and pCREB-immunolabeled cells and volume of the areas of interest were analyzed by one-way ANOVA with Fisher’s least significant difference (LSD) post hoc tests to determine which means differed from one another. Data are presented as mean ± standard error of mean (SEM). A p-value less than 0.05 was considered statistically significant. “N” represents the number of animals in each hormone treatment group. All analyses were performed with (SPSS) 12.0 for Windows.
Technical Considerations
The EB regimens selected in our study were based on those reported in the literature and ongoing studies in our laboratory. We based the 10 µg EB for 14 days protocol on our previously published data (e. g., Cohen and Pfaff, 1981; Carlstrom et al., 2001; Zhou et al., 2001, 2004, 2005), and reports of others (Gu et al., 1996), to optimize the detection of hormonal action on cellular responses and relate our finding to published data on estrogen effects on the CREB signaling cascade, including pCREB (Gu et al., 1996; Zhou et al., 1996; Ábrahám et al., 2003; Szegő et al., 2006; cf. Rønnekleiv et al., 2007). NeuN was selected as a marker because of its suitability for immunocytochemical visualization of neurons and analysis of neuronal number by stereology following ovarian steroid treatment (Hoffman et al., 2003, 2005; Fan et al., 2008a).
Results
Plasma Levels of Estrogen and Uterine Weights Following Different Estrogen Regimens
We reported plasma estradiol levels for these animals previously (cf. Figure 4, Fan et al., 2008a, for graph). Overall, EB treatments had a significant effect on plasma estradiol levels (F3,33 = 58.23, p<0.0001). The 2.5 µg for 4 and 14 days treatments resulted in levels of plasma estradiol (pg/ml) as determined by radioimmunoassay, and are consistent with levels obtained during proestrus in other studies (Goodman, 1978). Ten µg EB for 14 days resulted in plasma estradiol levels (129.30 ±11.06) that were approximately 2.5 times higher than those obtained with SO for 10 days + 2.5 µg EB for 4 days (51.13 ± 1.83 pg/ml; p<0.001) or 2.5 µg EB for 14 days (49.53 ± 4.01 pg/ml; p<0.001) and approximately five times higher than that seen with SO for 14 days (23.67 ± 2.37 pg/ml; p<0.001). The regimens of 2.5 µg EB for 14 days and for SO for 10 days + 2.5 µg EB for 4 days showed no differences in plasma levels of estradiol, themselves, but both regimens resulted in estradiol levels that were significantly higher than that seen with SO alone for 14 days (p<0.01 in both cases) (Fan et al., 2008a).
All groups receiving EB treatments had estradiol levels that were significantly higher than the vehicle, SO group; however, that group had estradiol levels of about 23.67 pg/ml. Plasma estradiol levels in the range of 20 pg/ml have been seen in other studies following injection of SO using the Coat-a-Count kit from DPC (Lee et al., 2004b; Nakamura et al., 2004). Lee et al (2004b) attributed the levels to the sensitivity of the kit; however, in our case, the extraction and recovery procedures, and sensitivity of the assay make this explanation unlikely. Dowsett and Folkerd (2005) suggest that inaccuracies in plasma levels using direct methods of measurement (antiserum added directly to plasma) may be due to competition by hormone-binding globulin for the antibody or high concentrations of water-soluble conjugated estrogen in the blood, which may cross-react with the antiserum. Extraction of estradiol with organic solvent, as was performed in the present study, eliminates these errors (Dowsett and Folkerd, 2005).
In addition, levels of estradiol following SO treatment alone may come from extragonadal sources, such as adipose tissue or liver (Zhao et al., 2005). Zhao et al. (2005) noted a rise in plasma estradiol after ovariectomy (at six months), that may be attributed to extragonadal aromatization (although the source of substrate is unknown). Moreover estradiol levels in ovariectomized rats given SO alone are likely not due to injections, themselves, or contamination by SO. In another study (Hanbury et al., 2007), we demonstrated that ovariectomized rats adapted to the same conditions, and perfused without having had any SO or other injections also exhibited estradiol levels in the 20 pg/ml range.
As validation of the treatment regimens for the various EB regimens used in our study, we measured their effects on parameters known to be affected by estrogen (data published in Fan et al., 2008a). EB treatments also caused a significant effect on uterine weight. There was a significant difference in uterine weight (g) between SO/14d, 10/14d, 2.5µg/14d and 2.5µg/4d. No differences were seen among any of the other groups.
Effect of EB on Mean Number of NeuN-immunolabeled Neurons and Mean Volume as Defined by NeuN immunolabeling in the Whole Hippocampus and Rostral Hippocampus
We measured dose and time-dependent effects of EB on mean number of NeuN-immunolabeled neurons in the entire hippocampus. In addition, we measured these effects in the rostral hippocampus. The morphology of NeuN-immunolabeled cells was characteristic of neurons and was similar in labeling to those cells labeled with this antibody in other studies using immunoperoxidase techniques (Lyck et al., 2006). The nucleus, cell body and proximal parts of the cell processes exhibited labeling.
For the analysis of the whole CA1 region, overall, there were significant differences in mean number of NeuN-immunolabeled neurons among groups (F3, 15=16.01; P<0.0001) (Figure 3A). Specifically, there were significant differences between all of the treatment groups compared to SO (2.5 µg/4d: P<0.01; 2.5µg/14d: P<0.001; 10µg/14d: P<0.0001). In addition, there was a significant difference between the 10µg/14d versus the 2.5µg/4d groups (P<0.05). For the CA2 region, overall, there were significant differences in mean number of NeuN-immunolabeled neurons among groups (F3, 15=8.86; P<0.01) (Figure 3A). All treatment groups showed significant differences compared to SO (P<0.01 in all cases), but no differences were seen among any of the treatment groups. For the analysis of the whole CA3 region, overall, there were significant differences in mean number of NeuN-immunolabeled neurons among groups (F3, 15=6.54; P<0.01) (Figure 3A). Whereas, in this region, there were significant differences between all of the treatment groups compared to SO (P<0.01 in all cases), there were no differences among the treatment groups, themselves. The same situation held for the DDG, VDG and hilus. In the DDG, overall, there were significant differences in mean number of NeuN-immunolabeled neurons among groups (F3, 15=48.27; P<0.0001) (Figure 3A). In this region, there were significant differences between all of the treatment groups compared to SO (P<0.0001 in all cases). Similarly, In the VDG, overall there were significant differences in mean number of NeuN-immunolabeled neurons among groups (F3, 15=20.28; P<0.0001) (Figure 3A). There were also significant differences between all of the treatment groups compared to SO (P<0.0001 in all cases), but no differences between treatment groups. In the hilus, overall, there were significant differences in mean number of NeuN immunolabeled neurons among groups (F3, 15=13.34; P<0.001) (Figure 3A). There was a significant difference between each of the treatment groups and SO (P<0.001 in all cases), but no differences between any of the treatment groups.
Figure 3.
(A) Whole hippocampus shows little or no heterogeneity in response to different EB regimens in regard to NeuN-labeled neuronal counts. For CA1, there were differences in mean number of labeled neurons among groups, between all treatment groups compared to SO, and between the 10µg/14d vs the 2.5µg/4d groups. For the other regions, there were differences in mean number of labeled neurons among groups and all treatment groups showed differences compared to SO; no differences were seen among any of the treatment groups. Results are expressed as mean ± SEM (n= 4–5 per group) (For differences among groups: CA1: F3, 15=16.01; P<0.0001; CA2: F3, 15=8.86; P<0.01 CA3: F3, 15=6.54; P<0.01; DDG: F3, 15=48.27; P<0.0001; VDG: F3, 15=20.28; P<0.0001; hilus: F3, 15=13.34; P<0.001. *: P<0.01, ^: P<0.001, †: P<0.0001, compared with SO; ⊥: P<0.05, compared with 2.5µg/4d.)
(B) Whole hippocampus shows little or no heterogeneity in response to different EB regimens in regard to volume defined by NeuN. For CA1, there were differences in mean volume among groups, between all treatment groups compared to SO, and between the 10µg/14d versus the 2.5µg/4d groups. For the other regions, there were differences in mean volume among groups and all treatment groups showed differences compared to SO; no differences were seen among any of the treatment groups. Results are expressed as mean ± SEM (n= 4–5 per group). (For differences among groups: CA1: F3, 15=18.14; P<0.0001; CA2: F3, 15=16.74; P<0.0001; CA3: F3, 15=16.17; P<0.0001; DDG: F3, 15=36.02; P<0.0001; VDG: F3, 15=14.00; P<0.001; hilus: F3, 15=13.67; P<0.001. ^: P<0.001, †: P<0.0001, compared with SO; ⊥: P<0.05, compared with 2.5µg/4d.)
In terms of volume as defined by NeuN-immunolabeling of the whole CA1 region, there were significant differences in mean volume among groups (F3, 15=18.14; P<0.0001) (Figure 3B). All treatment groups showed significant differences in mean volume compared with vehicle group (2.5 µg/4d: P<0.001; 2.5µg/14d: P<0.001; 10µg/14d: P<0.0001). The 10µg/14d group was significantly different compared with 2.5µg/4d (P<0.05); there were no other differences among treatment groups. For CA2, there were significant differences in mean volume among groups (F3, 15=16.74; P<0.0001) (Figure 3B). All treatment groups showed significant differences compared to SO (2.5 µg/4d: P<0.0001; 2.5µg/14d: P<0.001; 10µg/14d: P<0.0001), but there were no differences between any of the treatment groups.
For CA3, there were significant differences in mean volume among groups (F3, 15=16.17; P<0.0001) (Figure 3B). All groups were significantly different compared to SO (2.5 µg/4d: P<0.0001; 2.5µg/14d: P<0.0001; 10µg/14d: P<0.001), but no differences were seen among treatment groups. For the mean DDG volume as defined by NeuN immunolabeling, there was a significant difference among treatment groups (F3, 15=36.02; P<0.0001) (Figure 3B). All groups showed significant differences compared to SO (P< 0.0001 in all cases), but no differences were seen among treatment groups. For the mean VDG volume, there was a significant difference among treatment groups (F3, 15=14.00; P<0.001). Similarly, all treatment groups were significantly different than SO (2.5µg/4d: P<0.001; 2.5 µg/14d: P<0.001; 10 µg /14d: P<0.0001), but no differences were seen between any of the treatment groups. For the mean hilus volume, there was a significant difference among treatment groups (F3, 15=13.67; P<0.001) (Figure 3B). All treatment groups were significantly different than SO (2.5 µg /4d: P<0.001; 2.5 µg /14d: P<0.001; 10µg /14d: P<0.0001), but no differences were seen between any of the treatment groups. In summary, the whole hippocampus displays almost no heterogeneity in response to all of the estrogen regimens. That is, all the hippocampal areas showed an increase in mean numbers of NeuN-immunolabeled cells and mean volume as defined by NeuN immunolabeling compared to SO, with only the CA1 showing differences between the 2.5µg/4d and 10µg/14d groups. These results differ from those in the rostral hippocampus described below, where there is a heterogeneity in responsiveness to the various estrogen regimens in regard to these parameters among and within hippocampal subregions.
We also examined the aforementioned parameters in the rostral hippocampus. For the CA1 region, overall, there were significant differences in mean number of NeuN-immunolabeled neurons among groups (F3, 15=29.05; P<0.0001) (Figure 4A). All treatment groups showed significant differences compared to SO (2.5µg /4d: P<0.0001; 2.5µg /14d: P<0.0001; 10µg /14d: P<0.001). Differences were also seen among treatment groups, but a disparate pattern was seen compared to that in the whole hippocampus. Here, both the 2.5/4d and 2.5/14d regimens displayed significantly higher mean NeuN-labeled neuronal numbers compared to the high regimen (P<0.01 in both cases). For rostral CA2, there was a significant difference among treatment groups (F3, 15=7.01; P<0.01) (Figure 4A). Here, however, differences were seen only between the treatment groups and SO (P<0.01 in all cases), but not between any of the treatment groups, themselves. The rostral CA3 presented a pattern different from any of the other groups for this parameter in that there were no differences between the treatment groups compared to SO or compared to each other (F3, 15=3.00; P>0.05) (Figure 4A). For the rostral DDG, on the other hand, overall, there were significant differences in mean number of NeuN-immunolabeled neurons among groups (F3, 15=78.38; P<0.0001) (Figure 4A). All treatment groups showed significant differences compared to SO (2.5µg /4d: P<0.0001; 2.5µg /14d: P<0.0001; 10µg /14d: P<0.05). Both moderate regimens also resulted in values that were significantly higher that 10µg/14d (P<0.0001 in both cases). For the rostral VDG, overall, there were significant differences in mean number of NeuN immunolabeled neurons among groups (F3, 15=82.75; P<0.0001) (Figure 4A). All treatment groups showed significant differences when compared with SO (2.5µg /4d: P<0.0001; 2.5µg /14d: P<0.0001; 10µg /14d: P<0.05). Both moderate regimens showed significant increases in this parameter compared with 10µg/14d (P<0.0001 in both cases). Also, there was a difference between the two moderate regimen (P<0.05).
Figure 4.
(A) Rostral hippocampus shows heterogeneity in response to different EB regimens in regard to NeuN-labeled neuronal counts. For all regions, except the CA3, there were differences among groups. In CA2, differences were seen only between the treatment groups and SO. In CA1, DDG and VDG, both the 2.5µg/4d and 2.5 µg/14d regimens displayed significantly higher mean labeled cell numbers compared to 10µg/14d, although all groups showed higher mean numbers than SO. Also in VDG, there was a difference between the two moderate regimens. Results are expressed as mean± SEM (n= 4–5 per group). (For differences among groups: CA1: F3, 15=29.05; P<0.0001; CA2: F3, 15=7.01; P<0.01; CA3: F3, 15=3.00; P>0.05; and DDG: F3, 15=78.38; P<0.0001; VDG: F3, 15=82.75; P<0.0001). †: P<0.0001, ^: P<0.001, *: P<0.01, compared with SO; *’: P<0.01; †”: P<0.0001, compared with 10ug/14day; ⊥”: P<0.05, compared with 2.5ug/14d.) (B) Rostral hippocampus shows heterogeneity in response to different EB regimens in regard to volume defined by NeuN. For all regions, except the CA1 and CA2, there were differences among groups. For CA3 and DDG, only the two moderate regimens were significantly different from SO and 10µg/14d, no differences were seen between 10µg/14d and SO. For the VDG, all treatment groups displayed differences compared to SO and both moderate regimes showed differences compared to 10µg/14d. Results are expressed as mean ± SEM (n= 4–5 per group) (For differences among groups: CA1: F3, 15=2.34; P>0.05; CA2: F3, 15=2.67; P>0.05; CA3: F3, 15=31.07; P<0.0001; DDG: F3, 15=11.49; P<0.001; VDG: F3, 15=31.42; P<0.0001; †: P<0.0001, ^: P<0.001, ⊥P<0.05, compared with SO; †”: P<0.0001, *: P<0.01, ⊥’: P<0.05, ; ^’: P<0.001, compared with 10ug/14d.)
In terms of NeuN volume in the rostral hippocampus, no differences among any of the groups were seen in the CA1 or CA2 regions (F3, 15=2.34; P>0.05; F3, 15=2.67; P>0.05, respectively) (Figure 4B). For the rostral CA3, overall, there were significant differences in mean volume among groups (F3, 15=31.07; P<0.0001) (Figure 4B). Only the two moderate regimens were significantly different from SO (P<0.0001 in both cases). The moderate regimens were also different than the 10µg/14d regimen (P<0.0001 in both cases). A similar pattern was seen in the rostral DDG to that in the CA3 region. Overall, there were significant differences in mean volume among groups (F3, 15=11.49; P<0.001) (Figure 4B). Only the moderate regimens were significantly different from SO (2.5µg /4d: P<0.0001; 2.5µg /14d: P<0.001). Both moderate regimens resulted in values that were significantly different than 10µg/14d (2.5µg /4d: P<0.01; 2.5µg /14d: P<0.05). For the rostral VDG, overall, there were significant differences in mean volume among groups (F3, 15=31.42; P<0.0001) (Figure 4B). All treatment groups displayed differences compared to SO (2.5µg /4d: P<0.0001; 2.5µg /14d: P<0.0001; 10µg /14d: P<0.05). Both moderate regimens showed differences compared to 10µg/14d (2.5µg /4d: P<0.0001; 2.5µg /14d: P<0.001). In contrast to the whole hippocampus, the rostral hippocampus displays heterogeneity in response to the estrogen regimens. Whereas, in regard to mean numbers of NeuN-immunolabeled neurons, all regions, except the CA3, displayed significant increases compared to the SO group; the response of the moderate regimens was also increased compared to the high regimen. In terms of volume defined by NeuN immunolabeling, the CA1 and CA2 regions showed no response to any of the regimens and, in the DDG and VDG, the moderate regimens were increased compared to the high regimen. These results differ from those in the whole hippocampus described above, where there is a uniformity in responsiveness to the various estrogen regimens in regard to these parameters.
Effect of EB on Mean Number of pCREB-immunolabeled Cells and Mean Volume as Defined by pCREB immunolabeling in the Whole Hippocampus and Rostral Hippocampus
We measured dose and time-dependent effects of EB on mean number of pCREB immunolabeled nuclei in the whole hippocampus. For the analysis of the whole CA1 region, overall, there were significant differences in mean number of pCREB- immunolabeled nuclei among groups (F3, 15=9.34; P<0.01) (Figure 5A). All treatment groups showed significant differences compared to SO (2.5 µg/4d: P<0.01; 2.5µg/14d: P<0.001; 10µg/14d: P<0.001), but no differences were seen between any of the treatment groups. For the CA2 region, overall, there were significant differences in mean number of pCREB-immunolabeled cells among groups (F3, 15=5.50; P<0.01), but no differences were seen between any of the treatment groups (Figure 5A). All treatment groups showed significant differences compared to SO (P< 0.01 in all cases). For the CA3 region, overall, there were significant differences in mean number of pCREB-immunolabeled cells among groups (F3, 15=5.1; P<0.05) (Figure 5A). All treatment groups showed significant differences compared to SO (2.5 µg/4d: P<0.05; 2.5µg/14d: P<0.01; 10µg/14d: P<0.01), but no differences were seen between any of the treatment groups. For the DDG region, overall there were significant differences in mean number of pCREB immunolabeled cells among groups (F3, 15=87.45; P<0.0001). All treatment groups showed significant differences compared to SO (P<0.0001 in all cases), but no differences were seen between any of the treatment groups. For the VDG, overall, there were significant differences in mean number of pCREB-immunolabeled cells among groups (F3, 15=32.65; P<0.0001) (Figure 5A). All treatment groups showed significant differences compared to SO (P<0.0001 in all cases), but no differences were seen between any of the treatment groups. For the hilus, overall there were significant differences in mean number of pCREB-immunolabeled nuclei among groups (F3, 15=22.24; P<0.0001) (Figure 5A). All treatment groups showed significant differences compared to SO (P<0.0001 in all cases), but no differences were seen between any of the treatment groups.
Figure 5.
(A) Whole hippocampus shows no heterogeneity in response to different EB regimens in regard to counts of pCREB-labeled cells. Overall, for all groups, there were differences in mean numbers among groups and between all treatment groups compared to SO, but no differences were seen among any of the treatment groups. In all regions, all treatment groups showed differences compared to SO, but no differences were seen among any of the treatment groups. Results are expressed as mean ± SEM (n= 4–5 per group). (For differences among groups: CA1: F3, 15=9.34; P<0.01; CA2: F3, 15=5.50; P<0.01; CA3: F3, 15=5.1; P<0.05; DDG: F3, 15=87.45; P<0.0001; VDG: F3, 15=32.65; P<0.0001; hilus: F3, 15=22.24; P<0.0001; *: P<0.01, ^: P<0.001, ⊥: P<0.05, †: P<0.0001, compared with SO.) (B) Whole hippocampus shows little or no heterogeneity in response to different EB regimens in regard to volume defined by pCREB. For all groups, there were differences in mean volume among groups and between all treatment groups compared to SO. In all regions, all treatment groups showed differences compared to SO. In DDG, there was also a significant difference between 10µg /14d and 2.5µg /4d. Results are expressed as ± SEM (n= 4–5 per group). (For differences among groups: CA1: F3, 15=16.02; P<0.0001; CA2: F3, 15=9.30; P<0.01; CA3: F3, 15=9.13; P<0.01; DDG: F3, 15=51.33; P<0.0001; VDG: F3, 15=20.39; P<0.0001; hilus: F3, 15=11.63; P<0.0001; *: P<0.01, ^: P<0.001, ⊥: P<0.05, †: P<0.0001, compared with SO; *’: P<0.01, compared with 2.5µg /4d)
In terms of volume of the whole CA1 region as defined by pCREB immunolabeling, overall, there were significant differences in mean volume among groups (F3, 15=16.02; P<0.0001) (Figure 5B). All treatment groups showed significant differences compared to SO (2.5µg/4d: P<0.001; 2.5µg/14d: P<0.001; 10µg/14d: P<0.0001), but no differences were seen between any of the treatment groups. For the CA2, overall, there were significant differences in mean volume among groups (F3, 15=9.30; P<0.01) (Figure 5B). All treatment groups showed significant differences compared to SO (P<0.01 in all cases), but no differences between any of the treatment groups. For the CA3, overall there were significant differences in mean volume among groups (F3, 15=9.13; P<0.01) (Figure 5B). All treatment groups showed significant differences compared to SO (2.5 µg/4d: P<0.01; 2.5µg/14d: P<0.01; 10µg/14d: P<0.001), but no differences were seen between any of the treatment groups. For the DDG, overall, there were significant differences in mean volume among groups (F3, 15=51.33; P<0.0001) (Figure 5B). Significant differences were seen between all of the treatment groups and SO (P<0.0001 in all cases). There was also a significant difference between 10µg/14d and 2.5µg/4d (P<0.01). For the VDG, overall, there were significant differences in mean volume among groups F3, 15=20.39; P<0.0001) (Figure 5B). All treatment groups showed significant differences compared to SO (P<0.0001 in all cases), but no differences were seen between any of the treatment groups. For the hilus, overall there were significant differences in mean volume among groups (F3, 15=11.63; P<0.0001) (Figure 5B). All treatment groups showed significant differences compared to SO (P<0.001 in all cases), but no differences were seen between any of the treatment groups. In summary, the whole hippocampus displays virtually no heterogeneity in response to all of the estrogen regimens in regard to mean numbers of pCREB-immunolabeled cells; that is, all the hippocampal areas showed an increase in mean numbers of pCREB-immunolabeled cells compared to SO and mean volume defined by pCREB immunolabeling, with only the DDG showing differences between differences between 2.5µg /4d and 10µg /14d groups. These results differ from those in the rostral hippocampus described below, where there is a heterogeneity in responsiveness to the various estrogen regimens in regard to these parameters.
For mean numbers of pCREB-immunolabeled cells in the rostral CA1, overall, there were significant differences in mean numbers of pCREB-positive cells among groups (F3, 15=9.87; P<0.01) (Figure 6A). All treatments showed significant differences compared to SO (2.5µg/4d: P<0.001; 2.5µg/14d: P<0.001; 10µg/14d: P<0.05), but no differences were seen between any of the treatment groups. For the rostral CA2, no differences were seen among any of the groups (F3, 15=2.40; P>0.05) (Figure 6A). For the rostral CA3, overall there were significant differences in mean numbers of pCREB-immunolabeled cells among groups (F3, 15=6.42; P<0.01) (Figure 6A). Only the two moderate regimens differed significantly from SO (P<0.01 in both cases). For the rostral DDG, overall, there were significant differences in mean numbers of pCREB-immunolabeled cells among groups (F3, 15=4.38; P<0.05) (Figure 6A). Both moderate regimens showed significant differences compared with SO (P<0.05 in both cases). Both moderate regimens also showed differences compared to the 10µg/14d regimen (P<0.05 in both cases). For the rostral VDG, overall there were significant differences in mean numbers of pCREB-immunolabeled cells among groups (F3, 15=5.74; P<0.01) (Figure 6A). Both moderate regimens showed differences compared to SO (2.5µg/4d: P<0.01; 2.5µg/14d: P<0.05). In addition, the 2.5µg/4d regimen was significantly different from the 10µg/14d regimen (P<0.05).
Figure 6.
(A) Rostral hippocampus shows heterogeneity in response to different EB regimens in regard to counts of pCREB-labeled cells. For all regions, except the CA2, there were differences among groups. In the CA1, all treatment groups were different than SO, but there were no differences among treatment groups. For CA3, DDG and VDG, the 10µg/14d group showed no differences compared to SO. Both moderate regimens showed differences from SO group. For DDG, both moderate regimens also showed differences from the 10µg/14d group. For VDG, 2.5µg/4d group showed a significance difference from 10µg/14d. Results are expressed as mean ± SEM (n= 4–5 per group). (For differences among groups: CA1: F3, 15=9.87; P<0.01; CA3: F3, 15=6.42; P<0.01; DDG: F3, 15=4.38; P<0.05; VDG: F3, 15=5.74; P<0.01; ⊥: P<0.05, ^: P<0.001, *: P<0.01 compared with SO; ⊥” P<0.05, compared with 10ug/14day.) (B) Rostral hippocampus shows heterogeneity in response to different EB regimens in regard to volume defined by pCREB. For all regions, except the CA1, there were differences among groups. In CA2 and DDG, all treatment groups showed differences in mean volume compared to SO, but no differences were seen among treatment groups. In CA3 both moderate regimens were different than SO and the 10µg/14d group. For the VDG, both moderate regimens were different than SO, but only the 2.5µg/4d group was different than the 10µg/14d group. Results are expressed as mean± SEM (n= 4–5 per group). (For differences among groups: CA1: F3, 15=2.87; P>0.05; CA2: F3, 15=14.27; P<0.001; CA3: F3, 15=13.32; P<0.001; DDG: F3, 15=7.57; P<0.01; VDG: F3, 15,=7.56; P<0.01; *: P<0.01, †: P<0.0001, ⊥: P<0.05, ^: P<0.001 compared with SO; ⊥” P<0.05, *’: P<0.01 compared with 10ug/14day.)
For volume of the rostral CA1 as defined by pCREB immunolabeling, no differences were seen among any of the treatment groups (F3, 15=2.87; P>0.05) (Figure 6B). For the rostral CA2, overall, there were significant differences in mean volume among groups (F3, 15=14.27; P<0.001) (Figure 6B). All treatment groups showed significant differences compared to SO (2.5µg/4d: P<0.01; 2.5µg/14d: P<0.0001; 10µg/14d: P<0.05), but no differences were seen between any of the treatment groups. In the CA2 of whole hippocampus, there were corresponding changes in mean number of NeuN-immunolabeled neurons and mean volume defined by NeuN immunolabeling (Figure 3A and B) and mean number of pCREB-immunolabeled cells and mean volume defined by pCREB immunolabeling ((Figure 5A and B). This phenomenon was not seen in the rostral hippocampus. Whereas, there was an overall increase in mean NeuN-labeled neuronal numbers in the rostral CA2, no changes were observed for mean volume defined by NeuN immunolabeling in this area (Figure 4A and B). On the other hand, there were no changes in mean number of pCREB-immunolabeled cells in rostral CA2, but there were overall changes in volume of this area as defined by pCREB immunolabeling. These results may be a function of the smaller size of the rostral CA2 region and sample size. For the rostral CA3, overall there were significant differences in mean volume among groups (F3, 15=13.32; P<0.001) (Figure 6B). Only the two moderate treatment groups showed differences compared to SO (P<0.001 for both cases) and 10µg/14d (P<0.01 for both cases). For the rostral DDG, overall there were significant differences in mean volume among groups (F3, 15=7.57; P<0.01) (Figure 6B). All three treatment groups showed significant differences compared to SO (2.5µg/4d: P<0.001; 2.5µg/14d: P<0.01; 10µg/14d: P<0.05), but no differences were seen between any of the treatment groups. For the rostral VDG, overall there were significant differences in mean volume among groups (F3, 15=7.56; P<0.01) (Figure 6B). The two moderate regimens showed an increase in this parameter over SO (P<0.01 in both cases). The 2.5µg/4d regimen showed an increase in this parameter compared with the 10µg/14d regimen (P<0.05). In contrast to the whole hippocampus, the rostral hippocampus displays heterogeneity in response to the estrogen regimens. Whereas, the rostral CA1 responded in a similar manner as that of the whole hippocampus in regard to mean number of pCREB-immunolabeled cells, none of the other hippocampal regions followed that pattern; the rostral CA2 showed no response to any of the hormone regimens and only the two moderate regimens elicited a response in the rostral CA3, DDG and VDG. In terms of volume in rostral hippocampus, there was no response to any of the regimens in CA1. Only the rostral CA2 and rostral DDG responded in a similar manner to the whole hippocampus in the CA3 and rostral VDG the high regimen did not elicit a response. These results differ from those in the whole hippocampus described above, where there is a uniformity in responsiveness to the various estrogen regimens in regard to these parameters.
Other Considerations
In general, the range of values for the mean numbers of pCREB-immunolabeled cells was higher than those for NeuN-immunolabeled neurons. For the whole hippocampus, the range for the mean numbers of pCREB-immunolabeled cells compared to the range for the mean numbers of NeuN-immunolabeled neurons for all four of the experimental groups combined is as follows for the various brain regions: CA1, 2.35 × 105 versus 1.86 × 105; CA2, 5.10 × 104 versus 4.08 × 104; CA3, 1.55 × 105 versus 1.03 × 105; DDG, 2.24 × 105 versus 1.78 × 105; VDG, 1.64 × 105 versus 1.25 × 105; Hilus, 7.02 × 104 versus 5.69 × 104. For the rostral hippocampus, the range for the mean numbers of pCREB-immunolabeled cells compared to the mean numbers of NeuN-immunolabeled neurons for all four of the experimental groups combined is as follows for the various brain regions: CA1, 6.55 × 104 versus 5.16 × 104; CA2, 1.69 × 104 versus 1.43 × 104; CA3, 5.08 × 104 versus 4.33 × 104; DDG, 9.66 × 104 versus 7.28 × 104; VDG, 9.10 × 104 versus 6.97 × 104.
Discussion
We demonstrate differences in responsiveness to moderate and high EB regimens depending upon hippocampal subregion and area examined. In general, in whole hippocampus, we observed similar responses to moderate and high EB doses and lengths of treatment in regard to the mean number of NeuN- or pCREB-immunolabeled neurons and cells and volume of hippocampal subregions. However, in the rostral hippocampus, there was a disparity in patterns of these parameters in response to various EB regimens. These results reflect neuroanatomical heterogeneity in responsiveness to EB within the entire hippocampus and its subregions, which may have implications in estrogen-induced changes in hippocampal function.
Dose and Time Effects of EB in Hippocampus
The 2.5 µg EB for 4 and 14 days regimens resulted in plasma estradiol levels comparable to that seen in intact animals during proestrus by Raval et al. (2009). Our results for pCREB immunolabeling in whole and rostral CA1 hippocampus are also consistent with their observations of increased pCREB protein content and immunolabeling in the CA1 region of animals in proestrus and estrus compared to diestrus and metestrus. With 10 µg/14d EB, levels of pCREB immunolabeling also increased over the vehicle in the rostral CA1 region, but not the rostral CA3 region, DDG or VDG. In those cases, neuroadaptive changes may have occurred in response to the higher dose. Neuroadaptive changes may also underlie the heterogeneity in responses for NeuN immunolabeling in the rostral hippocampus. Also, the anterior hippocampus functions mainly in emotional processing, whereas, the posterior portion is associated with memory processing, and the functional differentiation of the hippocampus may be masked when measuring whole hippocampal volume (Willard et al., 2009). This phenomenon may have occurred here, where differences in patterns of responsiveness to different regimens were seen in rostral, but not whole, hippocampus.
There was only one case, i. e., in rostral VDG, whereby, increasing the length of treatment time from 4 to 14 days resulted in a significant difference between the two groups receiving 2.5 µg EB. This contrasts with results seen in the medial amygdala where, using the same treatment paradigms, we reported increased mean numbers of NeuN- and pCREB-immunolabeled neurons and cells and volumes with the 2.5µg/14d, but not 2.5µg/4d, regimen (Fan et al., 2008a). Time is a factor in the induction and sustenance of pCREB in hippocampal primary cell cultures (Lee et al., 2004a). Also, animals chronically treated with continuous or cyclic estrogen showed no increase in cell proliferation in the DG three weeks after the onset of estrogen replacement (Tanapat et al., 2005).
EB-induced increases in mean numbers of NeuN- and pCREB-immunolabeled neurons and cells and volumes may result from an increase in neurogenesis and neurotrophic and/or survival factors, such as the CREB-related gene factor BDNF and the anti-apoptotic protein Bcl-2, respectively, and/or a decrease in neuronal death. Treatment with the 10µg/14d EB regimen increased levels of pCREB, BDNF mRNA and protein and CaMKIV protein expression in rostral CA1 and CA3 regions, but not rostral CA2 or DG, compared to SO (Carlstrom et al., 2001; Zhou et al., 2005). The effect of BDNF on cell survival may account for some of the increases in NeuN- and pCREB-immunolabeled neurons and cells and volumes. Increased pCREB may elicit an increase in Bcl-2, augmenting survival of cells expressing pCREB, thereby, upregulating the entire pathway (Fan et al., 2008a, b). Studies in rostral hippocampus showed EB effects on Bcl-2 immunolabeling in all subregions, with moderate regimens eliciting a greater response than vehicle or 10µg/14d EB in the CA2 region, DDG and VDG; Bcl-2 mRNA was increased in all regions with the 2.5µg/14d regimen (Fan, Pandey and Cohen, unpublished observations).
The increase in effectiveness with moderate EB regimens compared to the high regimen in some of the rostral hippocampal regions has precedence in other studies. Tanapat et al. (2005) demonstrated an increase in cell proliferation in the DG of ovariectomized rats following one week of hormone deprivation with acute treatment with a moderate, but not low or high, dose of estradiol. Animals subjected to four weeks of hormone deprivation showed no increase in cell proliferation and a decrease in cells exhibiting the neuronal phenotype following the acute, moderate regimen. We also waited approximately four weeks before hormone replacement. Gibbs (2000) reported that enhancement of the functional status of cholinergic projections to the hippocampus and frontal cortex depended on the estrogen regimen and that longer treatment was ineffective.
Possible Mechanisms of EB Regulation of NeuN and pCREB in Hippocampus
Whereas, some of the observed effects may be due to indirect effects of EB, others may be due to binding to its receptors. Studies indicate differences in ERα and ERβ distribution within the hippocampus. ER-immunolabeled cells (the antibody used may not cross react with ERβ) have been found in the hilus and stratum radiatum of the CA1 region in dorsal hippocampus and were increased in the rostral, ventral hilus, with fewer seen in the temporal-caudal region of the hilus (Weiland et al., 1997). ERβ increases from the rostral to caudal hippocampus (Shughrue et al., 1997) and is present in ventral CA2/CA3 regions (Milner et al., 2005). ERα is present in the ventral hippocampus (Shughrue et al., 1997). Increased ERβ toward the caudal region may account for the increase in mean number of NeuN- and pCREB-immunolabeled neurons and cells and/or volumes in whole hippocampus. Conversely, the lower number of ERβ receptors in the rostral region may account for the decreased mean number of NeuN- and pCREB-immunolabeled neurons and cells and/or volumes with 10µg/14d EB. Higher EB concentrations with fewer receptors may cause a down regulation of receptors and fewer opportunities for estrogen binding in the rostral hippocampus. The increase in ERβ from rostral to caudal hippocampus (Shughrue et al., 1997) may prevent ER downregulation with the higher doses and longer duration of treatment in whole hippocampus (Lauber et al., 1991; Medlock et al., 1991a, b; Tanapat et al., 2005).
Our data may also result from EB effects on membrane ERs, including mER-Gαq, the orphan G-protein-coupled receptor, GPR30/GPER1, and the plasma membrane-associated, putative ER-X (Toran-Allerand, 2005; Mermelstein and Micevych, 2008; Micevych and Mermelstein, 2008; Kelly and Rønnekleiv, 2009; Olde and Leeb-Lundberg, 2009; Kuo et al., 2010; Maggiolini and Picard, 2010). These receptors appear to mediate intracellular signaling pathways and are implicated in cell proliferation, neuroprotection, and growth and survival (Gingerich et al., 2010). Some of these receptors have been localized to the hippocampus and/or in hippocampal cell cultures and their activation may lead to CREB phosphorylation and transcription of CREB-related gene products (Mermelstein and Micevych, 2008; Kelly and Rønnekleiv, 2009). Immunoreactivity for GPR30 was localized in subregions of the rat hippocampus (Brailoiu et al., 2007). GPR30 mRNA and protein levels were observed in CA1-3 pyramidal cells and granule cells of the DG (Matsuda et al., 2008). Studies from the Mermelstein laboratory indicate that activation of ERα leads to mGluR1a signaling and phosphorylation of CREB via phospholipase C regulation of MAPK, whereas, stimulation of ERα or ERβ resulted in mGluR2/3 signaling, with a concomitant decrease in L-type channel-mediated phosphorylation of CREB (Boulware et al., 2005). These effects were sex-specific (Boulware et al., 2005) and are dependent upon calveolin proteins (Boulware et al., 2007; Mermelstein and Micevych, 2008).
EB effects on pCREB levels may be also due to estrogen effects on glia, as the mean number of EB-induced pCREB-immunolabeled cells is somewhat greater than the mean number of NeuN-immunolabeled neurons in whole and rostral hippocampus, suggesting that not all cells activated by estrogen are neurons. There is evidence for the co-localization of pCREB and the glial marker, glial fibrillary-acidic protein (GFAP) in the hippocampal CA1 region (Musholt et al., 2009). ERα has been found in rat glial cells following hippocampal lesions (García-Ovejero et al., 2002) and about 30% of ERβ-labeled profiles in rat hippocampal subregions are glia (Milner et al., 2005). Luquin et al. (1993) have shown no differences in the number of GFAP-immunoreactive cells in the hilus of the DG with different phases of the estrus cycle, after ovariectomy, and after pharmacological administration of estrogen and/or progesterone. However, within the rat hippocampal hilus, the surface density of GFAP-immunoreactive processes was highest on the afternoon of proestrus and morning of estrus. There was also a dose-dependent increase in surface density of immunoreactive cells ovariectomized rats given 17β-estradiol alone or with progesterone. Glia activation observed with variations in the estrus cycle may also affect neuronal responsiveness to estrogen (Struble et al., 2006).
Kuo et al. (2010) have indicated a mechanism, whereby mERs stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. Putative mERs, including the mERα and Gαq-mER, interact with the group I metabotrobic glutamate receptor 1a (mGluR1a) to set in motion cell signaling, including activation of PKC, increases in intracellular calcium concentrations, and CREB phosphorylation (Micevych and Mermelstein, 2008). In hypothalamic astrocytes, ERα is present in the membrane and has an extracellular component; levels of ERα in the membrane and its internalization are regulated by estradiol and mGluR1a ligands (Bondar et al., 2009). In our study, estrogen may have upregulated pathways that result in phosphorylation of pCREB in hippocampal glia, which should be investigated in future studies.
Significance
We show variability in responses of hippocampal cells to disparate estrogen regimens in subregions of hippocampus and throughout its extent. Activation of pCREB and duration of its activation in the hippocampus (Porte et al., 2008), is important for physiological and behavioral outcomes. Our results are relevant to the clinical use of low- versus high-dose hormone therapies. The disparity in responsiveness to estrogen within the hippocampus may depend on specific ERs, their distribution and other factors, which may confer responsiveness to estrogen with particular regimens.
Acknowledgments
Grant information: This work was supported by National Institute of Mental Health grant MH065990 and funds from the University of Illinois Earl M. Bane Charitable Trust (to R. S. C.), and the National Institute on Alcohol Abuse and Alcoholism grant AA-010005; AA-013341 and Veterans Affairs Merit grant and VA Career Scientist Award (to S. C. P.).
References
- Ábrahám IM, Han S, Todman MG, Korach KS, Herbison AE. Estrogen receptor {beta} mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barha CK, Dalton GL, Galea LA. Low doses of 17alpha-estradiol and 17beta-estradiol facilitate, whereas higher doses of estrone and 17alpha- and 17beta-estradiol impair, contextual fear conditioning in adult female rats. Neuropsychopharmacology. 2009;35(2):547–559. doi: 10.1038/npp.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Galea LA. Repeated estradiol administration alters different aspects of neurogenesis and cell death in the hippocampus of female, but not male, rats. Neuroscience. 2008;52(4):888–902. doi: 10.1016/j.neuroscience.2007.10.071. [DOI] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999;64:803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Bondar G, Kuo J, Micevych P. Estradiol-induced receptor-α trafficking. J Neurosci. 2009;29(48):15323–15330. doi: 10.1523/JNEUROSCI.2107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response-element binding protein. J Neurosci. 2005;25(20):5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Kordasiewicz H, Mermelstein PG. Calveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci. 2007;27(37):9941–9950. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- Carlstrom L, Ke ZJ, Unnerstall JR, Cohen RS, Pandey SC. Estrogen modulation of the cyclic AMP response element-binding protein pathway. Effects of long-term and acute treatments. Neuroendocrinology. 2001;74(4):227–243. doi: 10.1159/000054690. [DOI] [PubMed] [Google Scholar]
- Cohen RS, Pfaff DW. Ultrastructure of neurons in the ventromedial nucleus or the hypothalamus in ovariectomized rats with or without estrogen treatment. Cell Tissue Res. 1981;217(3):451–470. doi: 10.1007/BF00219357. [DOI] [PubMed] [Google Scholar]
- Dowsett M, Folkerd E. Deficits in plasma oestradiol measurements in studies and management of breast cancer. Breast Cancer Res. 2005;7:1–4. doi: 10.1186/bcr960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Hanbury R, Pandey SC, Cohen RS. Dose and time effects of estrogen on expression of neuron-specific protein and cyclic AMP response element-binding protein and brain region volume in the medial amygdala of ovariectomized rats. Neuroendocrinology. 2008a;88(2):111–126. doi: 10.1159/000129498. [DOI] [PubMed] [Google Scholar]
- Fan L, Pandey SC, Cohen RS. Estrogen affects levels of Bcl-2 protein and mRNA in medial amygdala of ovariectomized rats. J Neurosci Res. 2008b;86(16):3655–3664. doi: 10.1002/jnr.21801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA, Uban KA, Epp JR, Brummelte S, Barha CK, Wilson WL, Lieblich SE, Pawluski JL. Endocrine regulation of cognition and neuroplasticity: our pursuit to unveil the complex interaction between hormones, the brain, and behaviour. Can J Exp Psychol. 2008;62(4):247–260. doi: 10.1037/a0014501. [DOI] [PubMed] [Google Scholar]
- García-Ovejero D, Veiga S, García-Segura LM, DonCarlos LM. Glial expression of estrogen and androgen receptors after rat brain injury. J Comp Neurol. 2002;450:256–271. doi: 10.1002/cne.10325. [DOI] [PubMed] [Google Scholar]
- García-Segura LM, Cardona-Gomez P, Naftolin F, Chowen J. Estradiol upregulates Bcl-2 expression in adult brain neurons. Neuroendocrinology. 1998;9(4):593–597. doi: 10.1097/00001756-199803090-00006. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Effects of gonadal hormone replacement on measures of basal forebrain cholinergic function. Neuroscience. 2000;101(4):931–938. doi: 10.1016/s0306-4522(00)00433-4. [DOI] [PubMed] [Google Scholar]
- Gingerich S, Kim GL, Chalmers JA, Koletar MM, Wang X, Wang Y, Belsham DD. Estrogen receptor alpha and G-protein coupled receptor 30 mediate the neuroprotective effects of 17β-estradiol in novel murine hippocampal cell models. Neurosciece. 2010;170:54–66. doi: 10.1016/j.neuroscience.2010.06.076. [DOI] [PubMed] [Google Scholar]
- Goodman RL. A quantitative analysis of the physiological role of estradiol and progesterone in the control of tonic and surge secretion of luteinizing hormone in the rat. Endocrinology. 1978;102(1):42–150. doi: 10.1210/endo-102-1-142. [DOI] [PubMed] [Google Scholar]
- Gu G, Rojo AA, Zee MC, Yu J, Simerly RB. Hormonal regulation of CREB phosphorylation in the anteroventral periventricular nucleus. J Neurosci. 1996;16(9):3035–3044. doi: 10.1523/JNEUROSCI.16-09-03035.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB, Kiêu K, Nielsen J. The efficiency of systematic sampling in stereology--reconsidered. J Microsc. 1999;193(Pt 3):199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Hanbury R, Pandey SC, Cohen RS. Strain-dependent differences in plasma steroid horone levels and in phosphorylated CREB (pCREB) levels in limbic brain areas of intact and ovariectomized (OVX) female rats. Soc Neurosci Abs. 2007;729:17. [Google Scholar]
- Hoffman GE, Le WW, Schulterbrandt T, Legan SJ. Estrogen and progesterone do not activate Fos in AVPV or LHRH neurons in male rats. Brain Research. 2005;1054:116–124. doi: 10.1016/j.brainres.2005.06.082. [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Moore N, Fiskum G, Murphy AZ. Ovarian steroid modulation of seizure severity and hippocampal cell death after kainic acid treatment. Exp Neurol. 2003;182:124–134. doi: 10.1016/s0014-4886(03)00104-3. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Rønnekleiv OK. Control of CNS neuronal excitability by estrogens via membrane initiated signaling. Mol Cellul Endocrinol. 2009;308(1–2):17–25. doi: 10.1016/j.mce.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow LM, Pfaff DW. Induction of lordosis in female rats: two models of estrogen action and the effect of adrenalectomy. Horm Behav. 1975;6:259–276. doi: 10.1016/0018-506x(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J Neurosci. 2010;30(9):12950–12957. doi: 10.1523/JNEUROSCI.1158-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb SV. Endocrinology Laboratory of the Cornell New York State Animal Health Diagnostic Laboratory. Ithaca, NY: College of Veterninary Medicine; unpubl. observations. [Google Scholar]
- Lauber AH, Mobbs CV, Muramatsu M, Pfaff DW. Estrogen receptor messenger RNA expression in rat hypothalamus as a function of genetic sex and estrogen dose. Endocrinology. 1991;129(6):3180–3186. doi: 10.1210/endo-129-6-3180. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Campomanes CR, Sikat PT, Greenfield AT, Allen PB, McEwen BS. Estrogen induces phosphorylation of cyclic AMP response element binding (pCREB) in primary hippocampal cells in a time-dependent manner. Neuroscience. 2004a;124:549–560. doi: 10.1016/j.neuroscience.2003.11.035. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Romeo RD, Svenningsson P, Campomanes CR, Allen PB, Greengard P, McEwen BS. Estradiol affects spinophilin protein differently in gonadectomized males and females. Neuroscience. 2004b;127:983–988. doi: 10.1016/j.neuroscience.2004.05.049. [DOI] [PubMed] [Google Scholar]
- Lister JP, Tonkiss J, Blatt GJ, Kemper TL, DeBassio WA, Galler JR, Rosene DL. Asymmetry of neuron numbers in the hippocampal formation of prenatally malnourished and normally nourished rats: a stereological investigation. Hippocampus. 2006;16:946–958. doi: 10.1002/hipo.20221. [DOI] [PubMed] [Google Scholar]
- Lorenz B, García-Segura LM, DonCarlos LL. Cellular phenotype of androgen receptor-immunoreactive nuclei in the developing and adult rat brain. J Comp Neurol. 2005;492(4):456–468. doi: 10.1002/cne.20763. [DOI] [PubMed] [Google Scholar]
- Luquin S, Naftolin F, García-Segura LM. Natural fluctuation and gonadal hormone regulation of astrocyte immunoreactivity in dentate gyrus. J Neurobiol. 1993;24(7):913–924. doi: 10.1002/neu.480240705. [DOI] [PubMed] [Google Scholar]
- Lyck L, Jelsing J, Jensen PS, Lambertsen KL, Pakkenberg B, Finsen B. Immunohistochemical visualization of neurons and specific glial cells for stereological application in the porcine neocortex. J Neurosci Methods. 2006;152:229–242. doi: 10.1016/j.jneumeth.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Maggiolini M, Picard D. The unfolding stories of GPR30, a new membrane-bound estrogen receptor. J Endocrin. 2010;204:105–114. doi: 10.1677/JOE-09-0242. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Sakamoto H, Mori H, Hosokawa K, Kawamura A, Itose M, Nishi M, Prossnitz ER, Kawata M. Expression and intracellular distribution of the G protein-coupled receptor 30 in rat hippocampal formation. Neurosci Lett. 2008;441:94–99. doi: 10.1016/j.neulet.2008.05.108. [DOI] [PubMed] [Google Scholar]
- Medlock KL, Forrester TM, Sheehan DM. Short-term effects of physiological and pharmacological doses of estradiol on estrogen receptor and uterine growth. J Recept Res. 1991a;11(5):743–756. doi: 10.3109/10799899109064677. [DOI] [PubMed] [Google Scholar]
- Medlock KL, Lyttle CR, Kelepouris N, Newman ED, Sheehan DM. Estradiol down-regulation of the rat uterine estrogen receptor. Proc Soc Exp Biol Med. 1991b;196(3):293–300. doi: 10.3181/00379727-196-43191. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Micevych PE. Nervous system physiology regulated by membrane estrogen receptors. Rev Neurosci. 2008;19:423–424. doi: 10.1515/revneuro.2008.19.6.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Mermelstein PG. Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in the brain. Mol Neurobiol. 2008;38:66–67. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491(2):81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Dugger BN, Breedlove SM. Partial demasculinization of several brain regions in adult male (XY) rats with a dysfunctional androgen receptor gene. J Comp Neurol. 2005;487:217–226. doi: 10.1002/cne.20558. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AN. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Musholt K, Cirillo G, CAvaliere C, Bianco MR, Bock J, Helmeke C, Braun K, Papa M. Neonatal separation stress reduces glial fibrillary acidic protein- and S100-immunoreactive astrocytes in rat medial precentral cortex. Develop Neurobiol. 2009;69:203–211. doi: 10.1002/dneu.20694. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Chen J, Fujioka T, Malberg J, Tsuji S, Duman RS. Localization of phosphorylated cAMP response element-binding protein in immature neurons of adult hippocampus. J Neurosci. 2002;22(22):9868–9876. doi: 10.1523/JNEUROSCI.22-22-09868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura NH, Rosell DR, Akama KT, McEwen BS. Estrogen and ovariectomy regulate mRNA and protein of glutamic acid decarboxylases and cation-chloride cotransporters in the adult rat hippocampus. Neuroendocrinology. 2004;80:308–323. doi: 10.1159/000083657. [DOI] [PubMed] [Google Scholar]
- Olde B, Leeb-Lundberg LMF. GRP30/GPER1: searching for a role in estrogen physiology. Trends Endocrinol Metab. 2009;20(8):409–416. doi: 10.1016/j.tem.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Xu T. Deficits in amygdaloid cAMP-responsive element-binding protein signaling play a role in genetic predisposition to anxiety and alcoholism. J Clin Invest. 2005;115:2762–2773. doi: 10.1172/JCI24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Porte Y, Buhot MC, Mons NE. Spatial memory in the Morris water maze and activation of cyclic AMP response element-binding (CREB) protein within the mouse hippocampus. Learn Mem. 2008;15(12):885–894. doi: 10.1101/lm.1094208. [DOI] [PubMed] [Google Scholar]
- Raval AP, Saul I, Defazio KR, Perez-Pinzon MA, Bramlett H. Pretreatment with a single estradiol-17β bolus activates cyclic-AMP response element binding protein and protects CA1 neurons against global cerebral ischemia. Neuroscience. 2009;160(2):307–318. doi: 10.1016/j.neuroscience.2009.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers TJ, Lamb SV, Bartlett SA, Matamoros RA, Cowan RG, Engel JS. Effects of hemolysis and storage on quantification of hormones in blood samples from dogs, cattle, and horse. Am J Vet Res. 1991;7:1075–1080. [PubMed] [Google Scholar]
- Rønnekleiv OK, Malyala A, Kelly MJ. Membrane-initiated signaling of estrogen in the brain. Semin Reprod Med. 2007;25(3):165–177. doi: 10.1055/s-2007-973429. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Amaral DG. Stereological estimation of the number of neurons in the human amygdaloid complex. J Comp Neurol. 2005;491:320–329. doi: 10.1002/cne.20704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Leuner B. Estrogen-mediated effects on depression and memory formation in females. J Affect Disord. 2003;74(1):85–96. doi: 10.1016/s0165-0327(02)00428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388(4):507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Struble RG, Afridi S, Beckman-Randall S, Li M, Cady C, Nathan B, McAey ME. Neocortical and hippocampal glial fibrillary acidic protein immunoreactivity shows region-specific variation during the mouse estrous cycle. Neuroendocrinology. 2006;83:325–335. doi: 10.1159/000095340. [DOI] [PubMed] [Google Scholar]
- Szegő EM, Barabas K, Balog J, Szilagyi N, Korach KS, Juhasz G, Ábrahám IM. Estrogen induces estrogen receptor {alpha}-dependent cAMP response element-binding protein phosphorylation via mitogen activated protein kinase pathway in basal forebrain cholinergic neurons in vivo. J Neurosci. 2006;26:4104–4110. doi: 10.1523/JNEUROSCI.0222-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuma K, Matsuo A, Himeno Y, Hoshina Y, Ohno Y, Funatsu Y, Arai S, Kamei H, Mizoguchi H, Nagai T, Inoue M, Yamada K. 17beta-estradiol attenuates hippocampal neuronal loss and cognitive dysfunction induced by chronic restraint stress in ovariectomized rats. Neuroscience. 2007;146(1):60–68. doi: 10.1016/j.neuroscience.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Gould E. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J Comp Neurol. 2005;481(3):252–265. doi: 10.1002/cne.20385. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Estrogen and the brain. Beyond ER-α, ER-β and 17β-estradiol. Ann NY Acad Sci. 2005;1052:136–144. doi: 10.1196/annals.1347.009. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Effects of two estradiol regimens on anxiety and depressive behaviors and trophic effects in peripheral tissues in a rodent model. Gend Med. 2009;6(1):300–311. doi: 10.1016/j.genm.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland NG, Orikasa C, Hayashi S, McEwen BS. Distribution and hormone regulation of estrogen receptor immunoreactive cells in the hippocampus of male and female rats. J Comp Neurol. 1997;388(4):603–612. doi: 10.1002/(sici)1096-9861(19971201)388:4<603::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Willard SL, Friedman DP, Henkel CK, Shively CA. Anterior hippocampal volume is reduced in behaviorally depressed female cynomolgus macaques. Psychoneuroendocrinology. 2009;34(10):1469–1475. doi: 10.1016/j.psyneuen.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Horm Behav. 1998;34(2):140–148. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]
- Young KA, Holcomb LA, Yazdani U, Hicks PB, German DC. Elevated neuron number in the limbic thalamus in major depression. Am J Psychiatry. 2004;161:1270–1277. doi: 10.1176/appi.ajp.161.7.1270. [DOI] [PubMed] [Google Scholar]
- Zhao H, Tian Z, Hao J, Chen B. Extragonadal aromatization increases with time after ovariectomy in rats. Reprod Biol Endocrinol. 2005;3:6. doi: 10.1186/1477-7827-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Cohen RS, Pandey SC. Estrogen affects the expression of Ca2+/calmodulin-dependent protein kinase IV in amygdala. Neuroreport. 2001;12(13):2987–2990. doi: 10.1097/00001756-200109170-00046. [DOI] [PubMed] [Google Scholar]
- Zhou J, Pandey SC, Cohen RS. Estrogen decreases levels of calcineurin in rat amygdala and hippocampus. Neuroreport. 2004;15(15):2437–2440. doi: 10.1097/00001756-200410250-00027. [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhang H, Cohen RS, Pandey SC. Effects of estrogen treatment on expression of brain-derived neurotrophic factor and cAMP response element-binding protein expression and phosphorylation in rat amygdaloid and hippocampal structures. Neuroendocrinology. 2005;81(5):294–310. doi: 10.1159/000088448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Watters J, Dorsa D. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology. 1996;137:2163–2166. doi: 10.1210/endo.137.5.8612562. [DOI] [PubMed] [Google Scholar]