Abstract
The combination of highly efficient polymerizations with modular “click” coupling reactions has enabled the synthesis of wide variety of novel nanoscopic structures. Here we demonstrate the facile synthesis of a new class of clickable, branched nanostructures, polyethylene glycol (PEG)-branch-azide bivalent-brush polymers, facilitated by “graft-through” ring-opening metathesis polymerization (ROMP) of a branched norbornene-PEG-chloride macromonomer followed by halide-azide exchange. The resulting bivalent-brush polymers possess azide groups at the core near a polynorbornene backbone with PEG chains extended into solution; the structure resembles a unimolecular micelle. We demonstrate copper-catalyzed azide-alkyne cycloaddition (CuAAC) “click-to” coupling of a photocleavable doxorubicin (DOX)-alkyne derivative to the azide core. The CuAAC coupling was quantitative across a wide range of nanoscopic sizes (~6 – ~50 nm); UV photolysis of the resulting DOX-loaded materials yielded free DOX that was therapeutically effective against human cancer cells.
Introduction
The explosion of nanoscience is fuelled by problems that require nanoscale solutions and by the inherent challenges of nanoscale synthesis.1–3 In drug delivery, for example, plasma clearance time of a polymer-drug conjugate is closely linked to its nanoscopic size; materials smaller than 10 nm are excreted rapidly whereas larger materials often display significantly longer retention times.3–9 Nanoscopic architecture also plays an important role in determining in vivo retention time.10–12 Branched polymeric structures such as dendrimers, star polymers, bottle-brush polymers, and hyperbranched polymers generally display longer in vivo retention times compared to their linear polymer analogues.11,13 A wealth of research attention has focused on synthetic approaches to branched, stimuli-responsive, nanoscopic materials that possess orthogonal functionality for incorporation of therapeutic, imaging, and targeting molecules.14–25
The most widely studied branched macromolecules for drug delivery are dendrimers; their nanoscale, monodisperse, multi-valent structures provide functional handles for elaboration with bioactive groups of interest.26–28 One factor that limits widespread application of dendrimers is synthetic difficulty. Though recent synthetic advances are impressive,16,29–32 it remains a challenge to prepare functionally diverse dendrimers of variable sizes. To overcome this challenge, researchers have appended linear polymers to dendrimers33–36 or prepared linear polymers with dendritic fragments attached to their sidechains. The latter “dendronized linear polymers” have attracted attention as nanoscopic building blocks; their cylindrical shapes can provide important advantages in nanoscale fabrication when compared to spherical dendrimers.37–43 Similar nanoscale structures can be formed from bottle-brush-polymers that carry long chains grafted at high density to linear polymer backbones.44–63 Bottle-brush polymers are prepared by graft-to, graft-from, or graft-through methodologies. The graft-to strategy requires an efficient coupling reaction to attach a functional molecule to every monomer unit of a linear polymer; “click”64 reactions such as the copper-catalyzed azide-alkyne cycloaddition65,66 (CuAAC) and thiol-ene coupling have proven useful in this regard.43,46,67–70 Graft-from and graft-through methodologies require highly efficient polymerization reactions capable of initiation and propagation in sterically-demanding environments.48,51–53,71 Recent developments in efficient, controlled polymerization coupled with new click reactions provide materials chemists with the tools to generate novel functional nanoscopic materials.72–77
Ring-opening metathesis polymerization (ROMP) of strained alkene- (e.g. norbornene)-terminated macromonomers (MM) initiated by ruthenium N-heterocyclic carbene complexes (e.g. 1, Figure 1) has recently proven useful for graft-through synthesis of functional bottle-brush polymers;50,78–83 fast initiation leads to low polydispersities (PDIs) while release of ring strain provides the driving force for high conversion in these systems. Although catalyst 1 is incompatible with azides and alkynes, its functional group tolerance enables use of monomers bearing azide and alkyne precursors that are easily converted to the requisite click groups after polymerization. Binder and coworkers used ROMP combined with CuAAC to append various small molecules to clickable linear polymers.84,85 We have used ROMP in conjunction with CuAAC to generate cyclic polymers by end-to-end coupling.86 To our knowledge, there are no examples of water-soluble brush polymers prepared by graft-through ROMP that are subsequently functionalized via click reactions. Such a strategy combines the benefits of ROMP for graft-through brush polymer synthesis with the modularity and efficiency of CuAAC for clicking-to; branched nanoscopic polymers with easily addressable functional groups can be rapidly prepared over a wide range of sizes simply by altering the ratio of catalyst to MM.
Figure 1.
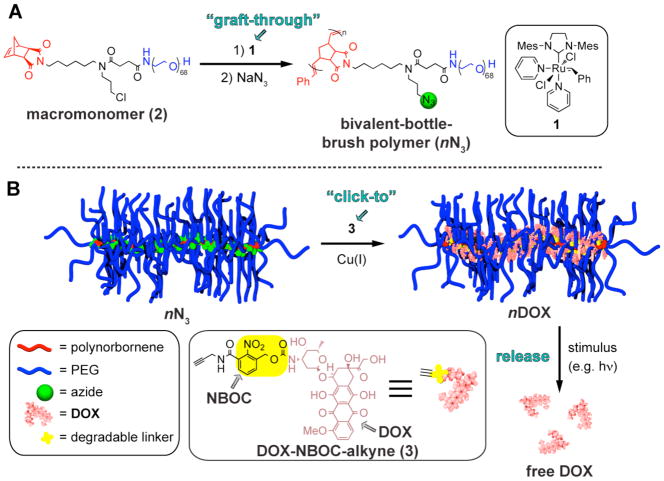
Schematic depiction of the “graft-through and click-to” approach described in this work. A: We start with a bivalent macromonomer (2) and perform “graft-through” ROMP with catalyst 1 followed by in situ chloride-azide exchange. B: The resulting azido-bivalent-brush polymer is then functionalized with an alkyne by click chemistry in a “click-to” step. If the alkyne partner is a drug molecule linked via a cleavable linker, then the resulting brush polymer can release the drug in response to an external stimulus. In this work we demonstrate controlled release of the anticancer agent doxorubicin (DOX) in response to 365 nm UV light.
In this report we demonstrate the sequential combination of graft-through- and click-to synthetic strategies to generate a clickable polyethylene glycol (PEG)-azide branched polymer material: a bivalent-bottle-brush polymer (Figure 1). Graft-through ROMP of a PEG-chloride macromonomer (Figure 1, Scheme 1, 6) gave bivalent-brush polymers of varying nanoscopic sizes. These materials resemble 1st-generation dendronized polymers with branch points located near the linear polymer backbone (Figure 1); attached on one side of the branch point is a linear polymer and on the other side a small molecule (e.g. a drug). This design incorporates a water-soluble PEG domain that extends into solvent and a hydrophobic alkyl chloride near the core. We hypothesize that PEG attachment to one branch of these bivalent-branch polymers will confer the biocompatibility widely observed for PEGylated systems.6,87 Core-functionalized nanomaterials have been elaborated with functional small molecules by click chemistry;88–92 in this work we convert the core alkyl chlorides to azides by treatment with sodium azide. Then, we utilize CuAAC to couple doxorubicin (DOX), to the core via a photo-cleavable nitrobenzyloxylcarbonyl (NBOC) linker. The latter enables controlled release of free DOX in response to long-wavelength (~365 nm) ultraviolet (UV) light; we demonstrate that released DOX is therapeutically active against human cancer cells in culture.
Scheme 1.
Synthesis of PEG-norbornene-chloride MM 6.
Results and Discussion
Synthesis of MM 6
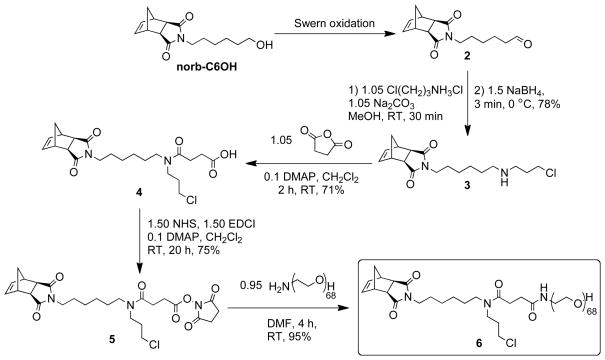
Clickable bivalent PEG brush polymers can be generated from an MM containing a strained norbornene derivative for ROMP, a PEG chain, and a functional group suitable for CuAAC (Figure 1, Scheme 1). Since neither azides nor alkynes are compatible with catalyst 1, we designed MM 6 to carry an alkyl chloride for facile conversion to an azide in a post-ROMP modification step. We incorporated a 6-carbon spacer between the strained norbornene moiety and the tertiary amide branch point to reduce steric hindrance between the growing polymer chain end and the branched side-chains. This spacer may also facilitate subsequent sidechain modification reactions, such as chloride to azide conversion and CuAAC. MM 6 was obtained in five steps from exo-N-(6-hydroxyhexyl)-5-norbornene-2,3-dicarboximide (norb-C6OH, Scheme 1). The synthesis commenced with Swern oxidation of norb-C6OH to give aldehyde 2. Reductive amination using 2 and 3-chloropropylamine hydrochloride gave secondary amine 3, which was readily converted to acid 4 by treatment with succinic anhydride. Reaction of 4 with N-hydroxysuccinimide (NHS) in the presence of N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride (EDC) and catalytic 4-(dimethylamino)pyridine (DMAP) gave NHS ester 5. We have prepared 5 on a multi-gram scale and expect it to be useful for the preparation of a variety of multifunctional ROMP monomers. In this work, commercially available 3 kDa PEG-NH2 was treated with a slight excess of 5 to give MM 6. Excess 5 was used to ensure complete conversion of every PEG-amine to amide 6; the only polymeric species in the final reaction mixture was 6 and it was easily purified via repeated precipitation in diethyl ether. The 1H NMR, 13C NMR, and MALDI spectra for MM 6 are provided in the supporting information.
Graft-through ROMP of 6 and halide-azide exchange to generate nN3 bivalent-brush polymers
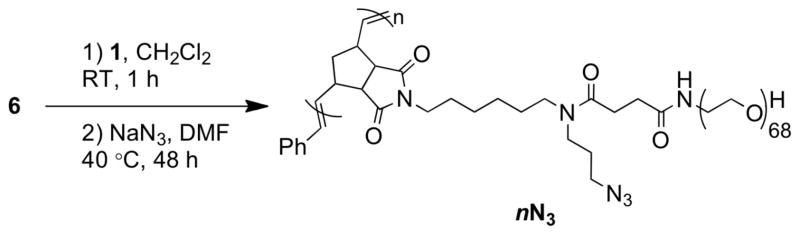
We pursued a one-pot synthetic strategy to rapidly generate a library of azide-functional brush polymers (nN3) with variable number-average degrees of polymerization (DP) and hydrodynamic radii. A strength of this approach is the ease of generating branched functional materials of variable sizes rapidly and efficiently; each of the polymers described here was prepared by adding an aliquot of catalyst 1 to a vial containing MM 6, allowing 60 min for ROMP to proceed, quenching the polymerization by addition of ethyl vinyl ether, solvent exchange, and finally treatment with sodium azide (Scheme 2). The first several steps were performed within a few hours whereas the final step, attaching azides to the polymer sidechains, was left for 48 h to ensure high conversion.
Scheme 2.
Synthesis of azido-bivalent-brush polymers (nN3) by ROMP and chloride-azide exchange.
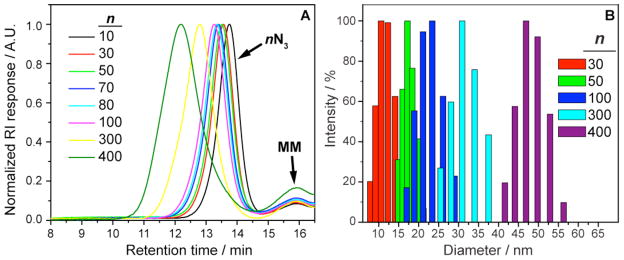
The polymer characterization data in Table I demonstrate that ROMP of 6 is well-controlled up to the highest DP tested; a small amount of catalyst deactivation and/or experimental error may explain the larger-than-expected DP for the 400N3 samples. In general, polymers with low polydispersities and molecular weights defined by the ratio of 6:1 were obtained. The GPC traces for all samples (Figure 2A) show monomodal molecular weight distributions and very high (>95%) MM conversions. All of the polymers were highly soluble (>100 mg/mL) in water; aqueous DLS measurements (Table I, Figure 3B) confirm that their nanoscopic dimensions increased with DP and were tunable over a broad range (radii from 3 – 25 nm) of relevant sizes for drug delivery applications. Furthermore, DLS (Figure 2B) shows that the particle size distributions are monomodal.
Table I.
Characterization of nN3 bivalent brush polymers by GPC (room temp., 0.2 M LiBr in DMF, 1 mL/min) and DLS (aqueous).
| n (theo.)[a] | DP[b] | Mn (GPC, kDa) | PDI | Rh[c]/nm |
|---|---|---|---|---|
| 10 | 10 | 34.1 | 1.11 | 3.1 (0.3) |
| 30 | 32 | 109 | 1.04 | 6.3 (0.8) |
| 50 | 53 | 180 | 1.07 | 6.9 (0.9) |
| 70 | 70 | 238 | 1.14 | 7.3 (0.3) |
| 80 | 80 | 272 | 1.14 | 7.5 (0.8) |
| 100 | 101 | 343 | 1.06 | 11 (1) |
| 300 | 295 | 1,000 | 1.11 | 15 (1) |
| 400 | 575 | 1,960 | 1.27 | 25 (2) |
ratio of MM to catalyst (i.e. theoretical DP). This number is used to identify nN3 and DOXN3 samples throughout the text,
DP observed derived from Mn(GPC)/Mn(MM),
hydrodynamic radii measured by dynamic light scattering (DLS). DLS correlation functions were fit using the CONTIN algorithm.
Figure 2.
A: Representative GPC traces for nN3 bivalent-brush polymer samples show monomodal molecular weight distributions, low polydispersities, and high conversions. B: Hydrodynamic diameter histograms for selected nN3 polymers obtained from DLS measurements using the CONTIN fitting algorithm. The particle sizes increase with increasing DP and are varied over a wide range by adjusting the ratio of MM to catalyst (6:1) before graft-through ROMP.
Figure 3.
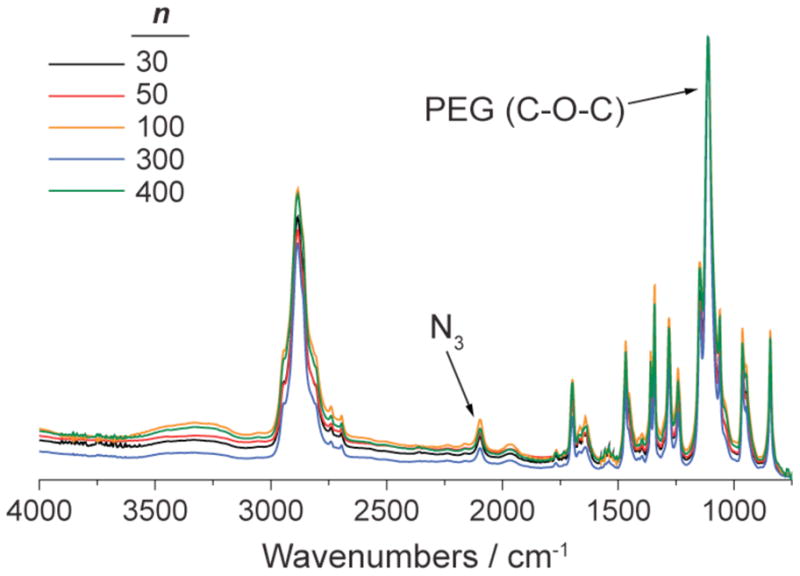
FTIR spectra of nN3 bivalent-brush polymers normalized to the strong PEG ether (C-O-C) antisymmetric stretch absorbance. The relative amount of azide is approximately the same for each n value which suggests that chloride-azide exchange is not sterically limited with increased DP.
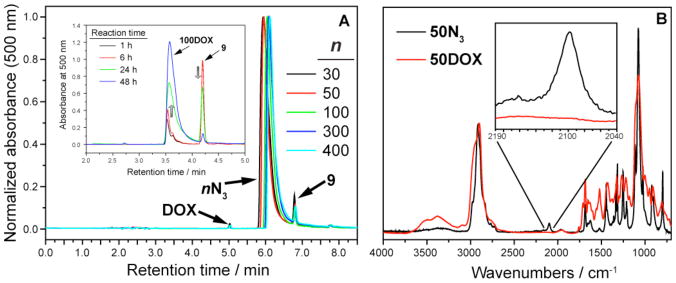
Graft-through ROMP ensures that every side chain unit possesses both a PEG chain and an alkyl chloride; the weight percentage of chloride is independent of DP. It follows that if every chloride is converted to an azide then the weight percentage of azide is also independent of DP. One may expect sidechain modification reactions with bivalent-brush polymers to display DP-dependent conversion; perhaps increased DP would lead to lower reaction yields because of increased steric hindrance. We have found that for both chloride-azide exchange and CuAAC reactions there is no apparent difference in reaction yield across the DP values studied here. Figure 3 shows the FTIR spectra for a subset of nN3 polymers normalized to the PEG ether (C-O-C antisymmetric stretch) absorbance at ~1100 cm−1. The intensities of the characteristic azide antisymmetric stretch (~2100 cm−1) are the same for each DP, which suggests that the azide:PEG ratio is the same for each sample and that the chloride-azide exchange reaction occurred with equal efficiency for each DP value.
Click chemistry using DOX-NBOC-alkyne 9
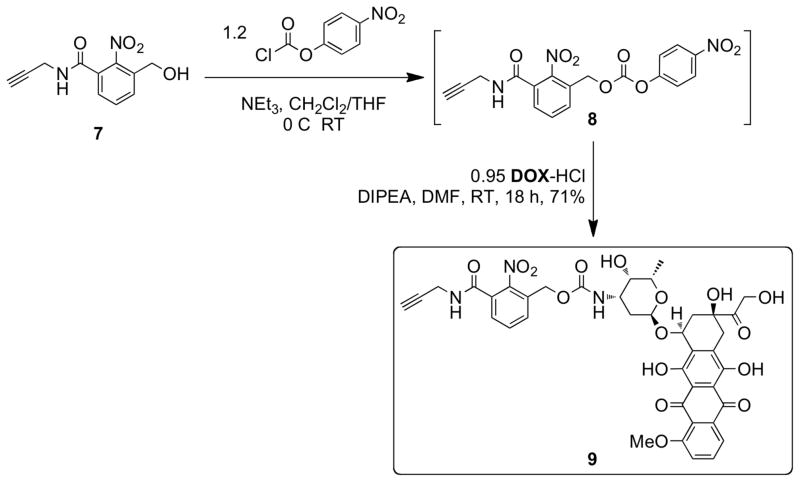
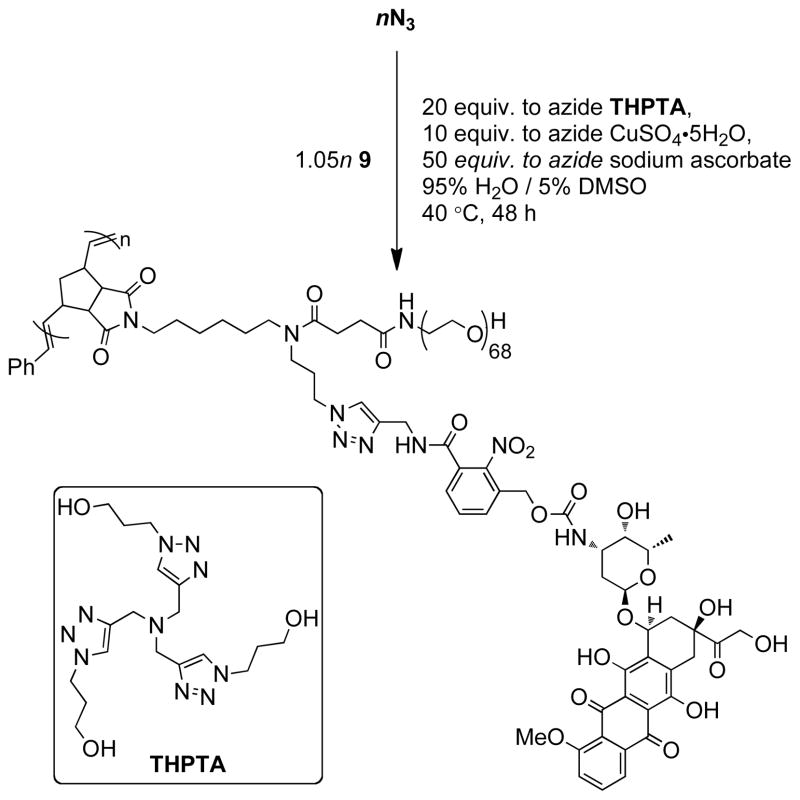
The ultimate application of these nN3 bivalent-brush polymers is defined by how they are decorated with functional groups. For drug delivery applications one can imagine clicking a drug-alkyne derivative attached via a cleavable linker group (Figure 1). The resulting polymer would undergo controlled drug release in response to an external stimulus leaving the brush polymer scaffold intact. In this work, we designed 9 for controlled release of DOX in response to UV light after click coupling to the nN3 polymer scaffold (Scheme 3). Photo-initiated release from macromolecules provides a means for site-specific, controlled drug delivery and could potentially complement traditional phototherapeutic methods.93–103 Though placement of azide groups near the core of these bivalent-brush polymers should allow PEG to extend into solution and increase the biocompatibility of these materials, a buried location may make them difficult to functionalize with subsequent reactions. Anticipating this challenge was one reason we chose two highly efficient reactions, halide-azide exchange and CuAAC, as the modification reactions; as described above CuAAC has proven useful for quantitative attachment of small molecules to the side chains of ROMP polymers84,85 and we expected that it would provide the best opportunity for high-yield coupling of sterically-demanding alkyne reagents to bivalent-branch polymers. There are a variety of CuAAC conditions throughout the literature and the catalyst/ligand/solvent system depends on the application;104–107 perhaps the most impressive applications of CuAAC are in the field of bioconjugation where the requisite azide and alkyne functional groups are present in very low concentration amongst a number of other reactive groups.108–111 The Finn laboratory recently reported optimized CuAAC conditions for bioconjugation which utilize a new accelerating ligand THPTA (Scheme 4).112 Aqueous CuAAC was found to proceed orders of magnitude faster with THPTA than in the absence of a ligand. Since these nN3 bivalent-brush polymers are nanoscopic, narrowly-dispersed, water-soluble materials with buried functional groups, we envisioned CuAAC reactions to them as similar to bioconjugation reactions to proteins bearing multiple azide or alkyne groups. After initial attempts without accelerating ligand, which led to < 20% conversion after several days, we were pleased to find that addition of THPTA to the reaction mixture gave quantitative conversions. In a typical reaction, the polymer, THPTA (20 equiv. to azide), and alkyne 9 (1.05 equiv. to azide) were dissolved in 95% H2O/5% DMSO such that the concentration of alkyne 9 was 2 mM. Aqueous sodium ascorbate (1.0 M) was added followed by copper(II) sulfate (1.0 M); two more aliquots of aqueous copper sulfate and sodium ascorbate were added over 2 days such that their final amounts were 10 and 50 equivalents, respectively, relative to azide. The reactions were performed at 40 °C to achieve the maximum rate possible without inducing potential azide-olefin cycloaddition side reactions.84
Scheme 3.
Synthesis of DOX-nitrobenzyloxycarbonyl (NBOC)-alkyne derivative 9 for click chemistry and photorelease of DOX.
Scheme 4.
CuAAC coupling of 9 to nN3 brush using tris(hydroxypropyltriazolylmethyl)amine (THPTA) ligand, copper(II) sulfate, and sodium ascorbate as an in situ reductant.
Figure 4A (inset) depicts the CuAAC coupling of 9 to 100N3 as monitored by liquid-chromatography mass-spectrometry (LC-MS). Compound 9 eluted at ~4.2 min while DOX-loaded polymer eluted from ~3.5 – ~4 min. The unique absorbance of DOX (~500 nm) enables facile monitoring of the CuAAC reaction; the 100N3 polymer does not absorb at 500 nm and therefore the growth of a new, broad polymer peak is attributed to CuAAC coupling of 9 to the polymer. The time-dependent LC-MS traces (Figure 4A, inset) show an increasing polymer absorbance at the expense of the alkyne 9 absorbance until a final, very high (>97%) conversion is reached. As for the case with chloride-azide exchange, one may expect the CuAAC reaction progress to depend on DP. Figure 4A shows preparatory high-performance liquid chromatography (prep-HPLC) 500 nm absorbance traces for several crude nDOX polymers; the total ratio of nDOX:9 is the same for each DP which again suggests that DP has little effect on the efficiency of reactions to these structures. The retention times for the nDOX polymers increase slightly for each DP indicating that polymer-column interactions are dependent on DP. The pure polymer fractions were collected and either lyophilized to dryness or concentrated before use in subsequent experiments. Figure 4B shows the FTIR spectrum of purified 50DOX compared to that of 50N3. The azide antisymmetric stretch at ~2100 cm−1, present in the spectrum of 50N3, is completely absent from the 50DOX spectrum which suggests very high consumption of the azide group. Integration of the broad aromatic resonances in the 1H NMR spectrum of 50DOX compared to the PEG methylene groups is in close agreement to that expected for fully DOX-functionalized polymer (Figure S5). The final DOX weight percentage for the functionalized polymers is 12.6%. As mentioned above, this value is independent of DP; it could be adjusted by varying the PEG length or the degree of branching in the MM precursor.
Figure 4.
A: Prep-HPLC traces of crude CuAAC mixtures which depict similar conversion for each n value. A (inset): Progress of CuAAC coupling of 9 to 100N3 monitored by LC-MS. B. FTIR spectra of 50N3 and 50DOX confirming complete loss of azide after CuAAC.
Photorelease of DOX and cell culture studies
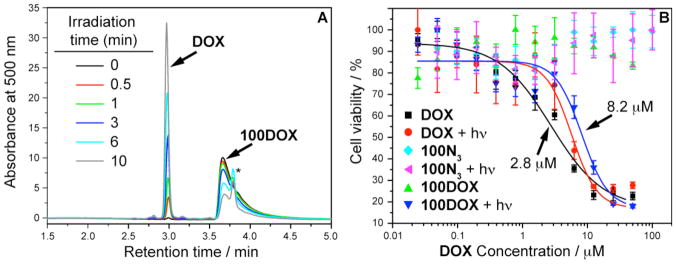
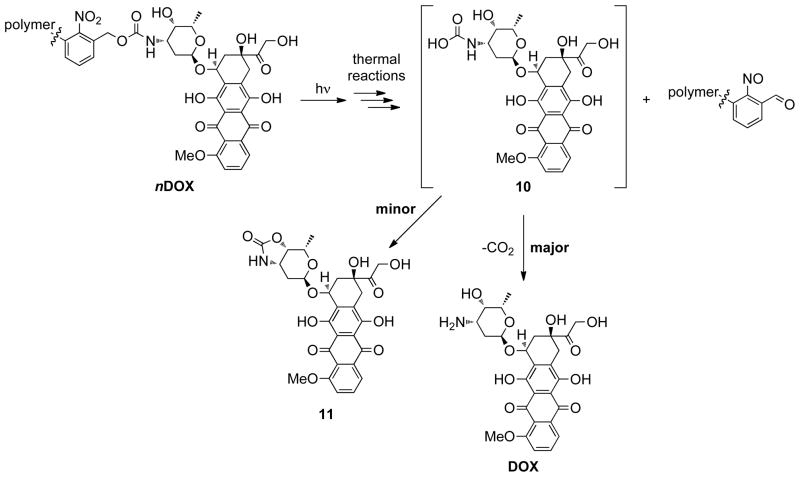
Having successfully coupled 9 to nN3 we next studied the release of free DOX from the brush polymer in response to UV light (~365 nm). An aqueous solution of purified nDOX polymer was irradiated at 365 nm for a given time and subjected to LC-MS analysis. The progress of photorelease was monitored over 10 min by repeated irradiation and LC-MS; the data for 100DOX are shown in Figure 5A. As irradiation time increased we observed a new peak at ~3 min that increased in intensity at the expense of the 100DOX polymer peak. The new peak corresponds to the retention time and mass of free DOX. We also observed a small side product at ~3.6 min with a mass of 569 Da (labelled “*” in Figure 5A), which is likely the result of intramolecular trapping of carbamic acid 10 to give cyclic carbamate 11. Nevertheless, the major product of UV photolysis was free DOX; the photolysis yield after 10 min irradiation was ~70% for all nDOX polymers which led us to examine the effectiveness of these materials against human cancer cells in cell culture.
Figure 5.
A: Time-dependent UV photolysis of 100DOX monitored by LC-MS; after 10 min irradiation ~70% of free DOX was released. A minor product (labelled “*”, see Scheme 5) was also observed at longer irradiation times. B: Viability of MCF-7 human breast cancer cells treated with free DOX, 100N3 bivalent-brush polymer, and drug-loaded 100DOX polymer both with and without UV irradiation. Data points were fit to a sigmoidal function and the half-maximum inhibitory concentrations (IC50) are shown for DOX and the photocleaved 100DOX. The x-axis labels refer to the concentration of both free and polymer-conjugated drug.
We performed cell viability experiments using MCF-7 human breast cancer cells. Cells were treated with aqueous solutions of either free DOX or the corresponding nDOX polymer at various concentrations and irradiated for 10 min using 365 nm light or kept in the dark. The cells were then incubated in the dark for 24 h, washed twice, and incubated for another 24 h in fresh, drug-free growth medium. Cell viability was assessed using the MTT assay (see methods and materials for details). Data for n = 100 polymers (100N3 and 100DOX) are shown in Figure 5B (cell viability data for other n values is given in the supporting information). Free DOX, with and without UV irradiation, effectively killed the cells at 3–5 μM while azide polymer 100N3 was non-toxic with and without light up to 100 μM; these data taken together suggest that under our conditions 10 min of 365 nm UV irradiation has no adverse affect on cell viability nor does it interrupt the cellular toxicity of DOX. None of the nDOX bivalent-brush polymers studied were toxic up to 50 μM in the absence of UV irradiation; irradiation for 10 min yielded IC50 values ~7–10 μM which confirms that free DOX generated by photorelease is therapeutically active. The IC50 values were not DP-dependent. This observation suggests that the nDOX polymers remain in the extracellular environment and upon photolysis free DOX is released and diffuses into the cell nucleus where it induces apopotosis. Though in vitro experiments do not show DP-dependent cytotoxicity, we expect that DP will affect in vivo trafficking and thus the overall utility of these materials in drug delivery. Targeting groups could be appended to the periphery of these materials to promote trafficking into the cell.113
Here we have demonstrated the utility of a combined graft-through/click-to strategy for the synthesis of a new class of clickable, branched nanostructures: PEG-branch-azide bivalent-brush polymers. This approach makes use of the remarkable efficiency of ROMP for graft-through polymerization and the modular coupling power of click chemistry for polymer functionalization. A wide range of nanoscale sizes is accessible which will enable detailed structure/function correlation in biological settings. We are currently exploring applications for these materials in drug delivery. Design and synthesis of novel clickable linkers (e.g. longer wavelength-, pH-, and redox-cleavable), attachment of targeting moieties at the PEG periphery, incorporation of degradable units within the polymer backbone, and use of chain-transfer agents to append orthogonal groups selectively to the ends of these polymers are being studied. Furthermore, copolymerization of MMs like 6 with other monomers (such as a norbornene-alkyl halide small molecule) will provide access to new multiblock polymer structures with the potential for higher drug loadings.
Supplementary Material
Scheme 5.
Photolysis of nDOX polymers gives carbamic acid 10. Decarboxylation of 10 yields free DOX as the major product while intramolecular trapping provides DOX-carbamate derivative 11 as a minor product (Figure 5A, labelled “*”). Reactive nitrosobenzaldehyde photoproducts remain bound to the polymer core after photolysis.
Acknowledgments
We thank Dr. S. Virgil and Mr. S. Presolski for helpful advice. This work was supported by the National Institutes of Health (NIH, R01-GM31332), the Beckman Institute at Caltech (postdoctoral fellowship for J.A.J.), and the MRSEC program of the National Science Foundation (NSF) under award number DMR-0520565.
Footnotes
Supporting information available. Synthetic procedures, spectral data, liquid chromatography methods, details of cell viability studies. This information is available free of charge via the Internet at http://pubc.acs.org/.
References
- 1.Whitesides GM. Small. 2005;1:172–179. doi: 10.1002/smll.200400130. [DOI] [PubMed] [Google Scholar]
- 2.Steigerwald ML, Brus LE. Acc Chem Res. 1990;23:183–188. [Google Scholar]
- 3.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 4.Matsumura Y, Maeda H. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 5.Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Science (Washington, DC, U S) 1994;263:1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 6.Gref R, Domb A, Quellec P, Blunk T, Mueller RH, Verbavatz JM, Langer R. Adv Drug Del Rev. 1995;16:215–233. doi: 10.1016/0169-409X(95)00026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards DA, Hanes J, Caponetti G, Hrkach J, Ben-Jebria A, Eskew ML, Mintzes J, Deaver D, Lotan N, Langer R. Science (Washington, DC, U S) 1997;276:1868–1871. doi: 10.1126/science.276.5320.1868. [DOI] [PubMed] [Google Scholar]
- 8.Langer R. Nature (London) 1998;392:5–10. [PubMed] [Google Scholar]
- 9.Mammen M, Chio S-K, Whitesides GM. Angew Chem, Int Ed. 1998;37:2755–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Qiu LY, Bae YH. Pharm Res. 2006;23:1–30. doi: 10.1007/s11095-005-9046-2. [DOI] [PubMed] [Google Scholar]
- 11.Fox ME, Szoka FC, Frechet JMJ. Acc Chem Res. 2009;42:1141–1151. doi: 10.1021/ar900035f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen B, Jerger K, Frechet JMJ, Szoka FC. J Controlled Release. 2009;140:203–209. doi: 10.1016/j.jconrel.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grayson SM, Godbey WT. J Drug Targeting. 2008;16:329–356. doi: 10.1080/10611860801969616. [DOI] [PubMed] [Google Scholar]
- 14.Duncan R. Nat Rev Drug Discovery. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 15.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Proc Natl Acad Sci U S A. 2007;104:15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu P, Malkoch M, Hunt JN, Vestberg R, Kaltgrad E, Finn MG, Fokin VV, Sharpless KB, Hawker CJ. Chem Commun (Cambridge, U K) 2005:5775–5777. doi: 10.1039/b512021g. [DOI] [PubMed] [Google Scholar]
- 17.Ihre HR, Padilla De Jesus OL, Szoka FC, Jr, Frechet JMJ. Bioconj Chem. 2002;13:443–452. doi: 10.1021/bc010102u. [DOI] [PubMed] [Google Scholar]
- 18.Breitenkamp K, Emrick T. J Am Chem Soc. 2003;125:12070–12071. doi: 10.1021/ja036561i. [DOI] [PubMed] [Google Scholar]
- 19.Becker ML, Remsen EE, Pan D, Wooley KL. Bioconj Chem. 2004;15:699–709. doi: 10.1021/bc049946e. [DOI] [PubMed] [Google Scholar]
- 20.Such GK, Tjipto E, Postma A, Johnston APR, Caruso F. Nano Lett. 2007;7:1706–1710. doi: 10.1021/nl070698f. [DOI] [PubMed] [Google Scholar]
- 21.Cengelli F, Grzyb JA, Montoro A, Hofmann H, Hanessian S, Juillerat-Jeanneret L. ChemMedChem. 2009;4:988–997. doi: 10.1002/cmdc.200800424. [DOI] [PubMed] [Google Scholar]
- 22.Tong GJ, Hsiao SC, Carrico ZM, Francis MB. J Am Chem Soc. 2009;131:11174–11178. doi: 10.1021/ja903857f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee S-M, Chen H, O’Halloran TV, Nguyen ST. J Am Chem Soc. 2009;131:9311–9320. doi: 10.1021/ja9017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J-S, Green JJ, Love KT, Sunshine J, Langer R, Anderson DG. Nano Lett. 2009;9:2402–2406. doi: 10.1021/nl9009793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen Y, Jin E, Zhang B, Murphy CJ, Sui M, Zhao J, Wang J, Tang J, Fan M, Van Kirk E, Murdoch WJ. J Am Chem Soc. 2010;132:4259–4265. doi: 10.1021/ja909475m. [DOI] [PubMed] [Google Scholar]
- 26.Helms B, Meijer EW. Science (Washington, DC, U S) 2006;313:929–930. doi: 10.1126/science.1130639. [DOI] [PubMed] [Google Scholar]
- 27.Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P. Polym J (Tokyo, Jpn) 1985;17:117–132. [Google Scholar]
- 28.Bosman AW, Janssen HM, Meijer EW. Chem Rev (Washington, DC, U S) 1999;99:1665–1688. doi: 10.1021/cr970069y. [DOI] [PubMed] [Google Scholar]
- 29.Montanez MI, Campos LM, Antoni P, Hed Y, Walter MV, Krull BT, Khan A, Hult A, Hawker CJ, Malkoch M. Macromolecules. 2010;43:6004–6013. [Google Scholar]
- 30.Antoni P, Robb MJ, Campos L, Montanez M, Hult A, Malmstrom E, Malkoch M, Hawker CJ. Macromolecules. 2010;43:6625–6631. [Google Scholar]
- 31.Killops KL, Campos LM, Hawker CJ. J Am Chem Soc. 2008;130:5062–5064. doi: 10.1021/ja8006325. [DOI] [PubMed] [Google Scholar]
- 32.Wu P, Feldman AK, Nugent AK, Hawker CJ, Scheel A, Voit B, Pyun J, Frechet JMJ, Sharpless KB, Fokin VV. Angew Chem, Int Ed. 2004;43:3928–3932. doi: 10.1002/anie.200454078. [DOI] [PubMed] [Google Scholar]
- 33.Lee CC, Gillies ER, Fox ME, Guillaudeu SJ, Frechet JMJ, Dy EE, Szoka FC. Proc Natl Acad Sci U S A. 2006;103:16649–16654. doi: 10.1073/pnas.0607705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gillies ER, Dy E, Frechet JMJ, Szoka FC. Mol Pharm. 2005;2:129–138. doi: 10.1021/mp049886u. [DOI] [PubMed] [Google Scholar]
- 35.Gillies ER, Frechet JMJ. J Org Chem. 2004;69:46–53. doi: 10.1021/jo035329s. [DOI] [PubMed] [Google Scholar]
- 36.Gillies ER, Frechet JMJ. J Am Chem Soc. 2002;124:14137–14146. doi: 10.1021/ja028100n. [DOI] [PubMed] [Google Scholar]
- 37.Grayson SM, Frechet JMJ. Macromolecules. 2001;34:6542–6544. [Google Scholar]
- 38.Boydston AJ, Holcombe TW, Unruh DA, Frechet JMJ, Grubbs RH. J Am Chem Soc. 2009;131:5388–5389. doi: 10.1021/ja901658c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang A, Zhang B, Waechtersbach E, Schmidt M, Schlueter AD. Chem--Eur J. 2003;9:6083–6092. doi: 10.1002/chem.200305142. [DOI] [PubMed] [Google Scholar]
- 40.Lee CC, Grayson SM, Frechet JMJ. J Polym Sci, Part A Polym Chem. 2004;42:3563–3578. [Google Scholar]
- 41.Yoshida M, Fresco ZM, Ohnishi S, Frechet JMJ. Macromolecules. 2005;38:334–344. [Google Scholar]
- 42.Canilho N, Kaseemi E, Mezzenga R, Schlueter AD. J Am Chem Soc. 2006;128:13998–13999. doi: 10.1021/ja065113i. [DOI] [PubMed] [Google Scholar]
- 43.Helms B, Mynar JL, Hawker CJ, Frechet JMJ. J Am Chem Soc. 2004;126:15020–15021. doi: 10.1021/ja044744e. [DOI] [PubMed] [Google Scholar]
- 44.Zhang M, Mueller AHE. J Polym Sci, Part A: Polym Chem. 2005;43:3461–3481. [Google Scholar]
- 45.Jiang X, Lok MC, Hennink WE. Bioconj Chem. 2007;18:2077–2084. doi: 10.1021/bc0701186. [DOI] [PubMed] [Google Scholar]
- 46.Tsarevsky NV, Bencherif SA, Matyjaszewski K. Macromolecules. 2007;40:4439–4445. [Google Scholar]
- 47.Lutz J-F, Boerner HG, Weichenhan K. Macromolecules. 2006;39:6376–6383. [Google Scholar]
- 48.Allen MJ, Wangkanont K, Raines RT, Kiessling LL. Macromolecules. 2009;42:4023–4027. doi: 10.1021/ma900056b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng G, Boeker A, Zhang M, Krausch G, Mueller AHE. Macromolecules. 2001;34:6883–6888. [Google Scholar]
- 50.Lu H, Wang J, Lin Y, Cheng J. J Am Chem Soc. 2009;131:13582–13583. doi: 10.1021/ja903425x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morandi G, Pascual S, Montembault V, Legoupy S, Delorme N, Fontaine L. Macromolecules. 2009;42:6927–6931. [Google Scholar]
- 52.Neugebauer D, Sumerlin BS, Matyjaszewski K, Goodhart B, Sheiko SS. Polymer. 2004;45:8173–8179. [Google Scholar]
- 53.Sumerlin BS, Neugebauer D, Matyjaszewski K. Macromolecules. 2005;38:702–708. [Google Scholar]
- 54.Gao H, Matyjaszewski K. J Am Chem Soc. 2007;129:6633–6639. doi: 10.1021/ja0711617. [DOI] [PubMed] [Google Scholar]
- 55.Hadjichristidis N, Pitsikalis M, Iatrou H, Pispas S. Macromol Rapid Commun. 2003;24:979–1013. [Google Scholar]
- 56.Tsukahara Y, Mizuno K, Segawa A, Yamashita Y. Macromolecules. 1989;22:1546–1552. [Google Scholar]
- 57.Tsukahara Y, Tsutsumi K, Yamashita Y, Shimada S. Macromolecules. 1990;23:5201–5208. [Google Scholar]
- 58.Dziezok P, Sheiko SS, Fischer K, Schmidt M, Moller M. Angew Chem, Int Ed. 1998;36:2812–2815. [Google Scholar]
- 59.Neiser MW, Okuda J, Schmidt M. Macromolecules. 2003;36:5437–5439. [Google Scholar]
- 60.Neiser MW, Muth S, Kolb U, Harris JR, Okuda J, Schmidt M. Angew Chem, Int Ed. 2004;43:3192–3195. doi: 10.1002/anie.200353259. [DOI] [PubMed] [Google Scholar]
- 61.Yuan Y-Y, Du Q, Wang Y-C, Wang J. Macromolecules. 2010;43:1739–1746. [Google Scholar]
- 62.Li C, Ge Z, Fang J, Liu S. Macromolecules. 2009;42:2916–2924. [Google Scholar]
- 63.Li A, Lu Z, Zhou Q, Qiu F, Yang Y. J Polym Sci, Part A Polym Chem. 2006;44:3942–3946. [Google Scholar]
- 64.Kolb HC, Finn MG, Sharpless KB. Angew Chem, Int Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 65.Tornoe CW, Christensen C, Meldal M. J Org Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 66.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem, Int Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 67.Sumerlin BS, Tsarevsky NV, Louche G, Lee RY, Matyjaszewski K. Macromolecules. 2005;38:7540–7545. [Google Scholar]
- 68.Gao H, Matyjaszewski K. J Am Chem Soc. 2007;129:6633–6639. doi: 10.1021/ja0711617. [DOI] [PubMed] [Google Scholar]
- 69.Tsarevsky NV, Bernaerts KV, Dufour B, Du Prez FE, Matyjaszewski K. Macromolecules. 2004;37:9308–9313. [Google Scholar]
- 70.Campos LM, Killops KL, Sakai R, Paulusse JMJ, Damiron D, Drockenmuller E, Messmore BW, Hawker CJ. Macromolecules. 2008;41:7063–7070. [Google Scholar]
- 71.Boerner HG, Beers K, Matyjaszewski K, Sheiko SS, Moeller M. Macromolecules. 2001;34:4375–4383. [Google Scholar]
- 72.Golas PL, Matyjaszewski K. Chem Soc Rev. 2010;39:1338–1354. doi: 10.1039/b901978m. [DOI] [PubMed] [Google Scholar]
- 73.Iha RK, Wooley KL, Nystrom AM, Burke DJ, Kade MJ, Hawker CJ. Chem Rev (Washington, DC, U S) 2009;109:5620–5686. doi: 10.1021/cr900138t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hawker CJ, Wooley KL. Science (Washington, DC, U S) 2005;309:1200–1205. doi: 10.1126/science.1109778. [DOI] [PubMed] [Google Scholar]
- 75.Johnson JA, Finn MG, Koberstein JT, Turro NJ. Macromol Rapid Commun. 2008;29:1052–1072. [Google Scholar]
- 76.Binder WH, Sachsenhofer R. Macromol Rapid Commun. 2007;28:15–54. [Google Scholar]
- 77.Hawker CJ, Fokin VV, Finn MG, Sharpless KB. Aust J Chem. 2007;60:381–383. [Google Scholar]
- 78.Xia Y, Kornfield JA, Grubbs RH. Macromolecules. 2009;42:3761–3766. [Google Scholar]
- 79.Xia Y, Olsen BD, Kornfield JA, Grubbs RH. J Am Chem Soc. 2009;131:18525–18532. doi: 10.1021/ja908379q. [DOI] [PubMed] [Google Scholar]
- 80.Li Z, Ma J, Cheng C, Zhang K, Wooley KL. Macromolecules. 2010;43:1182–1184. [Google Scholar]
- 81.Li Z, Zhang K, Ma J, Cheng C, Wooley KL. J Polym Sci, Part A: Polym Chem. 2009;47:5557–5563. doi: 10.1002/pola.23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Conrad RM, Grubbs RH. Angew Chem, Int Ed. 2009;48:8328–8330. doi: 10.1002/anie.200903888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Le D, Montembault V, Soutif JC, Rutnakornpituk M, Fontaine L. Macromolecules. 2010;43:5611–5617. [Google Scholar]
- 84.Binder WH, Kluger C. Macromolecules. 2004;37:9321–9330. [Google Scholar]
- 85.Binder WH, Kluger C, Josipovic M, Straif CJ, Friedbacher G. Macromolecules. 2006;39:8092–8101. [Google Scholar]
- 86.Clark PG, Guidry EN, Chan WY, Steinmetz WE, Grubbs RH. J Am Chem Soc. 2010;132:3405–3412. doi: 10.1021/ja9090337. [DOI] [PubMed] [Google Scholar]
- 87.Otsuka H, Nagasaki Y, Kataoka K. Adv Drug Delivery Rev. 2003;55:403–419. doi: 10.1016/s0169-409x(02)00226-0. [DOI] [PubMed] [Google Scholar]
- 88.O’Reilly RK, Joralemon MJ, Hawker CJ, Wooley KL. New J Chem. 2007;31:718–724. [Google Scholar]
- 89.O’Reilly RK, Joralemon MJ, Hawker CJ, Wooley KL. Chem--Eur J. 2006;12:6776–6786. doi: 10.1002/chem.200600467. [DOI] [PubMed] [Google Scholar]
- 90.O’Reilly RK, Joralemon MJ, Wooley KL, Hawker CJ. Chem Mater. 2005;17:5976–5988. [Google Scholar]
- 91.Joralemon MJ, O’Reilly RK, Hawker CJ, Wooley KL. J Am Chem Soc. 2005;127:16892–16899. doi: 10.1021/ja053919x. [DOI] [PubMed] [Google Scholar]
- 92.Prasuhn DE, Jr, Singh P, Strable E, Brown S, Manchester M, Finn MG. J Am Chem Soc. 2008;130:1328–1334. doi: 10.1021/ja075937f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dolmans DEJGJ, Fukumura D, Jain RK. Nat Rev Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 94.Bethea D, Fullmer B, Syed S, Seltzer G, Tiano J, Rischko C, Gillespie L, Brown D, Gasparro FP. J Dermatol Sci. 1999;19:78–88. doi: 10.1016/s0923-1811(98)00064-4. [DOI] [PubMed] [Google Scholar]
- 95.Mal NK, Fujiwara M, Tanaka Y. Nature (London, U K) 2003;421:350–353. doi: 10.1038/nature01362. [DOI] [PubMed] [Google Scholar]
- 96.Skwarczynski M, Noguchi M, Hirota S, Sohma Y, Kimura T, Hayashi Y, Kiso Y. Bioorg Med Chem Lett. 2006;16:4492–4496. doi: 10.1016/j.bmcl.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 97.McCoy CP, Rooney C, Edwards CR, Jones DS, Gorman SP. J Am Chem Soc. 2007;129:9572–9573. doi: 10.1021/ja073053q. [DOI] [PubMed] [Google Scholar]
- 98.Jiang MY, Dolphin D. J Am Chem Soc. 2008;130:4236–4237. doi: 10.1021/ja800140g. [DOI] [PubMed] [Google Scholar]
- 99.Agasti SS, Chompoosor A, You C-C, Ghosh P, Kim CK, Rotello VM. J Am Chem Soc. 2009;131:5728–5729. doi: 10.1021/ja900591t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee H-i, Wu W, Oh JK, Mueller L, Sherwood G, Peteanu L, Kowalewski T, Matyjaszewski K. Angew Chem, Int Ed. 2007;46:2453–2457. doi: 10.1002/anie.200604278. [DOI] [PubMed] [Google Scholar]
- 101.Pastine SJ, Okawa D, Zettl A, Frechet JMJ. J Am Chem Soc. 2009;131:13586–13587. doi: 10.1021/ja905378v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim H-C, Hartner S, Behe M, Behr Thomas M, Hampp Norbert A. J Biomed Opt. 2006;11:34024. doi: 10.1117/1.2209564. [DOI] [PubMed] [Google Scholar]
- 103.Haertner S, Kim H-C, Hampp N. J Polym Sci, Part A Polym Chem. 2007;45:2443–2452. [Google Scholar]
- 104.Lewis WG, Magallon FG, Fokin VV, Finn MG. J Am Chem Soc. 2004;126:9152–9153. doi: 10.1021/ja048425z. [DOI] [PubMed] [Google Scholar]
- 105.Rodionov VO, Fokin VV, Finn MG. Angew Chem, Int Ed. 2005;44:2210–2215. doi: 10.1002/anie.200461496. [DOI] [PubMed] [Google Scholar]
- 106.Rodionov VO, Presolski SI, Diaz DD, Fokin VV, Finn MG. J Am Chem Soc. 2007;129:12705–12712. doi: 10.1021/ja072679d. [DOI] [PubMed] [Google Scholar]
- 107.Rodionov VO, Presolski SI, Gardinier S, Lim Y-H, Finn MG. J Am Chem Soc. 2007;129:12696–12704. doi: 10.1021/ja072678l. [DOI] [PubMed] [Google Scholar]
- 108.Ngo JT, Champion JA, Mahdavi A, Tanrikulu IC, Beatty KE, Connor RE, Yoo TH, Dieterich DC, Schuman EM, Tirrell DA. Nat Chem Biol. 2009;5:715–717. doi: 10.1038/nchembio.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dieterich DC, Link AJ, Graumann J, Tirrell DA, Schuman EM. Proc Natl Acad Sci U S A. 2006;103:9482–9487. doi: 10.1073/pnas.0601637103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Speers AE, Cravatt BF. Chem Biol. 2004;11:535–546. doi: 10.1016/j.chembiol.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 111.Prescher JA, Bertozzi CR. Nat Chem Biol. 2005;1:13–21. doi: 10.1038/nchembio0605-13. [DOI] [PubMed] [Google Scholar]
- 112.Hong V, Presolski SI, Ma C, Finn MG. Angew Chem, Int Ed. 2009;48:9879–9883. doi: 10.1002/anie.200905087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kolonko EM, Kiessling LL. J Am Chem Soc. 2008;130:5626–5627. doi: 10.1021/ja8001716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.